- 1Department of Parasitology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
- 2Department of Microbiology, School of Biology, College of Science, University of Tehran, Tehran, Iran
- 3Foodborne and Waterborne Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: Blastocystis sp. is a common intestinal parasite, possibly responsible for diarrhea, vomiting and nausea, abdominal pain, and irritable bowel syndrome. However, many studies focused on this issue due to the uncertainty of its pathogenic potential. The extracellular vesicles (EVs) are significant mediators for cellular communication, carrying biological molecules such as proteins, lipids, and nucleic acids. Compared with other parasites, little is known about the Blastocystis EVs. Hence the present investigation was done.
Methods: The Blastocystis parasites were cultured in the DMEM medium, and a 550–585 bp fragment was amplified using PCR, and sequencing was done. A commercial kit was used for exosome extraction and dynamic light scattering (DLS), flow cytometry (CD63, CD81 markers), and electron microscopy tests to determine their morphology. The human leukemia monocytic cell line (THP-1) was exposed to Blastocystis EVs. Next, the expression of proinflammatory and anti-inflammatory cytokines, including IL-4, IL-6, IL-10, and tumor necrosis factor-alpha (TNF-α), were measured using quantitative PCR.
Results: Exosomes were extracted from ST1-3 Blastocystis sp. According to the DLS assay, the size of the exosomes was in the range of 30–100 nm. Electron microscopy images and CD63 and CD81 markers also confirmed the exosome's size, structure, and morphology. According to real-time PCR results, ST1-derived exosomes caused IL-6 and TNF-α upregulation and IL-10 and IL-4 downregulation, ST2- and ST3-derived exosomes downregulated IL-10, and ST3-derived exosomes caused IL-6 upregulation. There is a statistically significant difference (P ≤ 0.05).
Conclusion: To our knowledge, this is the first report of the release of exosome-like vesicles by the human parasite, Blastocystis, and the provided information demonstrates the role of this parasite, particularly ST1 on proinflammatory and anti-inflammatory cytokines and navigating the host response.
Introduction
Parasitic diseases contribute to high morbidity and mortality yearly, particularly in developing countries (1). The parasitic agents employ many molecular mechanisms to communicate and manipulate the host responses (2). The extracellular vesicles (EVs) have been recognized as novel mediators in maintaining intestinal homeostasis, principally in the intestinal mucosa (3). These nano-molecules are also implicated in coordinating the growth and development, horizontal gene transfer, and host-pathogen communications, particularly at the early stages of the infection. Several mechanisms exist for EV uptake by host cells (4, 5). Phagocytosis and macropinocytosis are primarily involved in EV uptake, surrounded by the plasma membrane (6). Other entry routes are receptor-mediated contact, fusion with the target cell plasma membrane, and delivery of the soluble cargo to the cells. Reportedly, the information carried by EVs isis involved in disease development (7). EVs have been known to possess clinical applications, both as diagnostic biomarkers and therapeutic agents (8).
There are different types of EVs based on size and biogenesis. Exosomes (30–100 nm) are composed of late endosomes containing vesicles that combine with the plasma membrane, (9) and are released into the extracellular environment, microvesicles (100–1,000 nm) are directly germinated from the plasma membrane and are produced by outward budding of the plasma membrane and apoptotic bodies (1,000–5,000 nm) result from the interaction of myosin-actin during programmed cell death or apoptosis and contain cell contents such as organelles (10). Specific proteins named calpins with cysteine-dependent calcium protease activity, found in most eukaryotes and some prokaryotes, are involved in the releasing processes of EVs (11). In parasitic agents, the EVs may play a critical role in modulating the host's immune responses (12, 13). The following proteins are consistently found within exosomes: heat shock proteins (HSP60, HSP70, and HSP90), nucleic acids (DNA, RNA, microRNA), tetraspanins (CD81, CD63, and CD37), anxins (I, II, V and VI), cytoskeletal proteins (actin and tubulin), metabolic enzymes, proteins involved in translation (elongation factors 1 and 2) and signaling proteins (14, 15). These biological macromolecules are particularly interested in the diagnosis of infectious disease pathology (16).
The extracellular protozoan parasite, Blastocystis sp., is a gastrointestinal inhabitant in animals and humans, being assigned to cause abdominal pain, diarrhea, vomiting, and nausea. It has also been speculated to be involved in the initiation and or progression of irritable bowel syndrome (IBS) (17, 18), inflammatory bowel disease (IBD), and colorectal cancer (19). The phylogenetic analysis has placed the Blastocystis within the Stramenopiles group, along with brown algae (20). Among 28 identified subtypes (STs) of Blastocystis, nine (ST1-ST9) have been found in humans (21). Based on the published literature, subtypes 1–3 have been linked to chronic urticaria and skin disorders (1, 22, 23). Moreover, Blastocystis can activate cytokine production and immune responses in vitro (24). Cysteine protease enzymes in Blastocystis (especially ST7) can induce myosin light chain phosphorylation, ZO1 protein degradation, and F-actin reorganization in the Caco-2 cell line (25, 26). Compared with other parasites, little is known about the characterization, function, and host-parasite interaction of the Blastocystis EVs (27). Therefore, the present experiment investigated the effects of the Blastocystis exosomes on the expression of proinflammatory and anti-inflammatory cytokines such as IL-6, TNFα, IL-4, and IL-10 using molecular techniques.
