
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 09 January 2023
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.930541
This article is part of the Research Topic Integrated Management of Chronic Kidney Disease Patients View all 24 articles
 Wenzhu Song1†
Wenzhu Song1† Yanfeng Liu2†
Yanfeng Liu2† Lixia Qiu1
Lixia Qiu1 Jianbo Qing2
Jianbo Qing2 Aizhong Li3
Aizhong Li3 Yan Zhao2
Yan Zhao2 Yafeng Li2,3,4,5
Yafeng Li2,3,4,5 Rongshan Li2,3*
Rongshan Li2,3* Xiaoshuang Zhou2*
Xiaoshuang Zhou2*Introduction: Chronic kidney disease (CKD) is a progressive disease with high incidence but early imperceptible symptoms. Since China’s rural areas are subject to inadequate medical check-ups and single disease screening programme, it could easily translate into end-stage renal failure. This study aimed to construct an early warning model for CKD tailored to impoverished areas by employing machine learning (ML) algorithms with easily accessible parameters from ten rural areas in Shanxi Province, thereby, promoting a forward shift of treatment time and improving patients’ quality of life.
Methods: From April to November 2019, CKD opportunistic screening was carried out in 10 rural areas in Shanxi Province. First, general information, physical examination data, blood and urine specimens were collected from 13,550 subjects. Afterward, feature selection of explanatory variables was performed using LASSO regression, and target datasets were balanced using the SMOTE (synthetic minority over-sampling technique) algorithm, i.e., albuminuria-to-creatinine ratio (ACR) and α1-microglobulin-to-creatinine ratio (MCR). Next, Bagging, Random Forest (RF) and eXtreme Gradient Boosting (XGBoost) were employed for classification of ACR outcomes and MCR outcomes, respectively.
Results: 12,330 rural residents were included in this study, with 20 explanatory variables. The cases with increased ACR and increased MCR represented 1,587 (12.8%) and 1,456 (11.8%), respectively. After conducting LASSO, 14 and 15 explanatory variables remained in these two datasets, respectively. Bagging, RF, and XGBoost performed well in classification, with the AUC reaching 0.74, 0.87, 0.87, 0.89 for ACR outcomes and 0.75, 0.88, 0.89, 0.90 for MCR outcomes. The five variables contributing most to the classification of ACR outcomes and MCR outcomes constituted SBP, TG, TC, and Hcy, DBP and age, TG, SBP, Hcy and FPG, respectively. Overall, the machine learning algorithms could emerge as a warning model for CKD.
Conclusion: ML algorithms in conjunction with rural accessible indexes boast good performance in classification, which allows for an early warning model for CKD. This model could help achieve large-scale population screening for CKD in poverty-stricken areas and should be promoted to improve the quality of life and reduce the mortality rate.
Chronic kidney disease (CKD) is defined as renal structural or functional abnormalities for 3 months, with a prevalence of 13.4% worldwide (1). In recent years, CKD has gradually moved away from the perception of “disease of the elderly,” with a growing trend toward age groups. Yet, imperceptible symptoms at the initial stages and lower public awareness are often responsible for the missing of golden treatment time (2). More seriously, it may develop into end-stage renal disease that requires renal replacement therapy, leading to a decrease in quality of life and an increase in mortality. What’s worse, it’s highly related to complications such as cardiovascular disease (3), becoming another “silent killer” after tumours and diabetes. As such, its early screening enables patients and their families to plan ahead, consult professional doctors for treatment, and discuss lifestyle modifications, which contribute to the alleviation of CKD.
Accurate diagnosis is closely related to the detection of CKD. Undoubtedly, albuminuria and α1-microglobulin serve as a good approach for early CKD screening in tertiary hospitals. Yet, when it comes to the rural areas in China, health care workers, critical care units, emergency facilities, health services and medical examinations are not necessarily guaranteed (4–6). In such a situation, it is not practical for primary health care institutions to make an early CKD screening using urine protein (7). Therefore, it is worth considering how to maximise the accessible medical parameters to construct a warning model for CKD in rural areas under the existing health care conditions.
Previous studies demonstrated that some risk factors, such as demographic, lifestyle, and blood biochemical parameters could be used to predict disease occurrence (8, 9). Also, it has been documented that the machine-learning approach allows for higher accuracy of disease risk prediction using routine clinical data, facilitating better decision support for clinicians (10). Accordingly, it is of great significance to combine machine learning algorithms with those easily accessible medical indexes to construct a warning model for CKD in poverty-stricken areas.
In this study, we aimed to employ machine learning algorithms for early screening of CKD and developed a warning model targeted at poverty-stricken areas using demographic, blood biochemical and physical examinations data from ten rural areas in Shanxi Province, intending to achieve a larger-scale CKD screening programme at a lower cost (reduced financial expenditure, reduced burden on medical staff) where possible, thus shifting forward its treatment window and improving quality of life.
