
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med., 07 September 2022
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.942751
 Kent Carpenter1
Kent Carpenter1 Ali Etemady-Deylamy1
Ali Etemady-Deylamy1 Victoria Costello1
Victoria Costello1 Mohammad Khasawneh1
Mohammad Khasawneh1 Robin Chamberland2,3
Robin Chamberland2,3 Katherine Tian1
Katherine Tian1 Maureen Donlin4
Maureen Donlin4 Brenda Moreira-Walsh4
Brenda Moreira-Walsh4 Emily Reisenbichler3
Emily Reisenbichler3 Getahun Abate1*
Getahun Abate1*Being introduced in 2010, fingolimod was among the first oral therapies for relapsing multiple sclerosis (MS). Since that time, postmarketing surveillance has noted several case reports of various cryptococcal infections associated with fingolimod use. To date, approximately 15 such case reports have been published. We present the first and unique case of cryptococcal chest wall mass and rib osteomyelitis associated with fingolimod use. The patient presented with left-side chest pain and was found to have a lower left chest wall mass. Computerized tomography (CT) showed chest wall mass with the destruction of left 7th rib. Aspirate from the mass grew Cryptococcus neoformans. The isolate was serotype A. Fingolimod was stopped. The patient received liposomal amphotericin B for 2 weeks and started on fluconazole with a plan to continue for 6–12 months. The follow-up CT in 6 weeks showed a marked decrease in the size of the chest wall mass. In conclusion, our case highlights the atypical and aggressive form of cryptococcal infection possibly related to immunosuppression from fingolimod use.
Cryptococcus neoformans is a common, encapsulated yeast from the Tremellomycetes class, known to commonly cause infections in immunosuppressed patients and rarely in immunocompetent patients (1). In immunocompetent patients, cryptococcal infection is often confined to the lungs that is commonly the primary infection site (2). This is mainly because of a robust Th1 immune response that is capable of controlling C. neoformans infection (2). In contrast, in immunocompromised patients, C. neoformans can cause severe disease with high mortality (3, 4). C. neoformans has capsular polysaccharides located externally to the cell wall with the flexibility to alter the composition, thereby altering antigenic properties and evading the immune response (5). The capsule is a virulence factor with immunomodulatory effects, affecting macrophages, neutrophils, and T cells. The capsule helps C. neoformans avoid phagocytosis, survive better in macrophages, and also induce widespread immunosuppression (6–10).
The most common presentations of cryptococcal infections include central nervous system (CNS) manifestations (meningoencephalitis), followed by pulmonary, and cutaneous manifestations (11). The increasing availability of fluconazole as well as the advent of highly active antiretroviral therapy for human immunodeficiency virus (HIV)-infected patients have decreased the incidence of cryptococcal infections.
While immunosuppression due to HIV is most classically associated with cases of cryptococcal infection, medication-induced immunosuppression is an increasingly recognized etiology of various types of cryptococcal infections (12). The immunomodulatory agent used to treat relapsed-remitting multiple sclerosis (MS), fingolimod, has been associated with at least 12 case reports to date of different types of cryptococcal infections, including primary meningoencephalitis (13–17) and primary cutaneous cryptococcosis (18–21).
We report a case of biopsy and culture-proven cryptococcal chest wall mass with pathologic fracture of rib in a patient on long-term fingolimod therapy for relapsed/remitting MS. Our case report represents a unique presentation of cryptococcal infection and highlights the importance of medication-induced cryptococcal infections and their subsequent management.
