- Hamad Medical Corporation, Doha, Qatar
Sickle cell disease (SCD) is a heterogeneous group of inherited disorders characterized by the production of sickle hemoglobin which is less soluble than an adult or fetal hemoglobin. Voxelotor is a hemoglobin S polymerization inhibitor that has been approved for sickle cell disease treatment in the adult and adolescent populations. It acts as a hemoglobin modulator by increasing its affinity to oxygen which prevents red blood cells from sickling. Chronic kidney disease is a common but under-reported complication of SCD and it is a leading cause of morbidity and mortality. The data about the safety and efficacy of voxelotor use in chronic kidney disease is limited. Herein we report a 49-year-old man, with sickle cell disease and stage IV chronic kidney disease, who was managed successfully with voxelotor and resulted in decreasing transfusion requirement and vaso-occlusive painful crisis without affecting kidney function.
Introduction
Sickle cell disease (SCD) is a heterogeneous group of inherited disorders characterized by the production of sickle hemoglobin, which polymerizes when deoxygenated resulting in rigid and very fragile red blood cells. Hemoglobin SS is the most common genotype, however, other genotypes like C, D, and E present with different phenotypes of chronic hemolysis and recurrent vaso-occlusive crisis (1, 2). SCD affects millions of people worldwide. It is estimated that the number of patients with SCD in the United States may approach 100,000 (3, 4). SCD is a multi-organ disorder that is associated with high morbidity and mortality. The major clinical features are chronic hemolysis, infection, vaso-occlusive events that cause pain, tissue ischemia, and sometimes infarction (5, 6).
Voxelotor is a hemoglobin S polymerization inhibitor that has been approved for sickle cell disease treatment by the United States Food and Drug Administration (FDA) November 2019 for the adult and adolescent population (≥12 years) (7). It reversibly binds to hemoglobin and increases its affinity to oxygen, stabilizing sickle hemoglobin and preventing polymerization. It can be considered for patients who did not tolerate hydroxyurea or can be prescribed as an additional treatment for patients who are refractory on maximum doses of hydroxyurea. The most common adverse effects are headache, abdominal pain, nausea, skin rash, and fever (8).
Chronic kidney disease is a common but under-reported complication of SCD and is a leading cause of morbidity and mortality. The annual incidence of acute renal failure and chronic kidney disease was found to be 2–3 times higher compared to the non-sickle group in a retrospective study (9). Renal impairment is a risk for drug toxicity as well as decreased drug efficacy.
The data about the safety and efficacy of voxelotor in chronic kidney disease is limited. Herein we report a case of a 49-year-old man, a known case of sickle cell disease and stage IV chronic kidney disease who was requiring frequent transfusion and suffering from severe body pain, managed successfully with voxelotor without affecting kidney function.
Case report
A patient is a 49-year-old man with SCD (hemoglobin S/D double heterozygous), diabetes mellitus, and hypertension. His clinical course was complicated by chronic kidney stage IV, due to sickle glomerulopathy (baseline creatinine 250–300 umol/L and estimated GFR around 25 ml/min/1.73 m2), splenectomy at age 20, osteonecrosis of the left femoral head status post left hip replacement at age of 25. The patient required recurrent hospital visits for vaso-occlusive pain crises and blood transfusions despite being compliant with hydroxyurea 1000 mg twice daily.
