- Complex Structure of Surgical Sciences and Technologies, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy
Coronavirus disease 2019 (COVID-19) primarily affects the respiratory tract, but also many other organs and tissues, leading to different pathological pictures, such as those of the musculoskeletal tissues. The present study should be considered as a speculation on the relationship between COVID-19 infection and some frequent musculoskeletal pathologies, in particular sarcopenia, bone loss/osteoporosis (OP) and fracture risk and osteoarthritis (OA), to hypothesize how the virus acts on these pathologies and consequently on the tissue regeneration/healing potential. The study focuses in particular on the modalities of interaction of COVID-19 with Angiotensin-Converting Enzyme 2 (ACE2) and on the “cytokine storm.” Knowing the effects of COVID-19 on musculoskeletal tissues could be important also to understand if tissue regenerative/reparative capacity is compromised, especially in elderly and frail patients. We speculate that ACE2 and serine proteases together with an intense inflammation, immobilization and malnutrition could be the responsible for muscle weakness, altered bone remodeling, increase in bone fracture risk and inflammatory joint pathologies. Future preclinical and clinical studies may focus on the regenerative/reparative properties of the musculoskeletal tissues after COVID-19 infection, toward a personalized treatment usually based on scaffolds, cells, and growth factors.
Introduction
Coronavirus disease 2019 (COVID-19) was first detected in Wuhan, China, in December 2019 and showed a rapid spread across the world; by March 11, 2020, the World Health Organization declared a global pandemic situation. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shows characteristics like the SARS-CoV, responsible for the SARS epidemic of 2002–2003 and it primarily infects the respiratory tract with pneumonia-associated symptoms, including fever, cough, difficulty breathing and fatigue (1). However, other pathological manifestations are found in other tissues of the human body including the musculoskeletal one. Therefore, in this regard, contrasting results are reported and studies are still in progress.
The aim of the present article is to investigate the relationship between COVID-19 infection and some frequent musculoskeletal pathologies, in particular sarcopenia, bone loss/osteoporosis (OP) and fracture risk and osteoarthritis (OA), to hypothesize how the virus acts on these pathologies and consequently on the tissue regeneration/healing potential, focusing in particular on the modalities of interaction of COVID-19 with Angiotensin-Converting Enzyme 2 (ACE2) and on the “cytokine storm.” Some aspects studied in this manuscript are to be considered a starting point to open a discussion and increase the orthopedic awareness on this topic.
There are many studies on the effects of COVID-19 in the most affected tissues, but what is known about the musculoskeletal tissues?
Some clinical studies reported significantly higher mortality rate, length of hospital stay (LOS), complication rate and ventilatory need in COVID-19 – positive patients undergoing surgery because of a hip (2–6) or proximal femur fracture (7, 8). On the other side, few studies investigated the fracture risk or the bone regenerative/reparative capability in COVID-19 positive patients. Some authors showed that the incidence of frailty fractures did not differ between infected and not infected patients and the real COVID-19 effect in patients affected by fractures, especially at the hip, still remains to be ascertained (9). However, a recent study showed a suppression in cell osteogenic differentiation with a low fracture healing rate and an increase in OP in COVID-19 positive patients due to an upregulation of miR-4485 (10).
Patients infected by COVID-19 show also weakness of skeletal muscles with associated fatigue, myalgia, muscle edema, rhabdomyolysis and injury, myopathy, polyneuropathy and Guillain-Barre syndrome (11, 12). An increase in proteins of sarcoplasmatic reticulum of the muscle fibers (creatinine kinase, lactate l, alanine aminotransferase, and aspartate aminotransferase) and a decrease in pH and oxygen levels have been observed in ischemic muscle during infection (13–20). Arthralgias localized in the joints, or myalgias in muscles, have been reported in patients infected with COVID-19 (21).
Osteoarthritis, rheumatoid arthritis and reactive arthritis are also observed. This latter is an acute aseptic arthritis that occurs in few weeks after COVID-19 infection and, in the last 2 years, several articles treated this COVID-associated pathology. It involves prevalently the lower limbs with a symptoms duration up to a month and a half (22).
What happens when the virus encounters the membrane-bound angiotensin-converting enzyme 2?
Angiotensin-converting enzyme 2 is used for virus entry in host cells and is a component of the ACE2/angiotensin (Ang) (1–7)/MAS axis, implicated in the regulation of body fluids, electrolytes and blood pressure. As observed in Figure 1, ACE2 converts angiotensin II (Ang II) into Ang (1–7), exerting opposite effects to Ang II (23).