From April 2019 to November 2019, Shanxi Provincial People’s Hospital carried out opportunistic screening for chronic kidney disease for residents over 40 years old in Ningwu County, Lu County, Yangqu County, Linxian County, Shouyang County, Zezhou County, Huozhou City, Hejin City, Linyi County and Ruicheng County in Shanxi Province. Up to 13,550 residents participated in this screening and 12,285 eventually enrolled in the study, including 5,206 men and 7,079 women aged 41–91 years. All study subjects signed informed consent forms and it was approved by the Shanxi Provincial Hospital Ethics Committee (No. 2021213).
Inclusion criteria: (1) Residents aged 40 years and above; (2) Participants without communication barriers; (3) Participants understanding the study significance and willing to sign a written informed consent; (4) participants without cognitive impairment or mental illness. Exclusion criteria: (1) incomplete information recorded; (2) poor compliance; (3) pregnant women or those with a history of drug abuse.
Data is collected through questionnaires, physical examinations, and laboratory analysis. The questionnaire included demographic information (including age, sex, annual income, educational levels), lifestyle (including smoking, alcohol consumption, diet and exercise). Questionnaires were conducted online and were completed by the subjects themselves or their family members. Physical examination comprised height, weight and systolic blood pressure (SBP), diastolic blood pressure (DBP), measured twice and averaged. All data were measured by medical professionals. Body mass index (BMI) was calculated by weight in kilograms divided by the square of height in meters.
Fasting venous blood was collected from subjects for fasting blood glucose (FPG), glycated haemoglobin (GHb), homocysteine (Hcy), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein (HDL-C). Urine specimens were collected from subjects. After centrifugation of 3,000 r/min for 10 min, the supernatant (low-speed centrifuge Anhui Zhongke Zhongke A SC3616) was extracted, and latex turbidity, sarcosine oxidase and immunoturbidimetry were employed for detection of α1-microglobulin (α1MG), urine creatinine (UCr) and microalbuminuria (MAU), respectively.
Study participants’ annual income, educational levels, health history, and lifestyle information was obtained from the questionnaire. Annual income was defined as <5K yuan, 5–10K yuan, 10–20K yuan, >20K yuan; educational attainment was defined as ≤ primary school, ≤ middle school, ≤ high school, ≥ college; smoking was classified as yes or no; alcohol consumption was classified as always (>100 g/time and 3 times/week), sometimes (<3 times/week or < 100 g/time) and rarely; exercise was classified as “none or a little” or “regular” (≥3 times/week, ≥30 min/time). BMI was defined as underweight (<18.5 kg/m2), normal (18.5–24.0 kg/m2), overweight (24.0–28.0 kg/m2), obesity (≥28 kg/m2). ACR was defined as MAU divided by UCr multiplied by 8.84; MCR was defined as α1MG divided by UCr multiplied by 8.84.
Explanatory variables include demographic information (age, sex, educational levels, annual income, residence), lifestyle (smoking, alcohol, exercise, salt consumption, diet), blood biochemistry (HDL, LDL, TG, TC, Hcy, FPG, GHb), physical examination indexes (SBP, DBP, BMI), a total of 20 variables. The response variables were defined as ACR outcomes or MCR outcomes with two classes (increased ACR or normal ACR and increased MCR or normal MCR) ACR ≥ 30 mg/g was defined as increased ACR; MCR > 23 mg/g was defined as increased MCR. The increased ones were assigned 1, and the normal ones were assigned as 0.
Since missing data is not an issue of great severity in this study, we excluded those samples with missing values, without making a data imputation. Then, Absolute Shrinkage and Selection Operator (LASSO) was used to select features that are more relevant to the response variables, the increased ACR and the increased MCR. Afterward, Synthetic Minority Over-Sampling Technique (SMOTE) was utilized to balance the classes to enable the machine learning models to better learn the data features, thus making the best classification. The workflow was shown in Figure 1.
LASSO represents one of the commonly used feature selection methods. It is characterized by adding L1 regularization penalty term when fitting generalized linear regression, so that the sum of absolute values of regression coefficients is less than a certain value. Its purpose is to minimize the sum of squares of residuals, and force the regression coefficients of variables that contribute less to the model to compress to zero, enabling a feature sparsity process (11, 12). It could eliminate predictors with autocorrelation or redundancy, allowing for automated variable selection within the model, and significantly contributing to the performance of classification models (13, 14).
Imbalanced datasets are not unusual in medical research, because the number of non-patients is extremely larger than that of patients, which serves as an obstacle to predictive performance (15). The Synthetic Minority Over-Sampling Technique (SMOTE) is an oversampling technique that is an effective algorithm for handling imbalances between data classes (16). It uses k-neighbour synthesis to amplify minority classes to obtain a balanced data set (17) that exhibits good performance in areas such as network intrusion detection systems and disease detection. In this study, there is a serious imbalance in the response variables, ACR outcomes and MCR outcomes (Figures 2A, B).
