
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Med. , 04 October 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.937963
This article is part of the Research Topic Clinical Teaching and Practice in Intensive Care Medicine and Anesthesiology View all 17 articles
Background: Reducing adverse effects during cesarean delivery and improving the quality of sensory blocks with appropriate doses of intrathecal hyperbaric bupivacaine can play an important role in the safe management of cesarean delivery. The aim of this study was to compare the doses of 10 and 12 mg of intrathecal hyperbaric bupivacaine 0.5% on sensory block level after first spinal failure in cesarean section (CS).
Methods: In this double-blind, randomized clinical trial, 40 candidates of CS after first spinal failure with class I-II based on American Society of Anesthesiologists (ASA) were randomly assigned into two equal groups (n = 20). Group A and B received the spinal anesthesia with 10 mg and 12 mg of hyperbaric bupivacaine (0.5%), respectively. Maximum levels of sensory block, motor block quality, and vital signs were measured in two groups by 60 min after SPA. Incidence of SPA complications during surgery were also recorded. Data were analyzed by SPSS ver.21 software using repeated measures analysis of variance at 95% confidence interval (CI) level.
Results: Excellent quality of sensory blocks and complete quality of motor blocks were achieved in all participants (100%). However, the mean time to onset of anesthesia (4.47 ± 0.69 vs. 3.38 ± 0.47, P < 0.001) and time to reach T10 level (60.73 ± 11.92 vs. 79.00 ± 19.21, P < 0.001) in the Group A, were significantly shorter than in the patients of Group B. The incidence of hypotension (P = 0.001), nausea/vomiting (P = 0.007) and bradycardia (P = 0.012) as well as administration of ephedrine and atropine were significantly higher in Group B compared to Group A.
Conclusion: Spinal anesthesia can be safely repeated with a 10 mg of hyperbaric bupivacaine 0.5% in a caesarean section after the initial spinal failure.
Clinical trial registration: [https://en.irct.ir/trial/40714], identifier [IRCT20120915010841N20].
Spinal anesthesia (SPA) is the most common, safest, and most rational choice for cesarean section (1). SPA is secure and effective, but not a 100% successful technique and complications have been part of the method (2), including failed or insufficient sensory block (3), postdural puncture headache (PDPH) (4), hypotension (5), bradycardia (6), nerve damage (7), nausea and vomiting (8). A specific level of sensory block is required in any surgery performed under SPA. In cesarean section (CS), the level of T4-T6 anesthesia is appropriate (9). Elevated level of sensory block (≥ T4) will cause hypotension, nausea and vomiting, decreased level of consciousness and maternal discomfort. Conversely, lower level of sensory block (≤ T6) will not provide adequate anesthesia for CS, and causes discomfort and dissatisfaction in the patient (10). Intrathecal anesthetic spread has an unpredictable extent and duration that can be related to various factors such as dosage, patient variables, cerebrospinal fluid (CFS) volume, injection rate, and injection site (11).
One of the issues of SPA is the failure of spinal anesthesia, which means that SPA has been performed but not enough sensory block has been provided for surgery (12). Failed SPA can be identified as partial or incomplete spinal block within 10 minutes after hyperbaric bupivacaine injection and 25 minutes after isobaric bupivacaine anesthesia (13). Failure rates in SPA have been reported from 1 to 17% in various 1-17% (14). However, major studies have reported a prevalence range of 2 to 4% (12, 15). Obesity, dry cerebrospinal fluid (CSF), bloody CSF, improper dose, incorrect anesthesia distribution, multiple lumbar puncture attempts, use of the L4/L5 interspace, history of previous anesthesia and technical errors are significantly associated with failed SPA (3, 16). Complete failure of the SPA can be managed by switching to general anesthesia or by repeating the SPA procedure (12). However, since most pregnant patients are at risk for aspiration and intubation problems, general anesthesia carries a relatively higher risk for this population and re-performing SPA is a better and safer choice (17).
Administration of an appropriate dose hyperbaric bupivacaine can minimize potential side effects while improving block quality (18). However, the optimal intrathecal dose of hyperbaric bupivacaine for SPA is still being debated. Previous studies have investigated and demonstrated the effects of different doses on sensory and motor blocks in different ways (19, 20). In addition, very few studies have been found on the appropriate dose of hyperbaric bupivacaine for cesarean section during repeated spinal anesthesia (21). Therefore, we conducted this study to compare the doses of 10 and 12 mg of intrathecal hyperbaric (0.5%) bupivacaine on sensory block level and PDPH after first spinal failure in cesarean section.
This prospective, double blind, parallel-group, randomized clinical trial study was conducted in Fatemiyeh Hospital in Hamadan, Iran, from July 2018 to July 2019. The protocol study was reviewed and approved by the Ethics Committees of Hamadan University of Medical Sciences (IR.UMSHA.REC.1398.232). This study Registered at Iranian Registry of Clinical Trials (IRCT20120915010841N20). Written informed consent were obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association (22). This study was performed and reported in accordance with the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement (23).
The study population consisted of parturient with class I-II of American Society of Anesthesiologists (ASA), aged 18 to 46 years, the height range 170-155 cm, a second SPA candidate after the first failed SPA (Bromage score 0 and no sensory block even at L4 dermatome after 10 min of first hyperbaric bupivacaine injection). Patients with a history of hypertensive pregnancy disorders, heart disease, Bromage scale >0, lack of pinprick sensation below umbilicus after spinal anesthesia, and of allergies to the study drug were excluded from the trial.
Forty parturient were selected by a convenience sampling method based on inclusion criteria. Patients were then randomized into two SPA groups containing 10 mg of hyperbaric bupivacaine 0.5% (Group A) and 12 mg of hyperbaric bupivacaine 0.5% (Group B). Equal number of patients were assigned to each group using the block randomization method (n = 20). Patients were assigned to Group A or Group B on a computer-generated random number selected by the patient using Random Allocation Software © (RAS; Informer Technologies, Inc., Madrid, Spain). The level of spinal block and the duration of hemodynamic sensory variables were compared between the two groups. Both the patients and the evaluator were blind to the assignments.
The Pre-SPA procedure was started for each patient as follows: Lactated Ringer serum (10 ml/kg) was injected using an 18-gauge needle depending on the patient’s weight. Standard monitoring includes electrocardiography, pulse oximetry and non-invasive blood pressure (NIBP), vital signs such as systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate (HR) and oxygen saturation (SpO2) was measured and recorded using an X162 monitor (Saadat Company, Iran).
SPA contains 10 mg of hyperbaric bupivacaine 0.5% (AstraZeneca Company, France) plus 2.5 μg of sufentanil (Abu Rayhan Company, Iran) using Quinke needle size 25 through lower lumbar (L3-4 or L4-5) intervertebral spaces in a sitting position was administered. The patient immediately lay on his back with a wedge under his right hip and was monitored for vital sign (SBP, DBP, MBP, HR, and SpO2). The effects of sensory and motor blocks were observed within 5 min, those that did not show efficacy within 5 min were observed for an additional 5 min and tested for motor and sensory blocks again. Those ASA I-II patients with insufficient motor and sensory block (Bromage score 0 and no sensory block even at L4 dermatome after 10 min of first hyperbaric bupivacaine injection) were considered for inclusion in the present study and they randomly (as described above) allocated to either Group A (10 mg of hyperbaric bupivacaine 0.5%) or Group B A (12 mg of hyperbaric bupivacaine 0.5%).
