- 1Emergency Department, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 2Department of Nursing, Peking Union Medical College Hospital, Beijing, China
- 3Accident and Emergency Medicine Academic Unit, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China
Background: Blood products are commonly transfused in patients with acute upper gastrointestinal bleeding (UGIB). There exists considerable practice variation and less evidence to guide fresh frozen plasma transfusion in patients with UGIB. The aim of this study was to explore any association between fresh frozen plasma transfusion following acute UGIB and clinical outcomes.
Methods: This was a prospective, observational, multicenter study conducted at 20 tertiary hospitals in China. Patients with acute UGIB with an international normalized ratio ≤ 2.0 at emergency department admission were included. Multivariate logistic regression models were used to examine and quantify any clinical associations.
Results: A total of 976 patients (61.57 ± 15.79 years old, 73.05% male) were included, of whom 17.42% received fresh frozen plasma transfusion. The overall 90-day mortality and rebleeding rates were 10.20 and 12.19%, respectively. After adjusting for confounding factors, transfusion of fresh frozen plasma during hospitalization was associated with higher 90-day mortality [odd ratio (OR), 2.36; 95% confidence interval (CI), 1.36–4.09; p = 0.002] but not rebleeding (OR, 1.5; 95% CI; 0.94-2.54; p = 0.085). In a subgroup analysis, patients with an international normalized ratio <1.5 who were treated with fresh frozen plasma were associated with both significantly higher 90-day mortality (OR, 2.78; 95% CI, 1.49–5.21; p = 0.001) and rebleeding (OR, 2.02; 95% CI, 1.16–3.52; p = 0.013), whereas in patients with an international normalized ratio between 1.5 and 2, we did not find any significant correlation.
Conclusion: This study found an association between fresh frozen plasma transfusion following acute UGIB and elevated 90-day mortality. Both 90-day mortality and rebleeding risk were significantly higher in patients with an international normalized ratio < 1.5. Fresh frozen plasma transfusion in acute UGIB does not improve the poor outcomes (Chinese Clinical Trial registry, Number ChiCTR1900028676).
Introduction
Acute upper gastrointestinal bleeding (UGIB) is a relatively common medical emergency associated with significant morbidity and mortality worldwide, with an incidence of 80–150 per 100,000 people each year (1–4). Despite recent advancements in pharmaceuticals and endoscopic hemostasis, the 30-day mortality from UGIB remains 5–14% (1–5).
Transfusion of blood components is integral to the management of patients with acute UGIB, but blood transfusion practice for acute UGIB is mainly empirical, and the evidence base of crystal-restricted empirical blood transfusion algorithms mainly comes from the study of major bleeding in trauma (6–13). Practice guidelines about UGIB recommend urgent reversal in all patients presenting with serious, life-threatening bleeding (i.e., hemodynamic instability or shock), either in the case of therapeutic or supratherapeutic international normalized ratio (INR) elevations (14). While the early benefits of fresh frozen plasma (FFP) infusion for patients with severe gastrointestinal bleeding are self-evident, its long-term effects on patients with severe UGIB and the benefits for less severe cases of UGIB remain unclear (6).
In most patients with UGIB, FFP infusions are not only used as part of resuscitation, but also are used to prevent or treat bleeding, usually guided by laboratory coagulation parameters (6). The causes of gastrointestinal hemorrhage and coagulopathy in patients with UGIB are different from those in trauma. In the case of gastrointestinal bleeding, the most common causes of coagulopathy are hepatic cirrhosis and oral anticoagulants (15, 16). However, prolonged INR in the setting of cirrhosis is not thought to be predictive of bleeding risk, so it is not clear what is the role of FFP in the setting of very mild prolongation of INR in cirrhotic patients (17, 18). In the case of coagulopathy caused by oral anticoagulant medication, prothrombin complex concentrate is most appropriate for reversing the effects of vitamin K antagonists. (6) The evidence for the use of blood components to reverse the anticoagulant effects of oral anticoagulants is poor.
In the absence of high-quality data, we performed a large prospective, real-world, observational study to determine the relationship between an FFP infusion within 72 h following presentation to an emergency department (ED) in patients with UGIB with an INR ≤2 at admission and key clinical outcomes (90-day mortality and rebleeding).
Materials and Methods
Study Design and Participants
This was a prospective, multicenter, non-interventional, real-world study (acute upper gastrointestinal real-world research, AUGUR) in China. The study sample included all non-trauma adult (age ≥18 years) patients diagnosed with UGIB who were admitted to an acute care hospital via the ED between 30 June 2020 and 10 February 2021. The diagnosis of UGIB was based on the presence of hematemesis, coffee ground emesis, and melena. Patients who refused to sign the participation consent form were excluded. In this study, to make the results more clinically meaningful, we excluded patients with an INR >2 at the time of admission. This study was conducted at 20 tertiary hospitals from 20 (out of an invited 31) different provinces, autonomous regions, or independent municipalities and was approved by the Institutional Ethics Review Boards of all 20 hospitals. Informed consent was obtained from all enrolled patients.