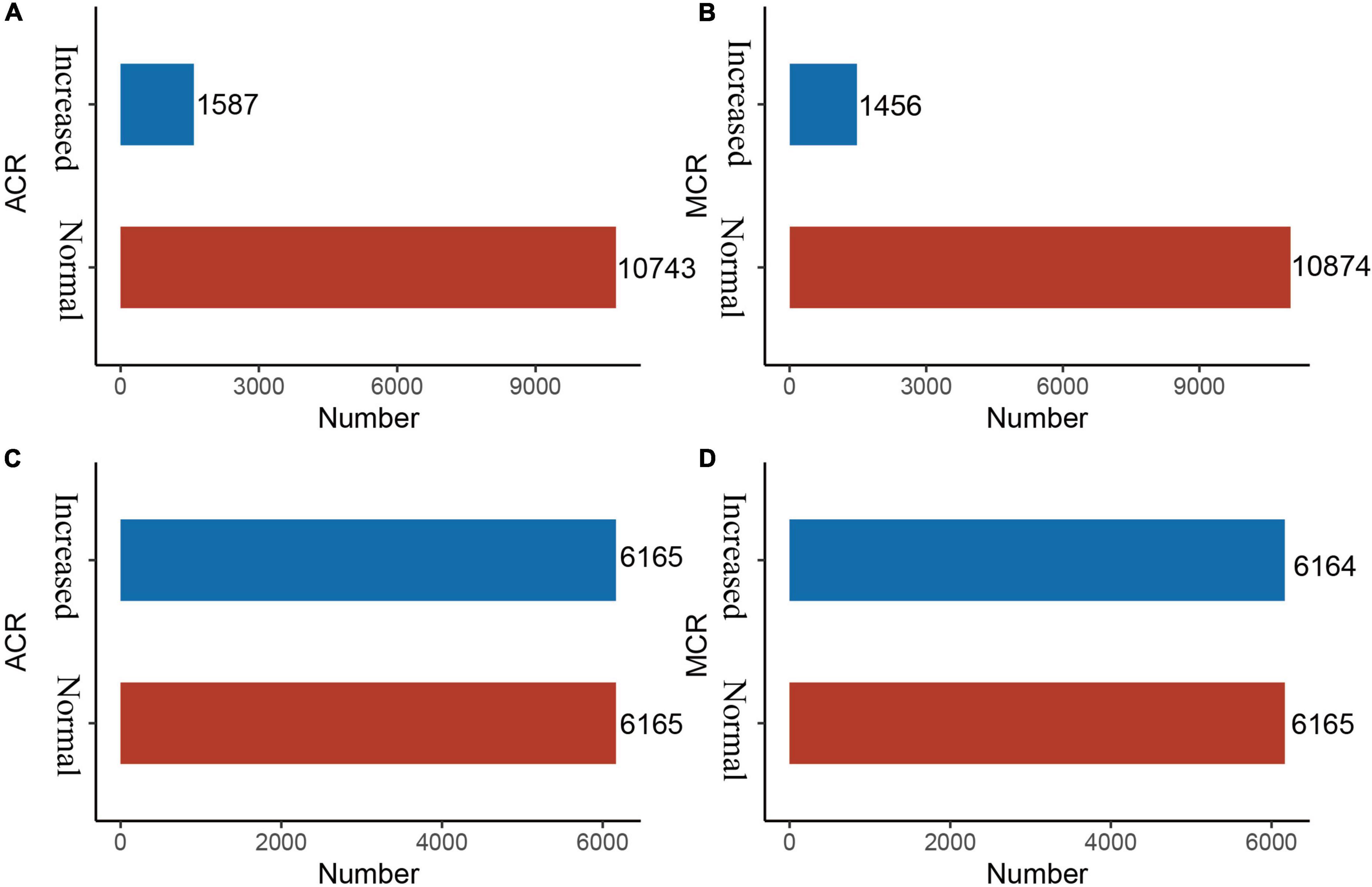
Figure 2. Before and after SMOTE of response variables for ACR and MCR outcomes. SMOTE, Synthetic Minority Over-Sampling Technique. It’s a good and powerful way to handle imbalanced data, and it was conducted under the parameters of k = 5, C.perc = “balance”, dist = “Overlap”. (A) ACR outcomes (before SMOTE); (B) MCR outcomes (before SMOTE); (C) ACR outcomes (after SMOTE); and (D) MCR outcomes (after SMOTE).
Three models, i.e., RF (18, 19), Bagging (20, 21) and XGBoost (22, 23) were employed to make a classification of ACR outcomes and MCR outcomes, respectively. More detailed information about the models could be obtained in the Supplementary material.