A 46-year-old female patient with medical history of relapsing-remitting MS who has been on fingolimod for more than 12 years was admitted on March 28 for left-side chest pain since the end of January. The pain was a dull aching type, becoming worse with deep breathing and touching. The timeline of her illness and workup is summarized in Figure 1. MS was diagnosed in May 2005 after presenting with numbness below the waist, loss of sensation in both of her feet, recurrent falls, and balance issues. At that time, she was treated with intravenous methylprednisolone for the initial flare, and a glatiramer daily injection was started after the initial flare. Her symptoms subsequently resolved on this therapy. She did not have any additional flares until the end of 2009 when she presented with problems of walking and lower extremity weakness. She was hospitalized and treated with methylprednisolone for an acute MS flare. At that point, she was considered to have relapsing, remitting MS. When she followed with neurology again in early 2010, her MS therapy was switched from glatiramer to fingolimod. Since that time, she has not had another MS flare and continued to tolerate fingolimod well. In January 2022, she developed cough and chest pain and was diagnosed to have COVID-19. On February 6, she was seen at urgent care for left-side chest pain. A chest X-ray was obtained and she was told that she had pneumonia. She received a single dose of dexamethasone and an oral antibiotic. Her cough resolved in about 2 weeks. However, 3 weeks after the onset of illness, she was seen by her primary care physician for worsening left lower anterior chest pain. A chest X-ray obtained at that time showed a lytic lesion of the left 7th rib with no acute pulmonary process (Figure 2A). On February 24, computerized tomography (CT) of chest was obtained and showed left chest wall mass with an erosion of left 7th rib subtle pathologic fracture. On March 17, ultrasound-guided aspiration pus and core biopsy of left chest wall mass were obtained. Gram stain and AFB smears were negative but Grocott's Methenamine Silver (GMS) stain showed “yeast forms” (Figure 3). Aerobic bacterial cultures and fungal cultures grew C. neoformans. On March 28, culture results were released and the patient was admitted to hospital for further management.
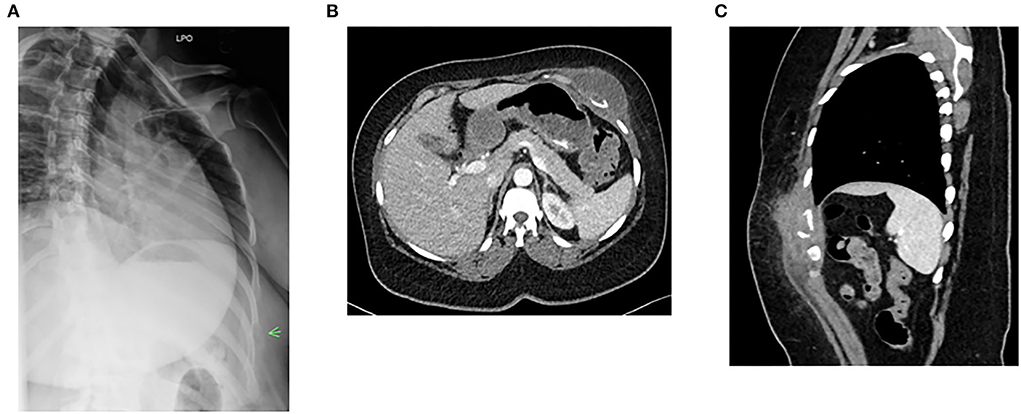
Figure 2. Imaging findings of chest wall mass and rib osteomyelitis. (A) Chest X-ray shows destruction of a rib. (B) Left chest wall mass (axial image). (C) Left chest wall mass (coronal view). Green arrow indicates the level of 7th rib fracture and mass.
