
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 04 January 2023
Sec. Geriatric Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.931565
Importance: It remains unknown whether Taiwanese veterans have a lower risk of subsequent cancer compared with non-veterans.
Objective: To examine whether veterans are associated with reduced cancer risk.
Methods: From January 2004 to December 2017, this study included 957 veterans and 957 civilians who were propensity score (PS) matched by years of birth, sex, residence, index year, days in the hospital, frequency of outpatient visits, and relevant comorbidities at baseline. Multivariate Cox proportional hazards regression analysis was applied to compare the risks of cancer, overall and by subgroup, and mortality. All the participants were cancer free at the baseline.
Exposures: Veterans retrieved from Taiwan National Health Insurance Research Database (NHIRD).
Main outcome: Cancer extracted from the Registry for Catastrophic Illness Patients Database (RCIPD).
Results: Overall, 1,914 participants were included, and 957 veterans with a mean (SD) age of 75.9 (6.79) years and 946 men (98.9%). The mean follow-up was about 10.5 (±4.51) years. Cancer was recorded in 6.68% (N = 64) and 12.12% (N = 116) of veterans and non-veterans, respectively. Veterans were associated with decreased risk [adjusted hazard ratio (aHR), 0.57; 95% CI: 0.41–0.78; P < 0.001] of cancer compared with civilians after controlling for age, sex, urbanization, hypertension, diabetes, hyperlipidemia, cardiovascular event, COPD, asthma, chronic liver disease, alcohol-related illness, and Parkinson’s disease. Cancer subgroup analyses verified this finding (HRs <1.0). The decreased incidence rate was predominantly for liver cancer (aHR, 0.18; 95% CI: 0.05–0.72; P < 0.05).
Conclusion: Taiwanese older veterans are associated with reduced overall cancer risk than individuals without veteran status.
Cancer is a major global public health issue and the second leading cause of death in the United States, with approximately 5,200 new cases expected to be diagnosed each day in the United States (US) (1). Research has established convincing evidence that much of the risk for cancers can be attributed to extrinsic exposures, with approximately 70–90% of cancer cases due to the patient’s lifestyle behaviors and environmental factors (2). Physically and mentally capable civilians are selected for military service, dedicate time in the military, such as 3–4 years in Scottish, and then leave the service with veteran status. The military population may differ from the general population in terms of exposure to factors associated with cancer risks, such as health behavior, smoking, alcohol consumption, diet, sun exposure, and physical fitness (3). Yet, there is a potential “healthy soldier effect” in which only those who are physically and mentally healthy are selected for the military, and there is evidence that this may carry over into the “healthy veteran effect” (3–6).
Studies of US veterans have shown that the most common malignancies are prostate, lung, and colorectal cancers, similar to trends in the general US population (7). Veterans with diabetes had a higher incidence of liver, pancreatic, and colorectal malignancies than non-diabetic veterans (8). Among Korean veterans who served in the Vietnam War, they have an increased risk of developing overall cancer and several specific cancers in the mouth, salivary glands, stomach, and small intestine (9), but other studies showed inconsistent findings (4, 10). In observational epidemiologic cancer studies, patient interactions with healthcare services, such as frequency of receiving outpatient care, the total length of stay at the baseline, and accessibility of services are important confounding factors (11). However, none of these previous studies included baseline comorbidities and medical accessibility to address detection bias in the analysis of subsequent cancer risk in veterans and non-veterans (4, 9, 10, 12–14).
There are some specific points about Taiwan’s veterans that need to be stated before the study. The most important eligibility issue to become a Taiwanese veteran is that the soldier has completed at least 10 years of military service, which is quite different compared with other countries (5). Veterans in Taiwan did not been through battles or wars that were fraught with the risk of exposure to toxic substances, such as Agent Orange (9, 10), or nerve agent, Sarin (12, 15), which are potentially associated with cancer. Veterans who are not working received a variety of benefits from the Veterans Affairs Council and are exempted from Medicare co-payment. Furthermore, veterans who are homeless or in need of support may be housed in a Veterans’ Home upon proper application. Each Veterans’ Home is geographically integrated into a network of care at a responsible Tertiary Veterans Hospital in Taiwan. Since Taiwanese veterans have a longer length of military service and comprehensive inpatient and discharge support from the national government, we hypothesize that veteran status has a prolonged health effect and lower risk of subsequent cancer development. We conducted this national population-based surveillance cohort study to examine whether participants with veteran status were at risk or protected against the development of cancer.
After obtaining approval from the Institutional Review Board (IRB) of the Taiwan Ministry of Health and Welfare, we identified medical records of veterans collected in the National Health Insurance Research Database (NHIRD) between 2000 and 2017. Universal Taiwan’s National Health Insurance (NHI) program administered by the Taiwan Government is single-payer and mandatory and includes >99% of Taiwan’s population (16). NHIRD is available for research purposes with appropriate applications. The validity of NHIRD for use in epidemiological cancer research has been shown in the previous publication (17–24). NHIRD includes patients treated at dedicated veterans’ hospitals. This study was a retrospective evaluation of patient information with no more than minimal risk to the subjects. As it is impossible to identify individual patients, informed consent is not required for this study. The encrypting procedure is identical and the linkage of the claims belonging to the exact patient is constant and feasible for continuous follow-up. The NHIRD records comprehensive and ongoing registration and claims information, including participants’ characteristics, disease diagnoses, outpatient visits, emergency department utility, inpatient information, diagnostic, treatment, operation codes, and prescribed medications. All claims can be linked in chronological order to provide a temporal sequence of all health services utilization. We used five data sources: beneficiary registries, ambulatory care expenditures by outpatient visits, inpatient expenditures by the number of admissions, registry of catastrophic illness patients database, and cause of death data. We used encrypted and unique personal identification numbers to obtain longitudinal medical histories of intake cases. Prior to 2016, the diagnosis codes used in this study were based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and from 2016 onward, the diagnosis codes were based on the ICD-10-CM. This study was approved by the China Medical University Hospital Research Ethics Committee with the certificated number CMUH109-REC2-031 (CR-2).
