- 1Anesthesia and Intensive Care, San Martino Policlinico Hospital, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) for Oncology and Neurosciences, Genoa, Italy
- 2Department of Medicine, University of Barcelona, Barcelona, Spain
- 3Griffith University School of Medicine, Gold Coast, QLD, Australia
- 4Critical Care Research Group, The Prince Charles Hospital, Brisbane, QLD, Australia
- 5Department of Anesthesiology and Perioperative Medicine, University of Utah, Salt Lake City, UT, United States
- 6Faculty of Medicine, University of Queensland, Brisbane, QLD, Australia
- 7Division of Neuroscience Critical Care, Departments of Neurology, Neurosurgery, and Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 8Hospital Sao Camilo de Esteio, Esteio, Brazil
- 9Section of Cardiac Surgery, Department of Surgery, Max Rady College of Medicine, University of Manitoba, Winnipeg, MB, Canada
- 10Cardiac Sciences Program, St. Boniface Hospital, Winnipeg, MB, Canada
- 11Department of Surgery, Division of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee, WI, United States
- 12Division of Cardiovascular Diseases, Lankenau Medical Center and Lankenau Institute of Medical Research, Wynnewood, PA, United States
- 13Jefferson Medical College, Philadelphia, PA, United States
- 14Laboratório de Pneumologia LIM-09, Disciplina de Pneumologia, Heart Institute (Incor), Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil
- 15Équipe de Recherche en Soins Intensifs (ERESI), Research Centre, Centre Intégré Universitaire de Santé et de Services Sociaux du Nord-de-l'île-de-Montréal, Hôpital du Sacré-Coeur-de-Montréal, 5400 boulevard Gouin Ouest, K-3000, Montreal, QC, Canada
- 16Division of Critical Care Medicine, McGill University Health Centre, Montreal, QC, Canada
- 17Department of Anesthesiology and Reanimation, Dr. Soetomo Academic Hospital, Surabaya, Indonesia
- 18Institute for Clinical Research and Health Policy Studies, Tufts Medical Center/Tufts University School of Medicine, Boston, MA, United States
- 19Pediatric Cardiac Intensive Care Division, National Cardiovascular Center Harapan Kita, Jakarta, Indonesia
- 20Kuwait Extracorporeal Life Support Program, Ministry of Health, Kuwait City, Kuwait
- 21Department of Anesthesia and Critical Care Medicine, Al-Amiri Hospital, Kuwait City, Kuwait
- 22Department of Medicine 2, University Hospital, Ludwig Maximilian University of Munich, Munich, Germany
- 23Department of Anesthesia and Critical Care, Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Milan, Italy
- 24Emergency Department, Azienda Socio Sanitaria Territoriale (ASST) Monza - San Gerardo Hospital, Monza, Italy
- 25University of Milano-Bicocca, Milan, Italy
- 26Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy
- 27Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
- 28Australian Centre for Health Services Innovation, Centre for Healthcare Transformation, School of Public Health and Social Work, Queensland University of Technology, Brisbane, QLD, Australia
- 29Institut d'Investigacions Biomediques August Pi i Sunyer, Barcelona, Spain
- 30Adult Intensive Care Services, The Prince Charles Hospital, Brisbane, QLD, Australia
Introduction: Neurological manifestations and complications in coronavirus disease-2019 (COVID-19) patients are frequent. Prior studies suggested a possible association between neurological complications and fatal outcome, as well as the existence of potential modifiable risk factors associated to their occurrence. Therefore, more information is needed regarding the incidence and type of neurological complications, risk factors, and associated outcomes in COVID-19.
Methods: This is a pre-planned secondary analysis of the international multicenter observational study of the COVID-19 Critical Care Consortium (which collected data both retrospectively and prospectively from the beginning of COVID-19 pandemic) with the aim to describe neurological complications in critically ill COVID-19 patients and to assess the associated risk factors, and outcomes. Adult patients with confirmed COVID-19, admitted to Intensive Care Unit (ICU) will be considered for this analysis. Data collected in the COVID-19 Critical Care Consortium study includes patients' pre-admission characteristics, comorbidities, severity status, and type and severity of neurological complications. In-hospital mortality and neurological outcome were collected at discharge from ICU, and at 28-days.
Ethics and Dissemination: The COVID-19 Critical Care Consortium main study and its amendments have been approved by the Regional Ethics Committee of participating sites. No further approval is required for this secondary analysis.
Trial Registration Number: ACTRN12620000421932.