Materials and methods
Parasite culture
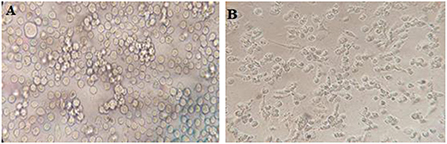
Positive Blastocystis samples were cultured in Dulbecco's Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and Penicillin-Streptomycin (1,000-unit penicillin and 4 mg/ml streptomycin) (Figure 1).
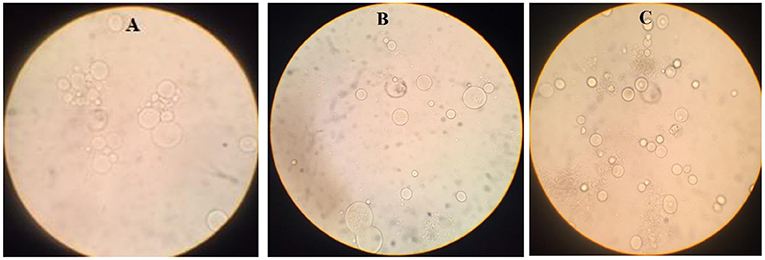
Figure 1. Blastocystis sp. in the medium (DMEM) with FBS and penicillin-streptomycin [(A): Subtype 3, (B): Subtype 2, (C): Subtype 1].
DNA extraction, polymerase chain reaction, and sequencing
Upon propagation of the parasites in the culture medium, DNA was extracted using DNA Extraction Kit (SINACLON), based on the manufacturer's protocol, and a ~600 bp fragment of the Blastocystis small subunit ribosomal DNA (SSU rDNA) gene was amplified using PCR method, according to the previous studies (Figure 2) (28). The amplification was done using a forward primer, RD5 (ATCTGGTTGATCCTGCCAGT) (29), and a reverse primer, BhRDr (GAGCTTTTTAACTGCAACAACG) (30).
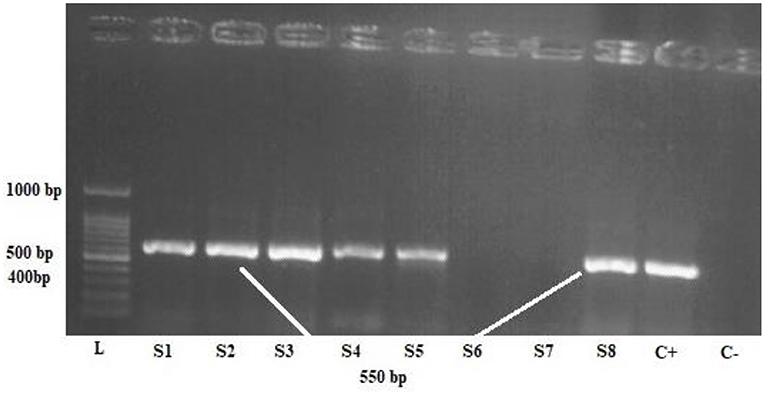
Figure 2. One percent agarose gel electrophoresis of the polymerase chain reaction product of Blastocystis samples, L: 100-bpDNA ladder, C + positive control, C- negative control, S1,2,3,4,5,8 positive samples.
The PCR amplicons were run on a 1% gel electrophoresis and visualized using ultraviolet (UV) illumination in a Gel-Doc apparatus. Sequencing was performed by the Sanger method. All obtained sequences were edited by SEQUNCHERTM software (ver. 5.4.5), and a comparison with other sequences deposited in the GenBank was performed using the basic local alignment tool (BLAST), available at http://blast.ncbi.nlm.nih.gov. Next, subtypes 1, 2, and 3 of Blastocystis sp. were selected for exosome extraction and further evaluation.
Exosome extraction
Initially, an FBS-free DMEM medium was used to culture the parasites for 48 h before extraction. A commercial kit, Exocib (Cib Biotech Co., IRI), was used for exosome extraction, and the extraction steps were done based on the manufacturer's guidelines. The purified exosomes were stored at 4°C for a few days or kept at −20°C or −80°C for a long time.
Quantification of exosome concentration
For this purpose, a kit-based Bradford test (Diba NoAvaran Azma Company, DNAbiotech) was used. The standard bovine serum albumin (BSA) was diluted with 1 mg/ml storage solution (100 μl BSA + 900 μl PBS or distilled water) and prepared based on the dilution kit protocol. A standard graph was plotted, and the trendline, the line equation, and the regression coefficient were determined on the graph. Finally, 20 μl of each sample was mixed with 180 μl of the reagent, and the optical density (absorbance) was read at 595 nm after 10 min.
Exosome characterization
Dynamic light scattering
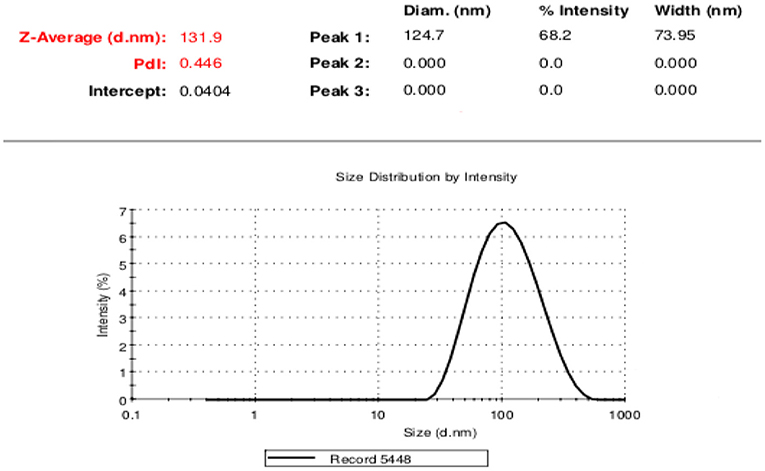
This procedure was done to determine the extracted exosomes' size distribution. For this aim, 50 μl of exosome sample for each subtype of interest was diluted in 950 μl of PBS. Then, the size distribution of the exosomes was read by Zetasizer Nano (ZS (Malvern Instruments, Southborough, UK) at 25 ° C with a refractive index of 1.38 and absorption of 0.01.