Definition of Outcomes
The primary outcome was the association between FFP transfusion and 90-day mortality. The secondary outcome was the association between FFP transfusion and rebleeding. Such outcomes were monitored from admission to the hospital or the onset of bleeding at admission to the hospital (for in-hospital patients) for up to 90 days. Mortality was defined as death from any cause within 90 days following the initial presentation. Rebleeding was defined by a recurrent episode of hematemesis, coffee ground emesis, melena, or both, with either shock or a decrease in hemoglobin concentration of at least 2 g/dl 24 h following the initial treatment and stabilization. All data were recorded for the full duration of the initial medical encounter.
Covariates
Recorded variables included age, gender, vital signs at triage, comorbidities, relevant past medical history, any concomitant intake of medications in 6 months preceding the bleeding episode, physical examination findings and laboratory data (hemodynamic data, complete blood count, and coagulation profile results), and any resuscitative measures employed. Endoscopic reports included the identification of any bleeding lesions or stigmata of recent hemorrhage. Both surgical and angiographic therapies, as well as any pharmacologic therapies administered for hemorrhage were recorded. Thrombotic in hospital refers to the occurrence of myocardial infarction, cerebral infarction, pulmonary embolism, or lower extremity thrombosis after admission.
Statistical Analysis
For baseline characteristics, variables are reported as mean ± standard deviation (s.d.) or proportions when they were continuous data or categorical data, respectively. The comparisons between different groups were performed using unpaired t-tests, chi-square, Fisher's exact, or Wilcoxon rank testing, as appropriate. Multiple logistic regression models were created to analyze possible independent relationships between FFP transfusion and mortality and rebleeding. We also listed different adjusted models as follows: unadjusted model 1; model 2: adjusted for age and gender; model 3 for fully adjustment (age, gender, systolic blood pressure, cirrhosis, hemoglobin at admission, hematemesis, peptic ulcer bleeding, thrombotic in hospital, red blood cells transfusion). Briefly, the potential confounding factors were based on clinical considerations that related to baseline patient characteristics or therapies administered and also both associated with FFP transfusion and clinical outcomes (90-day mortality and rebleeding). Independent variables were tested for multicollinearity and only included in the model if absent. Results are presented as odds ratios (OR) with 95% confidence intervals (95% CIs). P-values < 0.05 were considered statistically significant. Statistical analyses were performed with the R statistical software packages (The R Foundation, Vienna, Austria) and Empowerstats (Solutions, Inc., Boston, MA, USA).