Introduction
Coronavirus disease 2019 (COVID-19) presents with a wide spectrum of symptoms, from mild to severe, up to sequential organ failure and multiple-organ dysfunction (1). Reports of neurological manifestations associated with COVID-19 are increasing in the literature (2, 3). COVID-19 neurological signs can involve either the central nervous system (CNS), peripheral nervous system (PNS), or musculoskeletal system. Fatigue, myalgia, impaired sense of smell and taste, and headache are common neurological manifestations of COVID-19 (4, 5), whereas dizziness, confusion, delirium, agitation, stroke, hypoxic ischemic injury, seizures, encephalitis and coma among others have been reported neurological complications of hospitalized patients (4, 5). In some cases, neurological manifestations have been reported even without a primary respiratory involvement (4, 5). Several explanations have been proposed for the cause of neurological symptoms of COVID-19, but the underlying pathophysiology is not well defined. Putative mechanisms include viral neurotropism, a hyperinflammatory and hypercoagulable state, or pathological brain–lung crosstalk (6). Endothelial dysregulation (7–9) and pro-thrombotic state (10–12) have been widely suspected to be the possible main contributors of the increased risk of neurologic events. Indeed, COVID-19 patients are at high risk of hypoxia, hypotension, and microvascular abnormalities (13–15) which can promote neuroinflammation and excitotoxicity and increased permeability of the blood brain barrier (16). The risk is even more increased by the use of extracorporeal membrane oxygenation (ECMO) support that is a salvage option in COVID-19 critically ill patients with refractory hypoxemia (17). Prior studies suggested a possible association between neurological complications and mortality (18), but more information is required to delineate this association with respect to regional variation, as well as the risk factors associated to the occurrence of neurological complications (19). The aim of this study is to estimate the incidence of neurological complications in critically ill COVID-19 patients. Associations between neurological complications, patient-level variables and outcomes will also be assessed.
Methods and Analysis
Study Design
This is a pre-planned sub-analysis of a large international multicenter observational study of patients in participating intensive care units (ICUs) with COVID-19 of the COVID-19 Critical Care Consortium incorporating the ExtraCorporeal Membrane Oxygenation for 2019 novel Coronavirus Acute Respiratory Disease (ECMOCARD). The collaborative consists of investigators from the Asia-Pacific extracorporeal life support organization (APELSO) in collaboration with centers within the SPRINT-SARI and International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) Network. In Australia, this study is also supported by collaboration with the “National registry on the treatment and outcomes of patients requiring ECMO” (EXCEL Registry). A panel of 13 experts in neurocritical care was created in 2020 together with the main protocol of the COVID-19 Critical Care Consortium by the Steering committee of the consortium. The panel planned this subanalysis and the electronic case report form (eCRF) in February 2020 and followed it up through monthly meeting. The study will be conducted in compliance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) (20) (Supplementary Item 1). Trial registration number: ACTRN12620000421932.
Objectives
The primary objective is to identify and describe the type and incidence of neurological complications in COVID-19 patients before and after admission to ICU, for all ICU patients selected patient subgroups (sex, age, country, treatment, COVID-19 wave).
Secondary objectives include: To evaluate the effect of neurological complications on outcomes after COVID-19, i.e., mortality, duration of ICU and hospital stay, neurological outcome (modified Rankin scale, mRS) at discharge, incidence of delirium and cognitive outcome at discharge. To identify factors related to the occurrence of neurological complications (including neurological injury due to the antiviral therapy).
Specific Sub-analysis
Secondary sub-analyses will also include the investigation of (1) magnetic resonance images (MRI) or computed tomography (CT) features; (2) serum biomarkers [neuronal injury markers (S100B, neuron specific enolase, NSE), endothelial dysfunction markers, inflammatory markers].
Inclusion and Exclusion Criteria
The COVID-19 Critical Care Consortium included all COVID-19 patients (≥18 years) admitted to ICU for receiving critical care with confirmed or suspected COVID-19 respiratory disease. For this specific sub-analysis, further inclusion criteria will be available data on neurological complications/manifestations. Patients treated with mechanical ventilation or ECMO for other causes than COVID-19 will be excluded.
Study Procedures and Setting
The protocol of the main study has been previously published (21). Participants in the COVID-19 Critical Care Consortium Observational Study are recruited at multiple sites in over 52 countries from 1st January 2020 onwards.