Evaluation of exosome surface markers by flow cytometry technique
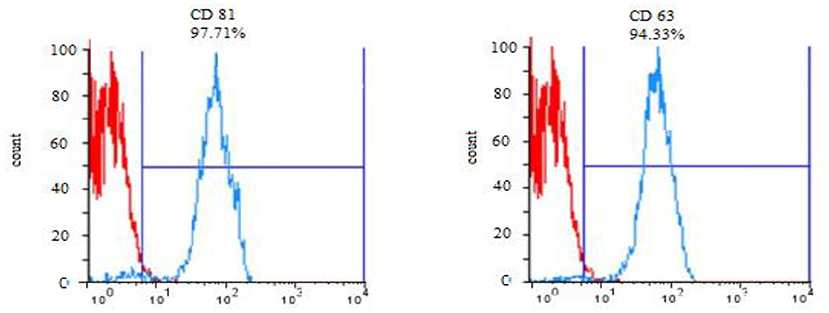
The exosome surface markers, CD63 and CD81, were evaluated using the flow cytometry technique. Initially, the exosomes were connected to sulfate aldehyde sulfate beads (size: 4 μm) to improve their size for the reader device. Briefly, 30 μg of the exosomes from each subtype was incubated with 90 μl of bead for 95 min at ambient temperature. Subsequently, the volume of the samples was increased to 1 ml with PBS and placed on a test tube rotator at 4°C for 16 h. Then the attachment process was terminated by adding 110 μl of 100 mM glycine solution and incubating for 30 min at room temperature. The exosome-coated beads were triple washed with PBS containing 0.5% BSA. Then, the exosome-bead complex was incubated with anti-CD63 and anti-CD81 antibodies separately for each group for about 1 h at 4°C. In the final step, the expression of the mentioned markers was examined by the flow cytometry method.
Evaluation the size, structure, and morphology of exosome
Scanning electron microscopy
The morphology and size of the exosomes were also monitored using SEM imaging. First, the samples were fixed using pre-cooled 2.5 % glutaraldehyde solution (Sigma–Aldrich, Germany), placed on the specimen stub, and snap-frozen in a freeze-dryer (Model 5006; Dena Vacuum Industry Co., Ltd). Then, the specimens were sputter-coated with gold-palladium and imaged by the SEM system.
Transmission electron microscopy
Purified exosomes were diluted with an equal volume of 4% paraformaldehyde at 4°C for 30 min, then carefully placed on a carbon-coated 300-mesh copper grid for 20 min, followed by a fixation step using 1% glutaraldehyde for 5 min. The mesh was contrasted with 2% uranyl acetate, washed twice, and the morphology of the isolated exosomes was observed. Exosome images retrieved through TEM (TEM, LEO 906, Zeiss, Germany) were analyzed by ImageJ software.
Expression of inflammatory and proinflammatory cytokines in the presence of exosomes
Cell culture
The Roswell Park Memorial Institute (RPMI 1,640) medium supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% L-glutamine was used to culture the human leukemia monocytic cell line (THP-1) (Figure 3). The cells were enumerated with trypan blue (0.4%), and 400 × 103 cells were seeded in each well of 12-well plates and subsequently treated by 50 ng phorbol myristate acetate (PMA) overnight, then washed three times. The cells were then rested for a day.
Exposure of THP-1 cells to exosomes
Prepared THP-1 cells were exposed to 10 μg of exosomes from each subtype, being incubated for 24 h. Next, the cells were trypsinized and collected in a microtube for the next steps.
RNA extraction
RNA extraction was performed manually according to the following protocol: First, 1 ml of Trizol (Thermo Scientific) was added to the collected samples and a pipette, then 250 μl of chloroform was added and mixed. The next step was incubated at room temperature for 15 min, then centrifuged at 15,000 rpm at 4° C for 15 min. In the next step, the supernatant was removed and transferred to a new microtube, then 600–800 μl of cold isopropanol was added to it and mixed together. The samples were kept at −20 °C for 24 h. They were then centrifuged at 13,000 rpm at 4 °C for 45 min. The supernatant was then removed, and 1 ml of 70% cold ethanol was added to the RNA precipitate (washing). The samples were centrifuged at 20,000 rpm at 4°C for 20 min. Remove the supernatant and allow the precipitate to dry for about 10 min (drying). Then 20 μl of sterile distilled water was added to the precipitate and gently pipetted to dissolve the RNA precipitate in water. The extracted RNA was stored at −70°C. The quality and concentration of RNA were evaluated with a nanodrop spectrophotometers (ND2000, Thermo Scientific). The OD 260/280 nm ratio was reported to be about 2.00.