Results
Patient Characteristics
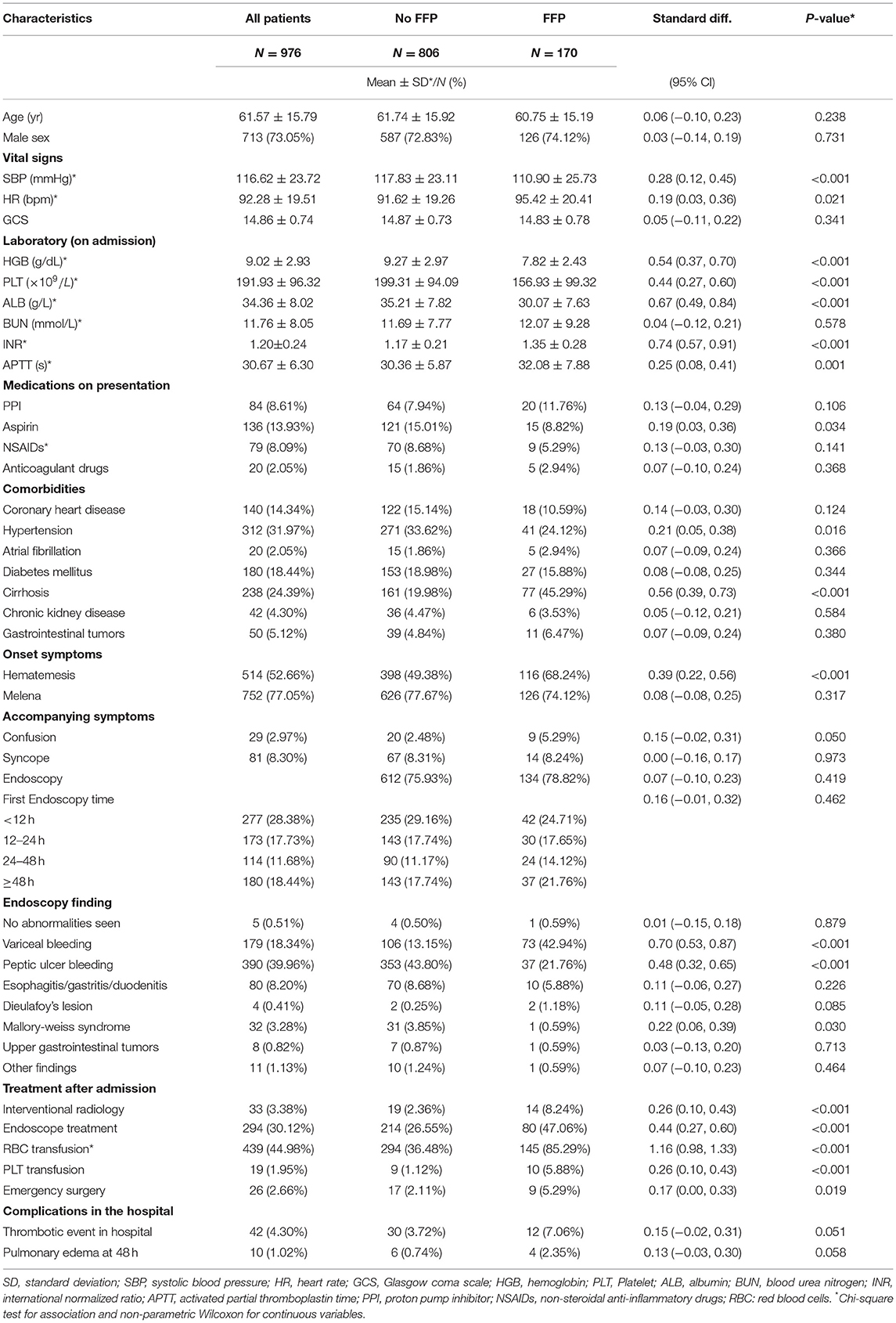
Of the initial sample, 60 patients were excluded due to lower gastrointestinal bleeding, 56 patients were excluded due to unavailable laboratory data, 12 patients were excluded due to loss to follow-up, and 96 patients were excluded due to an INR >2 at admission. Finally, a total of 976 patients with UGIB from 20 hospitals in 20 (out of a surveyed 31) provinces, autonomous regions, or independent municipalities were enrolled in this analysis. During the study period, all patients enrolled presented first to the ED (i.e., there were no enrolments of already admitted patients) and all were identified as having an UGIB. The overall mean age was 61.57 ± 15.79 years, 24.39% of patients had liver cirrhosis, and 5.12% of patients were taking oral anticoagulants. Among all patients, 76.43% of patients completed an endoscopic examination, and 17.42% of patients received FFP transfusion within 72 h after ED admission. Out of all the patients, 10.20% of died and 12.19% of experienced rebleeding. The characteristics of all patients, including those who received FFP transfusion within 72 h of admission and those who did not, are summarized in Table 1.
Comparison Between Patients Who Received FFP Transfusion and Those Without FFP Transfusion
Among the 170 patients who received FFP transfusion, 45.29% had liver cirrhosis, and the proportion of patients was significantly higher than that of the group who did not receive FFP transfusion. In addition, patients who received FFP transfusion were more likely to have hematemesis, and their admission systolic blood pressure, hemoglobin, and albumin average were lower than those who did not receive FFP transfusion. Overall, more patients in the FFP blood transfusion group showed signs of initial hemodynamic instability upon admission. Endoscopic results showed that the proportion of variceal bleeding was higher in the FFP transfusion group, while the proportion of peptic ulcers was higher in the non-plasma transfusion group. In examining treatments after admission, the proportion of the participants in the FFP transfusion group who received red blood cell transfusions, endoscopic treatments, or emergency surgery was higher than those in the non-FFP transfusion group (Table 1).
