- 1Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
- 2Pediatric Pulmonology Unit, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
High-flow nasal cannula (HFNC) oxygen therapy has rapidly become a popular modality of respiratory support in pediatric care. This is undoubtedly due to its ease of use and safety, which allows it to be used in a wide variety of settings, ranging from pediatric intensive care to patients' homes. HFNC devices make it possible to regulate gas flow and temperature, as well as allowing some nebulized drugs to be administered, features very useful in children, in which the balance between therapeutic effectiveness and adherence to treatment is pivotal. Although the physiological effects of HFNC are still under investigation, their mechanisms of action include delivery of fixed concentration of oxygen, generation of positive end-expiratory pressure, reduction of the work of breathing and clearance of the nasopharyngeal dead space, while providing optimal gas conditioning. Nevertheless, current evidence supports the use of HFNC mainly in moderate-to-severe bronchiolitis, whereas for asthma exacerbations and breath sleeping disorders there is a lack of randomized controlled trials comparing HFNC to continuous positive airway pressure (CPAP) and non-invasive ventilation (NIV), which are essentials for the identification of response and non-response predictors. In this regard, the development of clinical guidelines for HFNC, including flow settings, indications, and contraindications is urgently needed.
Introduction
High-flow nasal cannula (HFNC) oxygen therapy was first introduced into clinical practice in the early 2000s as an alternative to continuous positive airway pressure (CPAP) to manage apnea in premature neonates (1). Since then, its use in infants and children with respiratory failure has steadily grown. Indeed, nowadays HFNC is an extremely popular mode of respiratory support in pediatric care, due to a number of factors including the availability of easy-to-use devices that are exceptionally well tolerated by most of the patients, as compared to CPAP or other modes of non-invasive ventilation (NIV) (2). The HFNC apparatus is designed to provide heated and humidified gasses, usually air mixed with oxygen, at different flow rates and adjustable concentrations. The gas is inhaled via a soft and comfortable silicone nasal cannula that fits without occluding the nose. Although the term “high flow” is generally opposed to “low flow” used for conventional oxygen therapy (COT), there is no precise definition of what constitutes a high flow, as rates vary according to the age and weight of the patient, ranging from 2 to 60 L/min (3, 4).
While originally limited to pediatric intensive care units (PICU), because of its ease of use, HFNC has now expanded to a variety of settings, including emergency departments, inpatient pediatric wards and even patients' homes (4, 5). As a result, the body of literature on pediatric HFNC, despite still being scant compared to the broader range of adults, has steadily grown. Due to the proven safety and beneficial effects of heated humidified high flows, the future applications of HFNC in the pediatric setting will likely increase in the coming years. Therefore, the aim of this review is to report information about the most updated understanding on the action mechanism in children, addressing relevance and limitations of the current research, in order to provide an outlook on potential future perspectives.
Devices and Settings
Three types of HFNC devices are currently available for pediatric patients. The first type, utilized by Optiflow System® (Fisher and Paykel, Auckland, New Zealand) (Figure 1A), Precision Flow® (Vapotherm, Exeter, UK), and Comfort-Flo® (Teleflex Medical, Durham, NC, USA) consists of an air/oxygen blender that is connected to a system to humidify and heat the gas. The device can be equipped with a pressure relief valve that cuts off the flow when a predetermined pressure in the circuit is reached. The second type, employed by Airvo2® (Fisher and Paykel, Auckland, New Zealand) (Figure 1B), works through an integrated turbine generating the flow plus a heated humidifier with the advantage of not requiring an external source of gas, except from oxygen and nitric oxide. The third type is based on a CPAP or conventional ventilator with an HFNC breathing circuit connected to the humidifier.
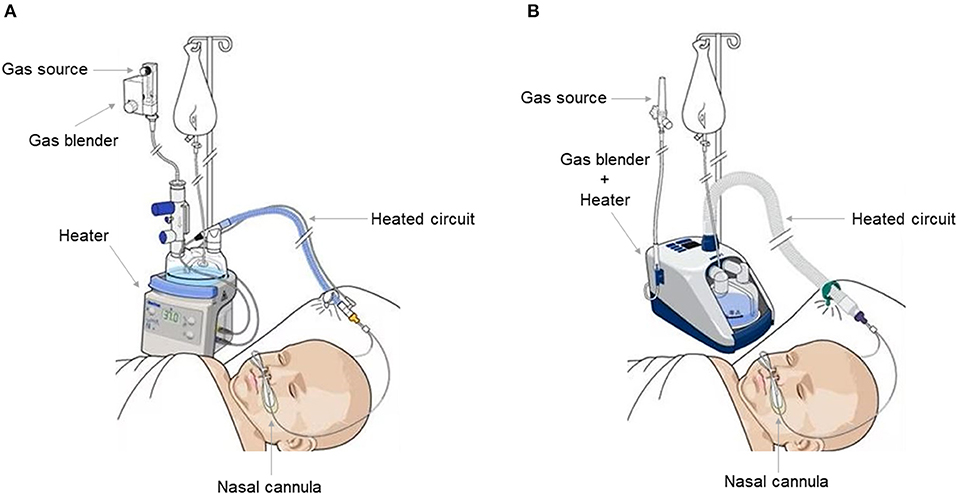
Figure 1. Fisher and Paykel Optiflow system ® (A) and Fisher and Paykel Airvo 2 ® system (B). Both allow an inhaled oxygen fraction of up to 100% and generate a flow of up to 60 L/min. The Fisher and Paykel Airvo 2 ® system combines a gas mixer and heater in one device.