We included people with veteran status as the participants of the study group from January 2004 to December 2015 and follow-up until 2017. The veteran status is determined with a code of the Registry for Beneficiaries in the NHIRD to identify ID1_UNIT = 61. The study database contains 2 million people, which was sampled by BNHI from the original claim data of NHIRD. There was no significant difference in the sex (P = 0.613) and age distribution between the subset data and the original NHIRD. The duration of 2000–2003 was set to be the baseline period to collect demographic and comorbidities information, and this period to be the look-back period to ensure all participants in both groups are free of cancer before this study. The reference group included patients who never had the status of veterans during the follow-up period. We matched one control civilian from the general population to each individual with veteran status on age, sex, and urbanization level of residence (i.e., the information about urbanization is based on the participants’ urbanization level of residence; level 1 denotes most urbanized and level 4 denotes least urbanized. This is a proxy for healthcare availability), index year, baseline disease severity (proxy by days in the hospital and frequency of outpatient visits at the baseline), and relevant comorbidities. To mitigate potential immortal time and survival bias, the index year was set 3 years after all participants of both groups entering NHIRD and the follow-up started subsequently. Control participants were alive on the index year. To estimate the risk of cancer, both groups were followed from the index year until the first cancer was diagnosed, death, or the end of the observation period (31 December 2017), whichever came first. The exclusion criteria were patients with missing information on age and sex, diagnosis of cancer before the index date, and a follow-up time of less than 1 year.
The outcome variables were overall cancer and prevalent specific cancer, ascertained by linking the unique identification number of each participant to the Registry for Catastrophic Illness Patients Database (RCIPD). Codes from the International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM), and Tenth Revision (ICD-10) were used to identify cancer. The positive predictive rate was 93% (19). Each participant was followed up until a diagnosis of cancer (ICD-9-CM code 140-208 and ICD-10-CM C00-C96) was made. We set all-cause mortality as the secondary outcome to examine the potential competing status of death on cancer.
The patient’s age and sex were recorded at the index year. Baseline comorbidities were extracted from at least three consistent medical claims records prior to the index year (i.e., 2000–2003). We identified the presence of the following comorbidities using ICD-9-CM codes: hypertension (ICD-9-CM codes 401-405), diabetes (ICD-9-CM code 250), hyperlipidemia (ICD9-CM code 272), cardiovascular disease (CVD) (ICD9-CM code 414-410 and 430-430), chronic obstructive pulmonary disease (COPD) (ICD-9-CM code 490-492 and 493-496), asthma (ICD-9-CM code 493), chronic hepatitis (ICD-9-CM code 571.4), chronic kidney diseases (CKD) (ICD-9-CM code 585), and Parkinson’s disease (PD) (ICD-9-CM code 332.0). To address detection bias, we included the length of hospital stay and frequency of outpatient department within 1 year before the index year into the study.
Baseline characteristics of participants are shown as event numbers and percentages for categorical variables and as means and standard deviations for continuous variables. Chi-square was adopted to examine the categorical variables and Student’s t-test for continuous variables. The matching policy in our study was individual propensity score matching rather than frequency matching. The veteran group was matched to the non-veteran group based on their propensity score (1:1 ratio) using the nearest neighbor matching process, initially for the eighth digit, and then for the first digit as needed. Thus, matches were first made within a caliper width of 0.0000001, and then the caliper width was gradually increased to 0.1 for unmatched cases. In this way, some variables may remain different between the two groups, such as the urbanization level in this study. The crude hazard ratio (cHR) was estimated by the univariable Cox proportional hazards model, and then repeated with adjustment for the relevant confounding effect of covariates. The incidence of cancer during follow-up was calculated by dividing the number of events by the respective person-years at risk and presented as the number of events per 1,000 person-years. Kaplan–Meier curve was used to describe the cumulative incidence of overall cancer in the study and reference groups with the log-rank test. We used multivariable Cox proportional hazards regression models to examine the potential effect of veteran status on the cumulative risk of cancer after adjustment for age, sex, and covariates list in Table 1 including hypertension, diabetes, hyperlipidemia, CVD, COPD, asthma, chronic liver disease, CKD, and PD. Schoenfeld residual analysis was adopted to confirm the assumption of no violation of proportionality risk. Analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc.). All the tests of significance were two-tailed with a P-value of <0.05 was considered statistically significant. Cox proportional hazards analysis was used to compare the risks of death between veterans and non-veterans.
We stratified the data by year of birth in our analysis, dividing the data into 10-year bands to improve statistical power and examine potential birth cohort effects. We conducted a subgroup analysis to assess the association of the presence or absence of comorbidity with cancer in terms of veteran status. Furthermore, we determined the potential effect of veteran status on the risk of new-onset specific cancer type. Cancer type of the small number of subjects will not be shown in our report for the political “Personal Information Protection Act.” Patients with diabetes are at risk for cancer, thus, we conducted a further subgroup analysis based on the status of diabetes or not among veterans in our study.
The data source used in this study was the claims data of NHIRD published by Taiwan National Health Insurance. Participants were not involved in the retrospective secondary cohort study. For Taiwan legal restrictions according to the “Personal Information Protection Act,” data in this study cannot be made publicly available. Data requests for revision can be made as a formal appointment according to the original proposal.
A total of 1,914 participants were enrolled between January 2004 and December 2015. Among them, we identified the veteran group (N = 957) and the non-veteran control group (N = 957) after propensity score matching. We selected controls based on strict criteria of the same age, sex, index year, and comorbidities distribution.