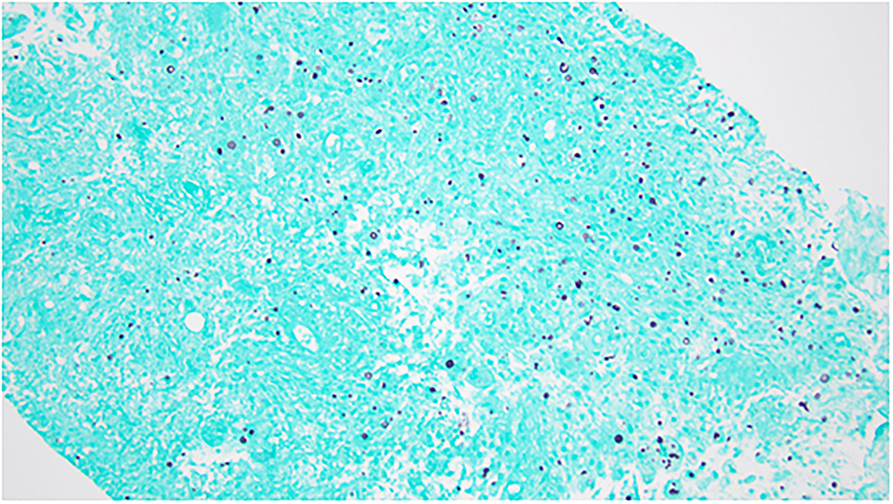
Figure 3. Grocott's Methenamine Silver (GMS) stain of aspirate showing yeast forms. Objective: 200×.
On admission, her home medication list did not include disease-modifying agents other than fingolimod. The patient also denied receiving steroids in the past at least 10 years other than a single dexamethasone injection she received 7 weeks prior to hospital admission. Her physical examination showed normal vital signs and a violaceous left anterior chest wall mass (approximately 5.5 cm length × 1.5 cm width) above the left 7th rib. The mass was soft and tender to palpation. CT of chest and abdomen showed left chest wall mass and destruction of 7th rib (Figures 2B,C). The patient was diagnosed to have cryptococcal chest wall mass with rib osteomyelitis and pathologic fracture. Further workup showed that HIV antigen and antibody test was negative and serum cryptococcal antigen titer was 1:80. Complete blood counts showed a total lymphocyte count of 300/μl (normal range: 1,100–3,900/μl) and complete metabolic panel was normal. Magnetic resonance imaging (MRI) of the brain showed previously known MS changes. This was followed by a lumbar puncture. The opening pressure was normal. Cerebrospinal fluid (CSF) cell counts, protein, and glucose were normal. CSF cryptococcal antigen and fungal culture were negative. As chest wall mass from C. neformans is very rare, the isolate was genotyped using the methods described previously and compared with other isolates (22). Supplementary Figure 1 performed shows that the isolate from our patient was serogroup A.
The patient was evaluated by a thoracic surgeon and interventional radiologist for possible debridement or placement of a drainage tube. There was no drainable residual abscess and extensive surgery was deemed unnecessary at this time. On admission to the hospital, fingolimod was discontinued. Due to the lack of literature on the management of extensive soft tissue and bone cryptococcal infection associated with fingolimod, we decided to start with the initial intensive treatment. The patient received 2 weeks of intravenous liposomal amphotericin B and flucytosine, followed by oral fluconazole with a plan to continue for 6–12 months. The patient has been adherent to medication. At 6 weeks of follow-up, chest pain has resolved and a follow-up CT showed a marked decrease in the size of chest wall mass.
Disease-modifying agents, including fingolimod, are used frequently and have been shown to reduce long-term disability in patients with relapsing-remitting MS. However, these agents are associated with a significant risk for opportunistic infections, including cryptococcosis with an incidence rate of about 9 per 100,000 person years (23). To date, we have noted 18 case reports of cryptococcal infections associated with the use of fingolimod, recently reviewed by Ma et al. (15) and at least four other new reports (19, 21, 24–26). These cases have described primary CNS cryptococcosis, primary cutaneous cryptococcosis, pulmonary cryptococcosis, and disseminated cryptococcosis (19, 21, 24–27). Among the 18 cases reported, 6 had subacute to chronic skin and/or soft tissue lesions (Table 1). The skin and soft tissue cryptococcal lesions reported in association with fingolimod include erythematous nodule from disseminated cryptococcus (29), ulcerative lesion from disseminated cryptococcosis (30), primary tender nodule (20), and primary non-healing ulcerated lesions (19, 21, 28). Our case is the first report of a cryptococcal chest wall mass with rib osteomyelitis following the use of fingolimod. Inflammatory pseudotumor responses have been reported in HIV-infected patients with disseminated cryptococcosis (31). Our case is HIV-negative and does not have the evidence of dissemination. The presence of a small cavitary pulmonary nodule in the left lower lobe raises the concern of direct extension to the chest wall, forming empyema necessitans.