There is no universal consensus among pediatricians about the optimal flow. As such, information about appropriate settings has been retrieved from the most relevant clinical studies in acute bronchiolitis (6–8). Patients younger than 24 months could tolerate a flow of 1–2 L/kg/min (up to 20 L/min). Superior flows had the same reported efficacy but resulted uncomfortable (8). Considering the child weight:
• 1–2 L/kg/min are recommended up to 10 kg;
• 1 L/kg/min from 10 up to 20 kg;
• 0.8–1 L/kg/min from 20 up to 40 Kg;
• 0.5–1.1 L/kg/min for >40 Kg.
Cannula size should also be chosen according to age and body weight. The cross-sectional area of the cannula should not exceed 50% of the nostrils because of the risk of unexpected increases in airway pressure and subsequent risk of air leakage.
Drugs Nebulization
Aerosolized drug delivery using HFNC is an attractive modality of administration since traditional nebulizer masks are often poorly tolerated by children (9). However, controversies emerged after some in vitro feasibility evaluation.
The HFNC presents two main limitations: (1) The aerosol administration via nasal cannulas increases the upper airways deposition in comparison to oral inhalation (10, 11); (2) High gas flow rate increases particle deposition by impaction (12–14). These researches suggest that aerosol particle distribution is only feasible at flows <6 L/min (9). Using vibrating mesh nebulizers placed immediately upstream or downstream of the humidification chamber, about 1–10% of the drug may be delivered to the lungs, a quantity significantly lower than amounts delivered with conventional interfaces (up to 25%) (15–17), but still sufficient to exert clinical effects in pediatric patients. To date, researches performed in vivo about drug nebulization using HFNC system are still few. In these studies, performed in children with respiratory distress due to asthma exacerbation or bronchiolitis, the inhalation of a bronchodilator through a nebulizer placed within the HFNC circuit was as effective as the inhalation through conventional devices, but the level of comfort was greater (18–20). The positive results observed with bronchodilators may not be generalized to other drugs (21). For example, the current HFNC devices are likely to be inefficient for antibiotics, wherein the drug volume deposited into the lung is an important factor of efficacy.
Physiological Effects of HFNC
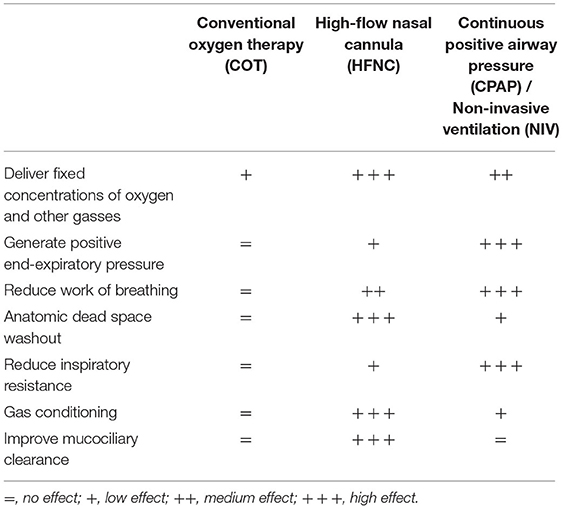
Studies are still ongoing to unravel the full spectrum of HFNC's mechanisms of action. It should be noted that HFNC has been rapidly introduced by clinicians into their daily practice, although some physiological aspects still remain to be clarified. A list of these effects in children is provided in Table 1.
Delivery of Fixed Oxygen Concentrations and Other Gasses
Physiologically, inspiratory flow varies with each breath and so does the fraction of inhaled oxygen (FiO2) administered via COT at low flows (22). In patients with respiratory distress, the inspiratory flow often exceeds the oxygen flow delivered by COT, resulting in oxygen concentration dilution (23, 24). The delivery of high flows allows matching patients' inspiratory peak flow (25). As a result, the FiO2 reaching the lower airways is closer to the FiO2 delivered by the HFNC device, enabling the possibility of a fine titration of oxygen administration (26).
This feature has also been exploited to deliver nitric oxide directly to the lungs. Inhaled nitric oxide (iNO) is a selective pulmonary vasodilator that decreases pulmonary arterial pressure and pulmonary vascular resistance without inducing systemic hemodynamic effects. The iNO therapy using HFNC has been reported successfully in infants with respiratory distress and post-extubation after the Fontan procedure (27, 28).