CDNA synthesis and real-time PCR
For cDNA synthesis, total RNA was reverse-transcribed using M-MLV reverse transcriptase according to the manufacturer's instructions (RT-ROSET Kit). Real-time PCR was performed in triplicate using 50 ng of cDNA with RealQ Plus 2x Master Mix Green reagent (Ampliqon) in a Rotor Gene-Q thermal cycler (Qiagen). Primer sequences are shown in Table 1. Relative gene expression values were normalized to β-actin and calculated using the comparative CT method (2∧–ΔΔCT). The values are presented as mean n-fold differences compared to the control (P ≤ 0.05 was reported to be significant).
Result
Subtyping of the Blastocystis sp.
The Blastocystis parasites were cultured in a DMEM medium enriched with 10% FBS and penicillin-streptomycin antibiotics, and a molecular investigation followed by sequencing of the propagated parasites revealed ST1-ST3. The sequences have been deposited in GenBank under accession numbers OL457221-OL457257. Multiple sequence alignments were performed using the ClustalW, and phylogenetic analyses using the maximum-likelihood (ML) method were carried out using the MEGA7 using all subtypes of Blastocystis. The tree was constructed (500 replicates) using the 18S rRNA gene sequence of Proteromonas laceratae (U37108) as an outgroup (Figure 4).
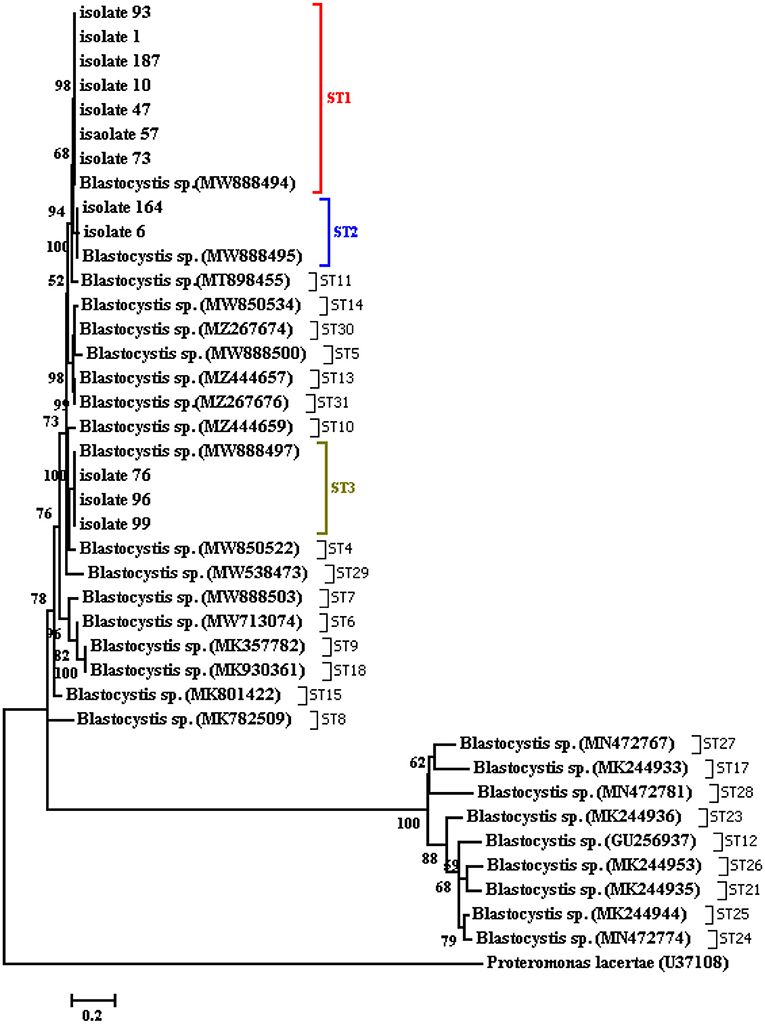
Figure 4. Phylogenetic tree of the SSU-rDNA gene sequences of Blastocystis isolates constructed by the neighbor-joining method using Mega4 software.
Determination of the concentration of extracted exosomes
Based on the Bradford assay, the concentrations of the exosomes for subtypes 1, 2, and 3 included 1.9 μg/μl (ST1), 2.3 μg/μl (ST2), and 2.2 μg/μl (ST3).
Confirmatory tests for exosome extraction
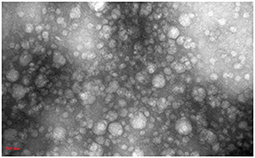
DLS test was performed to determine the size of the exosomes. The peak of EVs population was obtained in the range of exosomes, and the intensity was 68.2% (Figure 5). Furthermore, the electron microscopy studies confirmed the homogeneity of the population with a size of 30–100 nm and their spherical morphology (Figures 6, 7). Also, due to existing limitations, we were not able to measure exosomes in the range of 100–1,000 nm. The flow cytometry results showed the expression of CD63 (94.33%) and CD81 (97.71%) markers on the surface of exosomes (Figure 8).

Figure 6. SEM electron microscope image of exosome specimens of Blastocystis subtypes [(A): Exosomes without conjugation and (B): with sulfate aldehyde sulfate beads].
Expression of inflammatory and proinflammatory cytokines by THP-1 cells exposed to the exosomes
Next, the exosomes were exposed to THP-1 cells for 24 h, then cells were trypsinized, and RNA extraction, cDNA synthesis, and real-time PCR experiments were performed. Analysis of real-time PCR output and fold change calculation showed (Table 2) that in ST1, the expression of IL-6 and TNF-α was upregulated, compared to the control group. Also, ST1 showed a decrease in IL-4 and IL-10 expression, while IL-4 expression was not changed in ST2- and ST3-exposed THP-1 cells (Figure 9). It is noteworthy that IL-10 showed a decrease in expression in all three subtypes compared to the control group Notably, The expression of TNF-α in exosome-exposed cells in subtype 1 was upregulated compared to the control (LPS).
