
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 20 September 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.923282
This article is part of the Research Topic Eye in Systemic Diseases View all 21 articles
Serum magnesium levels have been reported to reflect the risk of diabetic retinopathy (DR); however, the effect of serum magnesium level on diabetic macular edema (DME) remains unclear. Here, we investigated the association between the serum magnesium levels and DME in patients with DR. Patients with DR were recruited between January 2018 and June 2021. A total of 519 such patients were included in this study. All patients underwent a standardized clinical ophthalmic examination by an experienced ophthalmologist, and an assay was conducted to determine the serum magnesium concentration. Compared with the non-DME group, the DME group had a higher proportion of insulin use and a higher level of serum ischemia-modified albumin and fasting plasma glucose. The serum magnesium and calcium levels were lower in the DME group than in the non-DME group (P < 0.05). Higher magnesium levels were negatively associated with DME after adjustment for relevant covariates. Compared with the participants in the lowest magnesium quartile, those in the fourth quartile showed a significantly lower risk of DME after adjustment [odds ratio (OR), 0.294; 95% confidence interval, 0.153–0.566; P < 0.0001]. Considering the potentially different effects of serum magnesium on the development of DME in patients with DR based on age, DR staging and insulin use, stratified analysis was performed by considering these factors. Among insulin-using patients with non-proliferative DR who were < 66 years of age, those in the third and fourth quartile of serum magnesium were less likely to develop DME than those in the lowest quartile of serum magnesium [OR (95% CI), 0.095 (0.014–0.620), 0.057 (0.011–0.305); P = 0.014, 0.001]. Overall, a higher serum magnesium level was associated with a lower risk of DME in patients with DR. Furthermore, patients with DR who used insulin were more likely to develop DME. Long-term studies on oral magnesium supplements are needed to determine whether maintaining the serum magnesium levels in a higher physiological range can reduce the risk of DME in patients with DR.
Diabetes is a global health problem with high incidence (1) and can lead to several complications. Diabetic retinopathy (DR) is a unique diabetic microvascular complication that is the main cause of adult vision loss in many countries (2). Diabetic macular edema (DME), a buildup of fluid in the central retina, can occur at various stages of DR. DME is the main cause of central vision loss in patients with diabetes and can affect activities of daily living, such as reading and driving, and severely decrease their quality of life (3). Increased retinal vascular permeability, followed by edema and hard exudate, are the main clinical features (4). The prevalence of DR and DME in patients with diabetes has been reported to be 35.4 and 7.4%, respectively (5).
Serum magnesium levels have been reported to reflect the risk of DR (6). Magnesium is the second largest intracellular cation in the human body, after potassium. It is a cofactor of more than 600 enzymes and an activator of more than 200 enzymes (7). Moreover, magnesium plays a role in many metabolic pathways, including glycolysis, β-oxidation, and insulin signal transduction (8, 9). However, the effect of serum magnesium level on DME remains unclear. Therefore, the purpose of this study was to determine the clinical value of serum magnesium levels in the diagnosis of DME by analyzing the relationship between the serum magnesium levels and DME in patients with DR.
This was a single-center retrospective study. A total of 519 patients with type 2 diabetes diagnosed with DR using color fundus imaging or fluorescein angiography who were admitted to the Department of Ophthalmology of the Affiliated Changshu Hospital of Xuzhou Medical University between January 2018 and June 2021 were enrolled in this study.
The exclusion criteria were as follows: (1) a history of eye trauma and eye surgery; (2) macular degeneration, macular anterior membrane, vitreomacular traction syndrome, and other fundus diseases; (3) pregnancy; (4) systemic diseases (neurological diseases; tumors; infections; severe hepatic, cardiac, and renal insufficiency; history of malignant tumors; history of hematology; history of autoimmune diseases; history of malabsorption; history of chronic diarrhea; and history of chronic kidney disease); (5) neuropsychiatric disorders leading to inability to cooperate with the examination; and (6) intake of thiazide diuretics, aminoglycoside antibiotics, amphotericin B, and other drugs that may affect serum magnesium excretion.
This study was approved by the Ethics Committee of the Affiliated Changshu Hospital of Xuzhou Medical University (Changshu, China; approval number: 2019082). All patients provided written informed consent upon admission.