Generation of Positive End-Expiratory Pressure, Work of Breathing Reduction and Washout of Anatomical Dead Space
The high flows produced by HFNC devices are often sufficient to prevail against children's expiratory flow and to generate a small positive end-expiratory pressure (PEEP) in the airways (29), albeit the pressure delivered by HFNC devices to the distal airways is difficult to measure. Esophageal manometry is considered the standard for detecting pleural pressure in spontaneously breathing patients (30). This technique may be feasible in adults, but difficult to adopt in children (31). As such, measurements are often obtained in the experimental settings (29) by various indirect methods including electrical impedance tomography on the surface of the chest (32) or electrical activity of the diaphragm (33). In addition, the generation of PEEP is linearly dependent on the amount of flow and is influenced by the weight of the patient, the size of the cannula and nostrils and the degree of mouth opening (34). Therefore, all these factors combined could influence results consistency. The few studies performed measuring pressure in the pharynx and esophagus reported that a limited PEEP of 2–4 cm H2O was generated by HFNC in children (35, 36).
The work of breathing (WOB) is the energy expended by the respiratory muscles to perform their activity. Theoretically, lowering the respiratory rate and improving thoraco-abdominal coordination should reduce WOB. The PEEP generated by high flows has been considered one of the factors contributing to reducing WOB, via matching intrinsic PEEP and increasing alveolar recruitment (33, 37). This concept has been challenged by a recent study from Guglielmo and co-workers. In their trial, HFNC applied to 22 children with bronchiolitis reduced breathing effort, without a consistent increase in end-expiratory lung volume and no significant change in tidal volume or transpulmonary pressure, raising the hypothesis that PEEP application is not the primary HFNC mechanism for reducing WOB in bronchiolitis (38).
HFNC also provides a washout of the “anatomical dead space,” namely the volume of air located in the first third of the respiratory tract which does not take part in the gas exchange process. Clearance of carbon dioxide (CO2) from nasopharyngeal dead space can also affect the WOB, by producing a more efficient ventilation (39–41). The only in vivo study designed specifically to provide insight into the mechanism of dead space washout was performed on adult volunteers using tracer gas. It showed effective clearance in the upper airways directly related to the HFNC flow rate and time, with subsequently a reduction in rebreathing of expired air, which could result in improvement of alveolar ventilation and gas exchange (40). To some extent, this mechanism might contribute to the HFNC effectiveness in treating sleep breathing disorders in children. Indeed, reducing CO2 levels has the potential to improve breathing patterns and correct apnea and hypopnea (42). Duong et al. showed that flows delivered at 20 L/min to 4–8-year-old child airway replicas reduced end-tidal carbon dioxide from baseline values, whereas delivery of CPAP through a sealed nasal mask increased end-tidal carbon dioxide from baseline values (43). Thus, further in vivo studies investigating HFNC therapy as an alternative to CPAP therapy for treating OSA, should consider potential beneficial effects of improved gas washout when administering HFNC distinctly from its use to produce PEEP.
Reduction of Inspiratory Resistance and Gas Conditioning
The nasopharynx facilitates humidification and warming of the inhaled gas by contact with its large mucosal surface area, but at the same time the passage of air through this anatomical region causes an increase in inspiratory resistance. HFNC minimizes this resistance by providing nasopharyngeal gas flows via nasal prongs that match or exceed a patient's peak inspiratory flow, with a positive effect on WOB (42).
Non-warmed, non-humidified air can have a detrimental impact on child upper and lower airways. First, Greenspan et al. demonstrated that respiration, even for a short time, of not warmed or humidified air resulted in a significant decrease in both pulmonary compliance and conductance in ventilated infants (44). Fontanari et al. provided a physiological explanation of this phenomenon in their study. They showed that receptors in the nasal mucosa respond to cold and dry gas to elicit a protective bronchoconstrictor response in both normal subjects (45) and asthmatics (46) mediated by the cholinergic system (47). The beneficial effects of warm, humidified air have been demonstrated in vivo in infants (48). In fact, although HFNC provided lower PEEP than CPAP, the pulmonary compliance was higher, corroborating the hypothesis that conditioning of respiratory gas could have an impact on the lung, particularly useful in case of asthma attack, wherein the inhalation of warm air could reduce bronchoconstriction.
Optimization of Mucociliary Clearance
In muco-obstructive lung diseases, delivery of heated flows at core temperature and water vapor saturation may improve airway clearance. An in vitro study has shown that inspired gas with low humidity even for short periods could impair the function of human airway epithelial cells (49). Furthermore, air temperature is also crucial for optimal cilia movement, which occurs at 37°C (50). HFNC devices can deliver flow gasses at 100% humidity and core temperature, features particularly advantageous in hypersecretory states requiring an optimization of airway clearance, such as bronchiolitis, cystic fibrosis and bronchiectasis. At the moment, data on the effect of HFNC on such conditions are lacking. Only 2 case reports have been published so far, both showing that long-term home HFNC reduced atelectasis, hospitalization frequency and improved mucus drainage in post-acute bronchiolitis and CHARGE syndrome (51, 52).
Clinical Applications
Bronchiolitis
Acute bronchiolitis is one of the most frequent diseases in children under 2 years (53). Multiple microorganisms are responsible for its clinical manifestations, but respiratory syncytial virus is by far the most common (53). In recent years, the use of HFNC has progressively gained popularity over the current standard of care with COT, especially in case of moderate-to-severe acute bronchiolitis. Indeed, this nosological entity represents the main indication for HFNC in patients older than neonates.