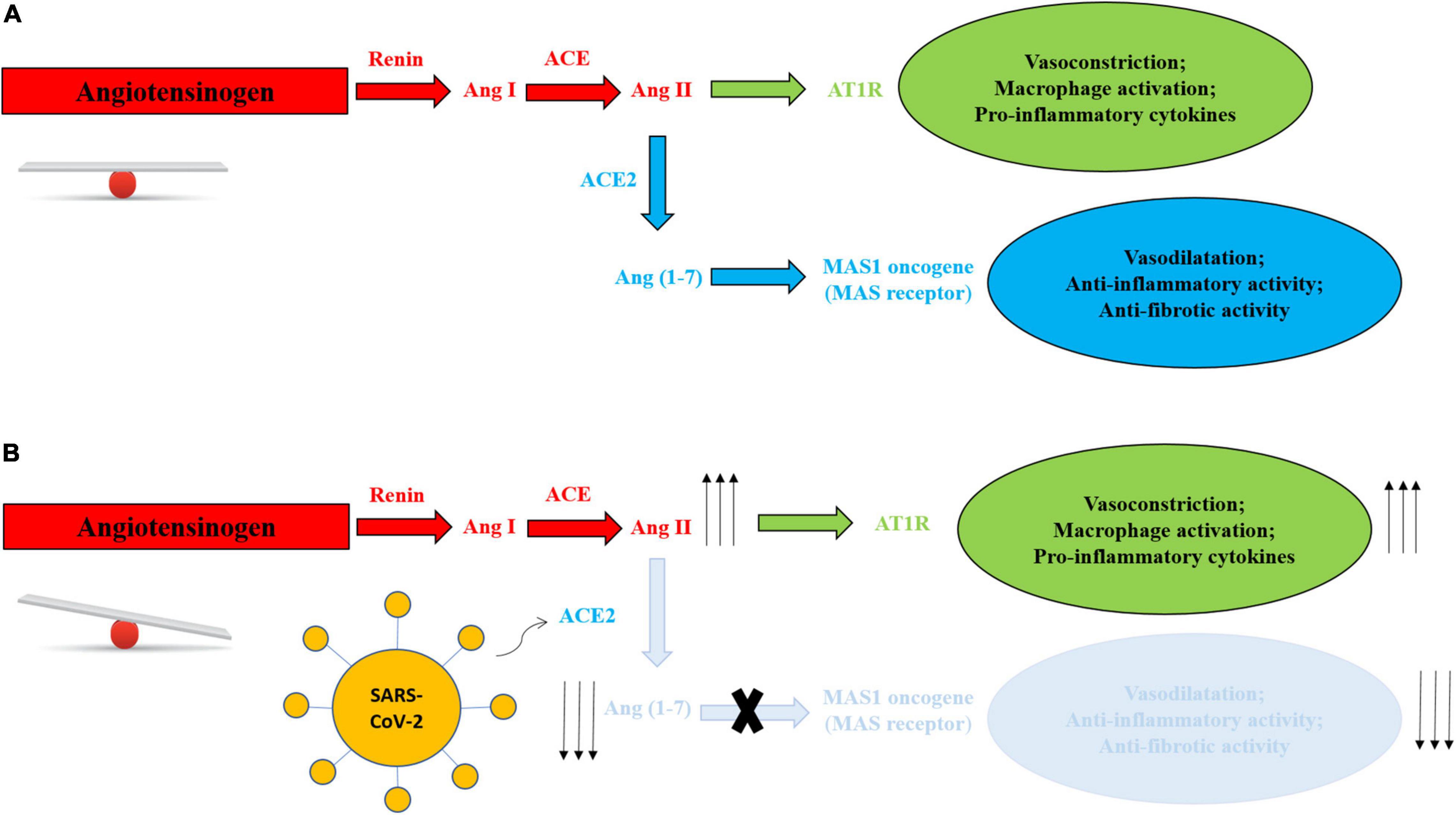
Figure 1. (A) Balanced RAS system in absence of SARS-CoV-2. (B) RAS system mechanism unbalance in presence of SARS-CoV-2. Virus entry leads to degradation of membranal ACE2 with depletion of Ang 1–7, blunting role of the Ang (1–7)/MAS axis and reducing its physiological and protective activities, and increasing Ang II-AT1R production and activities, with vasoconstriction, activation of macrophages and increase in inflammatory cytokines.
Angiotensin-converting enzyme 2 expression is downregulated after the binding of the virus, blunting its role in the ACE2/Ang (1–7)/MAS axis and reducing its physiological and protective activities (24) (Figure 1).
The entry and spread of the virus into the host cells are mediated by spike (S) protein, present on the virus surface, and facilitated by some proteases located on the host cell surface in proximity to the ACE2, such as the transmembrane serine protease 2 (TMPRSS2), a proteolytic enzyme. TMPRSS2 attacks the S1 unit of the virus S protein and detaches S1 from the S2 unit. The viral S2 unit merges with the host cell membrane, allowing the transfer of the viral content inside the cell through endocytosis or membrane fusion, causing all the clinical manifestation of the virus (Figure 2). TMPRSS2 and ACE2 are co-expressed in several tissues (25, 26).