Patients in Group A received 10 mg and patients in Group B received 12 mg of 0.5% high hyperbaric bupivacaine (AstraZeneca Company, France), respectively and 2.5 μg of sufentanil (Abu Rayhan Company, Iran). Taking the same precautions, a 25-gauge Quincke needle was placed above or below the gap in the first attempt and the submucosal block was performed again (one space above in patients where previous spinal was attempted at L4-5 interspace or one space below in those patients who had previous spinal at L3-4 interspace) by a senior anesthesiologist who have worked in anesthesiology for over 10 years. The patient immediately lay on his back, a wedge under his right buttock displaced the left uterus, and monitoring (sensory block, motor block, and vital signs) was initiated. Surgery could be started after the initiation of spinal anesthesia was confirmed by a proper movement block of the lower extremities without a pinching sensation. If the patient complained of a pin-stab sensation 10 minutes after repeated spinal cord administration, general anesthesia was given and the patient was excluded from the study.
The maximum level of sensory block was assessed by the pin-prick method using a 25-gauge needle, the time to reach the maximum level of anesthesia and the time to reach the T10 level as primary outcomes, were recorded for each patient. The sensory block quality and pain were assessed using the visual analog scale (VAS). VAS scores were recorded by creating a handwritten mark on a 10 cm line indicating the chain between “excellent” and “poor” (24), which described excellent postoperative quality as none (0), mild (< 3), moderate (3-6), or severe (7-10). The quality of the motion block was assessed using the Bromage Scale (25). A modified Bromage Scale was used: 0 = no motor block; 1 = able to flex knee free movement of feet, unable to raise extended leg (partial motor block); 2 = free movement of feet only (almost complete motor block); 3 = unable to move hips, knees, feet (complete motor block). Sedation was evaluated by Ramsay scale, it divides a patient’s level of sedation into six categories ranging from severe agitation to deep coma; 1 = anxious and agitated or restless or both; 2 = co-operative, oriented and tranquil; 3 = responding to commands only; 4 = brisk response to light glabellar tap or loud auditory stimulus; 5 = sluggish response to light glabellar tap or loud auditory stimulus; 6 = no response to stimulus (26).
Vitals parameters including SBP, DBP, MBP, HR, and SpO2 were measured at baseline (pre-SPA procedure), immediately after SPA procedure, and 2,4,6,8, 10, 15, 20, 30, 40, 50, and 60 min of post postoperatively. SBP less than 90 mmHg and bradycardia (heart rate less than 60 beats per minute) were treated with incremental intravenous doses of 10 mg ephedrine and 0.5 mg intravenous atropine, respectively. Finally, the amount of ephedrine and atropine used, the occurrence of nausea and vomiting during surgery, the time of onset of anesthesia and the maximum level of anesthesia (using a needle or pinprick), the quality of sensory (VAS scores) and motor block (Bromage Scale), the time of anesthesia to T10, sedation score (Ramsay scale) and Apgar score (in minutes 1 and 5) for infants was examined and recorded for each participants. Nausea and Vomiting, headache, hypotension (BP < 90/60mmHg), bradycardia (HR < 60/min), chills and high spinal were recorded during procedure for each patient. In addition, one week after surgery, patients were evaluated and questioned by researcher over the phone about the presence or absence of postdural puncture headache (PDPH).
Power calculations was done based on primary outcome, time to reach T10 level in two group of study (60.73 ± 11.92 in 10 mg of hyperbaric 0.5% vs. 79.00 ± 19.21 in 12 mg of hyperbaric 0.5%, P < 0.001). Analyses according to the sample size of 20 patients in each group with considering the type I error (α) set as two-sided 5% (Z1-α/2 = 1.96) and type II error (β) set as 20% (Z1-β = 0.84), estimated the power of the test equal to 100%. It should be noted that with a power level of 80 and 95% confidence interval (CI), a sample size equal to 13 patients in each group was sufficient to detect clinically significant differences between the two groups. Analyzing the power of the test using Stata 11 software. Variables were expressed as mean ± standard deviation (SD) or percentage (%) for continuous and discrete variables, respectively. Results were analyzed by independent t-test (between groups), and paired t-test (within group) for parametric data and Mann–Whitney U-test for non-parametric data. Fisher’s exact test and Chi-square test were used for categorical data as appropriate. The Shapiro-Wilk test was conducted to test whether the data were normally distributed. Using a general linear model, hemodynamic changes and complications between the two groups were compared using a repeated measurement ANOVA test, with the baseline values (age) used as covariates in the model. The assumption of sphericity was addressed by Mauchly’s test of sphericity, and when the assumption was not satisfied, the Greenhouse-Geiser correction of P-value were utilized. To assess the effect of intervention, the analysis of covariance (ANCOVA) was used after controlling for baseline measures and confounders in a two-step hierarchical model. Logistic regression analysis was used to predict incidence of complications according to influencing 12 mg dose of hyperbaric bupivacaine 0.5% compare to 10 mg, and the significant variables were reported as odds ratio (OR) with 95% confidence interval (CI). GraphPad Prism 9© (GraphPad Software Inc., La Jolla, CA) was used to show the changes of hemodynamic parameters in two groups of study (12 mg vs. 10 mg of hyperbaric 0.5%) over times. Statistical analysis was carried out using SPSS software (ver.21) (SPSS Inc., IL, Chicago, United States). In all analyses, P-values less than 0.05 were considered as significant.
A total of forty parturient were included in the study and Figure 1 shows the patient registration flow chart. Fifty parturient with class I-II of ASA, who candidate of SPA for non-emergent cesarean section after the first failed SPA with Bromage score 0 and no sensory block after 10 min of first hyperbaric bupivacaine injection were screened for eligibility criteria. Out of 50 cases, 40 patients met the inclusion criteria and randomly assigned into two equal groups (n = 20); Group A (received 10 mg of hyperbaric bupivacaine 0.5%) and Group B (received 12 mg of hyperbaric bupivacaine 0.5%). During the intervention and follow-up stages, only one patient in the group A underwent general anesthesia due to failed of SPA and was excluded from the study. Totally 39 patients were analyzed, 19 and 20 patients in the Group A and B, respectively.
Comparison of baseline demographics and hemodynamic parameters between the two groups of study is presented in Table 1. There were no statistically significant differences in demographic and baseline hemodynamic parameters of parturient such as; age (P = 0.722), SBP (P = 0.985), DBP (P = 0.398), MBP (P = 0.531), HR (P = 0.372) and SpO2 (P = 0.682) in the Group A and Group B.