The Association Between Transfusion and Mortality or Rebleeding
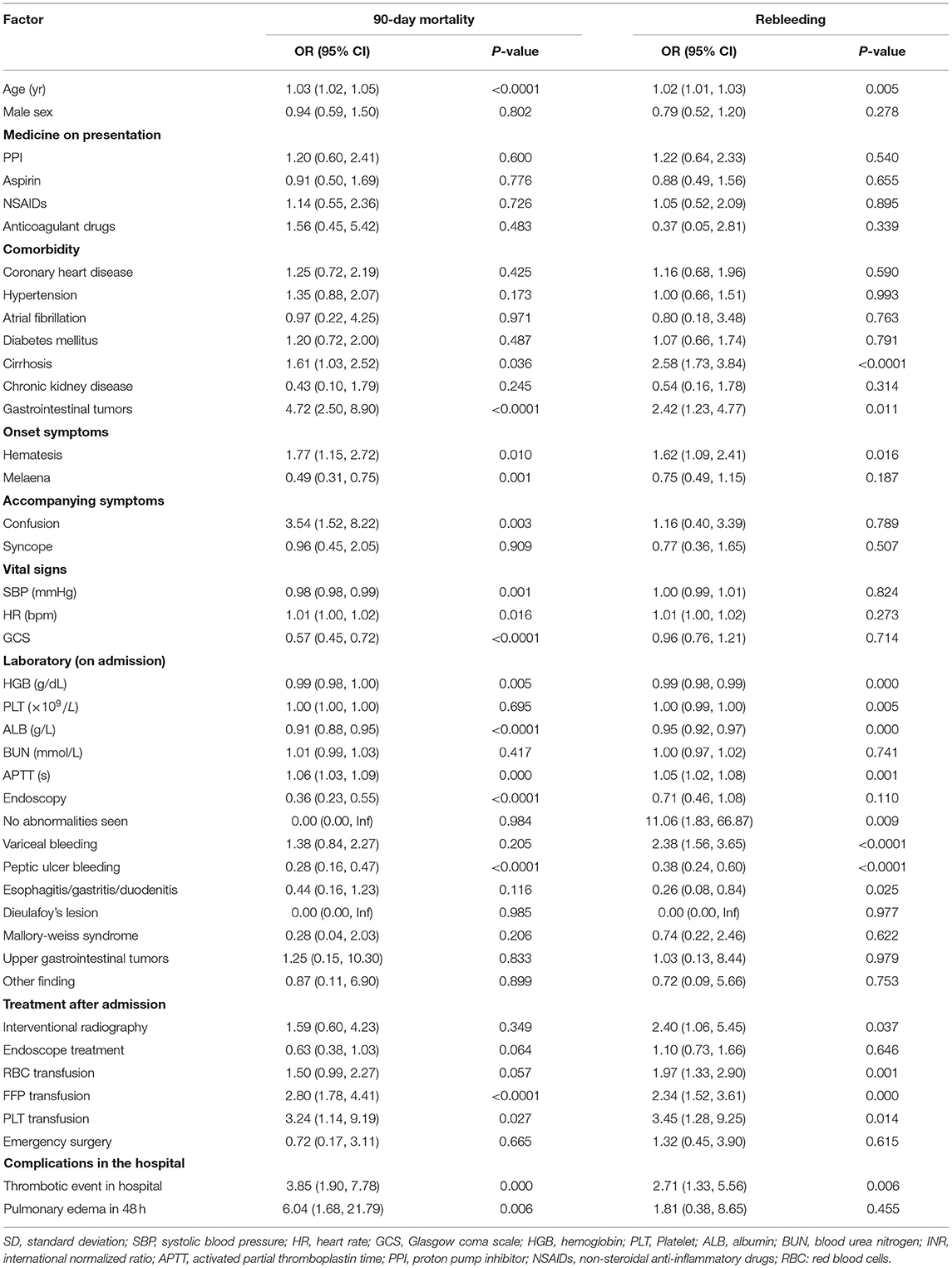
All-cause 90-day mortality and rebleeding were higher in FFP transfused vs. non-transfused patients (mortality: 20.00 vs. 8.19%, p < 0.001; rebleeding: 21.18 vs. 10.30%, p < 0.001) on univariate analysis. Other characteristics significantly associated with death and rebleeding in univariate analysis included age, liver cirrhosis, gastrointestinal tumors, hematemesis, systolic blood pressure (SBP), hemoglobin (HGB), albumin (ALB), peptic ulcer bleeding, and thrombotic events in hospital (Table 2). On multivariate analysis after adjusting for confounding variables, including age, gender, SBP, HGB, liver cirrhosis, hypertension, confusion, hematemesis, peptic ulcer bleeding, thrombosis in hospital, and RBC transfusion, an FFP transfusion was an independent predictor of 90-day mortality (OR, 2.36; 95% CI, 1.36–4.09; p = 0.002) but not for rebleeding (OR, 1.55; 95% CI, 0.94–2.54; p = 0.085) (Table 3).
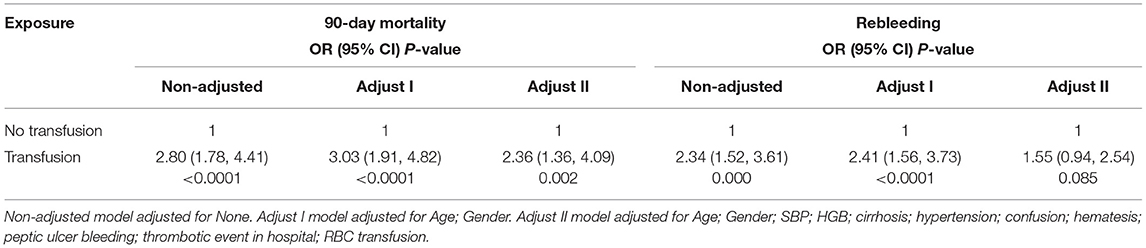
Table 3. Association between FFP transfusion and 90-day mortality or rebleeding by multiple regression.