Data Collection
Data collection started from the commencement of COVID-19 pandemic and is planned to continue until completion of COVID-19 pandemic, as judged by the World Health Organization. According to the COVID-19 Critical Care Consortium Observational Study protocol (21) and neurological sub-study protocol, the following data will be collected: general patient characteristics, age, gender, body mass index (BMI), country, previous chronic comorbidities, scores of severity; premorbid scores [modified Rankin scale (0–6 points), Figure 1; new neurological complications, laboratory findings, imaging, and management of neurological complications (Supplementary Item 2); patient outcome (mortality at discharge, at 28-days, withdrawal of life-saving therapy and reason; mRS at ICU discharge, mRS at 28 days after discharge). Main eCRF of the COVID-19 critical care consortium study and neuro sub-study are provided in the Supplementary Items 3, 4.
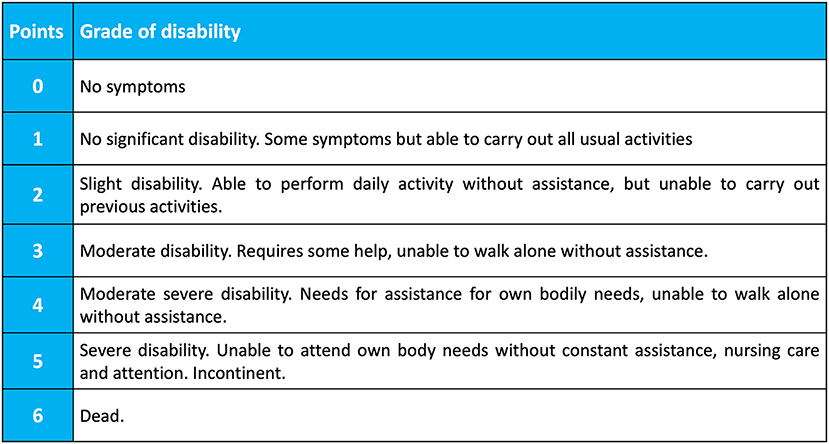
Figure 1. Modified Rankin Scale (mRS). The Modified Rankin Score (mRS) is a 6-point disability scale with possible scores ranging from 0 to 6 (from 0 = no symptoms to 6 = dead). A score of 0–3 indicate mild to moderate disability and a score of 4–5 indicate severe disability. From Wade (22).
Data Management
Data are stored in the central online eCRF database managed by the Oxford University in anonymized form, in order to preserve confidentiality of information in medical records. The Username and password will be assigned by the Oxford University during the registration process for individual Research Coordinators or Site Investigators. All electronic data transfer between study site and database will be username and password protected. The Participant List of the Neurology sub-study is maintained locally and is not to be transferred to any other location. confidentiality of the participant will be maintained unless disclosure is required by law.
Data entry and management will be coordinated by ISARIC and ECMOCARD steering committee, including programming and data management support. ANZIC-RC and ISARIC will act as custodian of the data. The University of Queensland (Australia) will receive data from the data custodians via data sharing agreements. The management committee of the trial will take responsibility for the content and integrity of any data.
Definition of Neurological Complications
Definition of neurological complications (23–32) is listed in Table 1.
Statistical Analysis Plan
Planned analyses will comprise of descriptive summaries and regression-based methods for estimating associations between patient-level variables, neurological complications, and outcomes. Descriptive statistics for summarizing the study cohort will be presented as medians with interquartile ranges and frequencies with percentages for continuous and categorical variables, respectively. As an observational study, missing data are expected; a data completeness summary will accompany descriptive summaries for all variables considered. The incidence of neurological complications will be calculated as the number of events per 1,000 ICU days and as the number of events divided by the total number of ICU admissions. Incidence will be estimated per complication using logistic and Poisson regression; Poisson models will include patient days as an offset to account for varying ICU exposure. Baseline models will be adjusted for patient-level variables (e.g., sex, age, country) and calendar time to account for the timing of different COVID-19 waves. Additional covariates will be informed by univariate analysis and penalized regression techniques to address the secondary objective related to incidence.
Analysis of associations between neurological complications and clinical outcomes will be examined using generalized linear mixed models for binary outcomes and parametric survival models for time-to-event outcomes. Evidence of potential associations, including patient demographics and clinical signs assessed during ICU admission, will initially be assessed using univariate analysis. Results of univariate analysis will be used to inform variable selection for multivariable analysis.
Multivariable models for all study objectives will be adjusted for known confounders as fixed or random effects, including study center, country, and calendar time. Model results will be presented as odds ratios (binary outcomes), relative risks (count outcomes) or hazard ratios (time-to-event outcomes) with 95% confidence intervals and p-values from hypothesis tests as appropriate.
