
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 01 July 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.938761
This article is part of the Research Topic Mechanism and Therapy of Autoimmune Skin Diseases View all 8 articles
Bullous pemphigoid (BP) is a life-threatening autoimmune disease of the skin that is mainly characterized by a large range of tension blisters and intense itching of the skin. The 1-year mortality rate of BP was 23.5%. Superinfection caused by skin lesion ulceration is one of the important causes of disease death. Therefore, it is challenging to control infection and improve skin wound healing. Here, we report the case of an elderly woman who presented with BP and involved the oral mucosa. The patient was successfully treated with hormones combined with topical berberine, and 95% of the patients’ lesions healed completely after 1 month. In addition, we inductively analyzed the current treatments for BP to provide a reference for BP clinical treatment.
Bullous pemphigoid (BP), a type of pemphigoid, is a rare, cutaneous autoimmune disease characterized by autoantibodies against hemidesmosome structural proteins at the dermal-epidermal junction. It is mainly characterized by a large range of tension blisters and intense itching (1). Minor oral mucosal lesions may be present in only 10–20% of these patients, and lesions in other mucosal areas are relatively rare (2). The 1-year mortality rate of pemphigoid is 23.5% (3). Superinfection caused by skin lesion ulceration is one of the important causes of disease death (2). Therefore, it is challenging to control infection and improve skin wound healing. Here, we report a case of an elderly woman who presented with BP and oral mucosa involvement. Based on clinical experience, we used steroids combined with topical berberine and achieved highly positive outcomes. We inductively analyzed the current treatment to provide a reference for BP clinical treatment.
The patient was a 76-year-old woman who had a 5-year history of Sjogren’s syndrome. The first uncomfortable symptom she experienced was a generalized erythematous rash on the skin around the eyes approximately for 10 days, accompanied by increasing intraocular secretions, itching, and blurred vision. A week following the onset of initial symptoms, lesions developed into generalized herpes with itching and partial ulceration on the trunk. Physical examination revealed erythema around the eyes and ulcers on the palate of the mouth, herpes wall laxity, Nikolsky’s sign (±), yellowish herpes discharge, and a thin, partially damaged bright red erosive surface. Laboratory examination revealed the following: desmoglein 1 (Dsg-1), 3.9 U/mL (0.1–200 U/mL); desmoglein 3 (Dsg-3), 100.30 U/mL (0.1–200 U/mL); anti-BP180 antibody, positive; anti-BP230 antibody, positive. Skin histopathology (Figure 1A) showed that the epidermis was separated from the dermis by blisters in the epidermis. Immunofluorescence staining (Figure 1B) revealed linear C3 and IgG deposits along the basement membrane. Combined with its clinical features, we confirmed the diagnosis of BP. After diagnosis, the patient was immediately prescribed the traditional therapy of glucocorticoid steroid methylprednisolone (40 mg per day ivgtt) for symptomatic treatment. Five days post-treatment, new blisters and erosive lesions still formed. Moreover, instead of reducing the methylprednisolone dose, it was also increased to 60 mg per day. According to clinical expertise, traditional Chinese medicine treatments were added to the treatment regimen. Considering the patient’s skin lesions, tongue, and pulse, this condition is classified as damp-heat accumulation skin pemphigus disease. The patient’s treatment timeline is shown in Figure 1C.