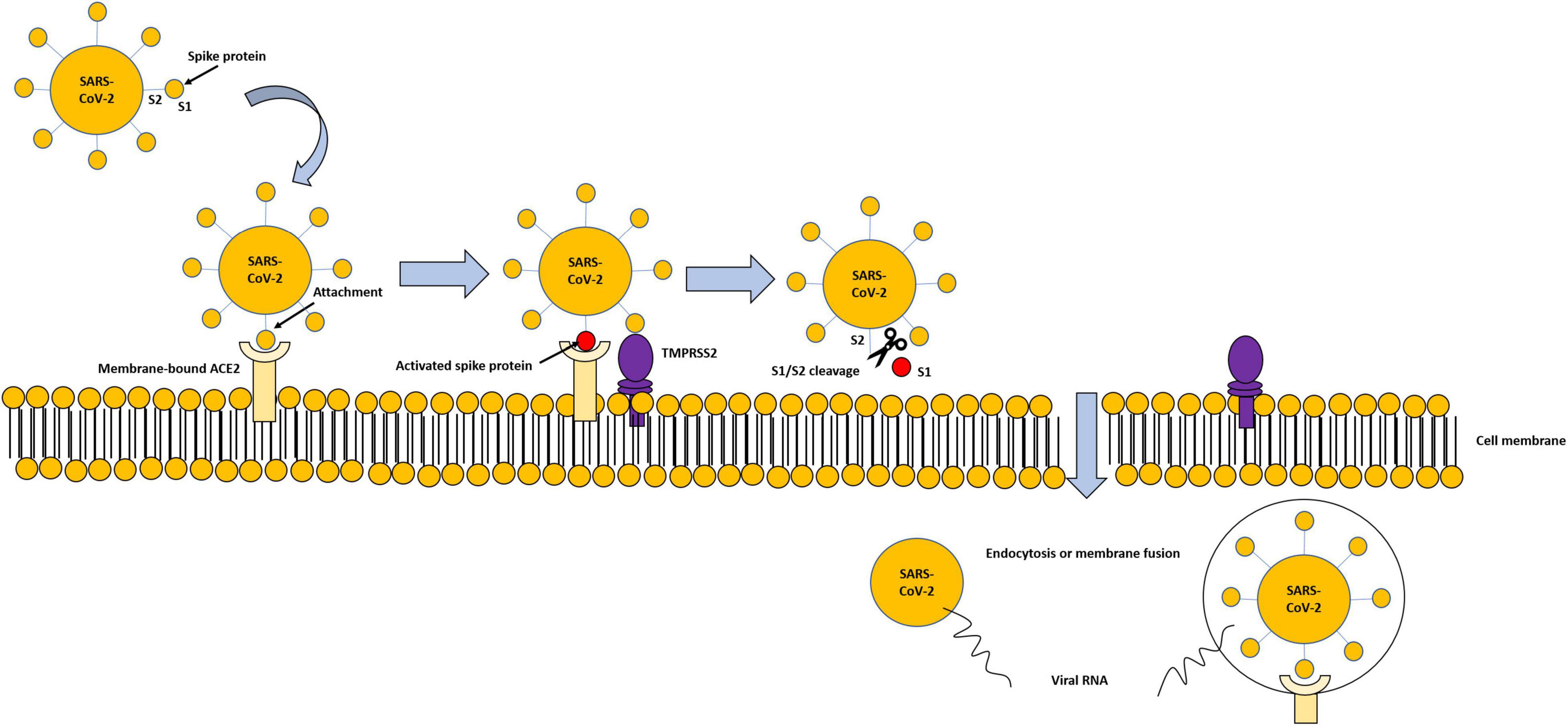
Figure 2. Modality of virus entry, through ACE2-TMPRSS2 interaction. Viral spike proteins enable attachment to cell-membrane-bound ACE2. TMPRSS2 attacks the S1 unit of the virus S protein and detaches S1 from the S2 unit. The viral S2 unit merges with the host cell membrane, allowing the transfer of the viral content inside the cell through endocytosis or membrane fusion, and subsequent degradation of internalized ACE2.
As regard bone tissue, in healthy conditions, the relationship between osteoblasts (OBs) and osteoclasts (OCs) is perfectly balanced for the maintenance of bone mass (27). OP is one of the most important causes of morbidity and functional decline in the elderly with consequent bone fractures and bone density is reduced with an imbalance between OCs and OB activity.
Osteoblasts and OCs possess ACE2, and the axis ACE2/Ang (1–7)/MAS seems to be involved in bone remodeling and anti-inflammatory activity, increasing OB metabolism, alkaline phosphatase activity, collagen I and osteocalcin production, and keeping low the level of OC precursors. This concurs to reduce osteoclastogenesis and bone loss in healthy conditions (28, 29).