As shown in Table 1, 98.9% of the veterans were male and 54.8% of them were born between 1917 and 1926. Gender differences were consistent with the gender distribution of the armed forces. Although the veteran group presented with more comorbidities such as hypertension, CVD, COPD, and asthma compared with the non-veteran group before the propensity score (PS) match. These comorbidities and frequency of medical utility were well-balanced after the PS match (Table 1, right column). The mean (SD) age was 75.9 (6.79) years. The mean (SD) follow-up time for cancer was 10.5 (4.51) years. The mean (SD) follow-up time for death was 10.6 (4.48) years. Although not all variables between the two cohorts were matched to statistically insignificant, we used the multivariate Cox proportional hazards regression model to adjust these variables.
The association between veteran status and cancer risk was shown in Table 2. By the end of the study, 64 (6.68%) veterans had a medical record of cancer diagnosis compared with 116 (12.12%) non-veterans. The veteran group had a lower incidence rate (IR) of cancer (IR, 6.38 per 1,000 person-years) than those in the control group (IR, 11.5 per 1,000 person-years). After adjustment for birth year, sex, urbanization, and comorbidities, the adjusted HR (aHR) of cancer for the veteran group relative to the control group was 0.57 [95% confidence interval (CI): 0.41–0.78; P < 0.001]. Among the comorbidities, the risk of cancer in participants with diabetes was 1.47 times greater than those without diabetes (95% CI: 1.04–2.07; P = 0.03). The risk of cancer in participants with chronic liver disease was 2.22 times greater than those without chronic liver disease (95% CI: 1.01–4.88; P = 0.046).
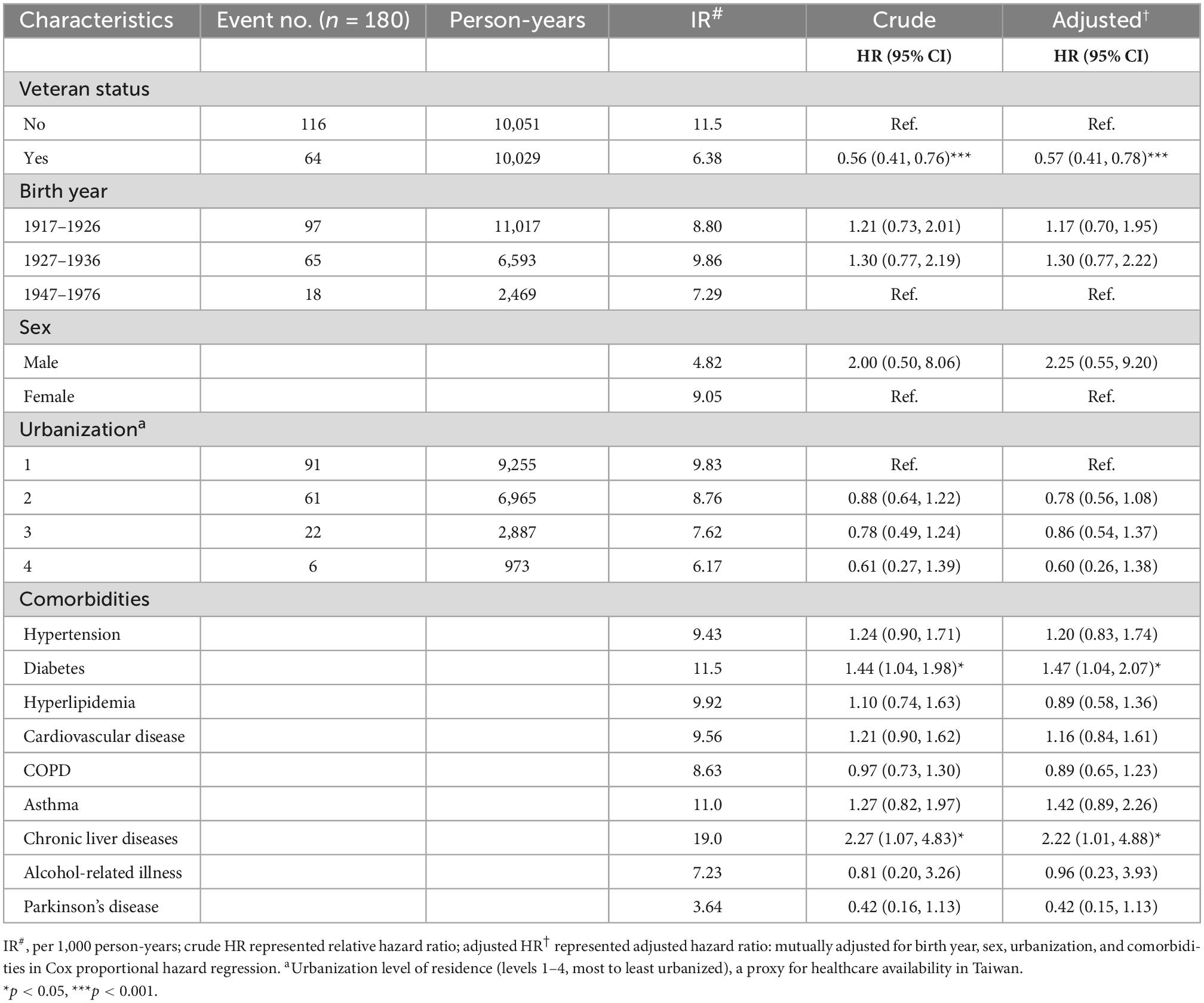
Table 2. Cox model measured hazard ratio and 95% confidence intervals of cancer associated with and without veteran status and covariates on the propensity score matched cohorts.