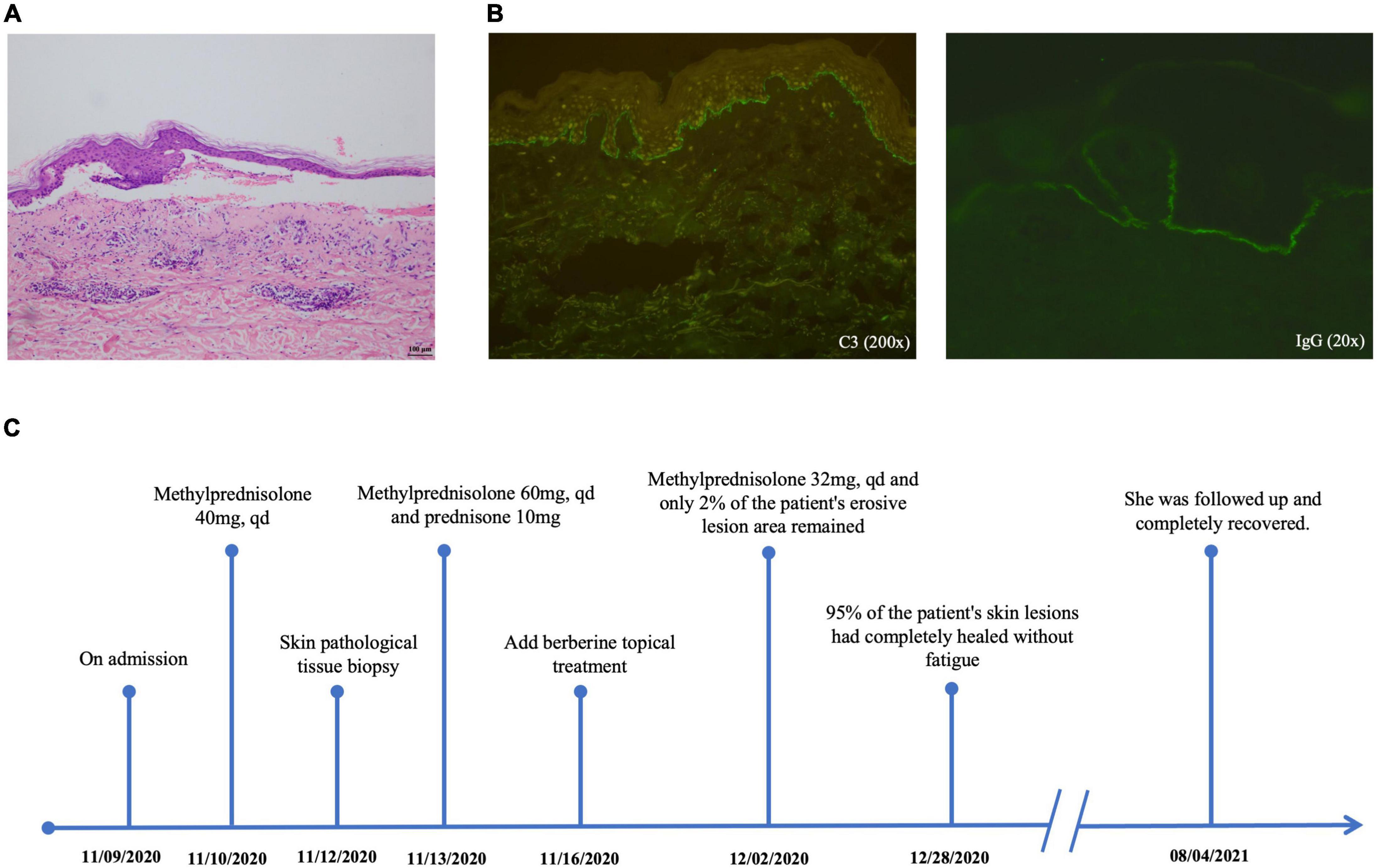
Figure 1. Skin histopathology (A) shows that the epidermis is separated from the dermis with blisters in the epidermis (10×). Immunofluorescence staining (B) reveals linear C3 and IgG deposits along the basement membrane. Patient timeline (C) demonstrates the therapeutic schedule and disease status.
Berberine is primarily derived from Coptis chinensis Franch and is typically administered in clinical practice as berberine hydrochloride tablets (Figures 2A,C). It is a common Chinese herbal medicine with the function of heat clearing, dampness drying, and analgesic. Berberine was ground into powder and blended with sesame oil (50 mg/mL) (Figure 2B). Small gauze was immersed in the mixture and was applied one by one to the cutaneous wound in the form of stamps (Figure 2D). Finally, a bandage was wrapped around the outside to prevent the small gauze from falling off (Figure 2E). The outer bandage was opened every 3 days, and most of the small gauze adhered to the eroded skin. Gauze that did not fall off was kept on the skin, and new berberine-soaked gauze was applied onto the skin. Two weeks later, the patient’s periocular and trunk skin lesions were significantly improved, comprising only 2% of the residual surface area of the erosive surface (Figures 3A–D). New epithelial tissue was observed, along with a remarkable pain reduction. Notably, the dose of methylprednisolone was reduced to 32 mg per day. After 1 month of hospitalization, 95% of the patient’s skin lesions had completely healed without fatigue. After hospital discharge, the patient completely recovered from BP and had a great quality of life during follow-up (Figures 3E,F).