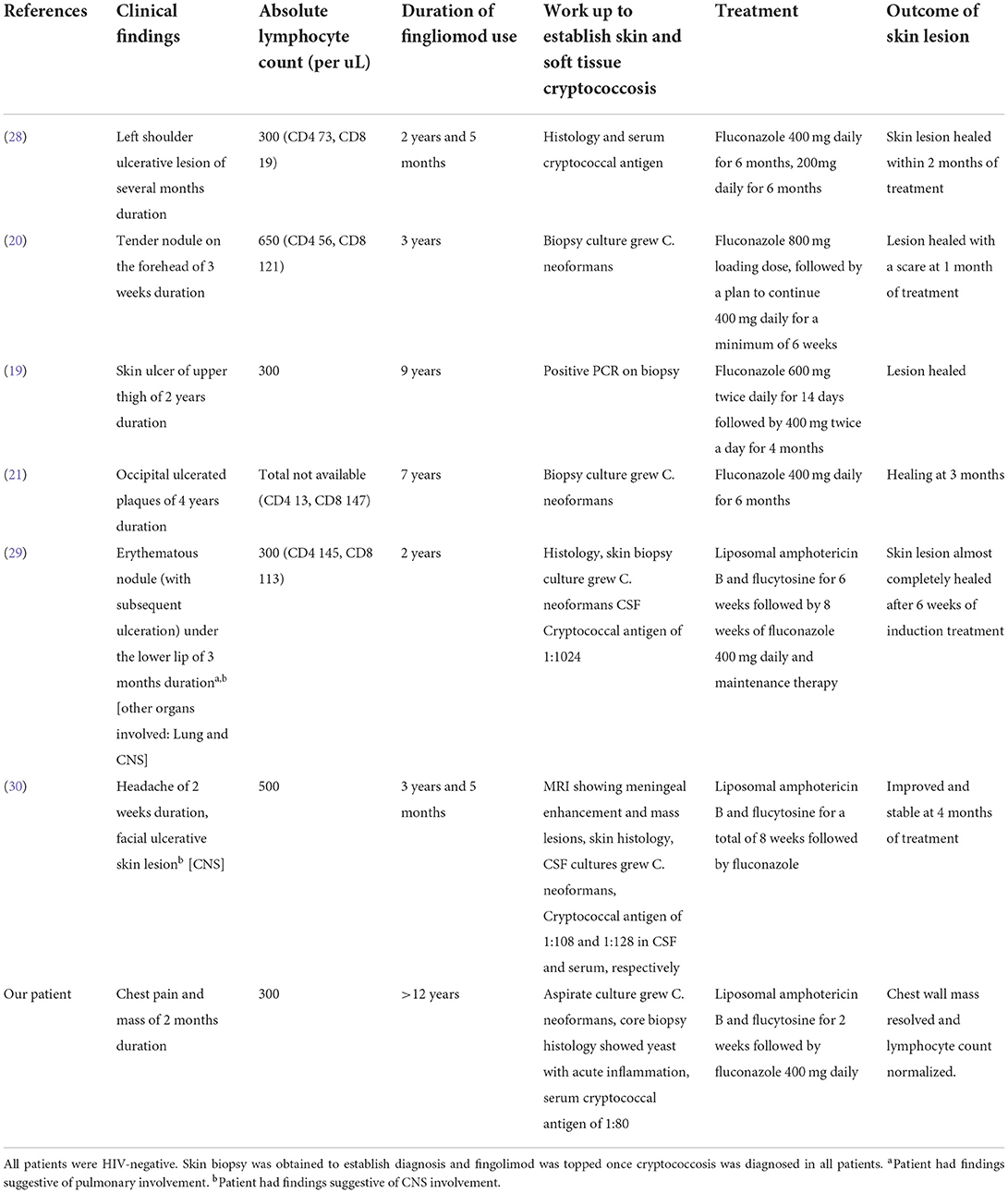
Table 1. Clinical characteristics, treatment, and outcome of patients with cryptococcal skin and/or soft tissue infection associated with the use of fingolimod.