Table 3 shows the results of the subgroup analysis. After adjustment for birth year, sex, urbanization, and comorbidities, veteran status was not associated with an increased risk of cancer development (all HR <1). The age-specific aHR of cancer was lowest for a patient with the birth year of 1917–1926 (aHR, 0.46; 95% CI: 0.30–0.73; P < 0.001). The aHRs of cancer for the veteran to the non-veteran group were universally reduced; aHR, 0.35 (95% CI: 0.13–0.89) in all participants without comorbidities and 0.61 (95% CI: 0.43–0.85) in all participants with comorbidities. Specifically, the risk of subsequent cancer in veterans was lower in the subgroup without diabetes (aHR 0.51; 95% CI: 0.35–0.75), but the potential protective effect appeared to be diminished in the subgroup with diabetes (aHR, 0.68; 95% CI: 0.38–1.21).
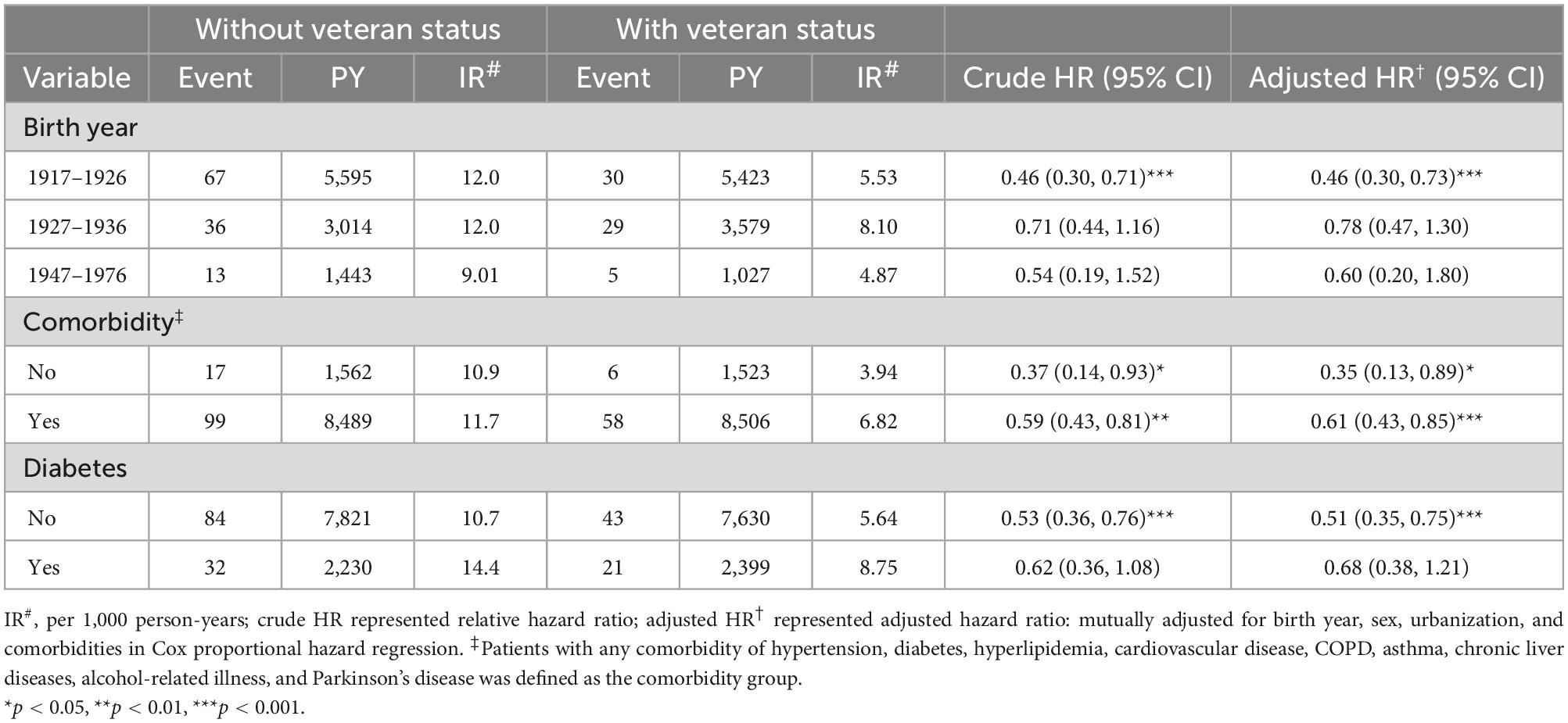
Table 3. Comparison of incidence and hazard ratio of cancer stratified by birth year and comorbidity between groups with and without veteran status on the propensity score matched cohorts.
Table 4 shows the comparison of the IR of cancer type between the veteran group and the non-veteran control group. The data illustrate that veteran status was significantly inversely associated with liver cancer (aHR, 0.18; 95% CI: 0.05–0.72; P < 0.05). There was no significant impact of veteran status on the risk of cancer, including stomach cancer (aHR, 0.76; 95% CI: 0.28–2.05), lung cancer (aHR, 0.90; 95% CI: 0.46–1.76), prostate cancer (aHR, 0.84; 95% CI: 0.36–1.96), and kidney and bladder cancer (aHR, 0.43; 95% CI: 0.14–1.40). There was a reduced risk for colorectal cancer with crude HR 0.46 (95% CI: 0.22–0.97) and almost achieved statistical significance, aHR 0.49, 95% CI: 0.23–1.05, although the number of cases was small.