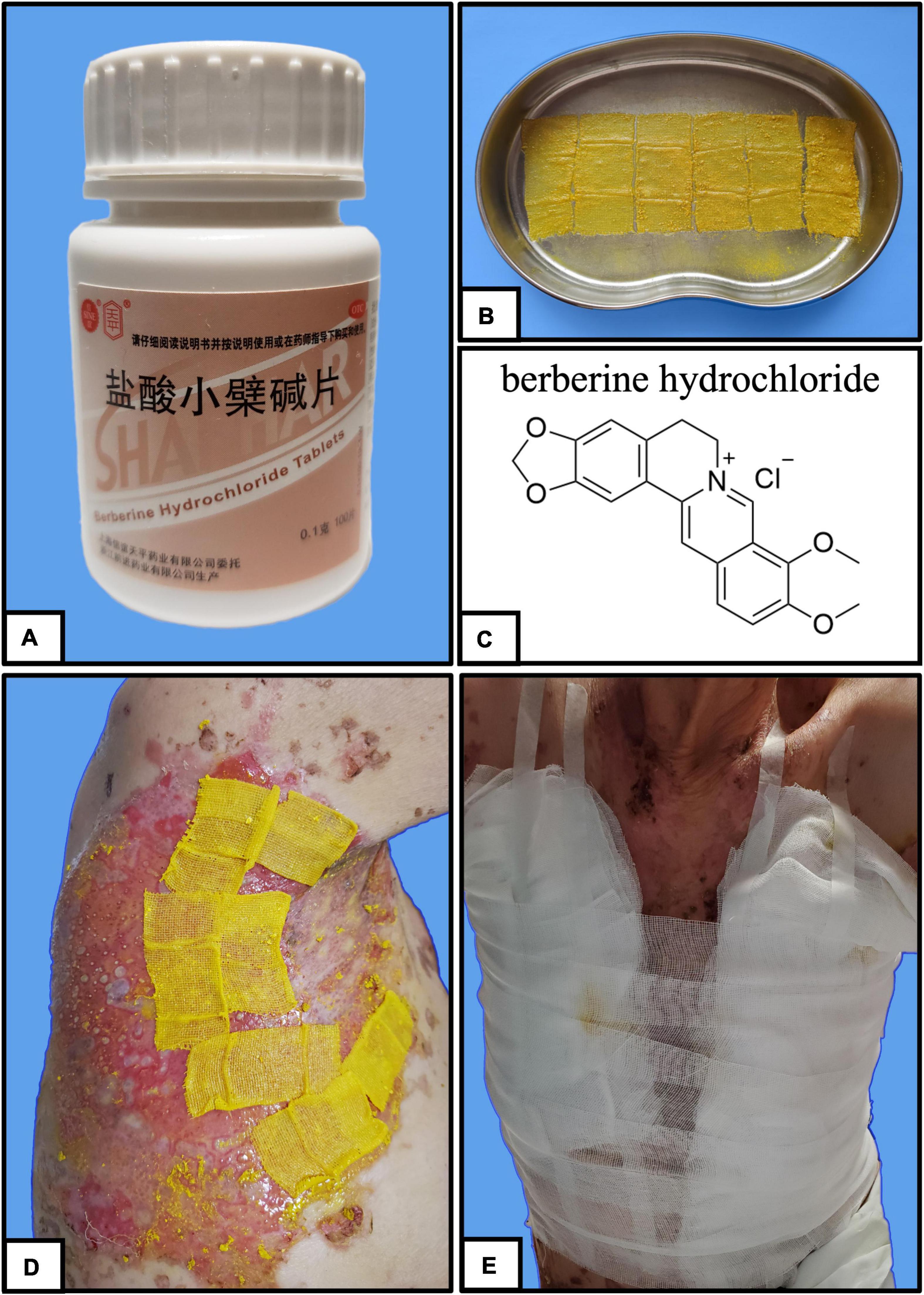
Figure 2. Stamp therapy. (A–C) Stamp gauze soaked with sesame oil mixed with berberine. (D) Stamp gauze is affixed thoroughly to the broken skin of the patient. (E) Bandage is wrapped around the periphery to prevent the gauze from falling.