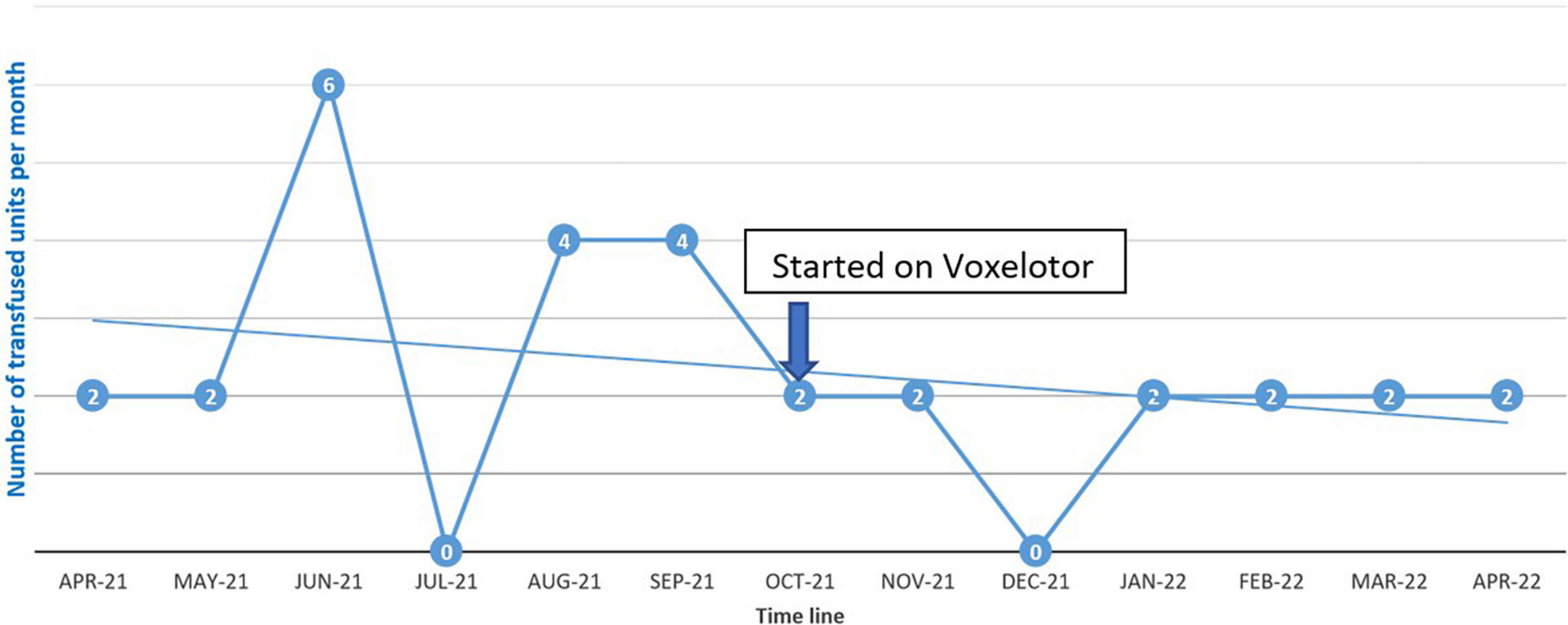
The patient was started on voxelotor 1,500 mg daily. After starting voxelotor, transfusion frequency decreased from every 2 weeks to every 4 weeks to maintain hemoglobin above 7 g/dL as can be seen in Figure 1. The patient reported that his body pain decreased by more than 70% and he did not report any of the known voxelotor side effects such as headache, nausea, diarrhea, or abdominal pain.
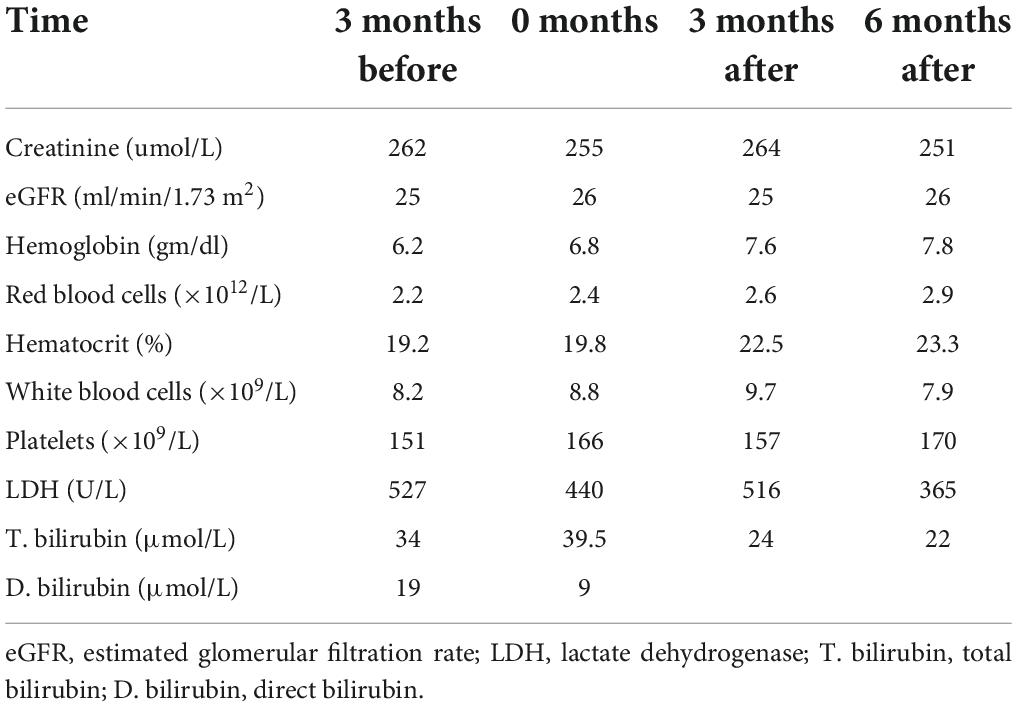
Close follow-up of kidney function over 6 months on voxelotor showed that creatinine and GFR remained stable, as seen in Table 1.