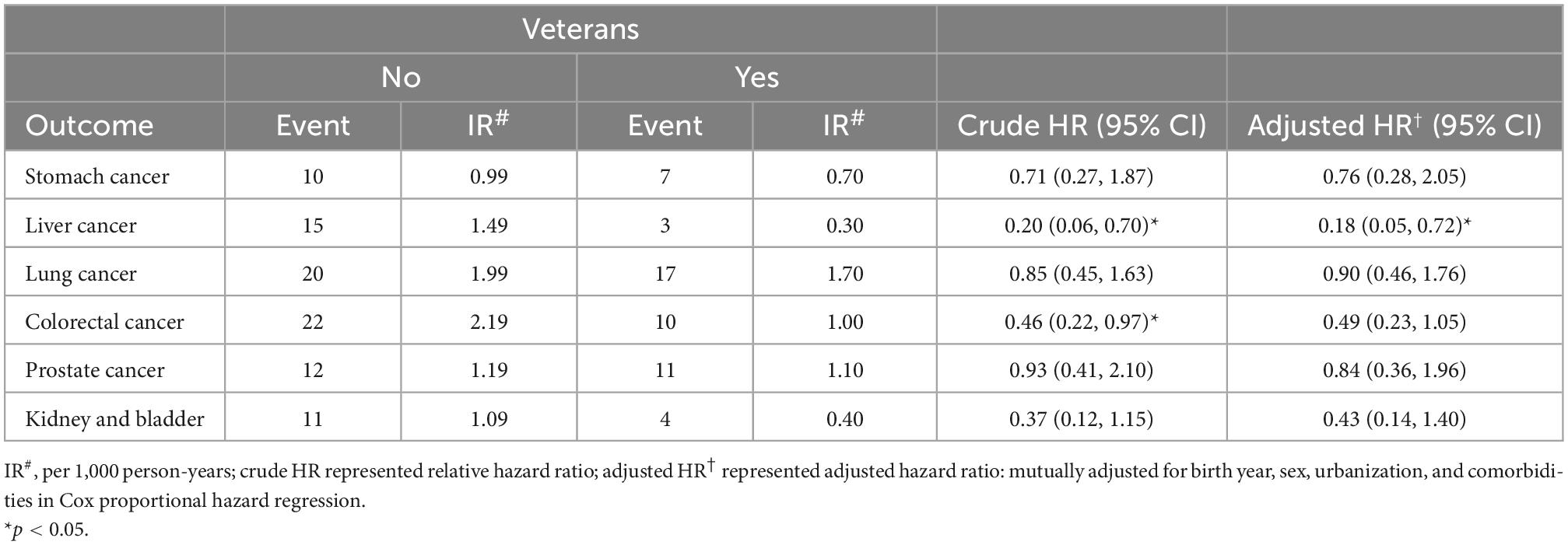
Table 4. Comparison of incidence and hazard ratio of cancer type on the propensity score matched cohorts.
Table 5 shows the comparison of the IR of death and estimation stratified by birth year and comorbidities. The risk of death in veterans and non-veterans did not differ significantly [aHR, 1.03; (95% CI: 0.93–1.14)]. In the age subgroup (born in 1917–1926 and 1937 onward), the aHR of all-cause mortality was 0.97 (95% CI: 0.85–1.11) and 1.09 (95% CI: 0.80–1.49), respectively. In the age subgroup (born in 1927–1936), the aHR of all-cause mortality was 1.51 (95% CI: 1.22–1.88).
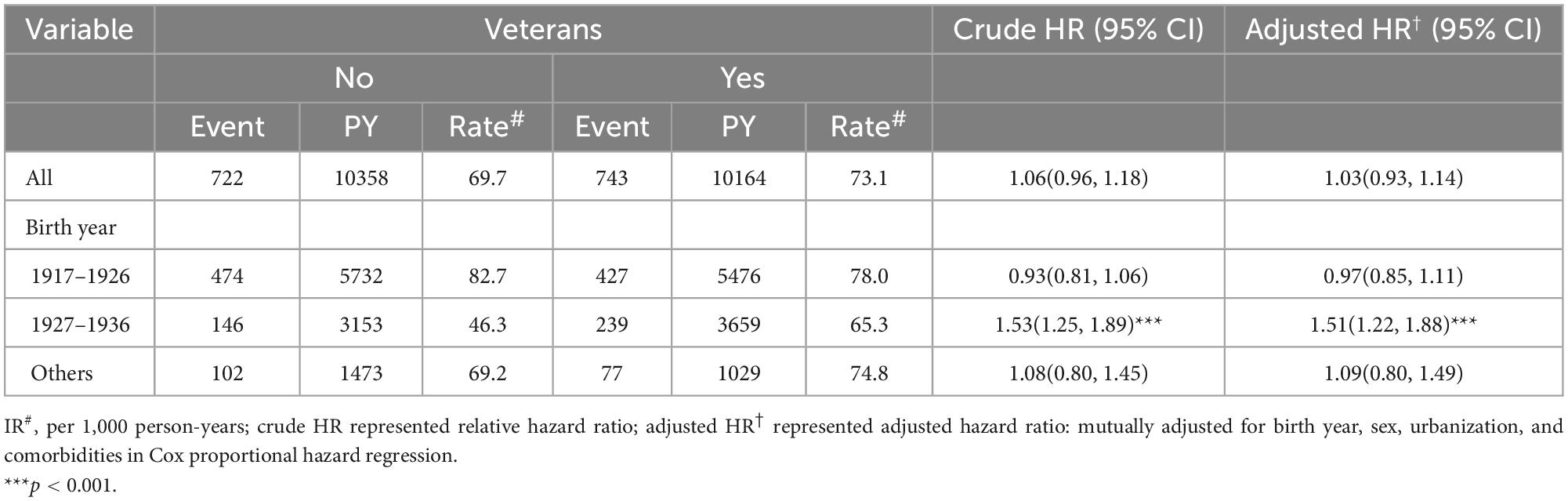
Table 5. Comparison of incidence and hazard ratio of all-cause mortality and stratified by birth year.