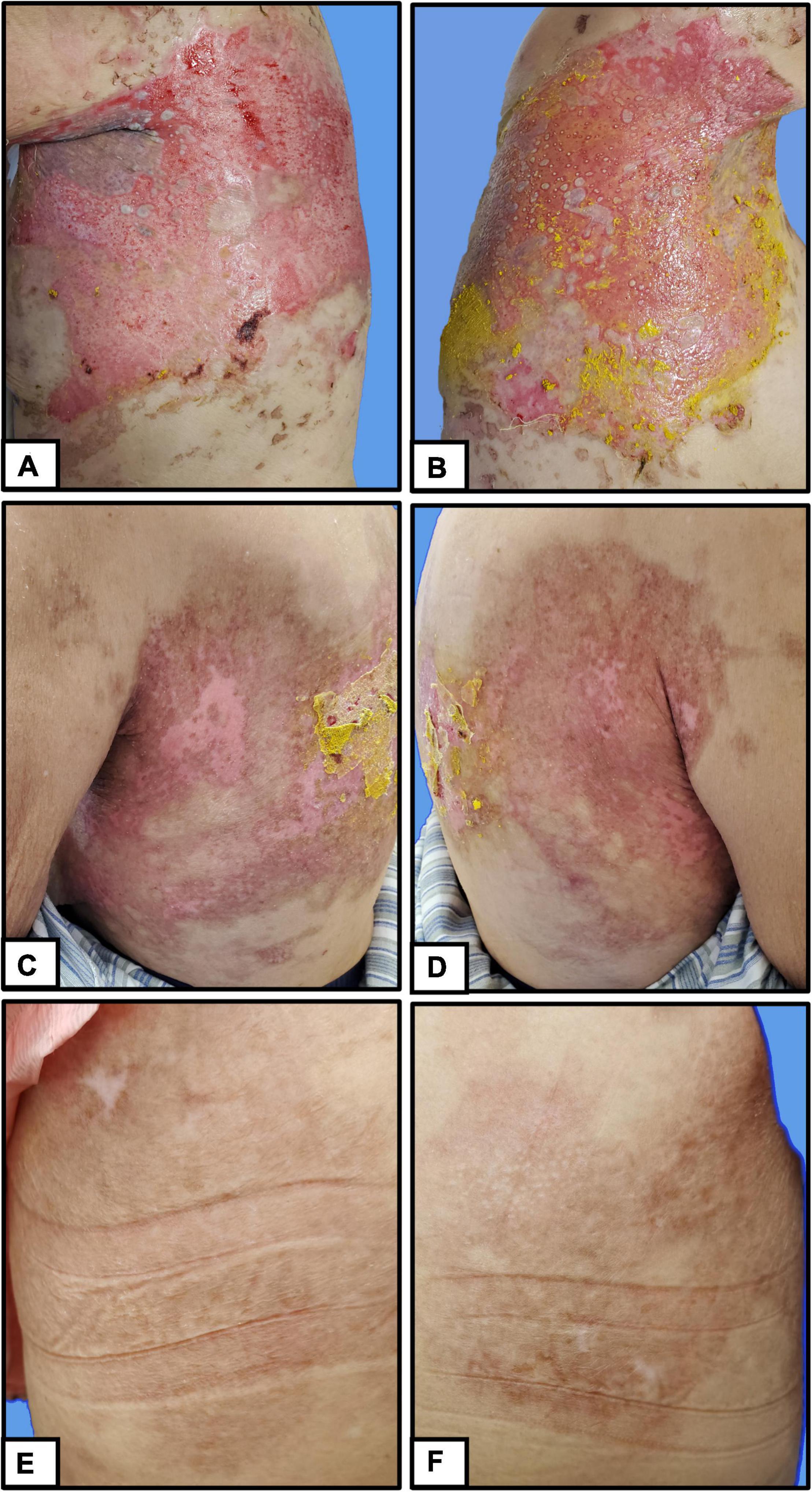
Figure 3. Clinical manifestations of patients with pemphigus. (A,B) Before treatment, (C,D) 2 weeks after treatment, (E,F) during follow-up.
In this case, we diagnosed it as BP with the syndrome of damp-heat accumulation skin based on clinical manifestations and skin biopsy. We tried a novel combination of drug and treatment-adjuvant therapy with the external application of berberine, a traditional Chinese medicine extract for clearing away heat, dryness, and dampness. The results showed that this traditional Chinese medicine external method is effective.