Discussion
Polymerization of the deoxygenated sickle hemoglobin is the main driver of the pathophysiology of sickle cell disease. Voxelotor acts as a hemoglobin modulator by increasing its affinity to oxygen which prevents red blood cells from sickling. Voxelotor received FDA approval in late 2019 for sickle cell disease treatment following a multicenter, phase 3, double-blind, randomized, placebo-controlled trial. This trial showed that voxelotor provided a sustained increase in hemoglobin and a decrease in hemolytic markers (10, 11).
Pharmacokinetics and pharmacodynamics studies of voxelotor in healthy people have shown it is a rapidly absorbed drug, has an oral bioavailability of 35%, binds to protein at 99.8%, and its post-peak primarily metabolism in the liver. Hepatic metabolism is via phase I (oxidation and reduction) and phase II (glucuronidation). Around two-thirds of voxelotor and its metabolites are excreted in feces (62.6%) while around one-third are excreted in the urine (35.4%) after oral administration (12).
The data about the safety and efficacy of voxelotor in chronic kidney disease is limited. Preston et al. reported the only study in this regard (13). Its two open-label, parallel-group, phase I clinical trials, studied the safety and pharmacokinetics of voxelotor in patients with liver or kidney impairment. Considering that impaired kidney function may change voxelotor exposure, a total of eight patients with SCD and chronic kidney disease (estimated GFR < 30 mL/min/1.73 m2) and eight healthy, matched controls were given voxelotor 900 mg daily following an overnight fast. Subjects with normal kidney function were matched to patients with SCD having severe kidney impairment based on age, sex, and body mass index. Mean creatinine was 394 μmol/L and creatinine clearance was 12.5 ml/min in the renal impairment group. End-stage renal disease patients on dialysis were excluded. The result showed no apparent effect of renal function on voxelotor excretion on the basis of similar post-peak mean plasma voxelotor concentration decline between the two groups. There were no serious adverse events (AE), mortality, or medication discontinuation due to AE. Based on these results, Preston et al. (13) concluded that; voxelotor is safe and tolerable in patients with SCD along with severe chronic kidney disease and no dose adjustment is needed.
In our case, the patient had a poor quality of life due to recurrent hospital visits for blood transfusion and a vaso-occlusive painful crisis. After starting a 1500 mg daily dose of voxelotor, his transfusion requirement decreased, and he had a subjective decrease in body pain. In the Preston study, they used the dose of 900 mg daily as it was expected to be within the upper range of the therapeutic dose and was well tolerated in healthy subjects, however, we used the dose of 1,500 mg daily based on the HOPE trial which showed a higher hemoglobin response compared to the dose of 900 mg and pl acebo (7). Indices of kidney function remained stable 6 months after starting treatment which supports the previously reported study. Up to our knowledge, this is the first real-world reported case of voxelotor use in sickle cell disease patients with stage IV chronic kidney disease with the approved dose of voxelotor 1500 mg daily.
Conclusion
In conclusion, considering the limited data, this case report indicates that voxelotor is safe and tolerable, and dose adjustment is not required in patients with SCD along with severe renal impairment; however, further studies are required to confirm this finding.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Research Council, Hamad Medical Corporation. The patients/participants provided their written informed consent to participate in this case study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AA contributed to acquisition of data and drafted the manuscript. Both authors equally contributed to writing and editing.