Clinical data, including age, sex, history of hypertension, systolic blood pressure, diastolic blood pressure, and use of hypoglycemic drugs, were collected. The height and weight of all patients were measured while being in the standing position at admission. The body mass index was calculated by dividing the weight by the height squared (kg/m2). Blood pressure was read as the patient’s sitting blood pressure measured in a quiet state by a professional nurse at admission. Fasting (> 8 h) blood samples were collected for analysis. Blood (cell) count and serum biochemical variables (i.e., urea, uric acid, calcium, magnesium, ischemia modified albumin, retinol binding protein, fasting blood glucose) were assessed using a conventional automated blood analyzer (AU5800 Series Chemistry Analyzers; Beckman Coulter, Brea, CA, USA).
Each patient underwent a standardized clinical ophthalmic examination by an experienced fundus surgeon, including a review of ophthalmic history, measurement of visual acuity, and intraocular pressure, slit-lamp examination, and dilated fundus examination under a slit lamp. Color fundus imaging and optical coherence tomography were performed after pupil dilation, and fluorescein angiography was performed, where necessary. The diagnostic criteria for DR were based on the International Clinical Diabetic Retinopathy Disease Severity Scale (10). DME was defined according to the Early Treatment Diabetic Retinopathy Study report as any retinal thickening or hard exudate within the diameter of a disc in the fovea in the presence of DR features (11). Macular edema was confirmed by optical coherence tomography (Cirrus HD-OCT5000; Carl Zeiss, Jena, Germany) or fluorescein angiography (Spectralis HRA; Heidelberg, Germany).
The measurement data were normally distributed, represented by mean ± standard deviation values, and comparisons between the groups were made using the independent-sample t-test. The measurement data were skewness distribution data, represented by quartiles, and the Mann–Whitney U-test was used for intergroup comparison. Categorical variables are expressed as