Two large clinical trials benchmarked the HFNC vs. COT (6, 7). Both reported a lower treatment failure rate, defined as escalation of care during that hospital admission, in the HFNC group. However, they showed no differences in duration of hospital stay, duration of oxygen therapy, or PICU admission in comparison with COT. A recent systematic review by Lin et al. likened the effectiveness of HFNC, CPAP and COT in acute bronchiolitis (54). They found that HFNC and CPAP were both superior to COT, but treatment failure events were significantly more frequent in the HFNC group when compared to the CPAP group. However, the authors included patients with any degree of bronchiolitis severity, without performing a subgroup analysis in children with moderate-to-severe bronchiolitis. This limitation was addressed by Catano-Jaramillo and colleagues in their meta-analysis (55). They showed that both CPAP and HFNC reduced the risk for intubation, but a lower rate of therapeutic failures was found with CPAP, confirming the previous results also in this cluster of patients. Noteworthy, despite being superior to HFNC, CPAP produced more adverse events, such as skin lesions, and was less tolerated. Data available indicate that HFNC being superior to COT, despite inferior to CPAP, could play a role in the rescue therapy for children with moderate-to-severe bronchiolitis because of its ease of use and safety.
Asthma
Due to its beneficial effects on the respiratory system, HFNC treatment may reduce the WOB during asthma exacerbations. Furthermore, the use of heated and humidified gas limits the bronchoconstriction induced by cold dry gas and improves airways cilia movement, contributing to mobilization of mucus plugs, hallmark of acute asthma attacks (56). To date, few reports explored the use of HFNC during asthma exacerbation. Two retrospective studies (57, 58) showed that treatment with HFNC improved heart rate, respiratory rate, SpO2/FiO2 ratio, pH level, and CO2 tension after 3–24 h compared to COT. These findings were confirmed by a prospective pilot trial by Ballestero et al. (59), in which 62 children (1–14 years) with moderate-to-severe asthma exacerbations were randomly assigned to HFNC or COT. Two hours after treatment, 53% of the children in the HFNC group demonstrated a decreased pulmonary score by at least 2 points vs. 28% in the COT group (p = 0.01). However, no between-group differences were observed in terms of PICUs admission and hospital length of stay. Pilar et al. (60) compared the efficacy of HFNC vs. NIV in a retrospective analysis. Twenty children received HFNC and eight of them escalated to NIV whilst 22 received NIV without treatment failure (p < 0.001). These authors suggested cautions when using HFNC over NIV, since it could potentially delay the initiation of NIV, resulting in longer periods of respiratory support and hospital stay.
Respiratory Support in Case of Congenital Heart Diseases
It is widely known that high PEEP values impede venous return and increases central venous pressure (61). In contrast to CPAP, HFNC supports respiration generating minimal PEEP values (29), and therefore its effect on central venous pressure is negligible (42). This mechanism may be of particular interest for respiratory support in patients with delicate hemodynamic balance, in whom a high PEEP could exerts deleterious effects. In a randomized controlled trial (RCT) in pediatric patients with congenital heart disease undergoing procedural sedation, the use of HFNC compared with COT, reduced the incidence of desaturation, the need for NIV and the risk of CO2 retention without causing hemodynamic instability (62). A case report on a 10-years old patient with Fontan circulation showed that in comparison to COT, HFNC reduced heart rate, systemic vascular resistance, pulmonary vascular resistance, increased cardiac output and improved cerebral circulation, measured by near-infrared spectroscopy (63). These effects were likely due to optimal oxygenation achieved without an increase in central venous pressure which helped to suppress adrenergic activity. Naohiro et al. conducted a retrospective study (64) on HFNC versus NIV for acute respiratory failure after cardiac surgery in children with inborn cardiac defects. The reintubation rate within 28 days was significantly lower in the HFNC group (3 vs. 26%, p = 0.04). Furthermore, the HFNC group's PICU stays were significantly shorter than those of the NIV group (10 days vs. 17 days, p = 0.009).
Obstructive Sleep Apnea
Obstructive sleep apnea (OSA) is the result of upper airway obstruction during sleep (65, 66). Children with OSA are at increased risk for neurocognitive and cardiovascular conditions (67, 68). The current treatment options for OSA in children include adenotonsillectomy, when applicable, and CPAP (69) with the latter often impeded by limited adherence (70, 71). Already in 2009 Brian McGinley and colleagues delivered high flows (20 L/min) to 12 children with mild-to-severe OSA, showing that the reduction in the apnea-hypopnea index on HFNC was comparable to that on CPAP (72). More recently, in two observational studies (73, 74) conducted in CPAP-intolerant children with moderate-to-severe OSA, HFNC reduced nocturnal respiratory events and improved oxygen saturation. In a case series (75), long-term home HFNC was successful in treating five children with severe OSA. Despite this limited evidence, HFNC might be considered as a rescue option in children not compliant to CPAP treatment. However, RCTs comparing CPAP to HFNC are warranted to provide definitive results.