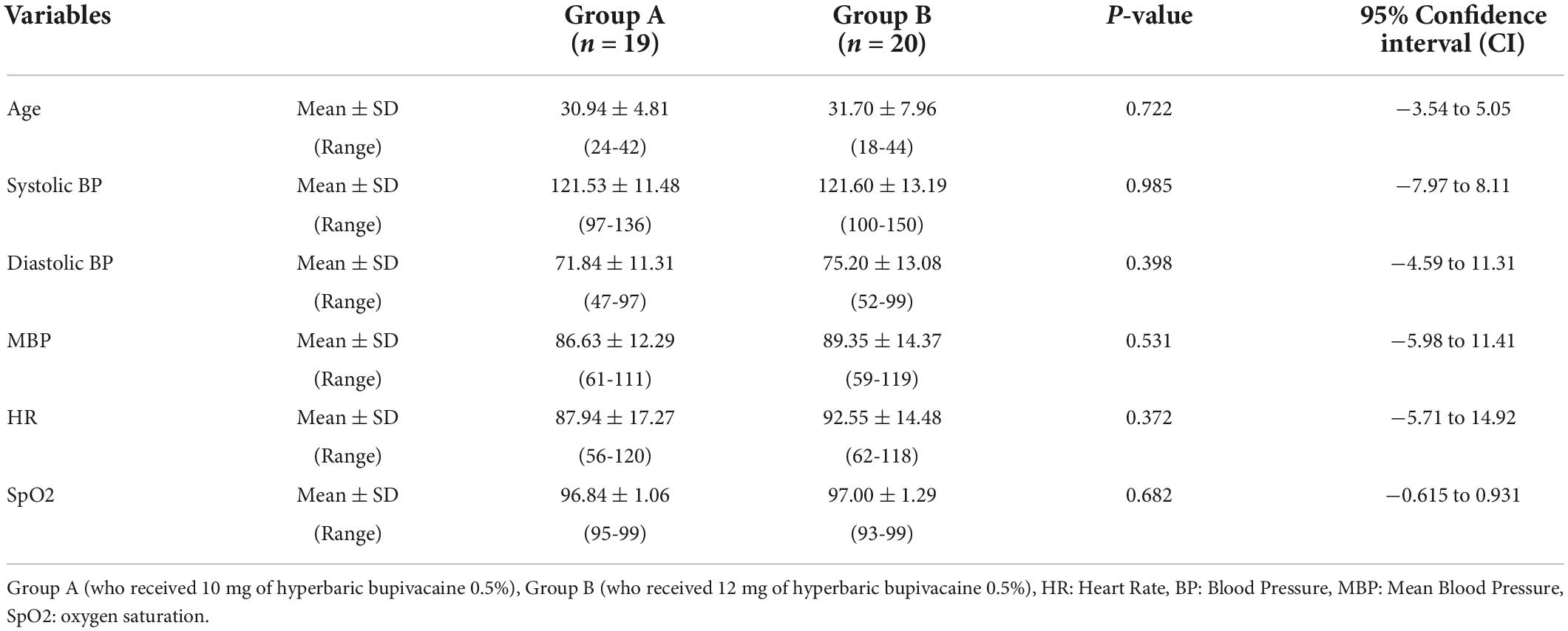
Table 1. Comparison of demographic and baseline hemodynamic parameters between the two study groups.
Comparison of spinal anesthesia characteristics and outcomes between the two groups of study are presented in Table 2. Excellent and complete quality of sensory and motor blocks was observed in all participants in the both groups. However, the mean time to onset of anesthesia (4.47 ± 0.69 vs. 3.38 ± 0.47, P < 0.001) and the mean time to reach T10 level (60.73 ± 11.92 vs. 79.00 ± 19.21, P < 0.001) in the Group A were significantly shorter than in the Group B. According to the results, in most patients in Group A the sensory level reached to T6 (n = 17, 89.5%), while the sensory level of more than half of the patients in Group B reached to T4 (n = 13, 65%). In terms of sensory level at recovery, nearly half of the patients (47.4%) in Group A had sensory level T12, while 50% of participants in the Group B had sensory level T8. There was statistically significant difference between two groups of the study in terms on maximum sensory level (P < 0.001) and also in sensory level in recovery (P < 0.001). The use of Ephedrine (85% vs. 31.6%, P < 0.001) and Atropine (30% vs. 0) in the group B who received SPA with 12 mg of hyperbaric bupivacaine 0.5% was significantly higher than the Group A. And finally, the satisfaction of patients in the Group B was significantly lower than the Group A. However, there was no significant difference in the Apgar scores of the neonates in the first minute (8.78 ± 0.71 vs. 8.95 ± 0.39, P = 0.387), and fifth minutes (9.94 ± 0.22 vs. 9.90 ± 0.31, P = 0.591) between two groups.
The time trend of hemodynamic parameters (SBP, DBP, MBP, HR, and SpO2) in the two study groups is presented in Table 3. This parameters were recorded at pre-SPA and immediately after SPA and then every 2 min up to 10-min, and then every 5 min up to 30-min, and then every 10 min up to 60-min after injection of anesthetic drug. Figure 2A shows the mean values of SBP changes in each group over time. The results showed that there was no significant difference in SBP between the two groups except for 4 and 8 min when SBP in Group A was significantly higher than Group B (P = 0.002 and P = 0.038, respectively), also in 30 min that the SBP in Group B was higher than Group A (P = 0.033). In within group, the effect of time on SBP in each group was statistically significant (a within-subject difference based on time effect) (P = 0.05). However, based on repeated measures analysis of variance (RMANOVA), the trend of changes in SBP levels between the two groups was not statistically significant (group * time interaction or an interaction effect) (P = 0.362).