frequency (percentage) and were compared between groups using the χ2-test. Spearman’s rank correlation was used to analyze the correlation between serum magnesium levels and other clinical variables. A strong correlation was defined as a correlation coefficient > 0.4 and a P-value < 0.01. Binary logistic regression models were used to examine the relationship between serum magnesium levels and DME outcome. Variables with a P-value < 0.1 in univariate logistic regression were included in the multivariate logistic regression model. Statistical analyses were performed using SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA). All tests were two-sided, and statistical significance was set at P < 0.05.
In total, 519 patients with DR participated in this study. The patients in the DME group were older than those in the non-DME group. Although the proportion of men in the DME group was higher than that in the non-DME group, the proportion of women was still higher than that of men. Compared to the non-DME group, the DME group had a higher proportion of insulin use to control blood glucose and a higher level of serum ischemia-modified albumin and fasting plasma glucose. The serum magnesium and calcium levels were lower in the DME than in the non-DME group (P < 0.05). The clinical characteristics of all patients are listed in Table 1.
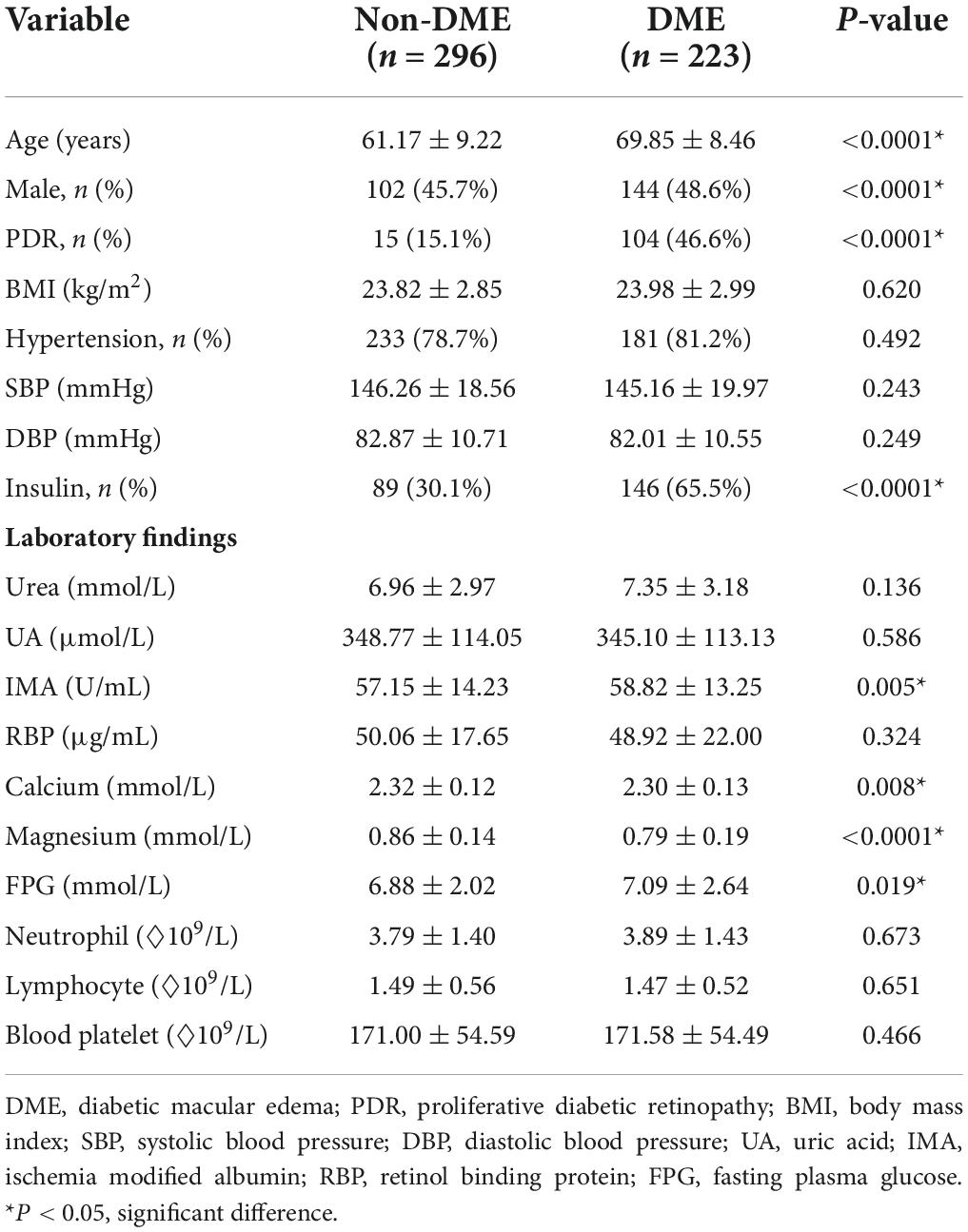
Table 1. Comparison of clinical characteristics between the diabetic macular edema (DME) and non-DME groups.
The serum magnesium levels of patients were divided into four groups according to the following quartiles: Q1 (≤ 0.78 mmol/L), Q2 (0.78–0.85 mmol/L), Q3 (0.85–0.91 mmol/L), and Q4 (> 0.91 mmol/L), respectively. The incidence of DME in all groups (58.1, 41.5, 41.7, and 26.9%, respectively) decreased with increasing serum magnesium levels. Compared with Q1, groups Q2, Q3, and Q4 showed statistically significant differences (P = 0.0051, P = 0.0121, P < 0.0001, respectively) (Figure 1). There was also a difference in the incidence of DME between the Q2, Q3, and Q4 groups (P = 0.0180, P < 0.0001, P = 0.0246, respectively).