Evaluation parameters include Accuracy (1), Specificity (2), Sensitivity (3) and Area under the receiver operating curve (AUC). When patients with kidney disease are classified as patients, the prediction is defined as True Positive (TP), and when a healthy person is classified as healthy, the prediction is defined as True Negative (TN). In addition, if healthy subjects are considered patients, the prediction is False Positive (FP); Similarly, False Negative (FN) if patients are considered healthy subjects. Accuracy is to evaluate how accurate the machine learning algorithms are to detect what it is supposed to measure. Specificity is the ability to correctly exclude those without renal conditions and Sensitivity is to correctly identify those with renal conditions.
Qualitative data are described as percentages (%), and quantitative data are expressed as mean standard deviation (M±SD) or median ± interquartile (P25, P75), as appropriate. Model construction: The datasets were divided into training set (80%) and testing set (20%). The former is used for model training, i.e., e, RF, Bagging, and XGBoost, while the latter is used for model performance evaluation. The comparisons between training set and testing set were conducted using t-test or non-parameter test for quantitative variables, and Chi-square test for qualitative variables. All analyses were implemented in R software (version 4.0.3).
A total of 12,330 rural residents participated in this study, of whom 5,230 were men and 7,100 were women aged 40–91 years. There were 1,587 (12.8%) cases with increased ACR and 1,456 (11.8%) cases with increased MCR, as shown in Table 1. Features in the training set and testing set are comparable for both ACR outcomes (except TG and sex) and MCR outcomes (P < 0.05), as shown in Tables 2, 3.
As shown in Figures 3A, B, after LASSO feature selection, 14 and 15 explanatory variables remained in the two datasets, respectively. Dataset with ACR outcomes as the response variable excluded six variables of annual income, residence, LDL, HDL, smoking, and exercise; while dataset with MCR outcomes as the response variable excluded five variables of TC, LDL, HDL, exercise, and salt consumption. As shown in Figures 2C, D, after SMOTE resampling, the number of patients and normal ones were 6,165, 6,165 for ACR outcomes, and 6,165, 6,164 for MCR outcomes, respectively.
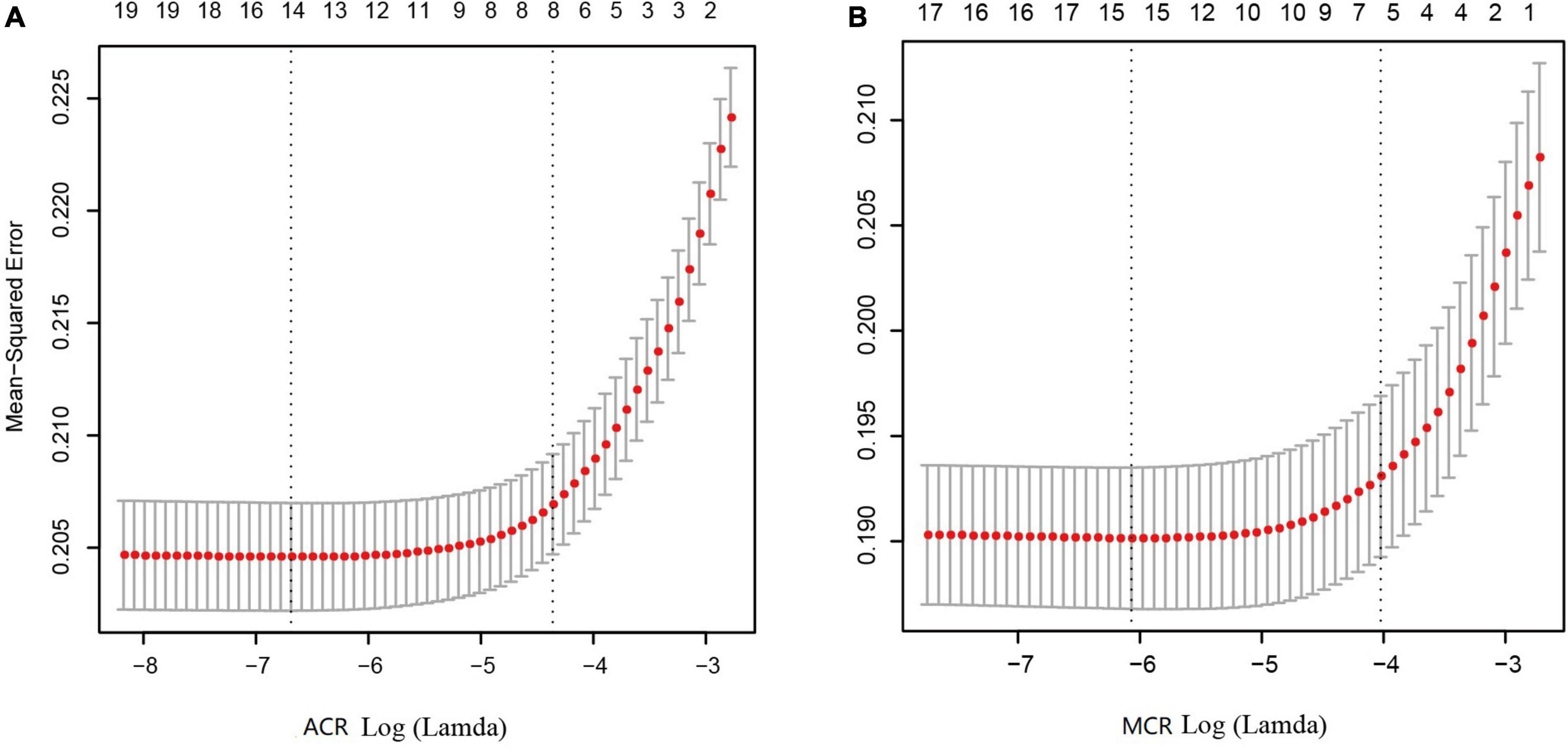
Figure 3. Results of feature selection using LASSO. When Lamda is minimum, corresponding features were taken into model construction, that is, 14 features for ACR outcomes (A) and 15 feature for MCR outcomes (B).