Figure 1 showed a statistically significant difference in the risk of cancer between patients with veteran status and those without veteran status. There was a reduction in cancer risk in the veteran group (log-rank test, P < 0.001). Figure 2 demonstrates no significant difference in the risk of death between the two groups.
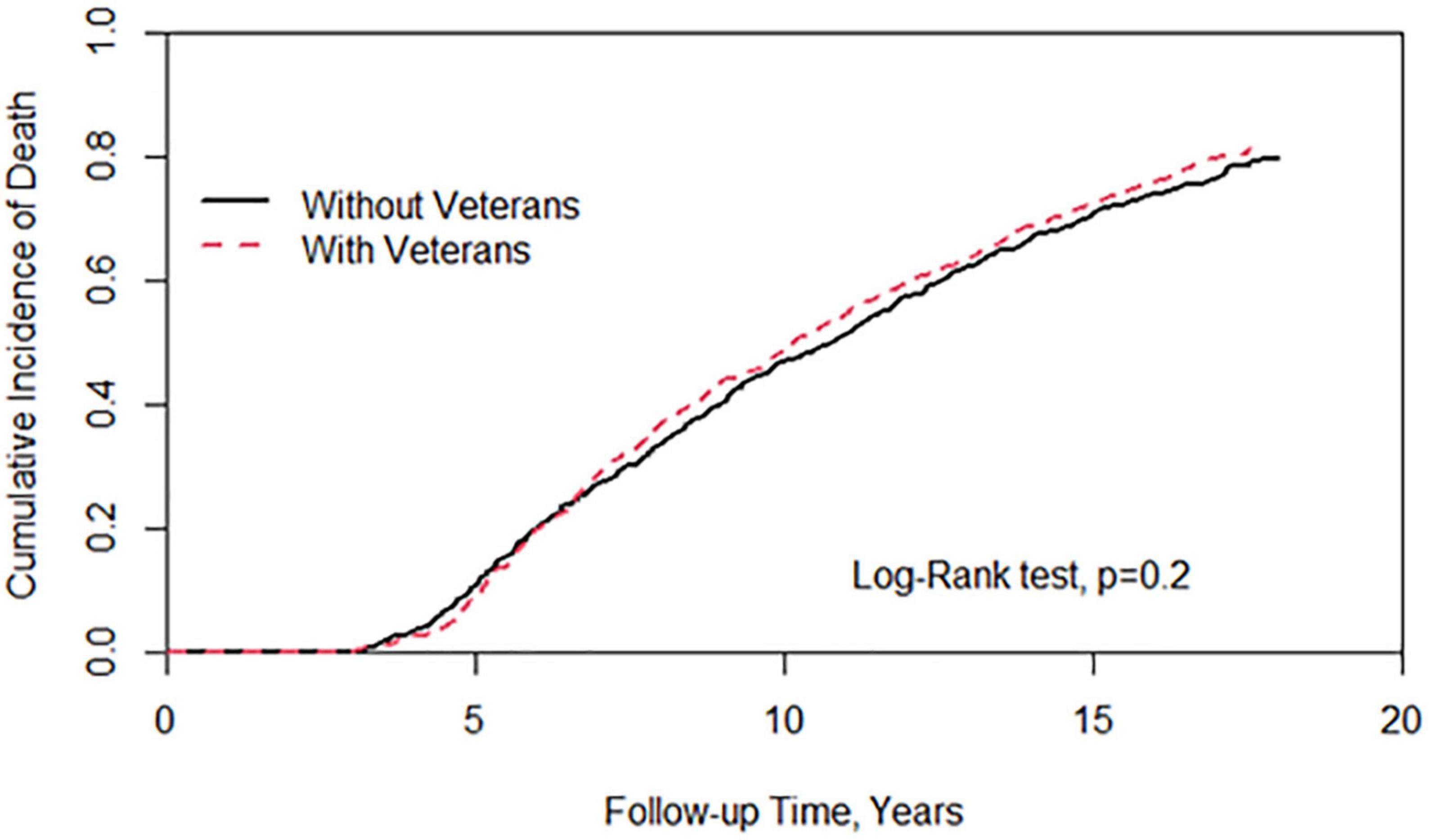
Figure 2. Cumulative incidence of all-cause mortality between individuals with and without veterans status.
To the best of our knowledge, this is the first countrywide large-scale study to investigate the association between veteran status and cancer in East Asians. Our study concludes that older military veterans (those born in 1917–1926) were at reduced risk of cancer in comparison with civilians without veteran status in Taiwan, after matching for birth year, sex, geographical region, medical accessibility, and comorbidities. The risk of all cancer among younger veterans (those born 1927 onward) did not have an increased risk experienced by non-veterans (all HR <1). Increased cancer risk in patients with diabetes and chronic liver disease were consistently reported in previous studies (25).
One study of the Korean veteran’s population described a higher incidence of lung, bladder, kidney, prostate, and colon cancer than in the general population (10). In another study on New Zealand Vietnam War veterans, the authors demonstrated that overall cancer risk was not significantly increased but hematologic malignancies (4). A recent study demonstrated that after considering the healthy worker effect, veterans exposed to herbicides decades ago have a higher risk of all cancer and an increased incidence of the esophagus, lung, liver, and gastric malignancies (9). Chemical exposure, such as tactical herbicides and Agent Orange, was a significant feature of the previous study. However, veterans in Taiwan did not attend Vietnam or Gulf War during their military service, and the overall risk of exposure to a human carcinogen, such as herbicides, is less likely. In addition, the previous study mentioned that US veterans have poorer health behaviors, such as current heavy alcohol and tobacco consumption (3), which have a role in cancer development (14). Although precise information on differences in chemical exposure and healthy lifestyle between Taiwan and other countries is not available, it is reasonable to presume that such disparity contributed in part to the almost opposite results of our study and previous cohorts in terms of veteran status and cancer risk. The reduction in cancer incidence in our study may be due to the fact that the effects of healthy soldiers were not offset by human carcinogen exposure in Taiwan.