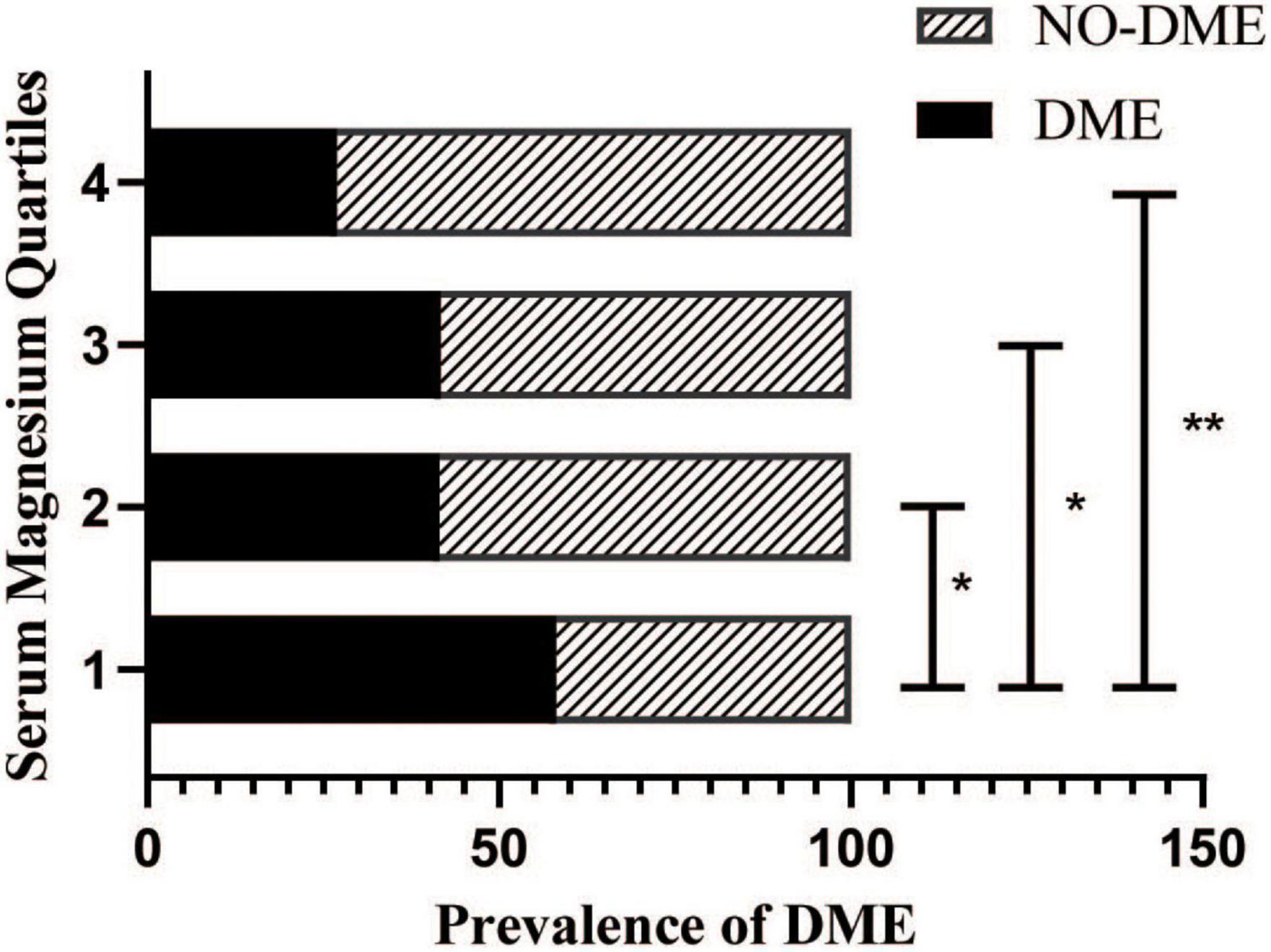
Figure 1. Prevalence of diabetic macular edema (DME) in patients with diabetic retinopathy stratified by the serum magnesium levels.
Table 2 shows the results from the univariate binary logistic regression model used to analyze the factors influencing DME, including serum magnesium, serum calcium, age, DR staging, and insulin use, in the multiple binary logistic regression model (Table 3). Multiple regression analysis (Table 3) showed that the serum magnesium levels in Q2 [odds ratio (OR), 0.512; 95% confidence interval (CI), 0.322–0.815], Q3 (OR, 0.515; 95% CI, 0.313–0.848), and Q4 (OR, 0.266; 95% CI, 0.153–0.461) were negatively correlated with the risk of DME compared with the serum magnesium levels in group Q1.
After adjusting for age, DR staging, and insulin use (Model 5), the negative correlation between serum magnesium and DME risk in the Q4 group was still statistically significant (Table 3). Higher serum magnesium levels were associated with a reduced risk of DME in patients with DR (OR, 0.310; 95% CI, 0.153–0.629). Insulin use (OR, 2.643; 95% CI, 1.683–4.152) and DR staging (OR, 9.308; 95% CI, 4.913–17.635) were associated with an increased risk of DME in patients with DR, whereas age was associated with a reduced risk of DME in patients with DR (OR, 0.918; 95% CI, 0.894–0.941).