Subgroup Analysis for the Association Between FFP Transfusion and Mortality or Rebleeding Based on INR
We then performed a subgroup analysis by dividing all patients into an INR <1.5 group and the INR 1.5–2 group, according to the admission data. After multivariate adjustment, we found that FFP transfusion in the INR <1.5 group was associated with a higher 90-day mortality rate (OR, 2.78; 95% CI, 1.49–5.21; p = 0.001) and rebleeding rate (OR, 2.02; 95% CI, 1.16–3.52; p = 0.013). There was no significant correlation between FFP transfusion and 90-day mortality or rebleeding in patients with an INR between 1.5 and 2 (Table 4).
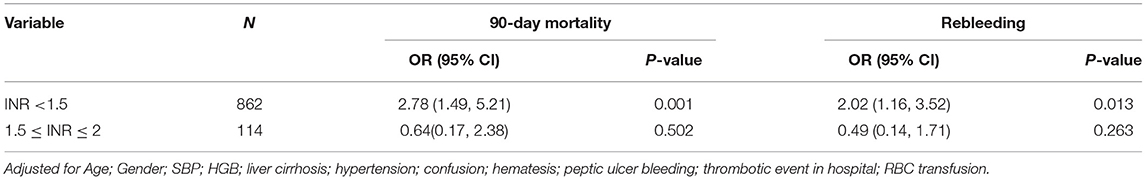
Table 4. Association between FFP transfusion of 90-day mortality or rebleeding in subgroup analysis.
Discussion
This large, prospective, observational, real-world study examined the association between FFP transfusions in patients with acute UGIB and the clinical outcomes of mortality and rebleeding. We found that any transfusion of FFP within 72 h of admission was associated with an increased risk of all-cause 90-day mortality. We also observed a trend toward increased rebleeding, which fell short of statistical significance, perhaps because of a lack of statistical power. Unlike the 30-day mortality or in-hospital mortality selected by most previous studies, we chose the 90-day mortality and rebleeding rate as our outcome indicators to avoid missing information on patients with adverse events after discharge. The sample size was relatively large with nearly 1,000 patients with UGIB included, which allowed us to examine the association of an FFP transfusion with the outcomes of patients with acute UGIB.
To ensure hemodynamic stability, many patients with acute gastrointestinal bleeding will receive high-density resuscitation [such as fluid resuscitation, FFP, and RBC transfusion(s)] within a short period of time after arrival in ED. In recent decades, there has been great progress in the exploration of fluid resuscitation and red blood cell infusion strategies, but so far, published data on the use of FFP in patients with UGIB are still limited and inconclusive (14, 17). UK NICE guidance on UGIB recommends transfusion of frozen plasma in actively bleeding patients if the INR is more than 1.5 (16). Australian guidelines also recommend that FFP transfusion in the setting of major RBC transfusion be guided by both the coagulation profile and the clinical scenario, with an INR of more than 1.5 likely reflecting a coagulopathy requiring correction by FFP and/or other coagulation factors (18). In our study, a total of 179 patients with UGIB received FFP transfusion within 72 h of admission. There are 67 patients admitted to the hospital with an INR between 1.5 and 2 who did not receive plasma transfusion. Among all 976 patients admitted to the hospital with an INR ≤2, 104 patients were admitted to the hospital with an SBP <90 mmHg, but only 29 of them underwent a plasma infusion. This captures the wide variability in medical management for patients with acute UGIB, indicating clinical uncertainty regarding optimal practice.
Coagulopathic patients with critical UGIB are a focus of ongoing clinical research. In most patients with UGIB without a coagulopathy, there are few studies on whether a FFP transfusion will cause harm or be ineffective to patients (19, 20). Hence, we only included patients with UGIB with an INR ≤2.0, as this population of patients would probably not benefit from FFP. In a multicenter study of non-variceal UGIB, there was an association between FFP with greater mortality (30-day and 1-year survival outcomes). These poor outcomes were independent of RBC transfusion and were dependent on the FFP dose (19). One small cohort study using a historical comparison group showed that aggressive volume resuscitation, including correction of coagulopathy (target = INR <1.8), led to an improved mortality (20).
In addition, the value of FFP on survival outcomes has also been questioned outside of UGIB cases in the trauma, intensive care, and cardiac surgery literature (21–23). Reported adverse effects of FFP transfusion include transfusion-associated lung injury and circulatory overload, with the risk of acute lung injury being higher in patients who received FFP and PLTs than in those who received only RBCs (24).