When constructing models for ACR outcomes, the number of increased ACR and normal ACR in the training set were both 4,932, and 1,233 in the testing set, respectively. When constructing model for MCR, the number of increased MCR and normal MCR in the training set were 4,973 and 4,891, respectively, and 1,273 and 1,192 in the testing set. The Accuracy, Sensitivity and Specificity of Bagging, RF and XGBoost reached over 99.00% in the training sets, and the AUC reached 0.99, as shown in Table 4. In the testing sets, XGBoost performed best, with Accuracy, Specificity and Sensitivity standing at 80.17, 77.05, and 83.29% for ACR outcomes and 82.27, 82.91, and 81.71% for MCR outcomes, and the AUC standing at 0.90. The performance of Bagging and RF is similar, as shown in Table 5.
Since XGBoost performs best in the classification, we indicated the contribution of the explanatory variables to the model by Gain, and the larger the Gain, the more important the variables were for the XGBoost model. The five variables that contributed most to the classification of ACR outcomes in the XGBoost model represented SBP, TG, TC, and Hcy, DBP. The five variables that were most important for the classification of MCR outcomes constituted age, TG, SBP, Hcy and FPG (Figure 4).

Figure 4. Contributions of explanatory variables to the XGBoost algorithm. The “Gain” means the relative contribution of the corresponding feature to the model calculated by taking the contribution of each feature to each tree in the model. The high value of this metric compared to other characteristics means that it is more important for generating predictions. Therefore, a larger value indicates that the variable is more important; ACR outcomes (A) and MCR outcomes (B).
To our knowledge, this study was the first one to employ machine learning algorithms in conjunction with existing indicators readily available in rural areas to construct an early warning model for CKD. In constructing the model, demographic information, physical examination and blood biochemical were taken as the explanatory variables; ACR (7) and MCR (24), early CKD screening parameters, collected and calculated from urine samples were taken as response variables. This study suggested that machine learning approaches show good performance in achieving early CKD-aided screening, as reflected by excellent Accuracy, Sensitivity, Specificity and AUC.
In 2012, Logistic Regression (LR) was employed for CKD prediction, but the model is accompanied by some defects (2), one of which concerns its sensitivity to multicollinearity. The second one relates to maximum likelihood estimation, unable to fit the true distribution of the data well. Recently, data-driven algorithms pick up pace, boasting great potential in cardiovascular diseases (25), tumors (26), immune diseases (27), and neurological diseases (28). Also, its application in renal diseases is increasing, ranging from acute kidney injury prediction (29) to kidney transplantation outcome prediction (30), interstitial fibrosis, and tubular atrophy detection (31).
In our previous work (32), we also employed LR, RF and Naive Bayes algorithms to make a classification of glomerular injury and tubular injury with the same population. The results suggested that RF performs best and could be employed as a novel auxiliary diagnostic approach for glomerular injury and tubular injury. To further compare the performance of other well-established algorithms, we, in this study, employed RF, Bagging and XGBoost to construct a warning model for CKD targeted at poverty-stricken areas with easily accessible parameters.
This study demonstrates that XGBoost represents the best classifier because the algorithm is a serial integrated learning algorithm based on Gradient Boosting Decision Tree that builds boosting trees in parallel by dependency generation (23). The objective function is improved by adding a regular term to the original function, thus reducing overfitting and speeding up convergence (33). Its extraordinary classification power in other diseases has also been demonstrated (34, 35). Besides, the five explanatory variables with the greatest output weight of XGBoost classifier for ACR outcomes represented SBP, TG, TC, and Hcy, DBP; and the five explanatory variables for MCR outcomes constituted age, TG, SBP, Hcy and FPG.