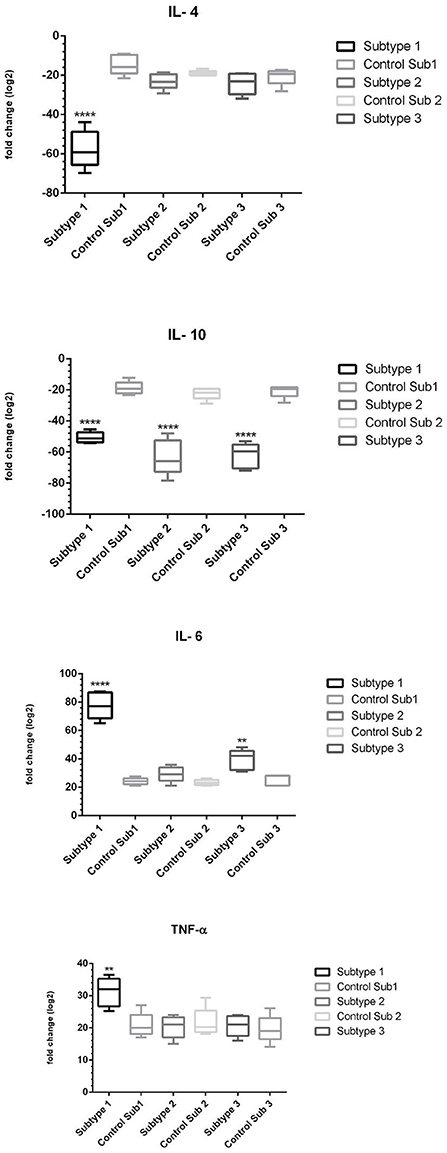
Figure 9. The expression of proinflammatory and anti-inflammatory cytokines in the presence of exosomes of subtypes 1, 2 and 3 of Blastocystis (The sign (*) in each subtype indicates which cytokine was statistically significant compared to control) (**p < 0.01; ****p < 0.0001).
Discussion
Blastocystis is an extracellular organism in the gut of animals and humans, which can suppress the inducible nitric oxide (iNOS) production and cleave the immunoglobulins, evading the host's immune system responses (34). This compromised molecular milieu also paves the way for other co-infections to infect the intestinal epithelium and cause disease (35). During the last decades, the molecular basis of the host-parasite cross-talk has been more elucidated, highlighting to be mediated by the EV nano-molecules (~30–5,000 nm) as tiny vehicles for cellular communication that may carry proteins, lipids, nucleic acids, and metabolites from their cellular origin, essential for genetic exchange, biomarker identification, and the diagnosis of diseases (36, 37).
Based on the in vitro experiments, Blastocystis parasites can elicit cytokine production and immune responses (38). Despite the availability of some Blastocystis subtype's genomes, our knowledge is still in its infancy on the biological and pathological mechanisms of the parasite (39). Curiously, membrane proteins and the integrity of the epithelial barrier could be substantially disrupted by the Blastocystis ST7 via degradation of tight proteins (3). So far, few studies have characterized EVs in Blastocystis, and the early evidence was provided by Tan through the TEM experiment (35). According to recent studies, Blastocystis-derived EVs were identified in ST7 B and H isolates, with cup-shaped morphology and an average size of 50 to 240 nm consistent with EVs derived from other parasites (40).
In recent years, a great deal of interest has been focused on the epidemiology, phylogeny, and cell biology of Blastocystis; only a few studies have addressed the parasite virulence and specific host responses (41). Based on the published literature, gastrointestinal symptoms similar to IBS, including diarrhea, abdominal pain, constipation, nausea, inflammation, and edema, have been reported in patients infected with Blastocystis (42). On the other hand, the parasite has been recognized mainly in asymptomatic individuals, thus possibly being non-invasive (43). Edema and infiltration of the inflammatory cells into the lamina propria have been reported in the cecum and colon of infected mice (44). An experiment showed that Blastocystis induces IL-8 production in colon T84 epithelial cells in a time-dependent manner, which provokes the inflammatory cells to invade the intestinal mucosa, resulting in tissue damage and gastrointestinal disorders (45).
Previously, no investigation was done to evaluate the association between IL-6 and TNF-α gene polymorphisms in susceptibility to diseases such as IBS (46). Here, we evaluated such an association and revealed a significant association between IL-6 gene expression in THP-1 cells exposed to ST1 exosomes. This may suggest that the presence of a parasite such as Blastocystis would play a substantial role in IBS by promoting the clinical outcomes. Another major finding in the current study was that no significant difference was observed regarding TNF-α expression in comparison with the control group, which is inconsistent with Iguchi et al. study, demonstrating significant upregulation of IFN-γ, IL-12, and TNF-α cytokines in the cecal mucosa (47). Downregulation of IFN-γ and TNF-α, along with the upregulation of IL-6 and IL-8, has been observed in colorectal cancer (48). This evidence indicates that the Blastocystis antigen (Blasto-Ag), an example of the extracellular allergen, has stimulated the humoral responses, leading to inflammatory reactions and cell propagation to combat the infection (49). Another study by Yakoob et al. demonstrated significantly lower IL-10 levels and colonic eosinophilic infiltration associated with IL-8 in the Blastocystis ST1 compared to ST3 and control (32). In the current study, we found a reduction in the expression of IL-10 in ST1, ST2 and ST3. It has been recognized that IL-4 and IL-13 cytokines mediate the goblet cell hyperplasia during the gut infection, which the latter plays a significant protective role against the infection (50). Our results demonstrated a decrease in IL-4 and IL-10 expression and an increase in IL-6 and TNF-α expression in Blastocystis ST1. Reportedly, IL-10 is essential in regulating inflammatory responses, as it reduces the production of chemotactic factors such as IL-8 (51). Altogether, the increased mucin layer and fluid secretion, goblet cell hyperplasia, and enhanced intestinal propulsive activity result in the expulsion of noxious agents from the gut lumen (52).