Considering the potentially different effects of serum magnesium on the development of DME in patients with DR based on age, DR staging, and insulin use, stratified analysis was performed based on these factors. Among insulin-using patients with non-proliferative DR who were < 66 years of age, those in the third and fourth quartile of serum magnesium were less likely to develop DME than those in the lowest quartile of serum magnesium [OR (95% CI), 0.095 (0.014–0.620) and 0.057 (0.011–0.305), respectively; P = 0.014 and 0.001, respectively].
As shown in Figure 2, marked Model 4 had the largest area under the curve (0.856; 95% CI, 0.823–0.890; Table 4) and a greater risk prediction value.
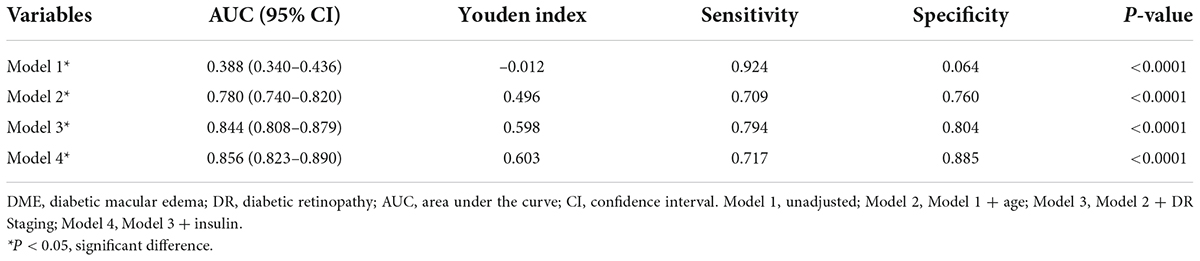
Table 4. The predictive value of serum magnesium alone and combined with age, DR staging, and insulin for the occurrence of DME.
The serum magnesium levels were positively correlated with age, DR staging and retinol binding protein but negatively with body mass index, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, and neutrophil count (all P < 0.05). There was no association between the serum magnesium levels and urea, uric acid, ischemia-modified albumin, calcium, lymphocyte count, platelet count (Table 5).
Diabetes is a serious public health concern in China. In 2018, the estimated prevalence of diabetes mellitus in China was 12.4%. Approximately 36.7% of the Chinese adults with diabetes reported being aware of their condition, 32.9% reported receiving treatment, and 50.1% of those receiving treatment had fully controlled disease. Compared with the United States, diabetes awareness, treatment, and control rates are lower in China (12). In many of our patients, diabetes was identified in the ophthalmology clinic after patients presented at the clinic with vision problems. Additionally, several patients with diabetes cannot be evaluated for fundus conditions due to severe cataract at the first ophthalmology visit. Without timely and effective treatment, DME may lead to severe vision loss or even blindness, which will impose a heavy economic burden on families and society. Therefore, timely detection and treatment of DME are important (3).
DME is a complex pathological condition caused by many factors. Retinal thickening in the macular area due to exudate accumulation (extracellular edema) caused by blood-retinal barrier dysfunction is considered the main pathological mechanism of DME (13). Magnesium is one of the major elements required to maintain normal metabolism and ionic balance in ocular tissues (14). A study involving 3,100 patients with diabetes with normal serum magnesium found that the incidence of diabetic retinopathy decreased with the increase of serum magnesium concentration. In patients with normal serum magnesium levels, the serum magnesium levels were negatively associated with the risk of diabetic microvascular complications (15). In another study involving 2,222 patients with diabetes, the authors stratified their serum magnesium levels into quartiles (Q1–Q4) and found that the group with low serum magnesium, Q1 (≤ 0.85 mmol/L), and Q2 (0.85–0.90 mmol/L), had significantly higher incidence rates of DR (50.9 and 30.2%, respectively) than the groups with high serum magnesium, Q3 (0.90–0.96 mmol/L), and Q4 (≥ 0.90 mmol/L) (23.5 and 21%, respectively). Lower serum magnesium levels were reportedly related to an increased risk of developing DR (9). However, the relationship between the serum magnesium levels and DME in patients with DR was not discussed in any of these studies. Our results suggest that within the normal range, higher serum magnesium level is a protective factor against DME in patients with DR, especially for those in non-proliferative DR period, aged < 66 years, who use insulin to control blood glucose.