The exact mechanism of fingolimod's effects on the risk of acquisition of cryptococcal infection remains unclear. One review suggested C. neoformans' unique ability to establish latent infection and evade immune escape mechanisms, combined with fingolimod's suppressive effects on multiple lines of the immune system needs further investigation (32). Another study in the murine model hypothesized that the reactivation of cryptococcal granulomas following the administration of fingolimod could be due to multiple mechanisms including profound CD4 and CD8 T-cells depletion, decreased macrophage phagocytosis, and decreased production of reactive oxygen species by macrophages (33).
A phase 3 clinical trial on fingolimod and a study after its introduction for clinical use showed that fingolimod causes lymphopenia in 8–13% of patients (34, 35), mostly within the 1st year of use (36). This is not surprising because fingolimod, a sphingosine-1-phosphate receptor (S1PR) modulator, works by reducing the recirculation of C-C chemokine receptor type 7+ (CCR7+) lymphocytes (37) and this may have relevance for protection against cryptococcal infection. Fingolimod does not affect CCR7+ T cells that include peripheral effector memory T cells and had no marked effect on T cells' ability to produce IFN-γ (37) and, therefore, the lymphopenia may not be severe enough to increase the risk of latent tuberculosis infection (LTBI) or affect the results of IFN-γ-based tests for LTBI (38). The effect of fingolimod on lymphocyte counts appears to be reversible but may take several months after holding the drug (39). As red blood cells (RBC) are the main regulators of serum sphingosine-1 phosphate concentration, a decrease in the number of RBCs may affect the severity of fingolimod-associated lymphopenia and reversal of counts (40).
It is believed that cryptococcal isolates vary in their virulence and tissue predilection. For instance, capsule-deficient Cryptococci cause a focal or dispersed granulomatous inflammatory reaction with areas of necrosis and minimal suppuration (41). The C. neoformans isolated from our patient was mucicarmine positive and, therefore, unlikely to be capsule deficient. Furthermore, genotyping study showed that the cryptococcal isolate was serogroup A (Supplementary Figure 1).
Due to the rarity of the cases of isolated cryptococcal infection associated with fingolimod use, no definitive treatment guidelines or consensus exist aside from those proposed in various case reports. We elected for a more aggressive treatment approach due to pulmonary and skeletal involvement and patient's immunocompromised state.
Due to increasing reports of fingolimod-associated cryptococcal infections, we believe it is important for MS and other neurological care providers to be aware of the risks associated with such immunomodulatory therapies. The features of our case and previously reported cryptococcosis highlight the need for future studies on the immunosuppressive effects of fingolimod and the exact mechanisms of how it increases the risk for opportunistic infections including cryptococcosis. Our case further highlights the need for a multidisciplinary team including internists or infectious disease specialists in conjunction with neurologists, thoracic surgeons, and interventional radiologists as the best approach to managing complex cases such as we have presented.
The strengths of this article include (i) description of a unique case of soft tissue and bone cryptococcal infection in a patient who was on fingolimod, (ii) presentation of follow-up data on clinical and radiological responses, while a patient is on anti-fungal treatment, and (iii) review of the literature with a focus on soft tissue cryptococcal infection and fingolimod. The limitation of this article includes difficulty in establishing a definite causal association between fingolimod and cryptococcal infection. However, the timeline and absence of other risk factors suggest that fingolimod is the likely risk for cryptococcal infection in our patient.