Funding
Open access funding was provided by the Qatar National Library.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yassin M, Soliman A, De Sanctis V, Nashwan A, Abusamaan S, Moustafa A, et al. Liver Iron Content (LIC) in adults with Sickle Cell Disease (SCD): correlation with serum ferritin and liver enzymes concentrations in Trasfusion Dependent (TD-SCD) and Non-Transfusion Dependent (NT-SCD) patients. Mediterr J Hematol Infect Dis. (2017) 9:e2017037. doi: 10.4084/MJHID.2017.037
2. Khamees I, Ata A, Choudry H, Soliman AT, De Sanctis V, Yassin MA. Manifestations of HbSE sickle cell disease: a systematic review. J Transl Med. (2021) 19:262.
3. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. (2010) 38:S512–21. doi: 10.1016/j.amepre.2009.12.022
4. Wali Y, Kini V, Yassin MA. Distribution of sickle cell disease and assessment of risk factors based on transcranial doppler values in the Gulf region. Hematol Amst Neth. (2020) 25:55–62. doi: 10.1080/16078454.2020.1714113
5. Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. (2010) 376:2018–31. doi: 10.1016/S0140-6736(10)61029-X
6. Yassin MA, Soliman AT, De Sanctis V, Abdula MAJ, Riaz LM, Ghori FF, et al. Statural growth and prevalence of endocrinopathies in relation to liver iron content (LIC) in adult patients with beta thalassemia major (BTM) and sickle cell disease (SCD). Acta Bio Medica Atenei Parm. (2018) 89(Suppl 2):33–40. doi: 10.23750/abm.v89i2-S.7085
7. Vichinsky E, Hoppe CC, Ataga KI, Ware RE, Nduba V, El-Beshlawy A, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. (2019) 381:509–19. doi: 10.1056/NEJMoa1903212
8. Estepp JH. Voxelotor (GBT440), a first-in-class hemoglobin oxygen-affinity modulator, has promising and reassuring preclinical and clinical data. Am J Hematol. (2018) 93:326–9. doi: 10.1002/ajh.25042
9. Yeruva SLH, Paul Y, Oneal P, Nouraie M. Renal failure in sickle cell disease: prevalence, predictors of disease, mortality and effect on length of hospital stay. Hemoglobin. (2016) 40:295–9. doi: 10.1080/03630269.2016.1224766
11. Ali MA, Ahmad A, Chaudry H, Aiman W, Aamir S, Anwar MY, et al. Efficacy and safety of recently approved drugs for sickle cell disease: a review of clinical trials. Exp Hematol. (2020) 92:11–8.e1. doi: 10.1016/j.exphem.2020.08.008
12. Hutchaleelaha A, Patel M, Washington C, Siu V, Allen E, Oksenberg D, et al. Pharmacokinetics and pharmacodynamics of voxelotor (GBT440) in healthy adults and patients with sickle cell disease. Br J Clin Pharmacol. (2019) 85:1290–302. doi: 10.1111/bcp.13896
Keywords: sickle cell disease, voxelotor, hemolysis, chronic kidney disease, vaso-occlusive crisis (VOC)
Citation: Alshurafa A and Yassin MA (2022) Case report: Safety and efficacy of voxelotor in a patient with sickle cell disease and stage IV chronic kidney disease. Front. Med. 9:931924. doi: 10.3389/fmed.2022.931924
Received: 24 May 2022; Accepted: 15 August 2022;
Published: 15 September 2022.
Edited by:
Hollie Marie Reeves, University Hospitals Cleveland Medical Center, United StatesReviewed by:
Salam Alkindi, Sultan Qaboos University, OmanNicola Conran, State University of Campinas, Brazil
Copyright © 2022 Alshurafa and Yassin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Awni Alshurafa, ZHIuYS5zaHVyYWZhQGdtYWlsLmNvbQ==; orcid.org/0000-0002-4454-5307; Mohamed A. Yassin, eWFzc2lubW9oYUBnbWFpbC5jb20=; orcid.org/0000-0002-1144-8076
 Awni Alshurafa
Awni Alshurafa Mohamed A. Yassin
Mohamed A. Yassin