No study concerning TMPRSS2 expression in bone cells has been performed. However, one study showed that other serine proteases, observed in OBs and osteocytes, control bone mineralization, and another study demonstrated that TMPRSS2:ERG fusion is involved in prostate cancer bone metastases, resulting in bone formation increase (30). Therefore, we hypothesize that the serine protease TMPRSS2 could be present in bone and be involved in the viral infection.
Even if ACE2 concentration in skeletal muscles is lower than in other tissues, the first evidence of the presence of ACE2 in skeletal muscles appeared in 2010 (31). ACE2, through the activation of ACE2/Ang (1–7)/MAS axis, seems to be important for the maintenance of skeletal muscle physiology and activity and for the protection against muscle weakness. In animal models the blockage of ACE2 activity, through the knockout of its gene, decreases grip strength, running distance and muscle fiber size, and increases senescence-associated gene, lipid accumulation, endoplasmic reticulum stress and mitochondrial dysfunction, accelerating the muscle weakness typically observed in the elderly (31). The increase in the amount of ACE2 induces the reduction of fibrosis and inflammatory infiltration associated with muscular dystrophies (32).
Transmembrane serine protease 2 seems not to be present in the skeletal muscle tissue. Conversely, Hepsin, a type II membrane-associated serine peptidase, which TMPRSS2 is part of, is expressed at high concentration in skeletal muscle and is highly expressed in muscle tissue affected by muscular dystrophy (33).
Sarcopenia prevalently affects elderly patients and is characterized by a decline in skeletal muscle fiber mass and strength, with consequent physical activity reduction and increase in falls, disability and oxidative stress (34). Since no study has yet explored the mechanism through which COVID-19 influences sarcopenia, we can only speculate. Our hypothesis is that COVID-19 infection, with the help of serine proteases, could reduce ACE2 physiological activity exerted on the skeletal muscle, leading to muscle weakness.
There is a lack of information on TMPRSS2 and ACE2 and cartilage, however, hepsin and TMPRSS4 are expressed in this tissue, inducing collagenase expression (35). Also, other serine proteases are implicated in cartilage destruction, metalloproteinase activity, aggrecan breaking and synovial inflammation (36).
What about inflammation, immobilization, and malnutrition?
Angiotensin-converting enzyme 2/Ang (1–7)/MAS axis cannot be assessed separately from the intense inflammation, immobilization, and malnutrition, that affect muscles and bones during COVID-19 infection.
After virus entry through ACE2, in the initial stages, cells of respiratory epithelium secrete cytokines that produce the immune response against virus, but after, when the severity of infection increases, the activated inflammatory pathways lead to the production of a storm of cytokines, the so called “cytokine storm” (37). The secretion of these pro-inflammatory cytokines leads to a dysregulation of the innate immune system, that in turn leads to the different tissue manifestations, including those of the musculoskeletal apparatus. These cytokines are considered the pathology biomarkers and are easily found in the serum of the affected patients (38).
The most important pro-inflammatory molecules produced by COVID-19 infection, that impact on musculoskeletal tissues, are interleukin-6 (IL6), IL1β, IL10, IL17, interferon-γ (INFγ), tumor necrosis factor-α (TNFα), C-reactive protein (CRP), soluble receptor for advanced glycation end products (sRAGE), receptor activator of nuclear factor-B ligand (RANKL), vascular endothelial growth factor (VEGF) and macrophage colony stimulating factor (M-CSF), that are high in serum of infected patients and induce fiber proteolysis and decrease protein synthesis. Among them, IL-6 should be considered as marker of severity of COVID-19 infection (37, 39).
In particular in bone, ACE2 has an anti-inflammatory property and reduces bone resorption. The blocking ACE2 with the virus, leads to decreased bone mass and increased joint inflammation (40).
Coronavirus disease 2019 infection increases IL6, IL1β, CXCL10, IL17, and TNFα that induce osteoclastogenesis and inhibit OB proliferation and metabolism causing increased bone fragility and risk of fractures in osteoporotic and elderly patients (41). Additionally, IL-6 is known to reduce nitric oxide (NO) bioavailability and to increase oxidative stress, leading to endothelial permeability, recruitment, and infiltration of the vascular wall by circulating leukocytes. In this context, a key aspect that cannot be underestimated is the interaction between bone tissue and vascular endothelial cells, through growth factors and chemokines. Thus, if the vascular network is damaged, the bone metabolism is also affected (42).
As regard muscles, elevated IL-6 concentration may be the sign of a vigorous immune and inflammatory response which could represent the link between severity of COVID-19 infection and muscular weakness development. IL-1β and IL-6 may cause fibrosis by inducing increased muscle fibroblast activity. IL-1β and TNF-α have been described to inhibit the differentiation and proliferation of satellite cells, the progenitor cells involved in muscle fiber growth (43).