Berberine, an alkaloid mainly extracted from the traditional Chinese medicine C. chinensis, has hypoglycemic, anti-inflammatory, antioxidant, and broad-spectrum antibacterial properties (4). In clinical trials, berberine has significant improvement effects on hypoglycemia, lipid-lowering, and insulin resistance (5). It also shows significant effects in treating cardiovascular, endocrine, gastrointestinal diseases, and cancer and is mostly used for gastrointestinal diseases such as enteritis and dysentery in clinical practice (1, 5). Berberine has been widely used in research and in the clinic for its anti-inflammatory properties. As early as, Kuo et al. (6) found that berberine can inhibit basal levels and TPA-mediated PGE2 levels and COX-2 expression by inhibiting AP-1 binding, confirming that berberine has a significant potential anti-inflammatory effect in vivo (6, 7). Furthermore, berberine may also act on vascular endothelial cells and immune cells via different mechanisms of action (7). It is well-documented that berberine is an effective medicine for the treatment of intestinal inflammatory diseases such as colitis, diarrhea, and dysentery due to its antibacterial activity against Escherichia coli, Helicobacter dysenteriae, and enterococci (4, 5). At the same time, berberine also has inhibitory effects on other Gram-positive and Gram-negative bacteria, including different strains of Staphylococcus, Actinobacillus pleuropneumoniae, Shigella dysenteriae, and Streptococcus agalactiae (5, 7). Moreover, its biological activities against dermatophytes such as Trichophyton violaceum and Microsporum canis have also been reported and studied (8). Berberine exhibits broad antibacterial effects, and as a natural alkaloid, it has unique advantages in counteracting bacterial resistance. Wojtyczka et al. (9) showed that berberine had significant synergistic effects with linezolid, cefoxitin, erythromycin, and other antibiotics. The authors also suggested that berberine may be an effective tool for the treatment of antibiotic-resistant bacterial infections. Based on the hypoglycemic and anti-inflammatory properties and broad-spectrum antibacterial ability of berberine, Zhang et al. (10) made berberine into the form of a nanohydrogel and explored its effect on wound healing in diabetic rats. The results showed that berberine could indeed effectively promote the repair of diabetic wounds. Samadian et al. (11) also chose berberine as a drug for wound dressing for nursing diabetic ulcers and resulted in a positive effect on promoting wound healing. Aside from antibacterial and anti-inflammatory effects, berberine may also have a certain effect on promoting wound healing.
Bullous pemphigoid is the most common form of pemphigoid, a rare condition affecting the elderly over 70 years of age. Typical clinical features include tension, serous or hemorrhagic bullae with a 1–3 cm diameter on normal or erythematous skin, accompanied by severe itching (12). BP usually occurs in the lateral limbs and lower abdomen. The mucous membrane is involved in 10–20% of patients, and the oral mucosa is more vulnerable than the conjunctiva, nose, pharynx, esophagus, and anus (2). Before forming blisters, there is usually non-bullous pruritus or signs of eczema, papules, and other skin lesions as prodromal symptoms. Such atypical symptoms can easily lead to misdiagnosis. Currently, a detailed medical history plays a vital role, including the specific circumstances of pruritus and the development of lesions, related neurological diseases, malignant tumors, autoimmune diseases and recently used drugs (2, 12). Takegami et al. (13) reported a case of the coexistence of Sjogren’s syndrome and BP in which tension blisters and erosive lesions of the skin were seen and oral mucosa was involved. Although there is no proven link between Sjogren’s syndrome and blistering disease, the patient’s long history of Sjogren’s syndrome and skin lesions around the eyes and mouth, in this case, may induce concern. According to Lucariello et al. (14), there is a certain correlation between BP and malignant tumors based on a meta-analysis. Last, aldosterone antagonists, DPP-4 inhibitors, anticholinergic medications, and dopaminergic medicines have all been linked to an increased risk of developing BP in other related studies (15). Chen et al. (16) meta-analysis concluded that dementia, stroke, heart disease, and diabetes are important factors contributing to the poor prognosis of patients with BP. As a result, special attention should be given to these patients with potential risks. Nevertheless, the diagnosis of BP is based mostly on skin histological biopsy and immunofluorescence confirmation.
At present, the treatment of BP is mainly glucocorticoids supplemented by antibiotics or steroids. According to the German guidelines, the severity of the disease is determined by the extent of skin involvement in BP, with 10% or less being defined as mild, 10–30% as moderate, and more than 30% as severe BP (12). Mild BP should be treated with topical corticosteroids alone, while moderate BP should be treated with other adjuvant therapies in combination with topical corticosteroids, and severe BP should be treated with systemic corticosteroids (12). Clobetine propionate cream (17) is a frequent topical steroid, while oral prednisolone is the most common systemic glucocorticoid therapy (12). In addition to hormones, drugs such as tetracycline and nicotinamide, azathioprine, doxycycline, mycophenolate mofetil, and dapsone are often used as adjuvant or alternative treatments (Table 1; 18–22). Furthermore, according to Engineer et al., intravenous immunoglobulin is an alternative hormone therapy worth investigating because it is effective in the clinic, particularly in resistant BP, with minimal adverse reactions (23). Other treatment options, such as immunoadsorption (24), biological monoclonal antibodies (25), targeted therapy (26), and other approaches, are also worthy of further exploration. In addition to Western medicine treatment, traditional Chinese medicine treatment is currently receiving increasing attention, and its efficacy is considerable. For example, Ge et al. (27) discovered that Tripterygium wilfordii Hook F was safe and effective in treating mild to moderate BP, with much fewer adverse effects than hormone treatment.