Pneumonia
The HFNC role in the management of children with acute respiratory failure due to pneumonia includes two RCTs (76, 77). In the first one (76), there was no difference in treatment failure between bubble CPAP and HFNC, but the study was stopped prematurely because of the high mortality rate in the COT group. Later, Cong Liu and co-workers (77) evaluated 84 children under 2 years of age in a RCT on HFNC vs. CPAP in the management of mild-to-moderate respiratory failure due to pneumonia. No differences were observed in terms of treatment failure necessitating intubation and transfer to the PICU, duration of hospital stay, non-invasive respiratory support and mortality. In addition, the HFNC group had a lower level of nasal injury, abdominal distension and better tolerance. However, since low PaO2/FiO2 ratio was associated with HFNC failure, the authors were cautious suggesting that HFNC should be considered as an intermediate level of respiratory support between COT and NIV.
Future Potential Use of the HFNC
Bronchiectasis and cystic fibrosis are associated with a chronic mucus secretion. For these conditions improving the muco-ciliary clearance is pivotal in order to prevent recurrent infections and therefore to preserve long-term function. The use of HFNC is particularly promising in the management of these conditions (78) for the aforementioned beneficial effects of humidified and heated gas flows on the airway cilia. To date, no studies have been published so far on pediatric patients with cystic fibrosis and only two case reports evaluated the effectiveness of long-term home HFNC in children with bronchiectasis with reduction in the frequency of pulmonary infection (51, 52).
Interhospital transport is a delicate moment for an ill child. Clinical deterioration following interhospital transport accounts for 30% of entire PICU admission, and is associated with increased rate of invasive ventilation use and prolonged PICU stay (79). An Australian study published in 2021 (80) reported that the implementation of HFNC on interhospital transport was associated with reduced PICU length of stay and respiratory support use, thus supporting its employment in this setting.
Further case reports have also described the effects of HFNC in children with acute pulmonary edema (81) and a pediatric burn patient with post extubation stridor (82).
Conclusion
The HFNC is a relatively safe and well-tolerated respiratory support suitable to a broad range of hospital and domiciliary settings. Several physiological mechanisms are responsible for its effectiveness. Studies published so far support their superiority over COT in almost every condition, with stronger evidence for rescue therapy for acute bronchiolitis. Notwithstanding, better designed and controlled studies are required to define the role of HFNC vs. CPAP and NIV, in order to better understand the predictors of non-response and avoid respiratory support escalation delay.
Author Contributions
All authors made substantial, direct, and intellectual contributions to the work and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CO2, carbon dioxide; COT, conventional oxygen therapy; CPAP, continuous positive airway pressure; FiO2, fraction of inhaled oxygen; HFNC, high-flow nasal cannula; iNO, inhaled nitric oxide; NIV, non-invasive ventilation; OSA, obstructive sleep apnea; PEEP, positive end-expiratory pressure; PICU, pediatric intensive care unit; RCT, randomized control trial; WOB, work of breathing.
References
1. Sreenan C, Lemke RP, Hudson-Mason A, Osiovich H. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics. (2001) 107:1081–3. doi: 10.1542/peds.107.5.1081
2. Mikalsen IB, Davis P, Øymar K. High flow nasal cannula in children: a literature review. Scand J Trauma Resusc Emerg Med. (2016) 24:93. doi: 10.1186/s13049-016-0278-4
3. Slain KN, Shein SL, Rotta AT. The use of high-flow nasal cannula in the pediatric emergency department. J Pediatr. (2017) 93(Suppl 1):36–45. doi: 10.1016/j.jped.2017.06.006
4. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. (2014) 2014:CD009850. doi: 10.1002/14651858.CD009850.pub2
5. Manley BJ, Owen L, Doyle LW, Davis PG. High-flow nasal cannulae and nasal continuous positive airway pressure use in non-tertiary special care nurseries in Australia and New Zealand: Non-tertiary high-flow nasal cannulae and CPAP use. J Paediatr Child Health. (2012) 48:16–21. doi: 10.1111/j.1440-1754.2011.02186.x
6. Kepreotes E, Whitehead B, Attia J, Oldmeadow C, Collison A, Searles A, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. (2017) 389:930–9. doi: 10.1016/S0140-6736(17)30061-2
7. Franklin D, Babl FE, Schlapbach LJ, Oakley E, Craig S, Neutze J, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. (2018) 378:1121–31. doi: 10.1056/NEJMoa1714855
8. Milési C, Pierre A-F, Deho A, Pouyau R, Liet J-M, Guillot C, et al. A multicenter randomized controlled trial of a 3-L/kg/min versus 2-L/kg/min high-flow nasal cannula flow rate in young infants with severe viral bronchiolitis (TRAMONTANE 2). Intensive Care Med. (2018) 44:1870–8. doi: 10.1007/s00134-018-5343-1
9. Ari A. Aerosol drug delivery through high flow nasal cannula. Curr Pharm Biotechnol. (2017) 18:877–82. doi: 10.2174/1389201019666171219104217
10. Croce C, Fodil R, Durand M, Sbirlea-Apiou G, Caillibotte G, Papon J-F, et al. In vitro experiments and numerical simulations of airflow in realistic nasal airway geometry. Ann Biomed Eng. (2006) 34:997–1007. doi: 10.1007/s10439-006-9094-8
11. Human respiratory tract model for radiological protection. A report of a Task Group of the International Commission on Radiological Protection. Ann ICRP. (1994) 24:1–482.