Finally, the results presented here highlighted the significance of the Blastocystis ST1 exosomes on the expression of IL-6, IL-10, IL-4 and TNF-α cytokines, strengthening the hypothesis that the Blastocystis parasite is a potent contributor to the inflammatory processes. To better understand the interaction of EVs of Blastocystis, other inflammatory cytokines such as MCP-1 and IL1B should be investigated. However, the actual pathogenicity of Blastocystis and its association with gastrointestinal symptoms is yet to be determined through extensive research, possibly in the field of EVs, their biogenesis, uptake, and cellular communication.
Conclusion
To our knowledge, this is the first report of the release of EVs by the human parasite Blastocystis, and our data demonstrate the role of this parasite, particularly ST1, on proinflammatory and anti-inflammatory cytokines and navigating the host responses. Further studies on the arrangement and function of these biologically-active vesicles could assist us in developing unprecedented therapeutic strategies, opening new doors toward the role of the Blastocystis parasite in gastrointestinal diseases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Tarbiat Modares University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MP and MN designed the study. EA and MP contributed to the methodology. EA and HM analyzed the data. AD, JS, and MN reviewed and edited the manuscript. EA was responsible for advising on controversial issues. MP is responsible for the overall content as a corresponding author. All authors have read and approved the final version of the manuscript.
Acknowledgments
This study is result of Ph.D. thesis that has been supported by Vice-Chancellor for Research of Tarbiat Modares University. We are very grateful for the help and assistance of Mr. Alireza Olyaiee.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TNF-α, tumor necrosis factor-alpha; DMEM, Dulbecco's Modified Eagle Medium; EVs, Extracellular vehicles; GI, gastrointestinal; IBS, Irritable Bowel Syndrome; IBD, Inflammatory Bowel Disease; BSA, Bovine Serum Albumin; SEM, Scanning Electron Microscope; TEM, Transmission Electron Microscopy; PMA, Phorbol Myristate Acetate; DLS, Dynamic Light Scattering.
References
1. Mahmoud MS, Saleh WA. Secretory and humoral antibody responses to Blastocystis hominis in symptomatic and asymptomatic human infections. J Egypt Soc Parasitol. (2003) 33:13–30.
2. Liu Y, Rhoads JM. Communication between B-cells and microbiota for the maintenance of intestinal homeostasis. Antibodies. (2013) 2:535. doi: 10.3390/antib2040535
3. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. (2009) 9:799–809. doi: 10.1038/nri2653
4. Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. (2014) 10:356–64. doi: 10.1038/nrrheum.2014.19
5. Evans-Osses I, Mojoli A, Monguio-Tortajada M, Marcilla A, Aran V, Amorim M, et al. Microvesicles released from Giardia intestinalis disturb host-pathogen response in vitro. Eur J Cell Biol. (2017) 96:131–42. doi: 10.1016/j.ejcb.2017.01.005
6. Olmos-Ortiz LM, Barajas-Mendiola MA, Barrios-Rodiles M, Castellano LE, Arias-Negrete S, Avila EE, et al. Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice. Parasite Immunol. (2017) 39:e12426. doi: 10.1111/pim.12426
7. Goll R, Granlund AV. Intestinal barrier homeostasis in inflammatory bowel disease, Scand. J Gastroenterol. (2015) 50:3–12. doi: 10.3109/00365521.2014.971425
8. Babatunde KA, Mbagwu S, Hernandez-Castaneda MA, Adapa SR, Walch M, et al. Malaria infected red blood cells release small regulatory RNAs through extracellular vesicles. Sci Rep. (2018) 8:884. doi: 10.1038/s41598-018-19149-9
9. Cheng IS, Sealy BC, Tiberti N, Combes V. Extracellular vesicles, from pathogenesis to biomarkers: the case for cerebral malaria. Vessel Plus. (2020) 2020:17. doi: 10.20517/2574-1209.2020.08
10. Shirley DAT, Farr L, Watanabe K, Moonah S. A review of the global burden, new diagnostics, and current therapeutics for amebiasis. Open Forum Infect Dis. (2018) 5:ofy161. doi: 10.1093/ofid/ofy161
11. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neuro-Oncol. (2013) 113:1–11. doi: 10.1007/s11060-013-1084-8
12. Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA Cargo selection, content, release, and uptake. Cell Mol Neurobiol. (2016) 36:301–12. doi: 10.1007/s10571-016-0366-z
13. Bebawy M, Roseblade A, Luk F, Rawling T, Ung A, Grau GER. Cell-derived microparticles: new targets in the therapeutic management of disease. J Pharm Pharm Sci. (2013) 16:238–53. doi: 10.18433/J3989X