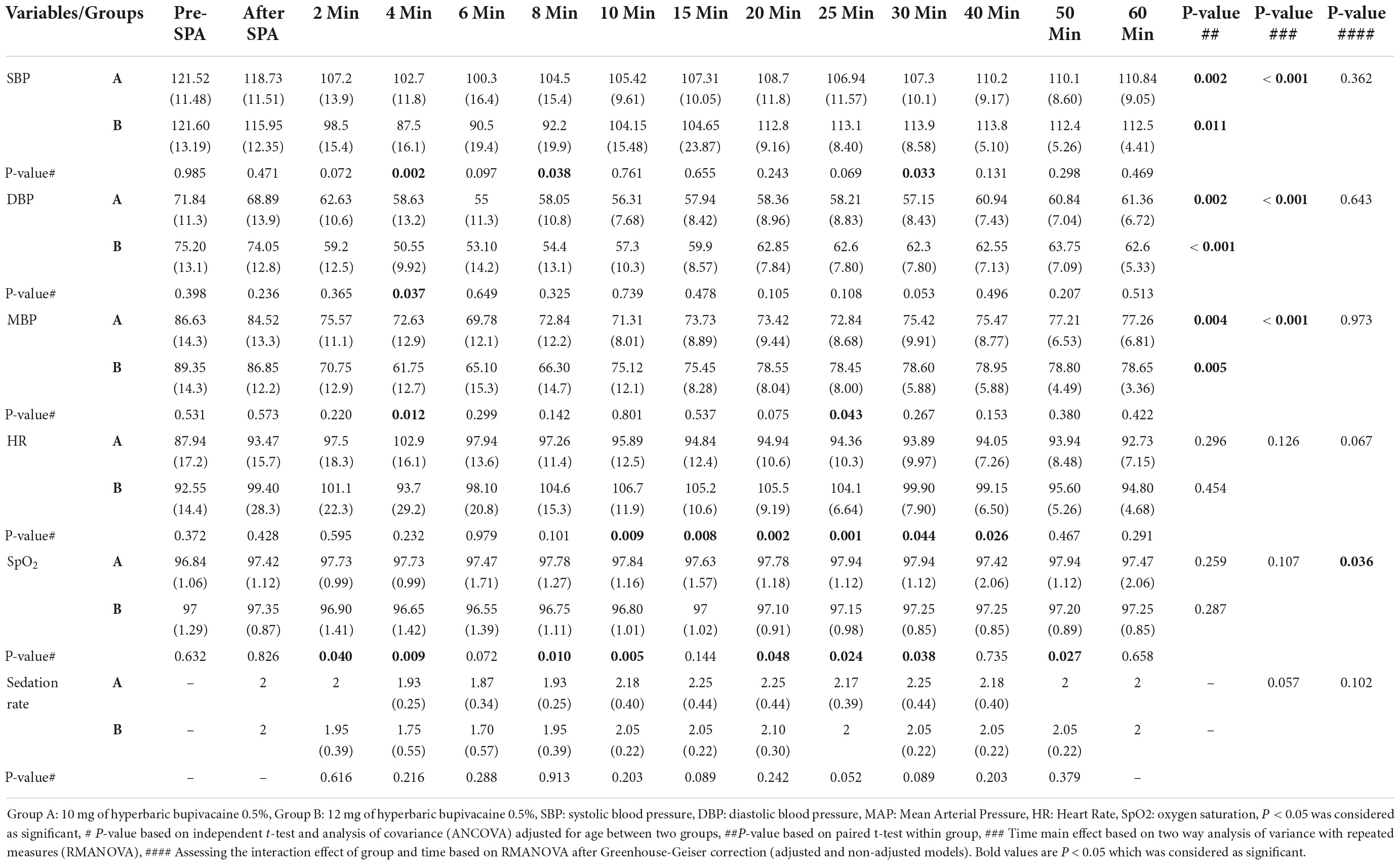
Table 3. Comparison of hemodynamic parameters and sedation rate based on Ramsay scale in two groups of study.
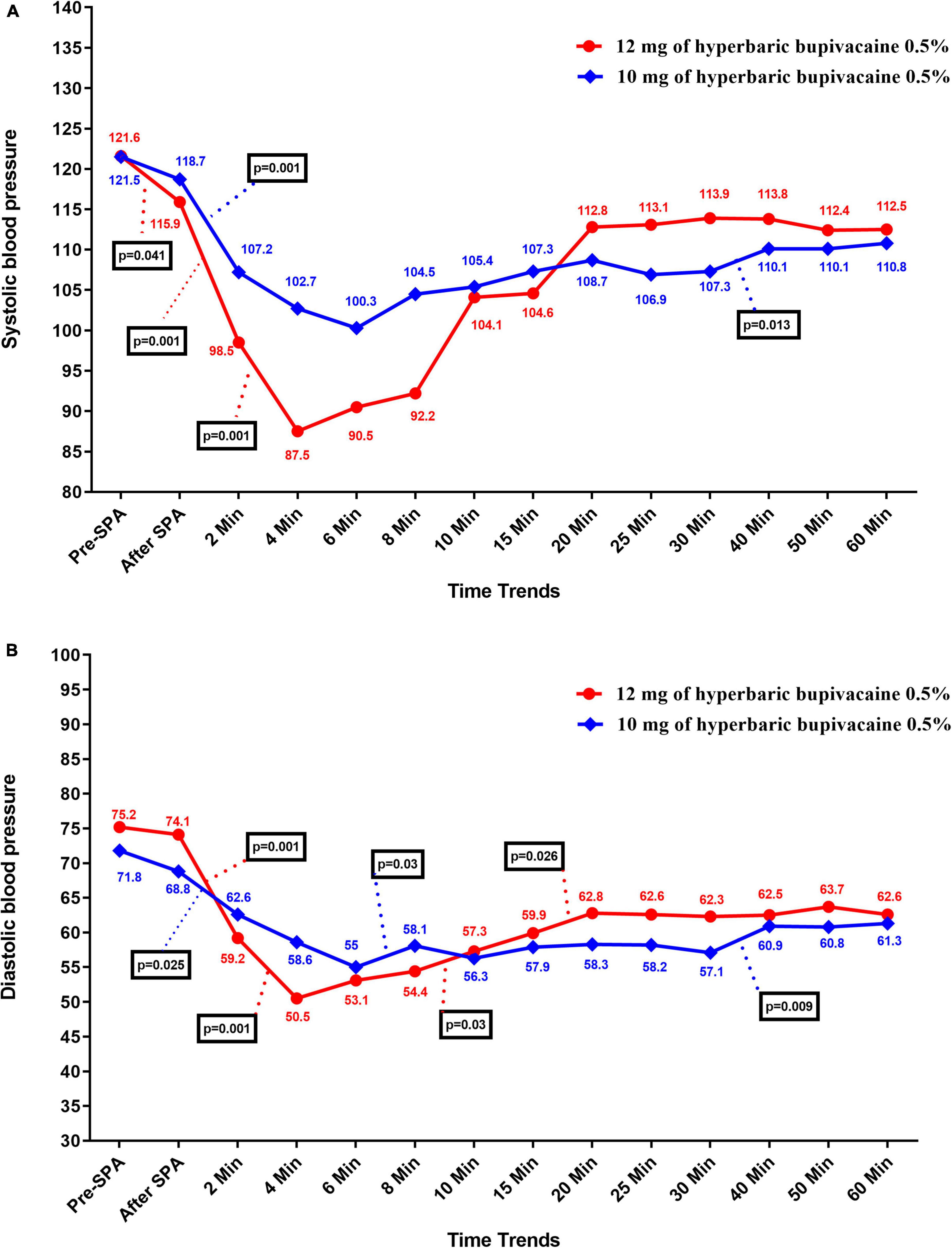
Figure 2. Changes (A) systolic and (B) diastolic blood pressure in two groups of study over times, *P-values shows statistically significant between two times within groups.
As shown in Figure 2B, there was no significant difference in DBP between the two groups except at 4 min, when DBP in Group A was significantly higher than Group B (58.63 ± 13.25 vs. 50.55 ± 9.92, P = 0.037). Within the group, the effect of time on DBP was statistically significant in each group (difference within the subject based on the effect of time) (P < 0.05). However, the trend of changes in DBP levels between the two groups (group * time interaction or an interaction effect) (P = 0.643).
Figure 3A shows the mean values for changes of MBP in each group over times. According to the results, there was no significant difference in MBP between the two groups except at 4 min (72.63 ± 12.95 vs. 61.75 ± 12.73, P = 0.012) and 25 min (72.84 ± 8.68 vs. 78.45 ± 8, P = 0.043) when MBP in Group A was significantly higher and lower than Group B, respectively. In within group, the effect of time on MBP was statistically significant in Group A (P = 0.004) and Group B (P = 0.005) (a within-subject difference based on time effect). However, the trend in changes in MBP levels was not statistically significant between two groups (group * time interaction or an interaction effect) (P = 0.935).