Study Status
The protocol version is 1.2.8 of the COVID-19 Critical Care Consortium Observational Study available at https://www.elso.org/COVID19/ECMOCARD.aspx. Data collection started from the commencement of COVID-19 pandemic and is planned to continue until completion of COVID-19 pandemic, as judged by the World Health Organization, as reported in the protocol.
Discussion
This neurological sub-analysis of the COVID-19 Critical Care Consortium Observational Study is designed with the aim to obtain a detailed overview on neurological complications in a large international multicenter cohort of critically ill COVID-19 patients admitted to ICU, to determine incidence and risk factors of neurological complications, and the association of neurological complications with outcome. This study will provide real-time global data without geographic restrictions.
In the latest 2 years, knowledge has increased regarding extra-pulmonary complications of COVID-19 and their effect on outcome. Severe COVID-19 disease potentially involves multiple organs, including pulmonary, coagulation, cardiac, neurological, renal, hepatic, and gastrointestinal manifestations (34). Many neurological manifestations have been described recently in small observational studies, but additional evidence is needed from large multicentric cohorts. For this reason, in the present study we aim to depict the incidence, risk factors, and impact on outcome of neurological complications in critically ill COVID-19 patients from a large observational multicentric cohort. Data regarding pre-admission neurological manifestations, in-hospital neurological complications as well as ICU-and-hospital length of stay, neurological outcome (mRS), and mortality are available in the eCRF. This sub-analysis of the COVID-19 Critical Care Consortium Observational Study was pre-planned during the first/second wave of the pandemic (late 2020), thus increasing the data quality and minimizing the chance of spurious results and limiting the potential of exploratory learning. The number of patients included in the main study is continuously growing since the beginning of pandemic, allowing to obtain a large sample size, which can provide important information on the current incidence and characteristics of neurological manifestations in COVID-19 patients, evaluating potential associations between predictors and development of neurological complications, and assessing outcomes at discharge from ICU and from hospital and 28-days patients' outcomes. The included patients will be from different countries and centers, including low incoming countries. The patients will be also included during different waves and years of the pandemic, before and after the advent of vaccination campaigns, and with different variants of COVID-19 (i.e., omicron, delta, etc.). This will provide interesting insights on the differences in epidemiology, management strategies, geographical, and economical characteristics of COVID-19 adult patients who manifest neurological complications admitted to ICU. This global research context will provide the lens through which the study as well as its methodological approaches, findings, conclusions, and recommendations can be viewed.
Incidence and Types of Neurological Manifestations and Complications of COVID-19
The importance of investigating neurological manifestations in COVID-19, assessing their risk factors, and association with outcome is justified by the increasing identification in the available literature of many studies which reported high morbidity and mortality and poor neurological outcome in COVID-19 patients who manifest neurological complications, with the need for identifying and investigating such alterations in a bigger cohort of COVID-19 critically ill patients. Indeed, regarding each of the identified neurological manifestations of COVID-19, the data are fragmentary and come from different small cohorts. Myalgia, dysgeusia, and taste dysfunction were frequently reported (33% of cases), altered mental status in 32%, headache 29%, encephalopathy 26%, alteration of consciousness 13%, stroke 12%, dizziness 10%, vision impairment 6%, intracerebral hemorrhage, 5%, seizure 4%, encephalitis 2%, and GBS 1% (35). Intracranial hemorrhage was identified in 477 patients with a prevalence of 0.85% and a mortality of 52% suggesting a very poor prognosis despite rare incidence (36). The prevalence of intracranial hemorrhage, ischemic stroke, and hypoxic ischemic brain injury was higher in patients with COVID-19 who underwent ECMO support (5.9%) with a mortality of 92% (17). Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis have been reported in 46 patients with COVID-19 only, of whom 32% died (37).
Risk Factors for Neurological Manifestations and Complications of COVID-19
Regarding risk factors and association of neurological manifestations with outcome, a systematic review revealed that patients who suffer from a severe COVID-19 have more CNS involvement, neurological symptoms, and association with stroke. More severe patients had higher D-dimer and C-reactive protein levels than non-severe patients and presented multiple organ involvement (38). Myalgia, acute cerebrovascular disease, elevated creatin kinase, and lactate dehydrogenase were associated with more severe disease (3), while delirium on admission is a good predictor of mortality outcome in COVID-19 (39). In a cohort of 1,072 patients, age, headache at presentation, preexisting neurologic disease, invasive mechanical ventilation, and neutrophil/lymphocyte ratio ≥ 9 were independent predictors of new neurologic complications (40). In another study, the CT lung disease severity score was predictive of acute abnormalities on neuroimaging in patients with COVID-19 with neurologic manifestations (41). In a retrospective analysis, previous neurological history did not impact mortality, whereas new neurological manifestations were predictors of death (42). In a large cohort of 3,055 COVID-19 patients, preexisting neurological disorders were associated with higher risk of developing new neurological manifestations (2).