As regard cartilage and joint, COVID-19, increasing inflammatory mediators, leads to an acceleration and worsening of OA. In addition, the clinical manifestation of reactive arthritis could be related to the increase of cytokines in the synovial fluid of patients affected by COVID-19, especially of IL17, and the activation of immune T cells. IL-1β, IL6, and TNF-α induce chondrolysis leading to arthalgia and/or the progression of OA (22).
In patients affected by COVID-19, Wingless/int1 (WNT) pathway has a role in ACE2 activity in muscles and bone. This is one of the pathways that is clearly important during skeletal development and bone homeostasis (44). In particular, after COVID-19 entry, the decrease in ACE2 activity is associated with an upregulation of WNT pathway, inducing several pathologies, such as hypertension, heart disease, and cancer (45).
There is a positive correlation between WNT/β-catenin pathway and inflammation, through an activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway and an increase in Cyclooxygenase (COX), upregulated also in OA pathology (46).
Immobilization of the COVID-19 affected patients causes further proinflammatory signals that can lead to muscle and bone fragility with reduced bone mineral density (BMD) and the general hypoxia increases the production of RANKL, VEGF, and M-CSF, that activate OCs and block osteogenesis of OBs. Long duration of intensive care unit (ICU) stay, multiorgan system failure, systemic inflammatory response syndrome (SIRS), prolonged sedation, and prolonged endotracheal intubation are well-recognized risk factors for the development of neuromuscular complications (43, 47).
Finally, malnutrition as calcium deficiency is deleterious to muscle and bone tissues. Calcium is important in the muscular function, and a positive association is found between an inadequate nutritional status (low intake of protein, vitamin D, and calcium) and osteosarcopenia. Lower albumin, magnesium and calcium are also found in the serum of patients affected by COVID-19 than in healthy subjects, underling that the addition of calcium and magnesium may help to reduce the consequences of COVID-19, such as chronic fatigue-syndrome-like and physiosomatic symptoms (48–51).
Discussion
Although other pathologies, in COVID-19 infected patients, require most of the attention of the medical community, musculoskeletal diseases and their behavior is a topic that should not be overlooked. Indeed, sarcopenia, OP and OA, alongside other comorbidities, such as hypertension, cardiac diseases, dementia, cancer and diabetes, contribute to the frailty syndrome observed in elderly patients.
Currently, it is still unclear how the effects of COVID-19 on the musculoskeletal system are mediated, and if some common pathologies (sarcopenia, OP, bone fractures, and OA) could be worsened by COVID-19 infection.
Starting from the evidence that ACE2 and TMPRSS2 are necessary for SARS-CoV-2 entry in the host cells and are co-expressed in some human tissues, we investigated on their presence in musculoskeletal cells and tissues. In addition, the extremely high inflammation cascade, which derives from and accompanies the reduction of ACE2 activity after virus entry, immobilization and malnutrition, which often accompany the patient, probably also contribute to worsening the picture of the pathologies, through the production of so many cytokines.
Given the scarcity of studies on this topic, in the future it could be important to design and carry out preclinical and clinical studies on the impact of COVID-19 infection on muscle, bone and cartilage to test in practice the theories proposed in this work. Finally, preclinical and clinical studies may focus on the regenerative/reparative properties of the musculoskeletal tissues after COVID-19 infection. An important issue could be the investigation of differences between COVID-19 infected and not infected patients at cellular, molecular and tissue levels in muscle, bone and cartilage tissues. This will be translated to personalized surgical reconstructive clinical treatments usually based on scaffolds, cells, and growth factors. Alongside the mechanisms that could contribute to the onset or worsening of a musculoskeletal pathology, the altered tissue regenerative/healing potential may also be compromised by the virus and probably could have an impact on the therapeutic surgical procedures. This could lead to an impaired post-operative progression after reconstruction and regenerative techniques.
In addition, in vitro studies are needed to evaluate the presence of ACE2 and TMPRSS2 in human musculoskeletal tissues because this seems to be necessary for the infection and related tissue damage.