14. SchoreyJS. Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. (2008) 9:871–81. doi: 10.1111/j.1600-0854.2008.00734.x
15. Silverman JM, Clos J, de Oliveira CC, Shirvani O, Fang Y, Wang C, et al. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. (2010) 123:842–52. doi: 10.1242/jcs.056465
16. Atayde VD, Aslan H, Townsend S, Hassani K, Kamhawi S, Olivier M. Exosome secretion by the parasitic protozoan leishmania within the sand fly midgut. Cell Rep. (2015) 13:957–67. doi: 10.1016/j.celrep.2015.09.058
17. Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology (Basel). (2020) 9:21. doi: 10.3390/biology9010021
18. Barbosa CV, Barreto MM, Andrade RJ, Sodré F, d'Avila-Levy CM, Peralta JM, et al. Intestinal parasite infections in a rural community of Rio de Janeiro (Brazil): prevalence and genetic diversity of Blastocystis subtypes. PLoS ONE. (2018) 13:e0193860. doi: 10.1371/journal.pone.0193860
19. Hublin JS, Maloney JG, Santin M. Blastocystis in domesticated and wild mammals and birds. Res Vet Sci. (2021) 135:260–82. doi: 10.1016/j.rvsc.2020.09.031
20. Greige S, El Safadi D, Khaled S, Gantois N, Baydoun M, Chemaly M, et al. First report on the prevalence and subtype distribution of Blastocystis sp. in dairy cattle in Lebanon and assessment of zoonotic transmission. Acta Trop. (2019) 194:23–9. doi: 10.1016/j.actatropica.2019.02.013
21. Danišová O, Valenčáková A. First detection of Blastocystis sp. in pigs in Slovakia and in Europe. Parasitol Int. (2021) 81:102235. doi: 10.1016/j.parint.2020.102235
22. Dogruman-Al F, Kustimur S, Yoshikawa H, Tuncer C, Simsek Z, Tanyuksel M, et al. Blastocystis subtypes in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey, Mem. Inst Oswaldo Cruz. (2009) 104:724–7. doi: 10.1590/S0074-02762009000500011
23. Kumarasamy V, Kuppusamy UR, Jayalakshmi P, Samudi C, Ragavan ND, Kumar S. Exacerbation of colon carcinogenesis by Blastocystis sp. PLoS ONE. (2017) 12:e0183097. doi: 10.1371/journal.pone.0183097
24. Bahrami F, Babaei E, Badirzadeh A, Riabi TR, Abdoli A. Blastocystis, urticaria, and skin disorders: review of the current evidences. Eur J Clin Microbiol Infect Dis. (2020) 39:1027–42. doi: 10.1007/s10096-019-03793-8
25. Wu B, Yin J, Texier C, Roussel M, Tan KS. Blastocystis legumain is localized on the cell surface, and specific inhibition of its activity implicates a prosurvival role for the enzyme. J Biol Chem. (2010) 285:1790–8. doi: 10.1074/jbc.M109.049064
26. Wawrzyniak I, Texier C, Poirier P, Viscogliosi E, Tan KSW, Delbac F, et al. Characterization of two cysteine proteases secreted by Blastocystis ST7, a human intestinal parasite. Parasitol Int. (2012) 61:437–42. doi: 10.1016/j.parint.2012.02.007
27. Nourrisson C, Wawrzyniak I, Cian A, Livrelli V, Viscogliosi E, Delbac F, et al. On Blastocystis secreted cysteine proteases: a legumain-activated cathepsin B increases paracellular permeability of intestinal Caco-2 cell monolayers. Parasitology. (2016) 143:1713–22. doi: 10.1017/S0031182016001396
28. Hoevers JD, Snowden KF. Analysis of the ITS region and partial ssu and lsu rRNA genes of Blastocystis and Proteromonas lacertae. Parasitology. (2005) 131:187–96. doi: 10.1017/S0031182005007596
29. Howe EHY, Singh M. Do Blastocystis hominis colony forms undergo programmed cell death? Parasitol Res. (2001) 87:362–7. doi: 10.1007/s004360000364
30. Scanlan PD, Stensvold CR, Cotter PD. Development and application of a Blastocystis subtype-specific PCR assay reveals that mixed-subtype infections are common in a healthy human population. Appl Environ Microbiol. (2015) 81:4071–6. doi: 10.1128/AEM.00520-15
31. Loots GG, Locksley RM, Blank spoor CM, Wang ZE, Miller W, Rubin EM, et al. Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science. (2000) 288:136–40. doi: 10.1126/science.288.5463.136
32. Yakoob J, Abbas Z, Usman MW, Sultana A, Islam M, Awan S, et al. Cytokine changes in colonic mucosa associated with Blastocystis spp. subtypes 1 and 3 in diarrhoea-predominant irritable bowel syndrome. Parasitology. (2014) 141:957–69. doi: 10.1017/S003118201300173X
33. Knerr I, Beinder E, Rascher W. Syncytin, a novel human endogenous retroviral gene in human placenta: evidence for its dysregulation in preeclampsia and HELLP syndrome. Am J Obstet Gynecol. (2002) 186:210–3. doi: 10.1067/mob.2002.119636
34. Tan KS, Mirza H. Blastocystis–Host Interactions: Insights from In Vitro Model Systems. In Blastocystis: Pathogen or Passenger? Heidelberg: Springer, Berlin. (2012), pp. 51–63.