Outcome of COVID-19 Patients With Neurological Manifestations and Complications
Patients affected by COVID-19 with neurological manifestations were noted to have an impaired quality of life in 49% of cases, with a residual disability at 6-months in 52%, impaired cognition in 69%, and persistence of anxiety and depression in 32% (43). Neurological outcome in 135 patients with COVID-19 at 3-months follow-up was impaired (44), and a significant patient number still suffer from neurological sequelae 1 year after SARS-CoV-2 infection (45). A large multicentric study investigating delirium in 4,530 COVID-19 patients revealed that acute brain dysfunction was highly prevalent and prolonged in critically ill patients with COVID-19, with benzodiazepines and lack of family visitation identified to be risk factors for its development (46). After 6 months, in a cohort of 236,379 patients with COVID-19, neurological and psychiatric manifestations had an estimated incidence of 33.62 and 12.84%, respectively (47). Clinical outcome was evaluated in a cohort of 267 patients, concluding that patients with cerebrovascular disease had the worst prognosis (48).
Potential Pitfalls and Unintended Effects of This Study
Taken together, a large number of case reports and case series, despite coming mainly from small cohorts and local studies raise interest around the need for clarification about type and incidence of COVID-19 neurological manifestations, risk factors, and association with outcome on large scale, thus encouraging to better plan for possible management and therapeutics for neurological complications in critically ill COVID-19 patients. A limitation of current available data in the literature is that most of the data come from small cohorts, that could be addressed by using the larger COVID-19 Critical Care Consortium. Our study is unique in a way that we can address both limitations by studying the questions with international cohort with granular neurological variables. According to the design of our study, no unintended effects are expected. However, some limitations should be addressed. Being an observational study, it can be exposed to bias and confounding. Additionally, it cannot be used to demonstrate causality.
Conclusions
In conclusion the present study will provide new information on a global scale regarding the incidence and type of neurological complications, risk factors, and associated outcomes in COVID-19 with clinical applications.
Ethics Statement
The study will be conducted in compliance with the current version of the COVID-19 Critical Care Consortium and Neurologic sub-study protocol. Protocol version and subsequent amendment will be submitted and approved by the Local Ethics Committee in compliance to national standards. Sites wishing to participate will be required to provide the COVID-19 Critical Care Consortium Research Coordinator with an Institutional Review Board (IRB) approval certificate. The regulations of the COVID-19 Critical Care Consortium state that this study will not require individual patient consent as an observational study. Data of this study is already recorded as part of routine clinical care, therefore justifying participant enrolment using a waiver of consent. However, for any location that deems individual consent necessary, informed consent will be managed in accordance with the local regulations of each involved IRB. In particular, in patients who meet the inclusion/exclusion criteria, informed consent will be obtained directly from the patient, either before the study or retrospectively in case the patient is unconscious at the time of enrolment. If the patient is unable to provide a consent form upon admission, informed consent will be obtained by his/her next of kin.
Author Contributions
DB drafted the manuscript and planned the methodology and the outcomes. S-MC and CR revised the manuscript and supervised the methodology and outcomes. DB, LP, MGr, SH, JF, GW, DBP, RA, LD, EG, MA, VW, AN, SD, GS, BP, DK, EM, AA-F, S-SS, MP, MGi, GF, PP, AP, NW, GL, JS, CR, and S-MC helped in the revision and methodology and approved the final version. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Bill & Melinda Gates Foundation, Grant number INV-034765; The University of Queensland; The Wesley Medical Research; The Prince Charles Hospital Foundation; The Health Research Board of Ireland. GL was a recipient of the BITRECS fellowship; the BITRECS project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 754550 and from the La Caixa Foundation (ID 100010434), under the agreement LCF/PR/GN18/50310006. JS was funded by the Advance Queensland fellowship program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.930217/full#supplementary-material
Abbreviations
APELSO, Asia-Pacific extracorporeal life support organization; BMI, body mass index; CNS, central nervous system; COVID-19, Coronavirus disease 2019; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; ECMOCARD, ExtraCorporeal Membrane Oxygenation for 2019 novel Coronavirus Acute Respiratory Disease; eCRF, electronic case report form; ICU, intensive care unit; IRB, institutional review board; ISARIC, International Severe Acute Respiratory and emerging Infection Consortium; MRI, magnetic resonance images; mRS, modified Rankin scale; NSE, neuron specific enolase; PNS, peripheral nervous system.