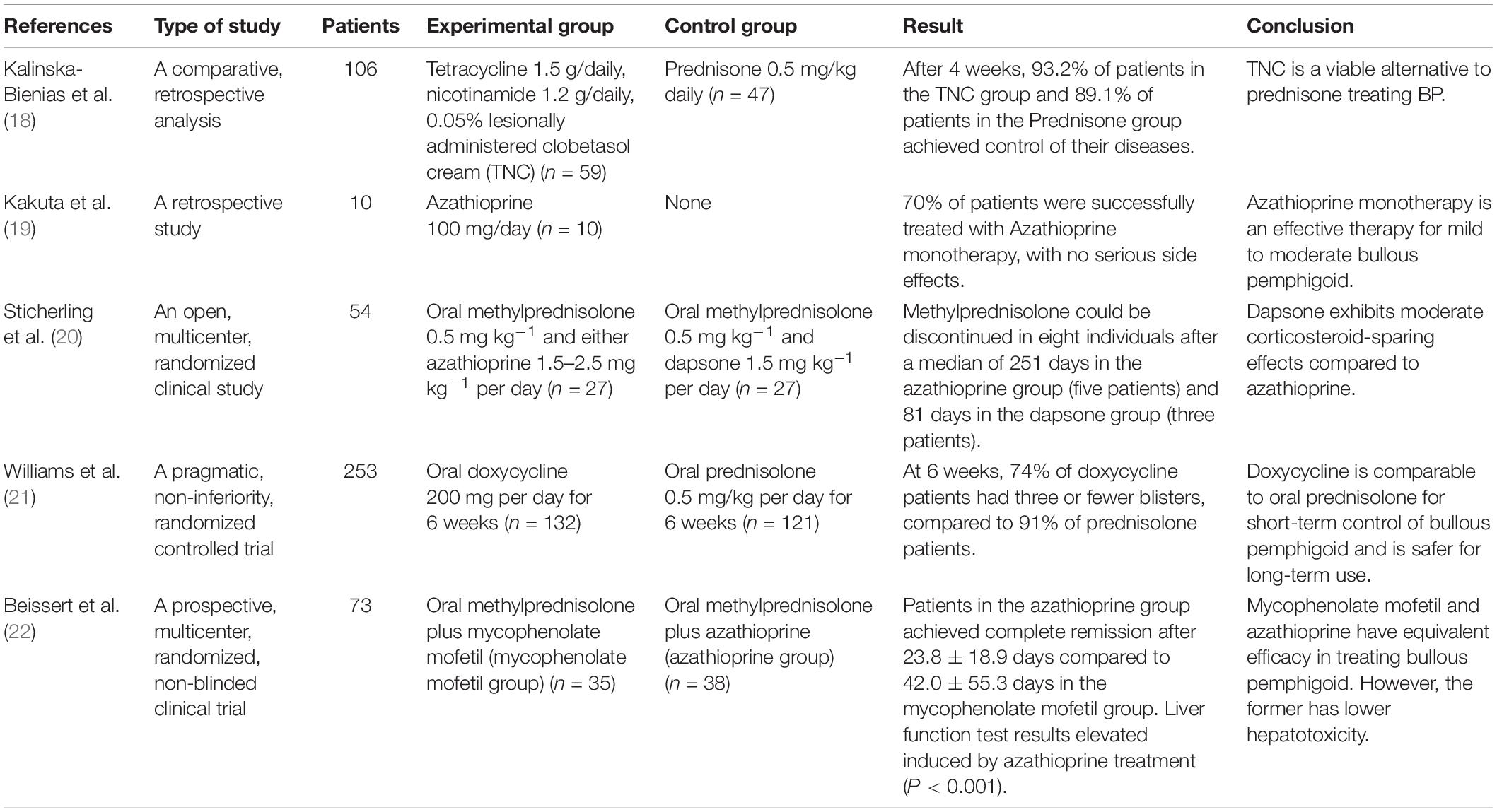
Table 1. Summary of clinical studies on adjunct and alternative hormone therapies for bullous pemphigoid (BP).