12. Amirav I, Borojeni AAT, Halamish A, Newhouse MT, Golshahi L. Nasal versus oral aerosol delivery to the “lungs” in infants and toddlers. Pediatr Pulmonol. (2014) 50:276–83. doi: 10.1002/ppul.22999
13. Laube BL, Adams GK 3rd, Norman PS, Rosenthal RR. The effect of inspiratory flow rate regulation on nebulizer output and on human airway response to methacholine aerosol. J Allergy Clin Immunol. (1985) 76:708–13. doi: 10.1016/0091-6749(85)90675-X
14. Brand P, Friemel I, Meyer T, Schulz H, Heyder J, Häubetainger K. Total deposition of therapeutic particles during spontaneous and controlled inhalations. J Pharm Sci. (2000) 89:724–31.
15. Dailey PA, Harwood R, Walsh K, Fink JB, Thayer T, Gagnon G, et al. Aerosol delivery through adult high flow nasal cannula with heliox and oxygen. Respir Care. (2017) 62:1186–92. doi: 10.4187/respcare.05127
16. Re'miniac F, Vecellio L, Heuze-Vourc'h N, Petitcollin A, Respaud R, Cabrera M, et al. Aerosol therapy in adults receiving high flow nasal cannula oxygen therapy. J Aerosol Med Pulm Drug Deliv. (2016) 29:134–41. doi: 10.1089/jamp.2015.1219
17. Réminiac F, Vecellio L, Loughlin RM, Le Pennec D, Cabrera M, Vourc'h NH, et al. Nasal high flow nebulization in infants and toddlers: An in vitro and in vivo scintigraphic study. Pediatr Pulmonol. (2017) 52:337–44. doi: 10.1002/ppul.23509
18. Valencia-Ramos J, Mirás A, Cilla A, Ochoa C, Arnaez J. Incorporating a nebulizer system into high-flow nasal cannula improves comfort in infants with bronchiolitis. Respir Care. (2018) 63:886–93. doi: 10.4187/respcare.05880
19. Morgan SE, Mosakowski S, Solano P, Hall JB, Tung A. High-flow nasal cannula and aerosolized β agonists for rescue therapy in children with bronchiolitis: a case series. Respir Care. (2015) 60:e161–5. doi: 10.4187/respcare.03996
20. Gates RM, Haynes KE, Rehder KJ, Zimmerman KO, Rotta AT, Miller AG. High-flow nasal cannula in pediatric critical asthma. Respir Care. (2021) 66:1240–6. doi: 10.4187/respcare.08740
21. Zainudin BM, Biddiscombe M, Tolfree SE, Short M, Spiro SG. Comparison of bronchodilator responses and deposition patterns of salbutamol inhaled from a pressurised metered dose inhaler, as a dry powder, and as a nebulised solution. Thorax. (1990) 45:469–73. doi: 10.1136/thx.45.6.469
22. Wagstaff TAJ, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates: oxygen delivery system performance. Anaesthesia. (2007) 62:492–503. doi: 10.1111/j.1365-2044.2007.05026.x
23. Renda T, Corrado A, Iskandar G, Pelaia G, Abdalla K, Navalesi P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br J Anaesth. (2018) 120:18–27. doi: 10.1016/j.bja.2017.11.010
24. Sim MAB, Dean P, Kinsella J, Black R, Carter R, Hughes M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated: performance of oxygen delivery devices during simulated respiratory failure. Anaesthesia. (2008) 63:938–40. doi: 10.1111/j.1365-2044.2008.05536.x
25. Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. (2011) 39:1103–10. doi: 10.1177/0310057X1103900620
26. Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. (2005) 50:604–9.
27. Tominaga Y, Iwai S, Yamauchi S, Kyogoku M, Kugo Y, Hasegawa M, et al. Post-extubation inhaled nitric oxide therapy via high-flow nasal cannula after Fontan procedure. Pediatr Cardiol. (2019) 40:1064–71. doi: 10.1007/s00246-019-02122-2
28. Ismail A, Sharara-Chami R, El-Khatib M. Combination of high-flow nasal cannula oxygen therapy and inhaled nitric oxide in a paediatric patient with respiratory distress. Anaesth Intensive Care. (2014) 42:521–3.