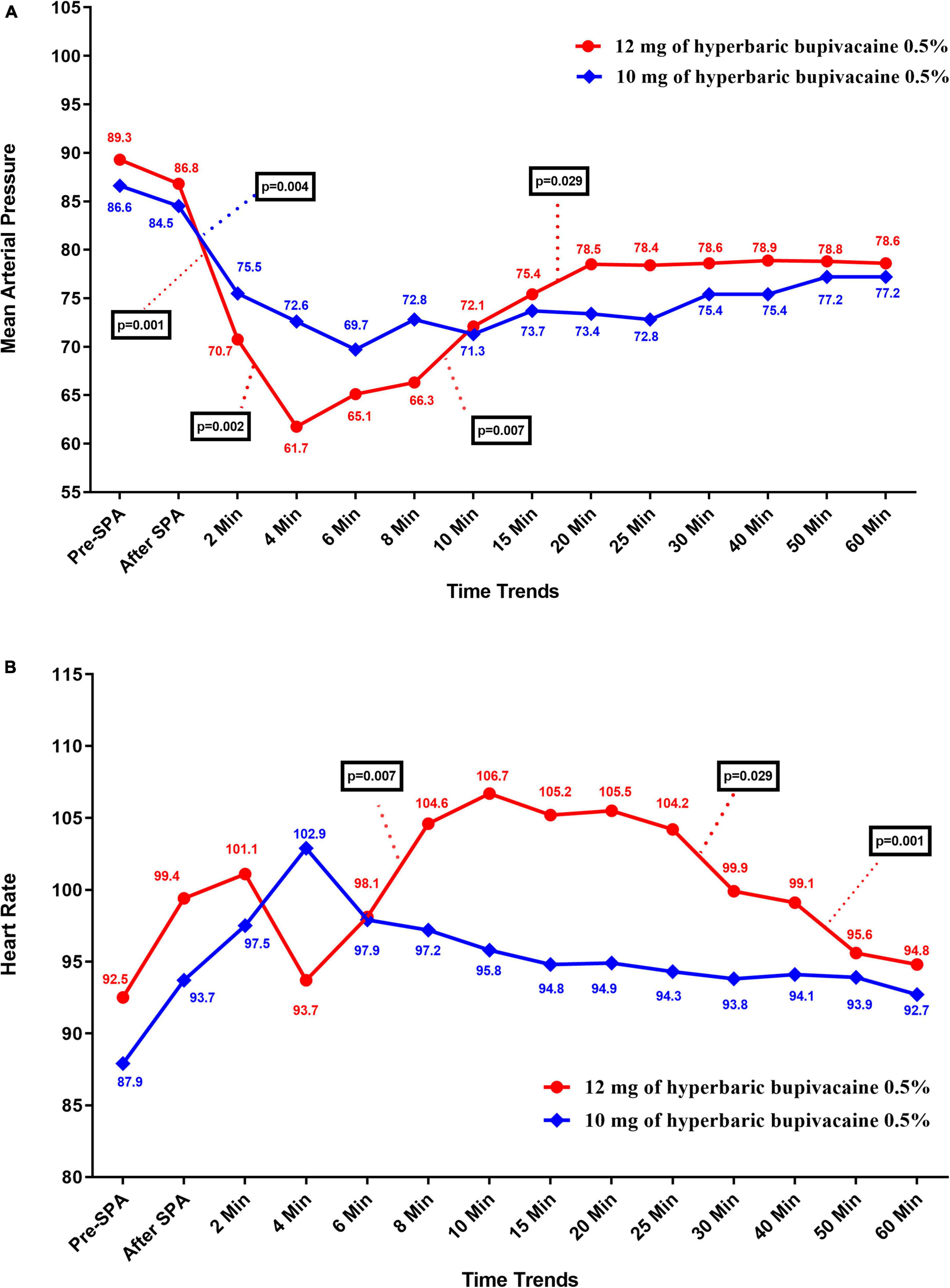
Figure 3. Changes (A) main blood pressure and (B) heart rate in two groups of study over times, * P-values shows statistically significant between two times within groups.
Figure 3B shows the mean values for changes of HR in each group over times. The results showed that there was a significant difference in HR between the two groups at 10 min to 40 min, when HR in Group B was significantly higher than Group A (P < 0.05). In within group, time effect on HR was not statistically significant in Group A (P = 0.296) and Group B (P = 0.454) (a within-subject difference based on time effect). Moreover, the trend in changes in HR levels was not statistically significant between two groups (group × * time interaction or an interaction effect) (P = 0.067).
Figures 4A,B shows the mean values for changes of SpO2 and sedation rate in each group over times, respectively. According to our findings, the mean SpO2 was significantly higher in the Group A than in the Group B (P < 0.05), except at pre-SPA (P = 0.682), immediately after SPA (P = 0.826), at 6 min (P = 0.072), 15 min (P = 0.144) and 60 min (P = 0.658). In within group, time effect on SpO2 was not statistically significant in each group (a within-subject difference based on time effect) (P > 0.05). While, the trend in changes in SpO2 levels was statistically significant between two groups (group × time interaction or an interaction effect) (P = 0.036). In terms of sedation rate, no significant difference was observed between the two groups and also within each group (P > 0.05). In addition, the trend in changes in sedation rate was not statistically significant between two groups (group * time interaction or an interaction effect) (P = 0.102).