Our study demonstrates the difference in overall cancer risk between veteran personnel and the general population. Incidence rates were lower in veteran personnel than in the general population for stomach, liver, lung, colorectal, prostate, kidney, and bladder cancer. But the differences in rates between the two groups were significant only for liver cancer. It is unclear why people with veteran status in Taiwan have a lower risk of cancer than other civilians, and the factors that may be associated with this difference are outlined below. Men who enter the military and leave as veterans are a specific group because those with certain diseases or conditions are ineligible for military service. In a 2015 national report, the prevalence of hepatitis B virus in Taiwan was 15–20%, with about 2.5 million adult carriers. We speculate that veterans are more likely to have been vaccinated against hepatitis, which is strongly associated with liver cancer, so the risk of liver cancer is significantly lower in those with veteran status than those without veteran status. One report noted a slight increase in the prevalence of smoking during military service and an even higher prevalence of smoking among younger military personnel compared with the general population (26). After 10 years of military anti-smoking health promotion programs, there have been some beneficial effects in reducing smoking rates among Taiwanese military personnel (27). However, the prevalence of smoking among veterans remains unclear and requires further investigation. Due to policy on developments of veteran health promotion and free access to medical care, the opportunity of veterans to receive adequate cancer screen is no less than the civilians. On the other hand, the all-cause mortality is no more than the civilians, which has been shown in our results in Tables 1, 5. Although the results were inconsistent with the previous studies (8, 15), our study used a more stringent approach, which included frequency of medical use and baseline comorbidities to avoid measurable confounding and detection bias, providing a more rigorous conclusion.
First, the specific biological basis of the retrospective cohort study is speculative. To minimize the observational confounding, we matched relevant comorbidities between the two groups at the baseline, which were not provided in the previous studies (4, 7, 8, 10, 13–15, 25). However, the urbanization level was not well-matched in this study. There is still a possibility of selection bias. Fortunately, we used this single-payer insurance health database with a well-validated coding for the outcome covariates (19), and this study was able to overcome the possible bias, and thus indicate a meaningful association.
Second, NHIRD cannot provide information on the dates the veteran entered and left the military, the battles in which they were involved, or the Navy, Army, or Air Force to which the veteran belonged at the time of initial service. However, randomized clinical trials are not feasible because there are different models of military services such as compulsory and voluntary soldiers. A major concern is the age of the study subjects. With a mean age of 75.9 years at the baseline, any potential subjects (veterans and non-veterans) who developed cancer in middle age will not have been captured as they will have been left-truncated either by mortality or by the exclusion of those who already had a cancer diagnosis at the baseline. Thus, both the veterans and non-veterans represent a “survivor population” at the baseline. This has the potential to introduce bias if the incidence of earlier-onset cancer differed between veterans and non-veterans.
Third, any issue of a small number of subjects (less than 3) will not be shown in our report for political restrictions according to the “Personal Information Protection Act” in Taiwan. Therefore, data and interpretation of female veteran-associated cancers are not available in this study.
There were other residual confounders in this study, such as obesity, alcohol, tobacco consumption, diet, exercise, and health behaviors. After accounting for the known limitations of observational studies, our results alleviate the public’s concern about the cancer risk profile of veterans after military service, however, the number of cases of cancer was small.
In the study, we provide assurance that older individuals with veteran status have a lower risk of developing cancer compared with those without veteran status. A more comprehensive study encompassing a larger cohort and a longer follow-up period is warranted.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the China Medical University Hospital Research Ethics Committee with certificated number CMUH109-REC2-031(CR-2). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
L-FP, RC, and K-HT: study conception and design, analysis and interpretation of data, and investigation. RC and CH: acquisition of data. L-FP and RC: writing—original draft preparation. CH, RC, and K-HT: writing—review and editing. All authors contributed to the article and approved the submitted version.
This study was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW111-TDU-B-212-134004) and China Medical University Hospital (DMR-111-105). We are grateful to Health Data Science Center, China Medical University Hospital for providing administrative, technical and funding support. The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
We thank Cheng-Li Lin for assistance with the study analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel R, Miller K, Fuchs H, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Wu S, Powers S, Zhu W, Hannun Y. Substantial contribution of extrinsic risk factors to cancer development. Nature. (2016) 529:43–7. doi: 10.1038/nature16166