Very few studies have been focused on the presence of ACE2 in muscle fibers, OBs, OCs and OC precursors, and no studies have been performed on ACE2 in cartilage tissue and on the presence of TMPRSS2 in the musculoskeletal tissues. In addition, few studies showed the presence of other serine proteases, of the same TMPRSS2 subfamily, in bone, muscle and cartilage; therefore, we could hypothesize that also TMPRSS2 is present in these tissues.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FV, DC, LM, AV, and MF have made substantial contributions to the conception and design of the study, acquisition and interpretation of data, drafting of the article, and final approval of the version to be submitted. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ministry of Health – Ricerca Corrente to IRCCS Istituto Ortopedico Rizzoli.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
COVID-19, Coronavirus disease 2019; ACE2, Angiotensin-converting enzyme 2; Ang, angiotensin; TMPRSS2, Transmembrane serine protease 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; S, spike; OP, osteoporosis; OA, osteoarthritis; IL6, interleukin 6; OBs, osteoblasts; OCs, osteoclasts; NO, nitric oxide; LOS, length of hospital stay; IL, Interleukin; INF γ, Interferon- γ; TNF α, Tumor Necrosis Factor- α; CRP, C-reactive protein; sRAGE, Soluble Receptor for Advanced Glycation End Products; RANKL, receptor activator of nuclear factor-B ligand; VEGF, vascular endothelial growth factor; M-CSF, macrophage colony stimulating factor; NF- κ B, nuclear factor kappa-light-chain-enhancer of activated B cells; COX, Cyclooxygenase; BMD, bone mineral density; ICU, intensive care unit; SIRS, systemic inflammatory response syndrome.
References
1. Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. (2020) 12:e7423. doi: 10.7759/cureus.7423
2. Muñoz Vives JM, Jornet-Gibert M, Camara-Cabrera J, Esteban PL, Brunet L, Delgado-Flores L, et al. Mortality rates of patients with proximal femoral fracture in a worldwide pandemic preliminary results of the Spanish HIP-COVID observational study. Bone Joint Surg Am. (2020) 102:e69. doi: 10.2106/JBJS.20.00686
3. Egol KA, Konda SR, Bird ML, Dedhia N, Landes EK, Ranson RA, et al. Increased mortality and major complications in hip fracture care during the COVID-19 pandemic: a New York City perspective. J Orthop Trauma. (2020) 34:395–402. doi: 10.1097/BOT.0000000000001845
4. Kayani B, Onochie E, Patil V, Begum F, Cuthbert R, Ferguson D, et al. The effects of COVID-19 on perioperative morbidity and mortality in patients with hip fractures. Bone Jt J. (2020) 102-B:1136–45. doi: 10.1302/0301-620X.102B9.BJJ-2020-1127.R1
5. Dallari D, Zagra L, Cimatti P, Guindani N, D’Apolito R, Bove F, et al. Early mortality in hip fracture patients admitted during first wave of the COVID-19 pandemic in Northern Italy: a multicentre study. J Orthop Traumatol. (2021) 22:15. doi: 10.1186/s10195-021-00577-9
6. Alcock H, Moppett EA, Moppett IK. Early mortality outcomes of patients with fragility hip fracture and concurrent SARS-CoV-2 infection: a systematic review and meta-analysis. Bone Jt Open. (2021) 2:314–22. doi: 10.1302/2633-1462.25.BJO-2020-0183.R1
7. Maniscalco P, Poggiali E, Quattrini F, Ciatti C, Magnacavallo A, Vercelli A, et al. Proximal femur fractures in COVID-19 emergency: the experience of two orthopedics and traumatology departments in the first eight weeks of the Italian epidemic. Acta Biomed. (2020) 91:89–96. doi: 10.23750/abm.v91i2.9636
8. Dupley L, Oputa TJ, Bourne JT, North West Covid Nof Study Group. 30-day mortality for fractured neck of femur patients with concurrent COVID-19 infection. Eur J Orthop Surg Traumatol. (2021) 31:341–7. doi: 10.1007/s00590-020-02778-0
9. Kayani B, Onochie E, Patil V, Begum F, Cuthbert R, Ferguson D, et al. Infographic: the effects of COVID-19 on perioperative morbidity and mortality in patients with hip fractures. Bone Jt J. (2020) 102-B:1279–80. doi: 10.1302/0301-620X.102B10.BJJ-2020-1774
10. Mi B, Xiong Y, Zhang C, Zhou W, Chen L, Cao F, et al. SARS-CoV-2-induced overexpression of miR-4485 suppresses osteogenic differentiation and impairs fracture healing. Int J Biol Sci. (2021) 17:1277–88. doi: 10.7150/ijbs.56657