We reported an atypical and aggressive form of cryptococcal infection in a patient who presented with left-side chest pain and left chest wall mass possibly related to immunosuppression from fingolimod use. Discontinuing fingolimod and antifungal treatment led to clinical improvement and marked a decrease in the size of chest wall mass.
The patient is happy with the care she is receiving. Her chest pain resolved but developed generalized itching after the initiation of fluconazole. On 6 weeks of follow-up visit, she agreed with the change of treatment to itraconazole.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
The patient provided written informed consent for performing genotyping studies on cryptococcal isolate and publishing all relevant clinical results.
KC, MK, VC, and KT prepared a draft manuscript. GA, AE-D, RC, MD, BM-W, and ER revised the manuscript. MD and BM-W performed genotyping of cryptococcal isolate.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.942751/full#supplementary-material
1. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Sorrell, Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. (2010) 50:291–322. doi: 10.1086/649858
2. Decote-Ricardo D, LaRocque-de-Freitas IF, Rocha JDB, Nascimento DO, Nunes MP, et al. Immunomodulatory role of capsular polysaccharides constituents of cryptococcus neoformans. Front Med. (2019) 6:129. doi: 10.3389/fmed.2019.00129
3. Diniz-Lima IF, Silva LM, Guimarães-de-Oliveira EB, Freire-de-Lima JC, Nascimento L, Morrot DO, et al. Cryptococcus: history, epidemiology, and immune evasion. App Sci. (2022) 12:7086. doi: 10.3390/app12147086
4. Hurtado JC, Castillo P, Fernandes F, Navarro M, Lovane L, Casas I, et al. Mortality due to cryptococcus neoformans and cryptococcus gattii in low-income settings: an autopsy study. Sci Rep. (2019) 9:7493. doi: 10.1038/s41598-019-43941-w
5. McFadden DC, Fries BC, Wang F, Casadevall A. Capsule structural heterogeneity and antigenic variation in cryptococcus neoformans. Eukaryot Cell. (2007) 6:1464–73. doi: 10.1128/EC.00162-07
6. Rocha JD, Nascimento MT, Decote-Ricardo D, Corte-Real S, Morrot A, Heise N, et al. Capsular polysaccharides from cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep. (2015) 5:8008. doi: 10.1038/srep08008
7. Ma H, May RC. Virulence in cryptococcus species. Adv Appl Microbiol. (2009) 67:131–90. doi: 10.1016/S0065-2164(08)01005-8
8. Vecchiarelli A, Pericolini E, Gabrielli E, Chow SK, Bistoni F, Cenci E, et al. Cryptococcus neoformans galactoxylomannan is a potent negative immunomodulator, inspiring new approaches in anti-inflammatory immunotherapy. Immunotherapy. (2011) 3:997–1005. doi: 10.2217/imt.11.86
9. Johnston SA, May RC. Cryptococcus interactions with macrophages: evasion and manipulation of the phagosome by a fungal pathogen. Cell Microbiol. (2013) 15:403–11. doi: 10.1111/cmi.12067
10. Zaragoza O, Taborda CP, Casadevall A. The efficacy of complement-mediated phagocytosis of cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol. (2003) 33:1957–67. doi: 10.1002/eji.200323848
11. Zavala S, Baddley JW. Cryptococcosis. Semin Respir Crit Care Med. (2020) 41:69–79. doi: 10.1055/s-0039-3400280
12. Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, et al. Dismukes, Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. (2001) 33:690–9. doi: 10.1086/322597