References
1. Wong CKH, Wong JYH, Tang EHM, Au CH, Wai AKC. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: a systematic review and meta-analysis. Sci Rep. (2020) 10:19765. doi: 10.1038/s41598-020-74988-9
2. Chou SH-Y, Beghi E, Helbok R, Moro E, Sampson J, Altamirano V, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. (2021) 4:e2112131. doi: 10.1001/jamanetworkopen.2021.12131
3. Yassin A, Nawaiseh M, Shaban A, Alsherbini K, El-Salem K, Soudah O, et al. Neurological manifestations and complications of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. BMC Neurol. (2021) 21:138. doi: 10.1186/s12883-021-02161-4
4. Huth SF, Cho S-M, Robba C, Highton D, Battaglini D, Bellapart J, et al. Neurological manifestations of coronavirus disease 2019: a comprehensive review and meta-analysis of the first 6 months of pandemic reporting. Front Neurol. (2021) 12:664599. doi: 10.3389/fneur.2021.664599
5. Misra S, Kolappa K, Prasad M, Radhakrishnan D, Thakur KT, Solomon T, et al. Frequency of neurologic manifestations in COVID-19. Neurology. (2021) 97:e2269–81. doi: 10.1212/WNL.0000000000012930
6. Battaglini D, Brunetti I, Anania P, Fiaschi P, Zona G, Ball L, et al. Neurological manifestations of severe SARS-CoV-2 infection: potential mechanisms and implications of individualized mechanical ventilation settings. Front Neurol. (2020) 11:845. doi: 10.3389/fneur.2020.00845
7. Sashindranath M, Nandurkar HH. Endothelial dysfunction in the brain. Stroke. (2021) 52:1895–904. doi: 10.1161/STROKEAHA.120.032711
8. Savarraj J, Park ES, Colpo GD, Hinds SN, Morales D, Ahnstedt H, et al. Brain injury, endothelial injury and inflammatory markers are elevated and express sex-specific alterations after COVID-19. J Neuroinflammation. (2021) 18:277. doi: 10.1186/s12974-021-02323-8
9. Krasemann S, Haferkamp U, Pfefferle S, Woo MS, Heinrich F, Schweizer M, et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep. (2022) 17:307–20. doi: 10.1016/j.stemcr.2021.12.011
10. Abbas Z, Chaudhary A. COVID-19 associated coagulopathy resulting in cerebral venous thrombosis and pulmonary embolism. Cureus. (2021) 13:e19602. doi: 10.7759/cureus.19602
11. Manolis AS, Manolis TA, Manolis AA, Papatheou D, Melita H. COVID-19 infection: viral macro- and micro-vascular coagulopathy and thromboembolism/prophylactic and therapeutic management. J Cardiovasc Pharmacol Ther. (2021) 26:12–24. doi: 10.1177/1074248420958973
12. Robba C, Battaglini D, Ball L, Valbusa A, Porto I, Della Bona R, et al. Coagulative disorders in critically ill COVID-19 patients with acute distress respiratory syndrome: a critical review. J Clin Med. (2021) 10:140. doi: 10.3390/jcm10010140
13. Waldrop G, Safavynia SA, Barra ME, Agarwal S, Berlin DA, Boehme AK, et al. Prolonged unconsciousness is common in COVID-19 and associated with hypoxemia. Ann Neurol. (2022) 91:740–55. doi: 10.1002/ana.26342
14. Rutkai I, Mayer MG, Hellmers LM, Ning B, Huang Z, Monjure CJ, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. (2022) 13:1745. doi: 10.1038/s41467-022-29440-z
15. Ambade V, Ambade S. SARS-CoV-2 infecting endothelial cells, biochemical alterations, autopsy findings and outcomes in COVID-19, suggest role of hypoxia-inducible factor-1. J Med Biochem. (2022) 41:14–20. doi: 10.5937/jomb0-30659
16. Chen J, Tan R, Mo Y, Zhang J. The blood-brain barrier in health, neurological diseases, and COVID-19. Fundam Res. (2022). doi: 10.1016/j.fmre.2022.03.003. [Epub ahead of print].