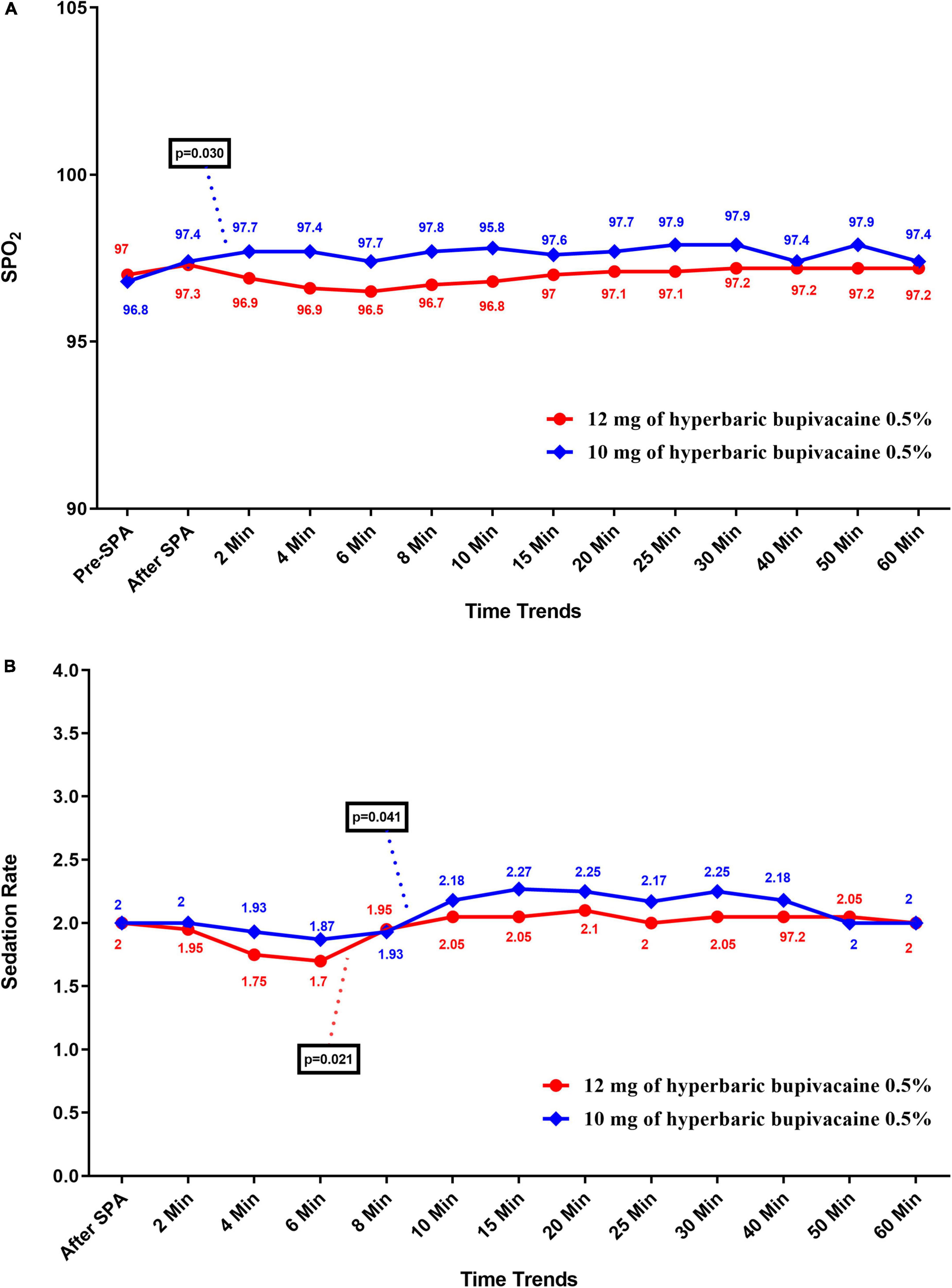
Figure 4. Changes (A) SpO2, and (B) sedation rate in two groups of study over times, * P-values shows statistically significant between two times within groups.
Table 4 shows comparison of complications related to the SPA procedure during operation, at recovery and after operation in two groups of study. According to our findings, hypotension was a common SPA side effect in both groups, which was occurring in 59% of the all participants. The results indicated that the incidence of hypotension (85 vs. 31.6%, P = 0.001), nausea/vomiting (70 vs. 26.3%, P = 0.007) and bradycardia (30% vs. 0, P = 0.012) were significantly higher in Group B compared to Group A. However, there was no significant difference in chills, headache, pain, high spinal and PDPH in the two groups (P > 0.05). Based on logistic regression analysis, 12 mg of hyperbaric bupivacaine (0.5%) can be increases the risk of hypotension (OR: 12.278, 95% CI: 2.573-58.589, P = 0.002), nausea/vomiting during operation (OR: 6.533, 95% CI: 1.613-26.469, P = 0.009) and ephedrine consumed (OR: 12.278, 95% CI: 2.573-58.589, P = 0.002) (Table 5).
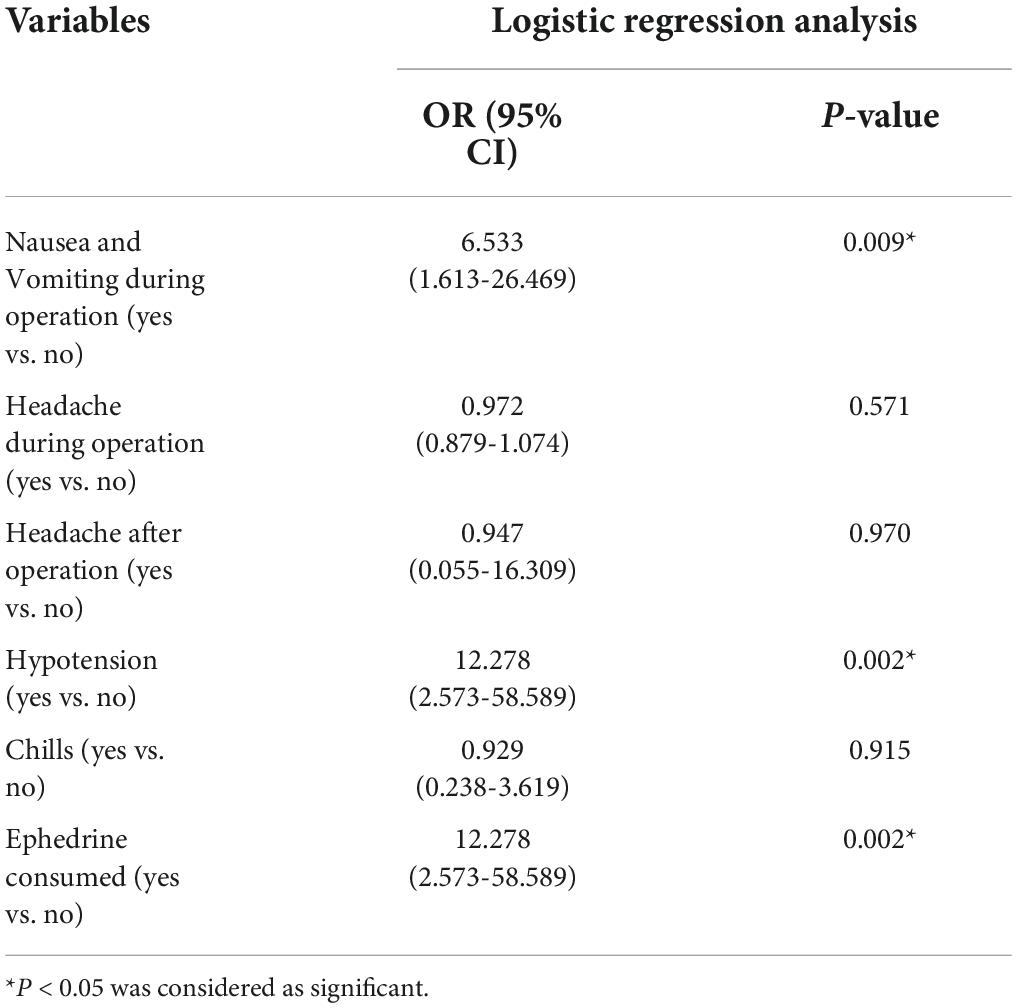
Table 5. Logistic regression analysis of influencing 12 mg dose of hyperbaric bupivacaine 0.5%, to predict incidence of complications.
The failure of a SPA to produce adequate block is not an uncommon occurrence in cesarean section. However, little information is available to provide guidance on duplicate dosing. The main purpose of this clinical trial was to compare the doses of 10 mg and 12 mg of intrathecal hyperbaric bupivacaine (0.5%) on sensory block level after first spinal failure in cesarean section. The excellent quality of sensory block and the complete quality of motor block were obtained in all participants. Although, both doses (10 mg and 12 mg) of intrathecal hyperbaric bupivacaine (0.5%) showed similar satisfactory block profiles. But our results revealed that the SPA with 12 mg hyperbaric bupivacaine (0.5%) can increase the mean anesthesia time and time to reach the T10 level.