In our study, the group of patients receiving FFP was much sicker (lower albumin level, lower blood pressure, more hemodynamic instability, more variceal bleeding). In these cases, bleeding may not be due to anticoagulation reasons, and transfusion with clotting factors cannot prevent or treat their underlying UGIB. Indeed, after adjusting for confounding factors, FFP did not reduce patient deaths, and, in fact, the FFP group had a higher 90-day mortality rate (but not a higher rebleeding risk). Among the adverse events recorded in our study, we also observed a correlation between plasma infusion and a trend of increased rates of pulmonary edema within 48 h and overall thrombotic events in the hospital, but these trends were not statistically significant, which may be due to a relatively small number of adverse events in our sample.
As in most studies, INR ≥1.5 is used as the criterion for judging coagulopathy, and coagulopathy is independently related to the death of patients with UGIB (16), we conducted a subgroup analysis of patients (admission INR <1.5 or INR between 1.5 and 2.0), and found that in patients with INR <1.5, transfusion of FFP within 72 h not only increased the 90-day mortality, but also increased the risk of rebleeding, but this was not found in the 1.5–2.0 INR group. This also indicates that there is a correlation between bleeding and anticoagulation in patients with INR 1.5–2, and FFP transfusion can prevent or treat their potential bleeding. Therefore, we speculate that in patients who have not developed coagulopathy, a “less is more” philosophy may be better.
Although it may seem counterintuitive to question the value of an FFP transfusion in UGIB, numerous studies in other disciplines that have assessed critically ill patient populations have suggested associations between FFP transfusions and adverse patient outcomes, including death, which persist after appropriate adjustment. In patients with UGIB, a restrictive blood transfusion strategy may be expanded to restrictive blood component transfusion strategy (red blood cells and plasma). Different from coagulopathy caused by trauma, most patients with UGIB have a coagulopathy associated with either oral anticoagulants or liver disease (7, 25). In the case of coagulopathy caused by oral anticoagulant medications, prothrombin complex concentrate is most appropriate for reversing the effects of vitamin K antagonism. The evidence for the use of blood components to reverse the anticoagulant effects of direct oral anticoagulants (DOACs) is still poor (26). Coagulopathy in liver disease is complex, traditionally viewed as a disorder of impaired hemostasis, but it is probably more accurate to consider the coagulopathy of liver disease as a “rebalanced hemostasis,” as it can be associated with thrombotic as well as hemorrhagic outcomes (27–29, 31). There is limited evidence to suggest that raising the INR in these patients is associated with an increased bleeding risk, and limited evidence to suggest that treatment with FFP either improves coagulation parameters or reduces bleeding risk in vivo (29). In addition to traditional coagulation tests, some studies suggest the use of point-of-care viscoelastic tests (e.g., thrombolastography, thromboelastometry, and sonoclot) to diagnose coagulation problems in patients with liver disease (30). Point-of-care viscoelasticity tests can provide actionable targets for correcting coagulation defects in patients with bleeding liver disease and may provide evidence-based algorithms for liver disease, but it is uncertain whether it can guide the management of patients with UGIB with liver disease (30). In our study, we did not perform viscoelastic testing on enrolled patients and therefore cannot provide evidence whether viscoelastic testing analysis affects transfusion requirements and alters clinical outcomes in this high-risk patient population.
Some limitations and strengths should be considered. First, to the best of our knowledge, this is a real-world prospective cohort study based on large-scale with a tremendous sample size. Second, we used comprehensive statistical analysis, including multiple logistic regression and subgroup analysis, to detect the association between FFP transfusion and mortality or rebleeding, which make our results become reliable. However, all clinical management decisions are still made by the attending physicians at the point of care. Although the participating hospitals in this study adopted standard clinical management protocols, some treatment options showed difference between the cases. Additionally, we only collected the results of whether or not patients died within 90 days and did not collect the specific time of death of each deceased patient after admission. If time was included for analysis, it may have more important clinical value. Finally, more prospective RCTs are now required to examine these findings and establish a possible causal link between FFP transfusion and adverse outcomes in patients with UGIB.