17. Kannapadi N V., Jami M, Premraj L, Etchill EW, Giuliano K, Bush EL, et al. Neurological complications in COVID-19 patients with ECMO support: a systematic review and meta-analysis. Hear Lung Circ. (2022) 31:292–8. doi: 10.1016/j.hlc.2021.10.007
18. Frontera JA, Yang D, Lewis A, Patel P, Medicherla C, Arena V, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. (2021) 426:117486. doi: 10.1016/j.jns.2021.117486
19. Davidescu EI, Odajiu I, Tulbǎ D, Sandu CD, Bunea T, Sandu G, et al. Prognostic factors in COVID-19 patients with new neurological manifestations: a retrospective cohort study in a romanian neurology department. Front Aging Neurosci. (2021) 13:645611. doi: 10.3389/fnagi.2021.645611
20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
21. Li Bassi G, Suen J, Barnett AG, Corley A, Millar J, Fanning J, et al. Design and rationale of the COVID-19 critical care consortium international, multicentre, observational study. BMJ Open. (2020) 10:e041417. doi: 10.1136/bmjopen-2020-041417
22. Wade DT. Measurement in Neurological Rehabilitation. New York, NY: Oxford University Press (1992).
23. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, (Buddy), Culebras A, et al. An updated definition of stroke for the 21st century. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
24. Stone JL, Rifai MHS, Sugar O, Lang RGR, Oldershaw JB, Moody RA. Subdural hematomas. Surg Neurol. (1983) 19:216–31. doi: 10.1016/S0090-3019(83)80005-6
25. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
26. Rodríguez Y, Rojas M, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Monsalve DM, et al. Guillain–Barré syndrome, transverse myelitis and infectious diseases. Cell Mol Immunol. (2018) 15:547–62. doi: 10.1038/cmi.2017.142
27. Fisher RS, Boas W van E, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia. (2005) 46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x
28. Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. (1999) 40:120–2. doi: 10.1111/j.1528-1157.1999.tb02000.x
31. Gutmann L, Gutmann L. Critical illness neuropathy and myopathy. Arch Neurol. (1999) 56:527. doi: 10.1001/archneur.56.5.527
32. Finsterer J, Stollberger C. Causes of hypogeusia/hyposmia in SARS-CoV2 infected patients. J Med Virol. (2020) 92:1793–4. doi: 10.1002/jmv.25903
33. Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. (2017) 21:90. doi: 10.1186/s13054-017-1670-9
34. Robba C, Battaglini D, Pelosi P, Rocco RMP. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. (2020) 14:865–8. doi: 10.1080/17476348.2020.1778470
35. He Y, Bai X, Zhu T, Huang J, Zhang H. What can the neurological manifestations of COVID-19 tell us: a meta-analysis. J Transl Med. (2021) 19:363. doi: 10.1186/s12967-021-03039-2
36. Schmidbauer ML, Ferse C, Salih F, Klingner C, Musleh R, Kunst S, et al. COVID-19 and intracranial hemorrhage: a multicenter case series, systematic review and pooled analysis. J Clin Med. (2022) 11:605. doi: 10.3390/jcm11030605
37. Manzano GS, McEntire CRS, Martinez-Lage M, Mateen FJ, Hutto SK. Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis following COVID-19. Neurol Neuroimmunol Neuroinflammation. (2021) 8:e1080. doi: 10.1212/NXI.0000000000001080
38. Severo Bem Junior L, do Rego Aquino PL, Nunes Rabelo N, do Rego Aquino MA, Veiga Silva AC, Ferreira Valenca Mota R de C, et al. SARS-CoV-2 and nervous system - neurological manifestations in patients with COVID-19: a systematic review. J Neurol Res. (2020) 10:113–21. doi: 10.14740/jnr602
39. Hariyanto TI, Putri C, Hananto JE, Arisa J, Fransisca V Situmeang R, Kurniawan A. Delirium is a good predictor for poor outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review, meta-analysis, and meta-regression. J Psychiatr Res. (2021) 142:361–8. doi: 10.1016/j.jpsychires.2021.08.031