In hypertension, the early glomerular filtration rate could remain normal, but when arterial pressure is constantly rising, exceeding the kidney’s ability to self-regulate, it leads to glomerular hyperperfusion, which leads to damage to the visceral epithelial cells of the renal tubules, increasing the permeability of the glomerular basement membrane and thus causing proteinuria (36), while leading to glomerular duct wall hardening thickening, lumen stenosis, resulting in renal parenchymal ischemia, which eventually leads to glomerulosclerosis. Hyperhomocysteinemia is an important player involved in the progression of end-stage renal disease, acting directly on glomerular cells, inducing glomerular dysfunction and tubular fibrosis (37). Renal dyslipidemia is characterized by the accumulation of TG, which accelerates damage to the glomerular and tubular interstitials (38). Hyperglycemia levels not only enhance oxidative stress and hemodynamic factors such as activation of the renin-angiotensin-aldosterone system and impaired self-regulation due to systemic hypertension, but also increase the load of glucose delivered to the proximal tubules, triggering maladaptive hypertrophy (39) and hyperplasia of cortical tubules, and upregulation of glucose transport (40), and activation of globular feedback, leading to hyperfiltration of glomeruli and tubular fibrosis.
We think our early warning model is practical and down-to-earth in rural China. As the largest developing country confronted with underdeveloped healthcare systems and aging population (41), China is struggling to address the issue of health care coverage in rural areas. Despite the tremendous achievements in health care in rural China over the past 30 years, the problem of “difficult and expensive access to health care” still exists in the countryside. Promoting the establishment of a digital healthcare system in rural areas can greatly enhance the efficiency and accuracy of the public service system. Our study involves 12,330 participants from rural areas and how to better use such large-scale collected data to make a warning model for CKD is of great significance. Rather than considering these available indicators in isolation, holistically exploring their full potential, and seeking to explore a warning model for rural areas is a thing of great significance. Thus, early interventions tailored to CKD in improvised areas could be made in advance to lower its progression.
Some limitations also stand out in this manuscript. First, this study was based on a cross-sectional survey, and we did not conduct a follow-up for patients with proteinuria. Second, we constructed the models with data from Shanxi Province, and there is no other data for verification. Our next step is to collect more data from other regions to test the models’ generalization. Additionally, cost-effectiveness was initially considered in this study, and other indicators reflecting CKD, such as blood creatinine, were not collected, which is our next focus. Besides, we did not collect a detailed history of smoking, alcohol consumption and dietary intake. More detailed information would allow for a more accurate and valid model.
In short, CKD has emerged as a global public health issue and its early diagnosis is of great importance. In rural China where primary health care service system and health education remain to be improved and strengthened, how to construct a warning model for CKD targeted at poverty-stricken areas is of great necessity and significance. In this study, we proposed a warning model using the available and accessible demographical, blood biochemical and lifestyle data in conjunction with machine learning approaches for rural areas. This model offers a leg-up for early CKD-assisted diagnosis in rural areas, which facilitates tailoring precise management and therapy for patients, thus, improving their quality of life and slowing the mortality rate.
Data supporting the conclusions in this manuscript would be made available from the corresponding author upon reasonable request.
The studies involving human participants were reviewed and approved by Ethics Committee of Shanxi Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
WS and YLu were responsible for the data analysis and the writing of the manuscript. LQ and JQ helped polish the manuscript. AL, YZ, and YLi gave precious advice on the statistical methods. RL and XZ were responsible for the conception and design of the research. All authors read and approved the final draft.
This work was supported by the Key Laboratory Project of Shanxi Province (201805D111020) and the Key Laboratory Construction Plan Project of Shanxi Provincial Health Commission (2020SYS01).
We appreciate all the authors and patients participating in this study. We are also indebted to my roommates, Fuliang Yi, Jiahui Ren, Jianguo Liang, and Zhenxing Zuo who encouraged me a lot during my writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.930541/full#supplementary-material
1. Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. (2019) 1165:3–15. doi: 10.1007/978-981-13-8871-2_1
2. Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. (2012) 379:815–22. doi: 10.1016/S0140-6736(12)60033-6
3. Wilson S, Mone P, Jankauskas SS, Gambardella J, Santulli G. Chronic kidney disease: definition, updated epidemiology, staging, and mechanisms of increased cardiovascular risk. J Clin Hypertens. (2021) 23:831–4. doi: 10.1111/jch.14186
4. Han J, Wu MC, Yang T. Challenge of China’s rural health. BMJ. (2016) 353:i2003. doi: 10.1136/bmj.i2003
5. Song S, Yuan B, Zhang L, Cheng G, Zhu W, Hou Z, et al. Increased inequalities in health resource and access to health care in rural China. Int J Environ Res Public Health. (2018) 16:49. doi: 10.3390/ijerph16010049
6. Ma C, Song Z, Zong Q. Urban-rural inequality of opportunity in health care: evidence from China. Int J Environ Res Public Health. (2021) 18:7792. doi: 10.3390/ijerph18157792
7. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Internal Med. (2013) 158:825–30. doi: 10.7326/0003-4819-158-11-201306040-00007
8. Wu Y, Fang Y. Stroke prediction with machine learning methods among older Chinese. Int J Environ Res Public Health. (2020) 17:1828. doi: 10.3390/ijerph17061828
9. Weng SF, Reps J, Kai J, Garibaldi JM, Qureshi N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. (2017) 12:e0174944. doi: 10.1371/journal.pone.0174944
10. Kalafi EY, Nor NAM, Taib NA, Ganggayah MD, Town C, Dhillon SK. Machine learning and deep learning approaches in breast cancer survival prediction using clinical data. Folia Biol. (2019) 65:212–20.