29. Ejiofor BD, Carroll RW, Bortcosh W, Kacmarek RM. PEEP generated by high-flow nasal cannula in a pediatric model. Respir Care. (2019) 64:1240–9. doi: 10.4187/respcare.06470
30. Pham T, Telias I, Beitler JR. Esophageal manometry. Respir Care. (2020) 65:772–92. doi: 10.4187/respcare.07425
31. Rubin S, Ghuman A, Deakers T, Khemani R, Ross P, Newth CJ. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. (2014) 15:1–6. doi: 10.1097/PCC.0000000000000011
32. Hough JL, Pham TMT, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. (2014) 15:e214–9. doi: 10.1097/PCC.0000000000000112
33. Pham TMT, O'Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. (2015) 50:713–20. doi: 10.1002/ppul.23060
34. Sivieri EM, Gerdes JS, Abbasi S. Effect of HFNC flow rate, cannula size, and nares diameter on generated airway pressures: an in vitro study: HFNC flow rate, cannula size, nares diameter on airway pressures. Pediatr Pulmonol. (2013) 48:506–14. doi: 10.1002/ppul.22636
35. Arora B, Mahajan P, Zidan MA, Sethuraman U. Nasopharyngeal airway pressures in bronchiolitis patients treated with high-flow nasal cannula oxygen therapy. Pediatr Emerg Care. (2012) 28:1179–84. doi: 10.1097/PEC.0b013e318271a671
36. Milési C, Baleine J, Matecki S, Durand S, Combes C, Novais ARB, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? a physiologic study intensive. Care Med. (2013) 39:1088–94. doi: 10.1007/s00134-013-2879-y
37. Lavizzari A, Veneroni C, Colnaghi M, Ciuffini F, Zannin E, Fumagalli M, et al. Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Arch Dis Child Fetal Neonatal Ed. (2014) 99:F315–20. doi: 10.1136/archdischild-2013-305855
38. Guglielmo RD, Hotz JC, Ross PA, Deakers TW, Diep JEL, Newth CJL, et al. High-flow nasal cannula reduces effort of breathing but not consistently via positive end-expiratory pressure. Chest. (2022). doi: 10.1016/j.chest.2022.03.008
39. Spicuzza L, Schisano M. High-flow nasal cannula oxygen therapy as an emerging option for respiratory failure: the present and the future. Ther Adv Chronic Dis. (2020) 11:2040622320920106. doi: 10.1177/2040622320920106
40. Möller W, Feng S, Domanski U, Franke K-J, Celik G, Bartenstein P, et al. Nasal high flow reduces dead space. J Appl Physiol. (2017) 122:191–7. doi: 10.1152/japplphysiol.00584.2016
41. Onodera Y, Akimoto R, Suzuki H, Okada M, Nakane M, Kawamae K, et al. A high-flow nasal cannula system with relatively low flow effectively washes out CO2 from the anatomical dead space in a sophisticated respiratory model made by a 3D printer. Intensive Care Med Exp. (2018) 6:7. doi: 10.1186/s40635-018-0172-7
42. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. (2009) 103:1400–5. doi: 10.1016/j.rmed.2009.04.007
43. Duong K, Noga M, MacLean JE, Finlay WH, Martin AR. Comparison of airway pressures and expired gas washout for nasal high flow versus CPAP in child airway replicas. Respir Res. (2021) 22:289. doi: 10.1186/s12931-021-01880-z
44. Greenspan JS, Wolfson MR, Shaffer TH. Airway responsiveness to low inspired gas temperature in preterm neonates. J Pediatr. (1991) 118:443–5. doi: 10.1016/S0022-3476(05)82165-1
45. Fontanari P, Burnet H, Zattara-Hartmann MC, Jammes Y. Changes in airway resistance induced by nasal inhalation of cold dry, dry, or moist air in normal individuals. J Appl Physiol. (1996) 81:1739–43. doi: 10.1152/jappl.1996.81.4.1739
46. Fontanari P, Zattara-Hartmann MC, Burnet H, Jammes Y. Nasal eupnoeic inhalation of cold, dry air increases airway resistance in asthmatic patients. Eur Respir J. (1997) 10:2250–4. doi: 10.1183/09031936.97.10102250
47. On On LS, Boonyongsunchai P, Webb S, Davies L, Calverley PM, Costello RW. Function of pulmonary neuronal M(2) muscarinic receptors in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2001) 163:1320–5. doi: 10.1164/ajrccm.163.6.2002129
48. Saslow JG, Aghai ZH, Nakhla TA, Hart JJ, Lawrysh R, Stahl GE, et al. Work of breathing using high-flow nasal cannula in preterm infants. J Perinatol. (2006) 26:476–80. doi: 10.1038/sj.jp.7211530
49. Kilgour E, Rankin N, Ryan S, Pack R. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med. (2004) 30:1491–4. doi: 10.1007/s00134-004-2235-3
50. Chidekel A, Zhu Y, Wang J, Mosko JJ, Rodriguez E, Shaffer TH. The effects of gas humidification with high-flow nasal cannula on cultured human airway epithelial cells. Pulm Med. (2012) 2012:380686. doi: 10.1155/2012/380686
51. Sato A, Hamada S, Ishigami T. Effect of long-term domiciliary high-flow nasal cannula use in a child with atypical CHARGE syndrome. Arch Bronconeumol. (2019) 55:219–20. doi: 10.1016/j.arbres.2018.06.010