40. Flores-Silva FD, García-Grimshaw M, Valdés-Ferrer SI, Vigueras-Hernández AP, Domínguez-Moreno R, Tristán-Samaniego DP, et al. Neurologic manifestations in hospitalized patients with COVID-19 in Mexico City. PLoS ONE. (2021) 16:e0247433. doi: 10.1371/journal.pone.0247433
41. Mahammedi A, Ramos A, Bargalló N, Gaskill M, Kapur S, Saba L, et al. Brain and lung imaging correlation in patients with COVID-19: could the severity of lung disease reflect the prevalence of acute abnormalities on neuroimaging? A global multicenter observational study. Am J Neuroradiol. (2021) 42:1008–16. doi: 10.3174/ajnr.A7072
42. Salahuddin H, Afreen E, Sheikh IS, Lateef S, Dawod G, Daboul J, et al. Neurological predictors of clinical outcomes in hospitalized patients with COVID-19. Front Neurol. (2020) 11:585944. doi: 10.3389/fneur.2020.585944
43. Chaumont H, Meppiel E, Roze E, Tressières B, de Broucker T, Lannuzel A. Long-term outcomes after NeuroCOVID: a 6-month follow-up study on 60 patients. Rev Neurol. (2022) 178:137–43. doi: 10.1016/j.neurol.2021.12.008
44. Rass V, Beer R, Schiefecker AJ, Kofler M, Lindner A, Mahlknecht P, et al. Neurological outcome and quality of life 3 months after COVID-19: a prospective observational cohort study. Eur J Neurol. (2021) 28:3348–59. doi: 10.1111/ene.14803
45. Rass V, Beer R, Schiefecker AJ, Lindner A, Kofler M, Ianosi BA, et al. Neurological outcomes 1 year after COVID-19 diagnosis: a prospective longitudinal cohort study. Eur J Neurol. (2022) 29:1685–96. doi: 10.1111/ene.15307
46. Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, Raman R, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. (2021) 9:239–50. doi: 10.1016/S2213-2600(20)30552-X
47. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. (2021) 8:416–27. doi: 10.1016/S2215-0366(21)00084-5
48. Ross Russell AL, Hardwick M, Jeyanantham A, White LM, Deb S, Burnside G, et al. Spectrum, risk factors and outcomes of neurological and psychiatric complications of COVID-19: a UK-wide cross-sectional surveillance study. Brain Commun. (2021) 3:fcab168. doi: 10.2139/ssrn.3767901
Appendix
Keywords: COVID-19, neurological complications, disability, stroke, neurological outcome
Citation: Battaglini D, Premraj L, Griffee M, Huth S, Fanning J, Whitman G, Bastos Porto D, Arora R, Durham L, Gnall E, Amato M, Williams V, Noel A, De Franca SA, Samoukovic G, Pujo B, Kent D, Marwali E, Al-Fares A, Stecher S-S, Panigada M, Giani M, Foti G, Pelosi P, Pesenti A, White NM, Li Bassi G, Suen J, Fraser JF, Robba C, Cho S-M and the COVID-19 Critical Care Consortium (2022) Neurological Manifestations of SARS-CoV-2 Infection: Protocol for a Sub-analysis of the COVID-19 Critical Care Consortium Observational Study. Front. Med. 9:930217. doi: 10.3389/fmed.2022.930217
Received: 27 April 2022; Accepted: 06 June 2022;
Published: 22 July 2022.
Edited by:
Savino Spadaro, University of Ferrara, ItalyReviewed by:
Ahmed Yassin, Jordan University of Science and Technology, JordanMustafa Bayraktar, Atatürk University, Turkey
Copyright © 2022 Battaglini, Premraj, Griffee, Huth, Fanning, Whitman, Bastos Porto, Arora, Durham, Gnall, Amato, Williams, Noel, De Franca, Samoukovic, Pujo, Kent, Marwali, Al-Fares, Stecher, Panigada, Giani, Foti, Pelosi, Pesenti, White, Li Bassi, Suen, Fraser, Robba, Cho and the COVID-19 Critical Care Consortium. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Denise Battaglini, YmF0dGFnbGluaS5kZW5pc2VAZ21haWwuY29t
†These authors share senior authorship
‡COVID-19 Critical Care Consortium Investigators are listed in Appendix
 Denise Battaglini
Denise Battaglini Lavienraj Premraj3,4
Lavienraj Premraj3,4 Samuel Huth
Samuel Huth Jonathon Fanning
Jonathon Fanning Glenn Whitman
Glenn Whitman Rakesh Arora
Rakesh Arora Lucian Durham
Lucian Durham Alexandre Noel
Alexandre Noel Bambang Pujo
Bambang Pujo David Kent
David Kent Eva Marwali
Eva Marwali Marco Giani
Marco Giani Paolo Pelosi
Paolo Pelosi Jacky Suen
Jacky Suen John F. Fraser
John F. Fraser Chiara Robba
Chiara Robba Sung-Min Cho
Sung-Min Cho