Magnesium may protect against DME through systemic and local mechanisms. The serum magnesium concentration in normal adults is 0.70–1.10 mmol/L. Approximately 20% of this magnesium is protein bound, 65% is ionized, and the rest is combined with various anions, such as phosphates and citrates (16). Hypomagnesemia occurs in 13.5–47.7% of the patients with type 2 diabetes (17). Insulin resistance could reduce the TRPM6 channel activity of the intestinal and renal tubular epithelium and reduce the absorption of magnesium in the intestinal and renal epithelium, resulting in low serum magnesium (18). Simultaneously, hypomagnesemia can aggravate insulin resistance, and both of them are mutually pathogenic factors (19). Hyperglycemia is a systemic risk factor for DME (3). Magnesium intake reduces oxidative stress responses and improves insulin and glucose metabolism (20, 21). Therefore, patients with DR having high serum magnesium levels may have a reduced occurrence of DME because magnesium improves blood glucose and insulin resistance. Increased oxidative stress levels in the context of low serum magnesium levels may also contribute to DME progression. Retinal nerve tissue is rich in polyunsaturated fatty acids and has the highest oxygen consumption among all tissues. During magnesium deficiency, insufficient antioxidant enzyme activity can damage membranes rich in lipid peroxidation by free radicals, consequently impairing retinal function (22).
Inflammation is another cause of DME. It has been reported that supplementation with MgO or Mg picolinate has the greatest impact on retinal function as it can improve lipid peroxidation and reduce the expression of pro-inflammatory cytokines, such as intercellular adhesion molecule and vascular endothelial growth factor, by preventing oxidative stress and lipid production (23). Magnesium can reduce retinal oxidative stress and neuronal inflammation (22). However, deficiency in magnesium causes systemic and local inflammatory states that may predispose patients with DR to DME.
Moreover, magnesium has been shown to protect against heart failure in patients with type 2 diabetes. This protective effect is mediated, in part, by factors, such as the control of blood sugar by magnesium and the reduction of endothelial inflammation (24). Oral magnesium supplementation can improve vascular endothelial function and reduce vascular endothelial inflammation (25, 26). Destruction of retinal vascular endothelial cells is considered to be the initial cause of DME (13). Therefore, decreased serum magnesium levels may lead to the aggravation of retinal vascular endothelial inflammation, resulting in endothelial dysfunction and increased permeability, which may make patients with DR more prone to DME. In addition, hypomagnesemia causes an imbalance between vasoconstriction and dilation (24). Retinal vasospasm caused by magnesium deficiency leads to retinal ischemia and hypoxia, which further accelerate retinal damage (22).
Our data also suggest that insulin-treated patients with DR are at a higher risk of developing DME. Many people with type 2 diabetes require insulin therapy because of the gradual loss of islet beta cell function. Insulin use may also increase the risk of DR and DME in some patients (27). Among more than 300 patients treated with insulin, a 100% increased risk of DME was reported in those treated with insulin compared with those treated with oral medications (27). In addition, some studies have found that the macular thickness of patients with type 2 diabetes treated with insulin was higher than that of people in the control group (28). Possible mechanisms of action include the upregulation of vascular endothelial growth factor expression, vascular activity of insulin itself, sudden improvement in glycemic control, and further damage to the already-impaired blood-retinal barrier (4). Insulin can also disrupt the tight junctions of retinal pigment epithelial cells by disrupting the outer barrier (29). Hypomagnesemia among diabetic patients was associated with diabetic retinopathy (30), and a decreased level of serum magnesium was associated with an increase in macular thickness and ellipsoid zone disruption in DME (31). However, the correlation between serum magnesium, DME, and insulin use was not discussed. Interestingly, in our data, elevated serum magnesium reduced the risk of DME in patients with non-proliferative DR aged < 66 years who were treated with insulin, but not in other age groups or in patients who were not treated with insulin. This may be related to the fact that in some of our patients, diabetes was discovered only after the first diagnosis in ophthalmology because of vision problems, and no standardized endocrine therapy had been previously performed. Nevertheless, in multiple quadratic regression, our data elucidated that the risk of DME was reduced in the highest quartile of serum magnesium compared with the lowest quartile, suggesting that maintaining adequate magnesium levels may reduce the risk of DME in patients with DR. Our results highlight the need for interventional studies to assess whether magnesium supplementation reduces the risk of DME in patients with DR.