11. Kang J, Choi YJ, Kim IK, Lee HS, Kim H, Baik SH, et al. LASSO-based machine learning algorithm for prediction of lymph node metastasis in T1 colorectal cancer. Cancer Res Treatment. (2021) 53:773–83. doi: 10.4143/crt.2020.974
12. Mullah MAS, Hanley JA, Benedetti A. LASSO type penalized spline regression for binary data. BMC Med Res Methodol. (2021) 21:83. doi: 10.1186/s12874-021-01234-9
13. Wang J, Zhang H, Wang J, Pu Y, Pal NR. Feature selection using a neural network with group lasso regularization and controlled redundancy. IEEE Trans Neural Netw Learn Syst. (2021) 32:1110–23. doi: 10.1109/TNNLS.2020.2980383
14. Jiang L, Greenwood CMT, Yao W, Li L. Bayesian hyper-LASSO classification for feature selection with application to endometrial cancer RNA-seq Data. Sci Rep. (2020) 10:9747. doi: 10.1038/s41598-020-66466-z
15. Geetha R, Sivasubramanian S, Kaliappan M, Vimal S, Annamalai S. Cervical cancer identification with synthetic minority oversampling technique and PCA analysis using random forest classifier. J Med Syst. (2019) 43:286. doi: 10.1007/s10916-019-1402-6
16. Chen PN, Lee CC, Liang CM, Pao SI, Huang KH, Lin KF. General deep learning model for detecting diabetic retinopathy. BMC Bioinformatics. (2021) 22(Suppl 5):84. doi: 10.1186/s12859-021-04005-x
17. Wang K, Tian J, Zheng C, Yang H, Ren J, Li C, et al. Improving risk identification of adverse outcomes in chronic heart failure using SMOTE+ENN and machine learning. Risk Manage Healthcare Policy. (2021) 14:2453–63. doi: 10.2147/RMHP.S310295
18. Sarica A, Cerasa A, Quattrone A. Random forest algorithm for the classification of neuroimaging data in Alzheimer’s disease: a systematic review. Front Aging Neurosci. (2017) 9:329. doi: 10.3389/fnagi.2017.00329
19. Song M, Jung H, Lee S, Kim D, Ahn M. Diagnostic classification and biomarker identification of Alzheimer’s disease with random forest algorithm. Brain Sci. (2021) 11:453. doi: 10.3390/brainsci11040453
20. Lin E, Lin CH, Lane HY. Applying a bagging ensemble machine learning approach to predict functional outcome of schizophrenia with clinical symptoms and cognitive functions. Sci Rep. (2021) 11:6922. doi: 10.1038/s41598-021-86382-0
21. Lin E, Lin CH, Lane HY. Prediction of functional outcomes of schizophrenia with genetic biomarkers using a bagging ensemble machine learning method with feature selection. Sci Rep. (2021) 11:10179. doi: 10.1038/s41598-021-89540-6
22. Davagdorj K, Pham VH, Theera-Umpon N, Ryu KH. XGBoost-based framework for smoking-induced noncommunicable disease prediction. Int J Environ Res Public Health. (2020) 17:6513. doi: 10.3390/ijerph17186513
23. Ogunleye A, Wang QG. XGBoost model for chronic kidney disease diagnosis. IEEE/ACM Trans Comput Biol Bioinformat. (2020) 17:2131–40. doi: 10.1109/TCBB.2019.2911071
24. Ishiwata S, Matsue Y, Nakamura Y, Dotare T, Sunayama T, Suda S, et al. Clinical and prognostic values of urinary alpha1-microglobulin as a tubular marker in acute heart failure. Int J Cardiol. (2021) 338:115–20. doi: 10.1016/j.ijcard.2021.06.041
25. Zheng X, Wang F, Zhang J, Cui X, Jiang F, Chen N, et al. Using machine learning to predict atrial fibrillation diagnosed after ischemic stroke. Int J Cardiol. (2021) 347:21–7. doi: 10.1016/j.ijcard.2021.11.005
26. Ruini C, Schlingmann S, Jonke Ž, Avci P, Padrón-Laso V, Neumeier F, et al. Machine learning based prediction of squamous cell carcinoma in Ex vivo confocal laser scanning microscopy. Cancers. (2021) 13:5522. doi: 10.3390/cancers13215522
27. Chen Y, Liao R, Yao Y, Wang Q, Fu L. Machine learning to identify immune-related biomarkers of rheumatoid arthritis based on WGCNA network. Clin Rheumatol. (2021) 41:1057–68. doi: 10.1007/s10067-021-05960-9