In this case, we chose berberine primarily based on traditional Chinese medicine’s basic theory and syndrome differentiation for patients. We considered berberine’s efficacy in clearing away heat and toxic substances, drying dampness, and relieving pain, and the symptomatic treatment of patients with pemphigus of the damp-heat accumulation type. In this case, we used a unique pharmaceutical dosage form called the “stamp therapy” method, from the way gauze adhered to the eroding skin like a stamp (Figure 2D; 28). This method was also a TCM nursing method distilled from clinical practical experience. Studies have shown that this method can effectively protect wounds, promote wound healing, and reduce the suffering herpes zoster patients (28). In this case, the patient’s wound recovery process was smooth, owing to the antibacterial and anti-inflammatory actions of berberine, and its wound healing effects. The usage of stamp therapy for wound care also protected the patient’s large area of blisters and ulcerating, and eroding wounds from serious infection and complications. Despite the lack of a clear understanding of the mechanistic role of berberine in wound healing, these ongoing investigations and clinical results provide a clinical foundation upon which berberine might be further studied and new therapeutic ideas for BP generation.
In conclusion, the advantages of traditional Chinese medicine and external treatment to treat skin diseases are becoming increasingly apparent. Whether as the primary treatment or as a supplementary nursing means, these treatment methods can achieve fantastic outcomes and significantly reduce the occurrence of adverse reactions and complications while reducing pain and improving quality of life.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LC and YW participated in writing the manuscript. WG and GY participated in patient management. DG, FL, and JZ contributed to critical revision. HL was responsible for the preparation of the tables and figures. All authors have read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Nos. 81803982 and 82074428), Clinical Research Plan of Shanghai Shenkang Hospital Development Center (No. SHDC2020CR4041), Shanghai Municipal Commission of Science and Technology (Nos. 20XD1423600 and 20DZ2301900), Sailing Program of Shanghai Rising-Star Program (No. 22YF1449700), the Project of Shanghai University of Traditional Chinese Medicine (Nos. Y2020064 and Y2021019), and Shanghai Municipal Commission of Economy and Information Technology, Shanghai Artificial Intelligence Innovation and Development Project-Intelligent Dermatology Clinic Based on Modern TCM Diagnostic Technology (No. 2020-RGZN-02038).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. (2013) 381:320–32. doi: 10.1016/S0140-6736(12)61140-4
2. Beek N, Zillikens D, Schmidt E. Bullous autoimmune dermatoses–clinical features, diagnostic evaluation, and treatment options. Dtsch Arztebl Int. (2021) 118:413–20. doi: 10.3238/arztebl.m2021.0136
3. Kridin K, Shihade W, Bergman R. Mortality in patients with bullous pemphigoid: a retrospective cohort study, systematic review and meta-analysis. Acta Derm Venereol. (2019) 99:72–7. doi: 10.2340/00015555-2930
4. Habtemariam S. Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol Res. (2020) 155:104722. doi: 10.1016/j.phrs.2020.104722
5. Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): a clinical review. Phytother Res. (2019) 33:504–23. doi: 10.1002/ptr.6252
6. Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. (2004) 203:127–37. doi: 10.1016/j.canlet.2003.09.002
7. Imenshahidi M, Hosseinzadeh H. Berberis vulgaris and berberine: an update review. BMC Complement Altern Med. (2016) 30:1745–64. doi: 10.1002/ptr.5693
8. Xiao CW, Ji QA, Wei Q, Liu Y, Bao GL. Antifungal activity of berberine hydrochloride and palmatine hydrochloride against microsporum canis –induced dermatitis in rabbits and underlying mechanism. BMC Complement Altern Med. (2015) 15:177. doi: 10.1186/s12906-015-0680-x
9. Wojtyczka RD, Dziedzic A, Kępa M, Kubina R, Kabała-Dzik A, Mularz T, et al. Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative staphylococcus strains in vitro. Molecules. (2014) 19:6583–96. doi: 10.3390/molecules19056583
10. Zhang P, He LB, Zhang J, Mei XF, Zhang YY, Tian H, et al. Preparation of novel berberine nano-colloids for improving wound healing of diabetic rats by acting Sirt1/NF-κb pathway. Colloids Sur. (2020) 187:110647. doi: 10.1016/j.colsurfb.2019.110647