However, our study had some limitations. First, this was a retrospective study, and further prospective studies are needed to investigate the association between the serum magnesium levels and microvascular complications in patients with diabetes. In this retrospective study, we investigated the serum magnesium levels in patients with DR, and the assessment of the systemic magnesium status was incomplete. Second, some of the enrolled patients did not know their specific diabetes history. Third, selection bias may have existed. Most patients in this study were residents of Changshu, China; therefore, caution should be exercised when applying the conclusions of this study to other patient groups. Prospective multicenter studies are needed to validate magnesium as a predictor of DME risk.
The higher the serum magnesium level, the lower the risk of DME in patients with DR. Furthermore, patients with DR who use insulin are more likely to develop DME. Long-term studies on oral magnesium supplements are needed to determine whether maintaining the serum magnesium levels within the physiological range can reduce the risk of DME in patients with DR.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Affiliated Changshu Hospital of Xuzhou Medical University (Changshu, China). The patients/participants provided their written informed consent to participate in this study.
XX: conception and design, data analysis and interpretation, and manuscript writing. ZJ and TJ: data collection and collation. ZH and JY: data interpretation and final review of the manuscript. All authors revised and approved the submitted manuscript.
Funding was received from the National Social Science Foundation of China (grant no. 19CGL062).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DME, diabetic macular edema; DR, diabetic retinopathy; OR, odds ratio.
1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. (2010) 87:4–14. doi: 10.1016/j.diabres.2009.10.007
2. Wong TY, Cheung CM, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. (2016) 2:16012. doi: 10.1038/nrdp.2016.12
3. Diep TM, Tsui I. Risk factors associated with diabetic macular edema. Diabetes Res Clin Pract. (2013) 100:298–305. doi: 10.1016/j.diabres.2013.01.011
4. Matsuda S, Tam T, Singh RP, Kaiser PK, Petkovsek D, Zanella MT, et al. Impact of insulin treatment in diabetic macular edema therapy in type 2 diabetes. Can J Diabetes. (2015) 39:73–7. doi: 10.1016/j.jcjd.2014.06.005
5. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Meta-analysis for eye disease (META-EYE) study group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:556–64. doi: 10.2337/dc11-1909
6. Xing B, Xu X, Li C, Zhao Y, Wang Y, Zhao W. Reduced serum magnesium levels are associated with the occurrence of retinopathy in patients with type 2 diabetes mellitus: a retrospective study. Biol Trace Elem Res. (2021) 200:2025–32. doi: 10.1007/s12011-021-02824-w
7. de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. (2015) 95:1–46. doi: 10.1152/physrev.00012.2014
8. Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. (2007) 2:366–73. doi: 10.2215/CJN.02960906
9. Chutia H, Lynrah KG. Association of serum magnesium deficiency with insulin resistance in type 2 diabetes mellitus. J Lab Physicians. (2015) 7:75–8. doi: 10.4103/0974-2727.163131
10. Wilkinson CP, Ferris FL III, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. (2003) 110:1677–82. doi: 10.1016/S0161-6420(03)00475-5
11. Kinyoun J, Barton F, Fisher M, Hubbard L, Aiello L, Ferris F III. Detection of diabetic macular edema. Ophthalmoscopy versus photography–early treatment diabetic retinopathy study report number 5. The ETDRS research group. Ophthalmology. (1989) 96:746–50. doi: 10.1016/s0161-6420(89)32814-4
12. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. JAMA. (2021) 326:2498–506.