11. Pires RE, Reis IGN, Waldolato GS, Pires DD, Bidolegui F, Giordano V. What do we need to know about musculoskeletal manifestations of COVID-19?: A systematic review. JBJS Rev. (2022) 10:e22.00013. doi: 10.2106/JBJS.RVW.22.00013
12. Fouad AM, Elotla SF, Elkaraly NE, Mohamed AE. Impact of COVID-19 pandemic on patients with rheumatic and musculoskeletal diseases: disruptions in care and self-reported outcomes. J Patient Exp. (2022) 9:23743735221102678. doi: 10.1177/23743735221102678
13. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol. (2020) 129:864–7. doi: 10.1152/japplphysiol.00321.2020
14. Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. (2020) 26:1618–20. doi: 10.3201/eid2607.200445
15. Kucuk A, Cure MC, Cure E. Can COVID-19 cause myalgia with a completely different mechanism? A hypothesis. Clin Rheumatol. (2020) 39:2103–4. doi: 10.1007/s10067-020-05178-1
16. Zhang H, Charmchi Z, Seidman RJ, Anziska Y, Velayudhan V, Perk J. COVID-19–associated myositis with severe proximal and bulbar weakness. Muscle Nerve. (2020) 62:E57–60. doi: 10.1002/mus.27003
17. Uysal BB, Ikitimur H, Yavuzer S, Islamoglu MS, Cengiz M. Case report: a COVID-19 patient presenting with mild rhabdomyolysis. Am J Trop Med Hyg. (2020) 103:847–50. doi: 10.4269/ajtmh.20-0583
18. Solıs JG, Esquivel Pineda A, Alberti Minutti P, Albarran Sanchez A. Case report: rhabdomyolysis in a patient with COVID-19: a proposed diagnostic-therapeutic algorithm. Am J Trop Med Hyg. (2020) 103:1158–61. doi: 10.4269/ajtmh.20-0692
19. Xu Y, Gu J. Cardiac and muscle injury might partially contribute to elevated aminotransferases in COVID-19 patients. Clin Gastroenterol Hepatol. (2020) 18:2847–8. doi: 10.1016/j.cgh.2020.04.042
20. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
21. Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am. (2020) 102:1197–204. doi: 10.2106/jbjs.20.00847
22. Slouma M, Abbes M, Mehmli T, Dhahri R, Metoui L, Gharsallah I, et al. Reactive. arthritis occurring after COVID-19 infection: a narrative review. Infection. (2022):1–9. doi: 10.1007/s15010-022-01858-z [Online ahead of print].
23. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. (2020) 126:1456–74. doi: 10.1161/CIRCRESAHA.120.317015
24. Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. (2020) 251:228–48. doi: 10.1002/path.5471
25. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271.e–80.e. doi: 10.1016/j.cell.2020.02.052
26. Salamanna F, Maglio M, Landini MP, Fini M. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front Med. (2020) 7:594495. doi: 10.3389/fmed.2020.594495
27. Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy. (2005) 4:325–8. doi: 10.2174/1568010054022015
28. Queiroz-Junior CM, Santos ACPM, Galvão I, Souto GR, Mesquita RA, Sá MA, et al. The angiotensin converting enzyme 2/angiotensin-(1-7)/Mas Receptor axis as a key player in alveolar bone remodeling. Bone. (2019) 128:115041. doi: 10.1016/j.bone.2019.115041
29. Obitsu S, Ahmed N, Nishitsuji H, Hasegawa A, Nakahama K, Morita I, et al. Potential enhancement of osteoclastogenesis by severe acute respiratory syndrome coronavirus 3a/X1 protein. Arch Virol. (2009) 154:1457–64. doi: 10.1007/s00705-009-0472-z
30. Delliaux C, Tian TV, Bouchet M, Fradet A, Vanpouille N, Flourens A, et al. TMPRSS2:ERG gene fusion expression regulates bone markers and enhances the osteoblastic phenotype of prostate cancer bone metastases. Cancer Lett. (2018) 438:32–43. doi: 10.1016/j.canlet.2018.08.027
31. Fernandes T, Hashimoto NY, Oliveira EM. Characterization of angiotensin-converting enzymes 1 and 2 in the soleus and plantaris muscles of rats. Braz J Med Biol Res. (2010) 43:837–42. doi: 10.1590/s0100-879x2010007500088
32. Riquelme C, Acuña MJ, Torrejón J, Rebolledo D, Cabrera D, Santos RA, et al. ACE2 is augmented in dystrophic skeletal muscle and plays a role in decreasing associated fibrosis. PLoS One. (2014) 9:e93449. doi: 10.1371/journal.pone.0093449
33. Sawada H, Kikukawa Y, Ban S, Kakudo T, Yokosawa H. Expression of trypsin-like proteases and proteasenexin-1 in mdx mouse muscles. Biochem Biophys Res Commun. (2004) 314:654–8. doi: 10.1016/j.bbrc.2003.12.143
34. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
35. Milner JM, Patel A, Davidson RK, Swingler TE, Desilets A, Young DA, et al. Matriptase is a novel initiator of cartilage matrix degradation in osteoarthritis. Arthritis Rheum. (2010) 62:1955–66. doi: 10.1002/art.27476