13. Wienemann T, Muller AK, MacKenzie C, Bielor C, Weyers V, Aktas O, et al. Cryptococcal meningoencephalitis in an IgG2-deficient patient with multiple sclerosis on fingolimod therapy for more than five years - case report. BMC Neurol. (2020) 20:158. doi: 10.1186/s12883-020-01741-0
14. Ward MD, Jones DE, Goldman MD. Cryptococcal meningitis after fingolimod discontinuation in a patient with multiple sclerosis. Mult Scler Relat Disord. (2016) 9:47–9. doi: 10.1016/j.msard.2016.06.007
15. Ma SB, Griffin D, Boyd SC, Chang CC, Wong J, Guy SD. Cryptococcus neoformans var grubii meningoencephalitis in a patient on fingolimod for relapsing-remitting multiple sclerosis: case report and review of published cases. Mult Scler Relat Disord. (2020) 39:101923. doi: 10.1016/j.msard.2019.101923
16. Chong I, Wang KY, Lincoln CM. Cryptococcal meningitis in a multiple sclerosis patient treated with Fingolimod: a case report and review of imaging findings. Clin Imaging. (2019) 54:53–6. doi: 10.1016/j.clinimag.2018.11.005
17. Achtnichts L, Obreja O, Conen A, Fux CA, Nedeltchev K. Cryptococcal meningoencephalitis in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol. (2015) 72:1203–5. doi: 10.1001/jamaneurol.2015.1746
18. Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. (2003) 41:177–88. doi: 10.1080/1369378031000137224
19. Dahshan D, Dessie SA, Cuda J, Khalil E. primary cutaneous cryptococcosis in a patient on fingolimod: a case report. Cureus. (2021) 13:e16444. doi: 10.7759/cureus.16444
20. Forrestel AK, Modi BG, Longworth S, Wilck MB, Micheletti RG. Primary cutaneous cryptococcus in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol. (2016) 73:355–6. doi: 10.1001/jamaneurol.2015.4259
21. Patil SM, Beck PP, Arora N, Acevedo BA, Dandachi D. Primary cutaneous cryptococcal infection due to fingolimod - Induced lymphopenia with literature review. IDCases. (2020) 21:e00810. doi: 10.1016/j.idcr.2020.e00810
22. Enache-Angoulvant A, Chandenier J, Symoens F, Lacube P, Bolognini J, Douchet C, et al. Molecular identification of cryptococcus neoformans serotypes. J Clin Microbiol. (2007) 45:1261–5. doi: 10.1128/JCM.01839-06
23. Sharma K, Chaudhary D, Beard K, Srivastava S, Khalid SH, Sriwastava S. A comprehensive review of varicella-zoster virus, herpes simplex virus and cryptococcal infections associated with sphingosine-1-phosphate receptor modulators in multiple sclerosis patients. Mult Scler Relat Disord. (2022) 59:103675. doi: 10.1016/j.msard.2022.103675
24. Cuascut FX, Alkabie S, Hutton GJ. Fingolimod-related cryptococcal meningoencephalitis and immune reconstitution inflammatory syndrome in a patient with multiple sclerosis. Mult Scler Relat Disord. (2021) 53:103072. doi: 10.1016/j.msard.2021.103072
25. Kaur P, Lewis A, Basit A, Cyr NS, Muhammad Z. Increased risk of disseminated cryptococcal infection in a patient with multiple sclerosis on fingolimod. IDCases. (2020) 22:e00961. doi: 10.1016/j.idcr.2020.e00961
26. Baghbanian SM, Amiri MRM. Cryptococcal meningoencephalitis in a multiple sclerosis patient after fingolimod discontinuation-a case report. Neurol Sci. (2021) 42:1175–7. doi: 10.1007/s10072-020-04728-4
27. Samudralwar RD, Spec A, Cross AH. Case report: fingolimod and cryptococcosis: collision of immunomodulation with infectious disease. Int J MS Care. (2019) 21:275–80. doi: 10.7224/1537-2073.2018-080
28. Carpenter AF, Goodwin SJ, Bornstein PF, Larson AJ, Markus CK. Cutaneous cryptococcosis in a patient taking fingolimod for multiple sclerosis: Here come the opportunistic infections? Mult Scler. (2017) 23:297–9. doi: 10.1177/1352458516670732