Conclusion
Blood is a scarce and expensive resource and there persist surprisingly large gaps in the evidence base to guide the effective use of FFP transfusion in patients with UGIB. This study suggests a clinically important association between FFP transfusion and an elevated risk of rebleeding and mortality, particularly in patients with an INR ≤1.5. FFP transfusion in acute UGIB does not improve the poor outcomes. Clinicians and expert consensus groups need to be aware of such considerations, as basic aspects of resuscitation are now being questioned and reassessed in many specialties across the world.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SL and XY: conception, design, and manuscript preparation and editing. SL: definition of intellectual content and final approval of the article. SL, XZ, and JW: literature search, analysis, and interpretation of the data. XZ: drafting of the article. XY and HZ: critical revision of the article for important intellectual content. All authors have read and approved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The AUGUR study was initiated by the Emergency Department of Peking Union Medical College Hospital, assisted by the Chinese College of Emergency Physicians and the Chinese Emergency Medical Partnership. The authors declare that this study received funding from AstraZeneca China. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The AUGUR thanks all the doctors and nurses participating in the study for their valuable assistance in completing the registry. The AUGUR investigators group includes Peking Union Medical Collage Hospital; The First Affiliated Hospital of Zhengzhou University; The Second people's Hospital of Guiyang; Renmin Hospital of Wuhan University; Shanxi Bethune Hospital; The First Affiliated Hospital of Wenzhou Medical University; China Medical University of Shengjing Hospital; BeiJing Hospital; Changhai Hospital; Qingdao Municipal Hospital; The First Affiliate Hospital of Xinjiang Medical University; West China Hospital Of Sichuan University; The Second Affiliated Hospital of Harbin Medical University; Guangdong Provincial Hospital of Chinese Medicine; The First Affiliated Hospital of Kunming Medical University; The Second Hospital of Hebei Medical University; Fujian Provincial Hospital; The Second Affiliated Hospital Of Guangxi Medical University; The First Affiliated Hospital Of The Force Medical University; The First Affiliated Hospital, Sun Yat-sen University.
References
1. Kamboj AK, Hoversten P, Leggett CL. Upper gastrointestinal bleeding: etiologies and management. Mayo Clin Proc. (2019) 94:697–703. doi: 10.1016/j.mayocp.2019.01.022
2. Fouad TR, Abdelsameea E, Abdel-Razek W, Attia A, Mohamed A, Metwally K, et al. Upper gastrointestinal bleeding in Egyptian patients with cirrhosis: post-therapeutic outcome and prognostic indicators. J Gastroenterol Hepatol. (2019) 34:1604–10. doi: 10.1111/jgh.14659
3. Hearnshaw SA, Logan RF, Lowe D, Travis SPL, Murphy MF, et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. (2011) 60:1327–35. doi: 10.1136/gut.2010.228437
4. Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. (2012) 107:1190–5. doi: 10.1038/ajg.2012.168
5. Zhong M, Chen WJ, Lu XY, Qian J, Zhu CQ. Comparison of three scoring systems in predicting clinical outcomes in patients with acute upper gastrointestinal bleeding: a prospective observational study. J Dig Dis. (2016) 17:820–8. doi: 10.1111/1751-2980.12433
6. Donovan K, Stanworth S, Jairath V. The optimal use of blood components in the management of gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. (2019) 42–43:101600. doi: 10.1016/j.bpg.2019.02.002
7. Barkun AN, Almadi M, Kuipers EJ, Laine L, Sung J, Tse F, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the international consensus group. Ann Intern Med. (2019) 171:805–22. doi: 10.7326/M19-1795
8. Leahy MF, Mukhtar SA. From blood transfusion to patient blood management: a new paradigm for patient care and cost assessment of blood transfusion practice. Intern Med J. (2012) 42:332–8. doi: 10.1111/j.1445-5994.2012.02717.x
9. Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. (2015) 313:471–82. doi: 10.1001/jama.2015.12
10. Haut ER, Kalish BT, Cotton BA, Efron DT, Haider A, Steven KA, et al. Prehospital intravenous fluid administration is associated with higher mortality in trauma patients: a national trauma data bank analysis. Ann Surg. (2011) 253:371e7. doi: 10.1097/SLA.0b013e318207c24f
11. Hussmann B, Lefering R, Waydhas C, Touma A, Kauther MD, Ruchholtz S, et al. Does increased prehospital replacement volume lead to a poor clinical course and an increased mortality? A matched-pair analysis of 1896 patients of the trauma registry of the german society for trauma surgery who were managed by an emergency doctor at the accident site. Injury. (2013) 44:611e7. doi: 10.1016/j.injury.2012.02.004
12. Maegele M, Lefering R, Yucel N, Tjardes T, Rizen D, Paffrath T, et al. Early coagulopathy in multiple injury: an analysis from the German trauma registry on 8724 patients. Injury. (2007) 38:298e304. doi: 10.1016/j.injury.2006.10.003
13. Wang CH, Hsieh WH, Chou HC, Huang YS, Shen JH, Yeo YH, et al. Liberal versus restricted fluid resuscitation strategies in trauma patients: a systematic review and meta-analysis of randomized controlled trials and observational studies*. Crit Care Med. (2014) 42:954e61. doi: 10.1097/CCM.0000000000000050
14. Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. (2015) 47:a1–46. doi: 10.1055/s-0034-1393172
15. Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. (2019) 42–43:101610. doi: 10.1016/j.bpg.2019.04.003
16. Jairath V, Kahan BC, Stanworth SJ, Logan RFA, Hearnshaw SA, Travis SPL, et al. Prevalence, management, and outcomes of patients with coagulopathy after acute nonvariceal upper gastrointestinal bleeding in the United Kingdom. Transfusion. (2013) 53:1069e76. doi: 10.1111/j.1537-2995.2012.03849.x
17. Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. (2019) 364:l536. doi: 10.1136/bmj.l536
18. National Blood Authority Australia. Patient Blood Management Guidelines: Module 1-Critical Bleeding/Massive Transfusion. Canberra: Australian Government (2011).