3. Hoerster K, Lehavot K, Simpson T, McFall M, Reiber G, Nelson K. Health and health behavior differences: US. military, veteran, and civilian men. Am J Prevent Med. (2012) 43:483–9. doi: 10.1016/j.amepre.2012.07.029
4. McBride D, Cox B, Broughton J, Tong D. The mortality and cancer experience of New Zealand vietnam war veterans: a cohort study. BMJ Open. (2013) 3:e003379. doi: 10.1136/bmjopen-2013-003379
5. Bergman B, Mackay D, Pell J. Dementia in scottish military veterans: early evidence from a retrospective cohort study. Psychol Med. (2021). [Epub ahead of print]. doi: 10.1017/S0033291721002440
6. Bergman B, Mackay D, Pell J. Suicide among scottish military veterans: follow-up and trends. Occup Environ Med. (2022) 79:88–93. doi: 10.1136/oemed-2021-107713
7. Zullig LL, Sims KJ, McNeil R, Williams CD, Jackson GL, Provenzale D, et al. Cancer incidence among patients of the U.S. veterans affairs health care system: 2010 update. Milit Med. (2017) 182:e1883–91. doi: 10.7205/MILMED-D-16-00371
8. Atchison E, Gridley G, Carreon J, Leitzmann M, McGlynn K. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. (2011) 128:635–43. doi: 10.1002/ijc.25362
9. Yi S, Ohrr H. Agent orange exposure and cancer incidence in korean vietnam veterans: a prospective cohort study. Cancer. (2014) 120:3699–706. doi: 10.1002/cncr.28961
10. Yi S. Cancer incidence in korean vietnam veterans during 1992-2003: the korean veterans health study. J Prevent Med Public Health. (2013) 46:309–18. doi: 10.3961/jpmph.2013.46.6.309
11. Nayan M, Hamilton RJ, Macdonald EM, Li Q, Mamdani MM, Earle CC, et al. Vasectomy and risk of prostate cancer: population based matched cohort study. BMJ. (2016) 355:i5546. doi: 10.1136/bmj.i5546
12. Bullman T, Mahan C, Kang H, Page W. Mortality in US army gulf war veterans exposed to 1991 khamisiyah chemical munitions destruction. Am J Public Health. (2005) 95:1382–8. doi: 10.2105/AJPH.2004.045799
13. Bergman B, Mackay D, Pell J. Lymphohaematopoietic malignancies in scottish military veterans: retrospective cohort study of 57,000 veterans and 173,000 non-veterans. Cancer Epidemiol. (2017) 47:100–5. doi: 10.1016/j.canep.2017.02.001
14. Bergman B, Mackay D, Morrison D, Pell J. Smoking-related cancer in military veterans: retrospective cohort study of 57,000 veterans and 173,000 matched non-veterans. BMC Cancer. (2016) 16:311. doi: 10.1186/s12885-016-2347-5
15. Barth S, Dursa E, Bossarte R, Schneiderman A. Trends in brain cancer mortality among U.S. gulf war veterans: 21 year follow-up. Cancer Epidemiol. (2017) 50:22–9. doi: 10.1016/j.canep.2017.07.012
16. Hsing A, Ioannidis J. Nationwide population science: lessons from the taiwan national health insurance research database. JAMA Intern Med. (2015) 175:1527–9. doi: 10.1001/jamainternmed.2015.3540
17. Lin J, Lin C, Lin M, Lai C, Lin H, Yang C, et al. Risk of leukaemia in children infected with enterovirus: a nationwide, retrospective, population-based, taiwanese-registry, cohort study. Lancet Oncol. (2015) 16:1335–43. doi: 10.1016/S1470-2045(15)00060-1
18. Chang R, Wei J, Lin M, Hung Y. The potential role of nontyphoidal salmonellosis in gastric cancer: a nationwide matched cohort study. Gastric Cancer. (2021) 24:292–301. doi: 10.1007/s10120-020-01132-x
19. Kao W, Hong J, See L, Yu H, Hsu J, Chou I, et al. Validity of cancer diagnosis in the national health insurance database compared with the linked national cancer registry in taiwan. Pharmacoepidemiol Drug Saf. (2018) 27:1060–6. doi: 10.1002/pds.4267
20. Shen Y, Hsu H, Lin T, Chang Y, Hu L, Chen L, et al. H1-antihistamines reduce the risk of hepatocellular carcinoma in patients with hepatitis b virus, hepatitis c virus, or dual hepatitis b virus-hepatitis c virus infection. J Clin Oncol. (2022) 40:1206–19. doi: 10.1200/JCO.21.01802
21. Huang C, Wei JC, Chien T, Kuo C, Lin S, Su Y, et al. Risk of breast cancer in females with hypothyroidism: a nationwide, population-based, cohort study. Endocr Pract. (2021) 27:298–305. doi: 10.1016/j.eprac.2020.09.007
22. Cai S, Perng W, Huang J, Chiou J, Dong L, Wei J. Neoplasm risk in rheumatic diseases has no correlation with conventional synthetic disease-modifying anti-rheumatic drugs usage-a population-based nested case-control study. Front Med. (2020) 7:473. doi: 10.3389/fmed.2020.00473
23. Lu Y, Hsin C, Lu Y, Wu M, Huang J, Huang C, et al. Risk of head and neck cancer in patients with peptic ulcers and the effect of Helicobacter pylori treatment. Sci Rep. (2021) 11:6229. doi: 10.1038/s41598-021-85598-4
24. Wu M, Leong P, Chiou J, Chen H, Huang J, Wei J. Increased risk of systemic lupus erythematosus in patients with Helicobacter pylori infection: a nationwide population-based cohort study. Front Med. (2019) 6:330. doi: 10.3389/fmed.2019.00330
25. Lin C, Chiang J, Li C, Liu C, Lin W, Hsieh T, et al. Cancer risks among patients with type 2 diabetes: a 10-year follow-up study of a nationwide population-based cohort in Taiwan. BMC Cancer. (2014) 14:381. doi: 10.1186/1471-2407-14-381
26. Chu N, Wu D, Shen M, Lin Y. Prevalence of adverse behaviors among young military conscripts in Taiwan. Milit Med. (2006) 171:301–5. doi: 10.7205/MILMED.171.4.301
Keywords: Taiwan, cohort, cancer, veterans, epidemiology
Citation: Pan L-F, Chang R, Hsu CY and Tsui K-H (2023) Older veterans associated with reduced risk of cancer: Retrospective nationwide matched cohort study in Taiwan. Front. Med. 9:931565. doi: 10.3389/fmed.2022.931565
Received: 29 April 2022; Accepted: 02 November 2022;
Published: 04 January 2023.
Edited by:
Tzvi Dwolatzky, Technion Israel Institute of Technology, IsraelReviewed by:
Meng-Che Wu, Taichung Veterans General Hospital, TaiwanCopyright © 2023 Pan, Chang, Hsu and Tsui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renin Chang, ✉ cmhhcHNvZHkxODgxQGdtYWlsLmNvbQ==; Kuan-Hao Tsui, ✉ a2h0c3VpNjBAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.