29. Seto H, Nishimura M, Minamiji K, Miyoshi S, Mori H, Kanazawa K, et al. Disseminated Cryptococcosis in a 63-year-old patient with multiple sclerosis treated with fingolimod. Intern Med. (2016) 55:3383–6. doi: 10.2169/internalmedicine.55.7255
30. D. Huang. Disseminated cryptococcosis in a patient with multiple sclerosis treated with fingolimod. Neurology. (2015) 85:1001–3. doi: 10.1212/WNL.0000000000001929
31. Sing Y, Ramdial PK. Cryptococcal inflammatory pseudotumors. Am J Surg Pathol. (2007) 31:1521–7. doi: 10.1097/PAS.0b013e318040ad0a
32. Grebenciucova E, Reder AT, Bernard JT. Immunologic mechanisms of fingolimod and the role of immunosenescence in the risk of cryptococcal infection: a case report and review of literature. Mult Scler Relat Disord. (2016) 9:158–62. doi: 10.1016/j.msard.2016.07.015
33. Bryan AM, You JK, McQuiston T, Lazzarini C, Qiu Z, Sheridan B, et al. FTY720 reactivates cryptococcal granulomas in mice through S1P receptor 3 on macrophages. J Clin Invest. (2020) 130:4546–60. doi: 10.1172/JCI136068
34. Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. (2014) 13:545–56. doi: 10.1016/S1474-4422(14)70049-3
35. Meca-Lallana JE, Oreja-Guevara C, Munoz D, Olascoaga J, Pato A, Ramio-Torrenta L, et al. Spanish, Four-year safety and effectiveness data from patients with multiple sclerosis treated with fingolimod: the Spanish GILENYA registry. PLoS ONE. (2021) 16:e0258437. doi: 10.1371/journal.pone.0258437
36. Ohtani R, Mori M, Uchida T, Uzawa A, Masuda H, Liu J, et al. Risk factors for fingolimod-induced lymphopenia in multiple sclerosis. Mult Scler J Exp Transl Clin. (2018) 4:2055217318759692. doi: 10.1177/2055217318759692
37. Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. (2008) 71:1261–7. doi: 10.1212/01.wnl.0000327609.57688.ea
38. Bouley AJ, Baber U, Egnor E, Samaan S, Sloane JA. Prevalence of latent tuberculosis in the multiple sclerosis clinic and effect of multiple sclerosis treatment on tuberculosis testing. Int J MS Care. (2021) 23:26–30. doi: 10.7224/1537-2073.2019-015
39. Ghadiri M, Fitz-Gerald L, Rezk A, Li R, Nyirenda M, Haegert D, et al. Reconstitution of the peripheral immune repertoire following withdrawal of fingolimod. Mult Scler. (2017) 23:1225–32. doi: 10.1177/1352458517713147
40. Hanazono A, Sanpei Y, Kamada S, Sugawara M, Iijima K. Hidden relationship between fingolimod and bleeding: possible novel management of fingolimod-associated lymphopenia. Med Hypotheses. (2020) 140:109635. doi: 10.1016/j.mehy.2020.109635
Keywords: cryptococcus, fingolimod, chest mass, osteomyelitis, immunosuppression
Citation: Carpenter K, Etemady-Deylamy A, Costello V, Khasawneh M, Chamberland R, Tian K, Donlin M, Moreira-Walsh B, Reisenbichler E and Abate G (2022) Cryptococcal chest wall mass and rib osteomyelitis associated with the use of fingolimod: A case report and literature review. Front. Med. 9:942751. doi: 10.3389/fmed.2022.942751
Received: 12 May 2022; Accepted: 08 August 2022;
Published: 07 September 2022.
Edited by:
Sam Donta, Falmouth Hospital, United StatesReviewed by:
Omid Mirmosayyeb, University at Buffalo, United StatesCopyright © 2022 Carpenter, Etemady-Deylamy, Costello, Khasawneh, Chamberland, Tian, Donlin, Moreira-Walsh, Reisenbichler and Abate. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Getahun Abate, Z2V0YWh1bi5hYmF0ZUBoZWFsdGguc2x1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.