Technical errors are common causes of failed spinal such as drug deposition at lower spinal level than surgical site, improper rate of injection, failure to detect dural puncture, needle from inside/outside the dural sac, patient co-operation, needle in ventral epidural space, and cerebrospinal fluid (CSF) tap. Therefore, due to the risk of aspiration and intubation problems in pregnant patients, repeating the procedure of SPA is the safer option (3, 27). Managing failure SPA or repeating the procedure is an event that is of concern to both the patient and the anesthesiologist, and several factors must be considered. Adequate dose of local anesthetic and the skills of anesthetist to prevent technical errors are of this factors. The superior quality of sensory blocks and the complete quality of motor blocks in both research groups may be attributed to the ability of anesthesia providers to prove their effectiveness with experienced hands (28). SPA is safer in skilled hands, but several factors are believed to affect SPA, including anatomical abnormalities such as kyphoscoliosis, sclerosis, and spinal stenosis following previous intrathecal surgery or chemotherapy and reduced anesthetic potency due to prolonged exposure to light (12, 29, 30).
In terms of dose of local anesthetic, previous studies have suggested that a dose of 12 mg (2.4 ml) of bupivacaine provides reliable anesthesia for cesarean section (27, 31). However, in the repetition of the SPA procedure there is a fear of over-expansion of the sensory block (12, 32). As in this study, high spinal block was occurred in 3 patients who received 12 mg hyperbaric bupivacaine (0.5%). So, our findings showed that the lower dose (10 mg) of anesthetic drug is safer and did not observed any high spinal block in Group A. On the other hand, by reviewing the literature, we found an association between hypotension and bradycardia in cesarean section with a higher dose of anesthetic, which was completely consistent with the results of this study (21, 33, 34). Our findings indicated that the higher dose of bupivacaine (12 mg) was related to higher nausea/vomiting, hypotension, and bradycardia as well as administration of more ephedrine and atropine, which ultimately reduces patient satisfaction. The high and very high satisfaction rate of parturients in this study was significantly higher in Group A compare to Group B (73.7 vs.30%, P < 0.05). Evidence suggests that overall satisfaction level of parturients decreases with number of attempt, pain during block, inadequate intraoperative analgesia, postoperative nausea/vomiting, hypotension, bradycardia and headache during operation and high level of PDPH (35). However, by ensuring the quality of spinal anesthesia, improving the clinical skills of anesthesiologists, preventing side effects, and educating mothers about cesarean section under local anesthesia and familiarity with the process, patients You can increase your satisfaction (36). Therefore, choosing the adequate dose of anesthetic drug can be increases the quality of local anesthesia and prevents side effects, and subsequently increase patient satisfaction. The results of this study are consistent with previous studies showing that reducing the dose of local anesthesia during repeated spinal anesthesia is safe and satisfactory (21, 32).
The limitations of our study were, we compared the only two doses of bupivacaine, based on the known optimal doses and low sample size. However, a large sample size study s need to be conducted to determine the optimal dose of hyperbaric bupivacaine that can be safely and successfully used to repeat SPA in parturient women. However, the important teaching concepts of this study are as follows; considering that failure in spinal anesthesia often happens to assistants and less experienced anesthesiologists, and the text books do not mention reducing the dose of bupivacaine in spinal re-injection, if these specialists regardless of reducing the dose of spinal drug, use bupivacaine with the same initial dose as mentioned in the text books, it can lead to an increase in the spinal level and cause problems for patients and anesthesiologists. Since we also work in the obstetric anesthesia training department and deal with spinal failure, we decided to investigate the reduction of bupivacaine dose following spinal failure so that less experienced specialists can use this experience and have fewer problems such as increasing the level of block and hypotension, etc.
Spinal anesthesia can be safely repeated with a 10 mg of hyperbaric bupivacaine 0.5% in a caesarean section after the initial spinal failure. SPA with 10 mg of hyperbaric bupivacaine 0.5% can be improves the quality of local anesthesia, prevents side effects and, as a result, increases patient satisfaction.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committees of Hamadan University of Medical Sciences (IR.UMSHA.REC.1398.232). The patients/participants provided their written informed consent to participate in this study.
FR-B, NM, and AP: study concept and design. FR-B, NM, and FS: analysis and interpretation of data, study concept and design, and Critical revision of the manuscript for important intellectual content. AP: acquisition of data and drafting of the manuscript. FS: statistical analysis. All authors contributed to the article and approved the submitted version.
The study was supported by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ghaffari S, Dehghanpisheh L, Tavakkoli F, Mahmoudi H. The effect of spinal versus general anesthesia on quality of life in women undergoing cesarean delivery on maternal request. Cureus. (2018) 10:e3715. doi: 10.7759/cureus.3715
2. Ring L, Landau R, Delgado C. The current role of general anesthesia for cesarean delivery. Curr Anesthesiol Rep. (2021) 11:18–27. doi: 10.1007/s40140-021-00437-6
3. Parikh KS, Seetharamaiah S. Approach to failed spinal anaesthesia for caesarean section. Indian J Anaesth. (2018) 62:691–7. doi: 10.4103/ija.IJA_457_18
4. Kwak KH. Postdural puncture headache. Korean J Anesthesiol. (2017) 70:136–43. doi: 10.4097/kjae.2017.70.2.136
5. Hofhuizen C, Lemson J, Snoeck M, Scheffer GJ. Spinal anesthesia-induced hypotension is caused by a decrease in stroke volume in elderly patients. Local Reg Anesth. (2019) 12:19–26. doi: 10.2147/lra.s193925
6. Lesser JB, Sanborn KV, Valskys R, Kuroda M. Severe bradycardia during spinal and epidural anesthesia recorded by an anesthesia information management system. Anesthesiology. (2003) 99:859–66. doi: 10.1097/00000542-200310000-00018
7. Kent CD, Bollag L. Neurological adverse events following regional anesthesia administration. Local Reg Anesth. (2010) 3:115–23. doi: 10.2147/lra.s8177
8. Jelting Y, Klein C, Harlander T, Eberhart L, Roewer N, Kranke P. Preventing nausea and vomiting in women undergoing regional anesthesia for cesarean section: challenges and solutions. Local Reg Anesth. (2017) 10:83–90. doi: 10.2147/lra.s111459
9. Kocarev M, Watkins E, McLure H, Columb M, Lyons G. Sensory testing of spinal anaesthesia for caesarean section: differential block and variability. Int J Obstet Anesth. (2010) 19:261–5. doi: 10.1016/j.ijoa.2010.02.002
10. Fakherpour A, Ghaem H, Fattahi Z, Zaree S. Maternal and anaesthesia-related risk factors and incidence of spinal anaesthesia-induced hypotension in elective caesarean section: a multinomial logistic regression. Indian J Anaesth. (2018) 62:36–46. doi: 10.4103/ija.IJA_416_17
11. Sushma KS, Ramaswamy AH, Shaikh SI. Correlation between Weight of the baby and the level of sensory blockade in spinal anaesthesia for caesarean section: an observational study. Anesth Essays Res. (2018) 12:318–21. doi: 10.4103/aer.AER_164_17
12. Fettes PDW, Jansson J-R, Wildsmith JAW. Failed spinal anaesthesia: mechanisms, management, and prevention. Br J Anaesth. (2009) 102:739–48. doi: 10.1093/bja/aep096
13. Ashagrie HE, Ahmed SA, Melesse DY. The incidence and factors associated with failed spinal anesthesia among parturients underwent cesarean section, 2019: a prospective observational study. Int J Surg Open. (2020) 24:47–51. doi: 10.1016/j.ijso.2020.03.009
14. Alabi AA, Adeniyi OV, Adeleke OA, Pillay P, Haffajee MR. Factors associated with failed spinal anaesthesia for caesarean sections in Mthatha general hospital, Eastern Cape, South Africa. S Afr Fam Pract. (2017) 59:128–32. doi: 10.1080/20786190.2017.1292696