11. Samadian H, Zamiri S, Ehterami A, Farzamfar S, Vaez A, Khastar H, et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: in vitro and in vivo studies. Sci Rep. (2020) 10:8312. doi: 10.1038/s41598-020-65268-7
12. Bağcı IS, Horváth ON, Ruzicka T, Sárdy M. Bullous pemphigoid. Autoimmun Rev. (2017) 16:445–55. doi: 10.1016/j.autrev.2017.03.010
13. Takegami Y, Makino T, Matsui K, Ueda C, Fukuda S, Hashimoto T, et al. Coexistence of antilaminin-332-type mucous membrane pemphigoid, lamina lucida-type linear IGA bullous dermatosis and sjogren syndrome. Clin Exp Dermatol. (2013) 38:194–6. doi: 10.1111/ced.12030
14. Lucariello RJ, Villablanca SE, Mascaró JM, Reichel M. Association between bullous pemphigoid and malignancy: a meta-analysis. Australas J Dermatol. (2018) 59:253–60. doi: 10.1111/ajd.12764
15. Liu SD, Chen WT, Chi CC. Association between medication use and bullous pemphigoid: a systematic review and meta-analysis. JAMA Dermatol. (2020) 156:891–900. doi: 10.1001/jamadermatol.2020.1587
16. Chen XX, Zhang YQ, Luo ZC, Wu YJ, Niu TX, Zheng JY, et al. Prognostic factors for mortality in bullous pemphigoid: a systematic review and meta-analysis. PLoS One. (2022) 17:e0264705. doi: 10.1371/journal.pone.0264705
17. Terra JB, Potze WJ, Jonkman MF. Whole body application of a potent topical corticosteroid for bullous pemphigoid. J Eur Acad Dermatol Venereol. (2014) 28:712–8. doi: 10.1111/jdv.12153
18. Kalinska-Bienias A, Kowalczyk E, Jagielski P, Kowalewski C, Wozniak K. Tetracycline, nicotinamide, and lesionally administered clobetasol as a therapeutic option to prednisone in patients with bullous pemphigoid: a comparative, retrospective analysis of 106 patients with long-term follow-up. Int J Dermatol. (2019) 58:172–7. doi: 10.1111/ijd.14270
19. Kakuta R, Yamagami J, Funakoshi T, Takahashi H, Ohyama M, Amagai M. Azathioprine monotherapy in autoimmune blistering diseases: a feasible option for mild to moderate cases. J Dermatol. (2018) 45:334–9. doi: 10.1111/1346-8138.14173
20. Sticherling M, Franke A, Aberer E, Glaser R, Hertl M, Pfeiffer C, et al. An open, multicentre, randomized clinical study in patients with bullous pemphigoid comparing methylprednisolone and azathioprine with methylprednisolone and dapsone. Br J Dermatol. (2017) 177:1299–305. doi: 10.1111/bjd.15649
21. Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec TR, Schmidt E, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet. (2017) 389:1630–8. doi: 10.1016/S0140-6736(17)30560-3
22. Beissert S, Werfel T, Frieling U, Böhm M, Sticherling M, Stadler R, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of bullous pemphigoid. Arch Dermatol. (2007) 143:1536–42. doi: 10.1001/archderm.143.12.1536
23. Engineer L, Ahmed AR. Role of intravenous immunoglobulin in the treatment of bullous pemphigoid: analysis of current data. J Am Acad Dermatol. (2001) 44:83–8. doi: 10.1067/mjd.2000.112288
24. Muller PA, Brocker EB, Klinker E, Stoevesandt J, Benoit S. Adjuvant treatment of recalcitrant bullous pemphigoid with immunoadsorption. Dermatology. (2012) 224:224–7. doi: 10.1159/000339071
25. Bishnoi A, De D, Handa S, Mahajan R. Biologics in autoimmune bullous diseases: current scenario. Indian J Dermatol Venereol Leprol. (2021) 87:611–20. doi: 10.25259/IJDVL_886_19
26. Garrido P, QueiróS C, Travassos A, Borges-Costa J, Filipe P. Emerging treatments for bullous pemphigoid. J Dermatolog Treat. (2022) 33:649–61. doi: 10.1080/09546634.2020.1782325
27. Ge XL, Li SZ, Jin X, Jin HZ, Zuo YG. Treatment of bullous pemphigoid in Chinese patients with Tripterygium wilfordii Hook F. Dermatol Ther. (2020) 33:e13721. doi: 10.1111/dth.13721
Keywords: bullous pemphigoid, autoimmune dermatoses, berberine, stamp therapy, treatment, case report
Citation: Cheng L, Wang Y, Lu H, Guo W, Yan G, Zhu J, Guo D and Li F (2022) Successful Treatment of Bullous Pemphigoid Lesions by Berberine Stamp Therapy: A Case Report and Literature Review. Front. Med. 9:938761. doi: 10.3389/fmed.2022.938761
Received: 08 May 2022; Accepted: 13 June 2022;
Published: 01 July 2022.
Edited by:
Meng Pan, Shanghai Jiao Tong University, ChinaReviewed by:
Maryam Nasimi, Tehran University of Medical Sciences, IranCopyright © 2022 Cheng, Wang, Lu, Guo, Yan, Zhu, Guo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongjie Guo, Z2RvbmdqaWUuMTk5MkAxNjMuY29t; Fulun Li, ZHJsaWZ1bHVuQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.