13. Zhang X, Bao S, Lai D, Rapkins RW, Gillies MC. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. (2008) 57:1026–33. doi: 10.2337/db07-0982
14. Kamińska A, Romano GL, Rejdak R, Zweifel S, Fiedorowicz M, Rejdak M, et al. Influence of trace elements on neurodegenerative diseases of the eye-the glaucoma model. Int J Mol Sci. (2021) 22:4323. doi: 10.3390/ijms22094323
15. Lu J, Gu Y, Guo M, Chen P, Wang H, Yu X. Serum magnesium concentration is inversely associated with albuminuria and retinopathy among patients with diabetes. J Diabetes Res. (2016) 2016:1260141. doi: 10.1155/2016/1260141
16. Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. (2000) 294:1–26. doi: 10.1016/s0009-8981(99)00258-2
17. Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes. (2016) 65:3–13. doi: 10.2337/db15-1028
18. Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. (2002) 31:166–70. doi: 10.1038/ng889
19. Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci. (2019) 20:1351. doi: 10.3390/ijms20061351
20. Castellanos-Gutiérrez A, Sánchez-Pimienta TG, Carriquiry A, da Costa THM, Ariza AC. Higher dietary magnesium intake is associated with lower body mass index, waist circumference and serum glucose in Mexican adults. Nutr J. (2018) 17:114. doi: 10.1186/s12937-018-0422-2
21. Jeong JW, Lee B, Kim DH, Jeong HO, Moon KM, Kim MJ, et al. Mechanism of action of magnesium lithospermate B against aging and obesity-induced ER stress, insulin resistance, and inflammsome formation in the liver. Molecules. (2018) 23:2098. doi: 10.3390/molecules23092098
22. Agarwal R, Iezhitsa L, Agarwal P. Pathogenetic role of magnesium deficiency in ophthalmic diseases. Biometals. (2013). [Epub ahead of print]. doi: 10.1007/s10534-013-9684-5
23. Orhan C, Er B, Deeh PBD, Bilgic AA, Ojalvo SP, Komorowski JR, et al. Different sources of dietary magnesium supplementation reduces oxidative stress by regulation Nrf2 and NF-κB signaling pathways in high-fat diet rats. Biol Trace Elem Res. (2021) 199:4162–70. doi: 10.1007/s12011-020-02526-9
24. Oost LJ, van der Heijden AAWA, Vermeulen EA, Bos C, Elders PJM, Slieker RC, et al. Serum magnesium is inversely associated with heart failure, atrial fibrillation, and microvascular complications in type 2 diabetes. Diabetes Care. (2021) 44:1757–65. doi: 10.2337/dc21-0236
25. Darooghegi Mofrad M, Djafarian K, Mozaffari H, Shab-Bidar S. Effect of magnesium supplementation on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Atherosclerosis. (2018) 273:98–105. doi: 10.1016/j.atherosclerosis.2018.04.020
26. Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. (2000) 102:2353–8. doi: 10.1161/01.cir.102.19.2353
27. Henricsson M, Nilsson A, Janzon L, Groop L. The effect of glycaemic control and the introduction of insulin therapy on retinopathy in non-insulin-dependent diabetes mellitus. Diabet Med. (1997) 14:123–31. doi: 10.1002/(SICI)1096-9136(199702)14:23.0.CO;2-U
28. Zapata MA, Badal J, Fonollosa A, Boixadera A, García-Arumí J. Insulin resistance and diabetic macular oedema in type 2 diabetes mellitus. Br J Ophthalmol. (2010) 94:1230–2. doi: 10.1136/bjo.2009.171702
29. Sugimoto M, Cutler A, Shen B, Moss SE, Iyengar SK, Klein R, et al. Inhibition of EGF signaling protects the diabetic retina from insulin-induced vascular leakage. Am J Pathol. (2013) 183:987–95. doi: 10.1016/j.ajpath.2013.05.017
30. Hamdan HZ, Nasser NM, Adam AM, Saleem MA, Elamin MI. Serum magnesium, iron and ferritin levels in patients with diabetic retinopathy attending Makkah Eye Complex, Khartoum, Sudan. Biol Trace Elem Res. (2015) 165:30–4. doi: 10.1007/s12011-015-0236-4
Keywords: magnesium, diabetic macular edema, diabetic retinopathy, diabetes mellitus, vision loss
Citation: Xiang X, Ji Z, Jiang T, Huang Z and Yan J (2022) Reduced serum magnesium is associated with the occurrence of diabetic macular edema in patients with diabetic retinopathy: A retrospective study. Front. Med. 9:923282. doi: 10.3389/fmed.2022.923282
Received: 19 April 2022; Accepted: 05 September 2022;
Published: 20 September 2022.
Edited by:
Anna Maria Roszkowska, University of Messina, ItalyReviewed by:
Changzheng Chen, Renmin Hospital of Wuhan University, ChinaCopyright © 2022 Xiang, Ji, Jiang, Huang and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengru Huang, aHpoZW5ncnVAMTYzLmNvbQ==; Jing Yan, eWo4NTA1QG1haWwudXN0Yy5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.