28. Yang S, Bornot JMS, Fernandez RB, Deravi F, Wong-Lin K, Prasad G. Integrated space-frequency-time domain feature extraction for MEG-based Alzheimer’s disease classification. Brain Informat. (2021) 8:24. doi: 10.1186/s40708-021-00145-1
29. Le S, Allen A, Calvert J, Palevsky PM, Braden G, Patel S, et al. Convolutional neural network model for intensive care unit acute kidney injury prediction. Kidney Int Rep. (2021) 6:1289–98. doi: 10.1016/j.ekir.2021.02.031
30. Coorey CP, Sharma A, Muller S, Yang JYH. Prediction modeling-part 2: using machine learning strategies to improve transplantation outcomes. Kidney Int. (2021) 99:817–23. doi: 10.1016/j.kint.2020.08.026
31. Ginley B, Jen KY, Han SS, Rodrigues L, Jain S, Fogo AB, et al. Automated computational detection of interstitial fibrosis, tubular atrophy, and glomerulosclerosis. J Am Soc Nephrol. (2021) 32:837–50. doi: 10.1681/ASN.2020050652
32. Song W, Zhou X, Duan Q, Wang Q, Li Y, Li A, et al. Using random forest algorithm for glomerular and tubular injury diagnosis. Front Med. (2022) 9:911737. doi: 10.3389/fmed.2022.911737
33. Hong WS, Haimovich AD, Taylor RA. Predicting hospital admission at emergency department triage using machine learning. PLoS One. (2018) 13:e0201016. doi: 10.1371/journal.pone.0201016
34. Jiang YQ, Cao SE, Cao S, Chen JN, Wang GY, Shi WQ, et al. Preoperative identification of microvascular invasion in hepatocellular carcinoma by XGBoost and deep learning. J Cancer Res Clin Oncol. (2021) 147:821–33. doi: 10.1007/s00432-020-03366-9
35. Xu Y, Yang X, Huang H, Peng C, Ge Y, Wu H, et al. Extreme gradient boosting model has a better performance in predicting the risk of 90-day readmissions in patients with ischaemic stroke. J Stroke Cerebrovasc Dis. (2019) 28:104441. doi: 10.1016/j.jstrokecerebrovasdis.2019.104441
36. Zenker M, Machuca E, Antignac C. Genetics of nephrotic syndrome: new insights into molecules acting at the glomerular filtration barrier. J Mol Med. (2009) 87:849–57. doi: 10.1007/s00109-009-0505-9
37. Small DM, Bennett NC, Roy S, Gabrielli BG, Johnson DW, Gobe GC. Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron Exp Nephrol. (2012) 122:123–30. doi: 10.1159/000350726
38. Attman PO, Samuelsson O, Alaupovic P. Progression of renal failure: role of apolipoprotein B-containing lipoproteins. Kidney Int Suppl. (1997) 63:S98–101.
39. Osterby R, Gundersen HJ. Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia. (1975) 11:225–9. doi: 10.1007/BF00422326
40. Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. (2005) 54:3427–34. doi: 10.2337/diabetes.54.12.3427
Keywords: machine learning, chronic kidney disease, albuminuria-to-creatinine ratio, α1-microglobulin-to-creatinine ratio, auxiliary diagnosis, warning model
Citation: Song W, Liu Y, Qiu L, Qing J, Li A, Zhao Y, Li Y, Li R and Zhou X (2023) Machine learning-based warning model for chronic kidney disease in individuals over 40 years old in underprivileged areas, Shanxi Province. Front. Med. 9:930541. doi: 10.3389/fmed.2022.930541
Received: 28 April 2022; Accepted: 19 December 2022;
Published: 09 January 2023.
Edited by:
Li Zuo, Peking University People’s Hospital, ChinaReviewed by:
Guisen Li, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, ChinaCopyright © 2023 Song, Liu, Qiu, Qing, Li, Zhao, Li, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongshan Li,  cm9uZ3NoYW5saTEzQDE2My5jb20=; Xiaoshuang Zhou,
cm9uZ3NoYW5saTEzQDE2My5jb20=; Xiaoshuang Zhou,  eGlhb3NodWFuZ3pob3U2NkAxNjMuY29t
eGlhb3NodWFuZ3pob3U2NkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.