36. Wilkinson DJ, Habgood A, Lamb HK, Thompson P, Hawkins AR, Desilets A, et al. Matriptase induction of metalloproteinase-dependent aggrecanolysis in vitro and in vivo promotion of osteoarthritic cartilage damage by multiple mechanisms. Arthritis Rheumatol. (2017) 69:1601–11. doi: 10.1002/art.40133
37. Choudhary S, Sharma K, Silakari O. The interplay between inflammatory pathways and COVID-19: a critical review on pathogenesis and therapeutic options. Microb Pathog. (2021) 150:104673. doi: 10.1016/j.micpath.2020.104673
38. Ye Q, Wang B, Mao J. Cytokine storm in COVID-19 and treatment. J Infect. (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037
39. Valle A, Lecarpentier Y, Valle JN. Interplay of opposing effects of the WNT/b-catenin pathway and pparg and implications for SARS-CoV2 treatment. Front Immunol. (2021) 12:666693. doi: 10.3389/fimmu.2021.666693
40. Hasan LK, Deadwiler B, Haratian A, Bolia IK, Weber AE, Petrigliano FA. Effects of COVID-19 on the musculoskeletal system: clinician’s guide. Orthop Res Rev. (2021) 13:141–50. doi: 10.2147/ORR.S321884
41. Liu P, Lee S, Knoll J, Rauch A, Ostermay S, Luther J, et al. Loss of menin in osteoblast lineage affects osteocyte-osteoclast crosstalk causing osteoporosis. Cell Death Dif. (2017) 24:672–82. doi: 10.1038/cdd.2016.16554
42. Carulli C, Innocenti M, Brandi ML. Bone vascularization in normal and disease conditions. Front Endocrinol. (2013) 4:106. doi: 10.3389/fendo.2013.00106
43. Bax F, Lettieri C, Marini A, Pellitteri G, Surcinelli A, Valente M, et al. Clinical and neurophysiological characterization of muscular weakness in severe COVID-19. Neurol Sci. (2021) 42:2173–8. doi: 10.1007/s10072-021-05110-8
44. Huybrechts Y, Mortier G, Boudin E, Hul WV. WNT signaling and bone: lessons from skeletal dysplasias and disorders. Front Endocrinol (Lausanne). (2020) 11:165. doi: 10.3389/fendo.2020.00165
45. Zhang Z, Li L, Li M, Wang X. The SARS-CoV-2 host cell receptor ACE2 correlates positively with immunotherapy response and is a potential protective factor for cancer progression. Comput Struct Biotechnol J. (2020) 18:2438–44. doi: 10.1016/j.csbj.2020.08.024
46. Ma B, Hottiger MO. Crosstalk between Wnt/b-Catenin and NF-kB Signaling Pathway during inflammation. Front Immunol. (2016) 7:378. doi: 10.3389/fimmu.2016.00378
47. Kress JP, Hall JB. ICU-Acquired weakness and recovery from critical illness. N Engl J Med. (2014) 370:1626–35. doi: 10.1056/nejmra1209390
48. Hampson G, Stone M, Lindsay JR, Crowley RK, Ralston SH. Diagnosis and management of osteoporosis during COVID-19: systematic review and practical guidance. Calcif Tissue Int. (2021) 109:351–62. doi: 10.1007/s00223-021-00858-9
49. Polito A, Barnaba L, Ciarapica D, Azzini E. Osteosarcopenia: a narrative review on clinical studies. Int J Mol Sci. (2022) 23:5591. doi: 10.3390/ijms23105591
50. Al-Hakeim HK, Al-Jassas HK, Morris G, Maes M. Increased ACE2, sRAGE, and immune activation, but lowered calcium and magnesium in COVID-19. Recent Adv Inflamm Allergy Drug Discov. (2022). doi: 10.2174/2772270816666220318103929 [Online ahead of print].
51. Al-Jassas HK, Al-Hakeim HK, Maes M. Intersections between pneumonia, lowered oxygen saturation percentage and immune activation mediate depression, anxiety, and chronic fatigue syndrome-like symptoms due to COVID-19: a nomothetic network approach. J Affect Disord. (2022) 297:233–45. doi: 10.1016/j.jad.2021.10.039
Keywords: COVID-19, ACE2, inflammation, bone, muscle, joint
Citation: Veronesi F, Contartese D, Martini L, Visani A and Fini M (2022) Speculation on the pathophysiology of musculoskeletal injury with COVID-19 infection. Front. Med. 9:930789. doi: 10.3389/fmed.2022.930789
Received: 28 April 2022; Accepted: 28 June 2022;
Published: 14 July 2022.
Edited by:
Zaid A. Abassi, Technion – Israel Institute of Technology, IsraelReviewed by:
Hussein Kadhem Al-Hakeim, University of Kufa, IraqSamuel Heyman, Hadassah Hebrew University Hospitals, Israel
Copyright © 2022 Veronesi, Contartese, Martini, Visani and Fini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deyanira Contartese, ZGV5YW5pcmEuY29udGFydGVzZUBpb3IuaXQ=
 Francesca Veronesi
Francesca Veronesi Deyanira Contartese
Deyanira Contartese Lucia Martini
Lucia Martini Milena Fini
Milena Fini