15. At A, So O. Failed spinal anaesthesia for caesarean section. J West Afr Coll Surg. (2011) 1:1–17.
16. Colish J, Milne AD, Brousseau P, Uppal V. Factors associated with failure of spinal anesthetic: an 8-year retrospective analysis of patients undergoing elective hip and knee joint arthroplasty. Anesth Analg. (2020) 130:e19–22. doi: 10.1213/ane.0000000000004304
17. Auroy Y, Benhamou D, Péquignot F, Jougla E, Lienhart A. [Survey of anaesthesia-related mortality in France: the role of aspiration of gastric contents]. Ann Fr Anesth Reanim. (2009) 28:200–5. doi: 10.1016/j.annfar.2008.12.018
18. Alimian M, Mohseni M, Faiz SHR, Rajabi A. The effect of different doses of intrathecal hyperbaric bupivacaine plus sufentanil in spinal anesthesia for cesarean sections. Anesthesiol Pain Med. (2017) 7:e14426. doi: 10.5812/aapm.14426
19. Chambers WA, Littlewood DG, Edstrom HH, Scott DB. Spinal anaesthesia with hyperbaric bupivacaine: effects of concentration and volume administered. Br J Anaesth. (1982) 54:75–80. doi: 10.1093/bja/54.1.75
20. Burlacu CL, Buggy DJ. Update on local anesthetics: focus on levobupivacaine. Ther Clin Risk Manag. (2008) 4:381–92. doi: 10.2147/tcrm.s1433
21. Bhar D, RoyBasunia S, Das A, Chhaule S, Mondal SK, Bisai S, et al. Repeat spinal anesthesia in cesarean section: a comparison between 10 mg and 12 mg doses of intrathecal hyperbaric (0.05%) bupivacaine repeated after failed spinal anesthesia: a prospective, parallel group study. Anesth Essays Res. (2016) 10:362–9. doi: 10.4103/0259-1162.172725
22. World Medical Association. Declaration of helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
23. Jayaraman J. Guidelines for reporting randomized controlled trials in paediatric dentistry based on the CONSORT statement. Int J Paediatr Dent. (2020) 31(Suppl. 1):38–55. doi: 10.1111/ipd.12733
24. Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. (2018) 2:e088. doi: 10.5435/JAAOSGlobal-D-17-00088
25. Graham AC, McClure JH. Quantitative assessment of motor block in labouring women receiving epidural analgesia. Anaesthesia. (2001) 56:470–6. doi: 10.1046/j.1365-2044.2001.01524-6.x
26. Rasheed A, Amirah M, Abdallah M, Parameaswari PJ, Issa M, Alharthy A. RAMSAY sedation scale and richmond agitation sedation scale (RASS): a cross sectional study. Health Sci J. (2018) 12:604. doi: 10.21767/1791-809X.1000604
27. Pokharel A. Study of failed spinal anesthesia undergoing caesarean section and its management. Postgrad Med J NAMS. (2011) 11:11-5.
28. Klimek M, Rossaint R, van de Velde M, Heesen M. Combined spinal-epidural vs. spinal anaesthesia for caesarean section: meta-analysis and trial-sequential analysis. Anaesthesia. (2018) 73:875–88. doi: 10.1111/anae.14210
29. Westphal M, Götz T, Booke M. Failed spinal anaesthesia after intrathecal chemotherapy. Eur J Anaesthesiol. (2005) 22:235–6. doi: 10.1017/s0265021505220409
30. Kumar R, Singh K, Prasad G, Patel N. Repeat spinal anesthesia after a failed spinal block in a pregnant patient with kyphoscoliosis for elective cesarean section. J Obstet Anaesth Crit Care. (2014) 4:84–6. doi: 10.4103/2249-4472.143879
31. Ginosar Y, Mirikatani E, Drover DR, Cohen SE, Riley ET. ED50 and ED95 of intrathecal hyperbaric bupivacaine coadministered with opioids for cesarean delivery. Anesthesiology. (2004) 100:676–82. doi: 10.1097/00000542-200403000-00031
32. Deshpande S, Idriz R. Repeat dose after an inadequate spinal block. Anaesthesia. (1996) 51:892. doi: 10.1111/j.1365-2044.1996.tb12639.x
33. Fitzgerald JP, Fedoruk KA, Jadin SM, Carvalho B, Halpern SH. Prevention of hypotension after spinal anaesthesia for caesarean section: a systematic review and network meta-analysis of randomised controlled trials. Anaesthesia. (2020) 75:109–21. doi: 10.1111/anae.14841
34. Somboonviboon W, Kyokong O, Charuluxananan S, Narasethakamol A. Incidence and risk factors of hypotension and bradycardia after spinal anesthesia for cesarean section. J Med Assoc Thai. (2008) 91:181–7.
35. Demilew BC, Getu D, Tesfaw D, Taye MG. Assessment of satisfaction and associated factors of parturients underwent cesarean section with spinal anesthesia at the General Hospital, Ethiopia; 2019. Ann Med Surg. (2021) 65:102282. doi: 10.1016/j.amsu.2021.102282
Keywords: motor block, cesarean section, bupivacaine, failed spinal, spinal anesthesia, sensory block
Citation: Manouchehrian N, Rahimi-Bashar F, Pirdehghan A and Shahmoradi F (2022) Comparison between 10 and 12 mg doses of intrathecal hyperbaric (0.5%) bupivacaine on sensory block level after first spinal failure in cesarean section: A double-blind, randomized clinical trial. Front. Med. 9:937963. doi: 10.3389/fmed.2022.937963
Received: 06 May 2022; Accepted: 22 August 2022;
Published: 04 October 2022.
Edited by:
Longxiang Su, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Ashraf Elagamy, Al Ain University, United Arab EmiratesCopyright © 2022 Manouchehrian, Rahimi-Bashar, Pirdehghan and Shahmoradi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farshid Rahimi-Bashar, ZnJfcmFoaW1pYmFzaGFyQHlhaG9vLmNvbQ==
†ORCID: Nahid Manouchehrian, orcid.org/0000-0003-1063-8537; Farshid Rahimi-Bashar, orcid.org/0000-0001-8276-1425; Azar Pirdehghan, orcid.org/0000-0001-9775-9504; Fatemeh Shahmoradi, orcid.org/0000-0002-5711-1233
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.