19. Subramaniam K, Spilsbury K, Ayonrinde OT, Latchmiah F, Mukhtar SA, Semmens JB, et al. Red blood cell transfusion is associated with further bleeding and fresh-frozen plasma with mortality in nonvariceal upper gastrointestinal bleeding. Transfusion. (2016) 56:816–26. doi: 10.1111/trf.13446
20. Baradarian R, Ramdhaney S, Chapalamadugu R, Skoczylas L, Wang K, Rivilis S, et al. Early intensive resuscitation of patients with upper gastrointestinal bleeding decreases mortality. Am J Gastroenterol. (2004) 99:619–22. doi: 10.1111/j.1572-0241.2004.04073.x
21. Li G, Rachmale S, Kojicic M, Shahjehan K, Malinchoc M, Kor DJ, et al. Incidence and transfusion risk factors for transfusion-associated circulatory overload among medical intensive care unit patients. Transfusion. (2011) 51:338–43. doi: 10.1111/j.1537-2995.2010.02816.x
22. Mitra B, Cameron PA, Gruen RL. Aggressive fresh frozen plasma (FFP) with massive blood transfusion in the absence of acute traumatic coagulopathy. Injury. (2012) 43:33–7. doi: 10.1016/j.injury.2011.10.011
23. Watson GA, Sperry JL, Rosengart MR, Minei J, Harbrecht BG, Moore EE, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. (2009) 67:221–7; discussion 228–30. doi: 10.1097/TA.0b013e3181ad5957
24. Khan H, Belsher J, Yilmaz M, Afessa B, Winters JL, Moore SB, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. (2007) 131:1308–14. doi: 10.1378/chest.06-3048
25. Marlu R, Hodaj E, Paris A, Albaladejo P, Crackowski JL, Pernod G. Effect of nonspecific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Hemostasis. (2012) 108:217e24. doi: 10.1160/TH12-03-0179
26. Tripodi A, Primignani M, Chantarangkul V, Viscardi Y, Dell'Era A, Fabris FM, et al. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res. (2009) 124:132e6. doi: 10.1016/j.thromres.2008.11.008
27. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. (2011) 365:147e56. doi: 10.1056/NEJMra1011170
28. Tripodi A. The coagulopathy of chronic liver disease: is there a causal relationship with bleeding? No Eur J Intern Med. (2010) 21:65e9. doi: 10.1016/j.ejim.2010.02.001
29. Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion. (2010) 50:1370–83. doi: 10.1111/j.1537-2995.2010.02630.x
30. Premkumar M, Kulkarni AV, Kajal K, Divyaveer S. Principles, interpretation, and evidence-based role of viscoelastic point-of-care coagulation assays in cirrhosis and liver failure. J Clin Exp Hepatol. (2022) 12:533–43. doi: 10.1016/j.jceh.2021.05.001
Keywords: acute upper gastrointestinal bleeding, fresh frozen plasma, mortality, rebleeding, blood transfusion
Citation: Liu S, Zhang X, Walline JH, Yu X and Zhu H (2022) Fresh Frozen Plasma in Cases of Acute Upper Gastrointestinal Bleeding Does Not Improve Outcomes. Front. Med. 9:934024. doi: 10.3389/fmed.2022.934024
Received: 02 May 2022; Accepted: 13 June 2022;
Published: 14 July 2022.
Edited by:
Dawesh P. Yadav, Banaras Hindu University, IndiaReviewed by:
Anurag Kumar Singh, Alabama State University, United StatesVivek Kumar Pandey, University of Kentucky, United States
Ashish Agarwal, All India Institute of Medical Sciences Jodhpur, India
Copyright © 2022 Liu, Zhang, Walline, Yu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuezhong Yu, eXh6cHVtY2gmI3gwMDA0MDsxMjYuY29t; Huadong Zhu, emh1aHVhZG9uZzE5NzAmI3gwMDA0MDsxMjYuY29t
 Shuang Liu
Shuang Liu Xiaoming Zhang
Xiaoming Zhang Joseph Harold Walline3
Joseph Harold Walline3 Xuezhong Yu
Xuezhong Yu Huadong Zhu
Huadong Zhu