35. Tan KS. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev. (2008) 21:639–65. doi: 10.1128/CMR.00022-08
36. Allen AR, Skuce RA, Byrne AW. Bovine tuberculosis in Britain and Ireland–a perfect storm? The confluence of potential ecological and epidemiological impediments to controlling a chronic infectious disease. Front Vet Sci. (2018) 5:109. doi: 10.3389/fvets.2018.00109
37. Liangsupree T, Multia E, Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. (2021) 1636:461773. doi: 10.1016/j.chroma.2020.461773
38. Ullah M, Qiao Y, Concepcion W, Thakor AS. Stem cell-derived extracellular vesicles: role in oncogenic processes, bioengineering potential, and technical challenges. Stem Cell Res Ther. (2019) 10:1–0. doi: 10.1186/s13287-019-1468-6
39. Nievas YR, Lizarraga A, Salas N, Cóceres VM, de Miguel N. Extracellular vesicles released by anaerobic protozoan parasites: current situation. Cell Microbiol. (2020) 22:e13257. doi: 10.1111/cmi.13257
40. Kumarasamy V, Anbazhagan D, Subramaniyan V, Vellasamy S. Blastocystis sp, parasite associated with gastrointestinal disorders: an overview of its pathogenesis, immune modulation and therapeutic strategies. Curr Pharm Des. (2018) 24:3172–5. doi: 10.2174/1381612824666180807101536
41. Wu ZN, Mirza H, Teo JDW, Tan KSW. Strain-dependent induction of human enterocyte apoptosis by Blastocystis disrupts epithelial barrier and ZO-1 organization in a caspase 3-and 9-dependent manner. Biomed Res Int. (2014) 1–11. doi: 10.1155/2014/209163
42. Carrera-Bravo C, Koh EY, Tan KS. The roles of parasite-derived extracellular vesicles in disease and host-parasite communication. Parasitol Int. (2021) 83:102373. doi: 10.1016/j.parint.2021.102373
43. Moe KT, Singh M, Howe J, Ho LC, Tan SW, Chen XQ, et al. Experimental Blastocystis hominis infection in laboratory mice. Parasitol Res. (1997) 83:319–25. doi: 10.1007/s004360050256
44. Li J, Deng T, Li X, Cao G, Li X, Yan Y, et al. rat model to study Blastocytis subtype 1 infections. Parasitol Res. (2013) 112:3537–41. doi: 10.1007/s00436-013-3536-7
45. Puthia MK, Lu J, Tan KS. Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human intestinal epithelial cells in an NF-κB-dependent manner. Eukaryot Cell. (2008) 7:435–43. doi: 10.1128/EC.00371-07
46. Jimenez-Gonzalez DE, Martinez-Flores WA, Reyes-Gordillo J, Ramirez-Miranda ME, Arroyo-Escalante S, Romero-Valdovinos M, et al. Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol Res. (2012) 110:1269–75. doi: 10.1007/s00436-011-2626-7
47. Iguchi A, Yoshikawa H, Yamada M, Kimata I, Arizono N. Expression of interferon gamma and proinflammatory cytokines in the cecal mucosa of rats experimentally infected with Blastocystis sp. strain RN94-9. Parasitol Res. (2009) 105:135–40. doi: 10.1007/s00436-009-1373-5
48. Chandramathi S, Suresh K, Kuppusamy UR. Solubilized antigen of Blastocystis hominis facilitates the growth of human colorectal cancer cells, HCT116. Parasitol Res. (2010) 106:941–5. doi: 10.1007/s00436-010-1764-7
49. Zhang J, Zhu S, Ma N, Johnston LJ, Wu C, Ma X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: a therapeutic target to control intestinal inflammation. Med Res Rev. (2021) 41:1061–88. doi: 10.1002/med.21752
50. Khan WI, Collins SM. Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol. (2004) 26:319–26. doi: 10.1111/j.0141-9838.2004.00715.x
51. Jinquan T, Larsen CG, Gesser B, Matsushima K, Thestrup-Pedersen K. Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J Immunol. (1993) 151:4545–51.
Keywords: exosomes, Blastocystis subtypes, proinflammatory, anti-inflammatory, cytokines
Citation: Norouzi M, Pirestani M, Arefian E, Dalimi A, Sadraei J and Mirjalali H (2022) Exosomes secreted by Blastocystis subtypes affect the expression of proinflammatory and anti-inflammatory cytokines (TNFα, IL-6, IL-10, IL-4). Front. Med. 9:940332. doi: 10.3389/fmed.2022.940332
Received: 10 May 2022; Accepted: 27 July 2022;
Published: 11 August 2022.
Edited by:
Md Robiul Karim, Bangabandhu Sheikh Mujibur Rahman Agricultural University, BangladeshReviewed by:
Milad Badri, Qazvin University of Medical Sciences, IranMrigya Babuta, Beth Israel Deaconess Medical Center and Harvard Medical School, United States
Copyright © 2022 Norouzi, Pirestani, Arefian, Dalimi, Sadraei and Mirjalali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Majid Pirestani, cGlyZXN0YW5pQG1vZGFyZXMuYWMuaXI=
 Mojtaba Norouzi
Mojtaba Norouzi Majid Pirestani
Majid Pirestani Ehsan Arefian
Ehsan Arefian Abdolhossein Dalimi
Abdolhossein Dalimi Javid Sadraei
Javid Sadraei Hamed Mirjalali
Hamed Mirjalali