52. Singh D, Rajbanshi A, Giri PP. A case of post adenoviral bronchiectasis being managed at home with humidified high flow nasal cannula (HHFNC). Respir Med Case Rep. (2020) 31:101233. doi: 10.1016/j.rmcr.2020.101233
53. Ralston SL, Lieberthal AS, Meissner HC, Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. (2014) 134:e1474–e1502. Pediatrics. (2015) 136:782. doi: 10.1542/peds.2015-2862
54. Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. (2019) 104:564–76. doi: 10.1136/archdischild-2018-315846
55. Cataño-Jaramillo ML, Jaramillo-Bustamante JC, Florez ID. Continuous positive airway pressure vs. high flow nasal cannula in children with acute severe or moderate bronchiolitis a systematic review and meta-analysis. Med Intensiva. (2022) 46:72–80. doi: 10.1016/j.medin.2020.09.008
56. Dunican EM, Watchorn DC, Fahy JV. Autopsy and imaging studies of mucus in asthma. Lessons learned about disease mechanisms and the role of mucus in airflow obstruction. Ann Am Thorac Soc. (2018) 15:S184–91. doi: 10.1513/AnnalsATS.201807-485AW
57. Baudin F, Buisson A, Vanel B, Massenavette B, Pouyau R, Javouhey E. Nasal high flow in management of children with status asthmaticus: a retrospective observational study. Ann Intensive Care. (2017) 7:55. doi: 10.1186/s13613-017-0278-1
58. Martínez G, Sánchez G, Del Castillo T, Moreno P, Muñoz M, Jiménez R. Treatment with high-flow oxygen therapy in asthma exacerbations in a paediatric hospital ward: experience from. An Pediatr. (2012) 90:72–8. doi: 10.1016/j.anpede.2018.06.008
59. Ballestero Y, De Pedro J, Portillo N, Martinez-Mugica O, Arana-Arri E, Benito J. Pilot clinical trial of high-flow oxygen therapy in children with asthma in the emergency service. J Pediatr. (2018) 194:204–10.e3. doi: 10.1016/j.jpeds.2017.10.075
60. Pilar J, Modesto I, Alapont V, Lopez-Fernandez YM, Lopez-Macias O, Garcia-Urabayen D, et al. High-flow nasal cannula therapy versus non-invasive ventilation in children with severe acute asthma exacerbation: an observational cohort study. Med Intensiva. (2017) 41:418–24. doi: 10.1016/j.medin.2017.01.001
61. Hong SH, Choi JH, Lee J. The changes of central venous pressure by body posture and positive end-expiratory pressure. Korean J Anesthesiol. (2009) 57:723–8. doi: 10.4097/kjae.2009.57.6.723
62. Duan X, Wei N, Wei J, Zhu Y, Kang Y, He Y, et al. Effect of high-flow nasal cannula oxygen therapy on pediatric patients with congenital heart disease in procedural sedation: a prospective, randomized trial. J Cardiothorac Vasc Anesth. (2021) 35:2913–9. doi: 10.1053/j.jvca.2021.03.031
63. Kuwata S, Kurishima C, Kim J, Iwamoto Y, Saiki H, Ishido H, et al. Clinical evaluation of the hemodynamic effects of the high-flow nasal cannula therapy on the Fontan circulation. Clin Med Insights Cardiol. (2015) 9:109–11. doi: 10.4137/CMC.S26137
64. Shioji N, Kanazawa T, Iwasaki T, Shimizu K, Suemori T, Kuroe Y, et al. High-flow nasal cannula versus noninvasive ventilation for postextubation acute respiratory failure after pediatric cardiac surgery. Acta Med Okayama. (2019) 73:15–20. doi: 10.18926/AMO/56454
65. Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossus activity during sleep in normal children. Am J Respir Crit Care Med. (2006) 173:902–9. doi: 10.1164/rccm.200509-1450OC
66. Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. (1996) 153:866–78. doi: 10.1164/ajrccm.153.2.8564147
67. Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. (2010) 33:1447–56. doi: 10.1093/sleep/33.11.1447
68. Ehsan Z, Ishman SL, Kimball TR, Zhang N, Zou Y, Amin RS. Longitudinal cardiovascular outcomes of sleep disordered breathing in children: a meta-analysis and systematic review. Sleep. (2017) 40:zsx015. doi: 10.1093/sleep/zsx015
69. Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. (2012) 130:e714–55. doi: 10.1542/peds.2012-1672
70. Hawkins SMM, Jensen EL, Simon SL, Friedman NR. Correlates of pediatric CPAP adherence. J Clin Sleep Med. (2016) 12:879–84. doi: 10.5664/jcsm.5892
71. Marcus CL, Rosen G, Ward SLD, Halbower AC, Sterni L, Lutz J, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. (2006) 117:e442–51. doi: 10.1542/peds.2005-1634
72. McGinley B, Halbower A, Schwartz AR, Smith PL, Patil SP, Schneider H. Effect of a high-flow open nasal cannula system on obstructive sleep apnea in children. Pediatrics. (2009) 124:179–88. doi: 10.1542/peds.2008-2824
73. Ignatiuk D, Schaer B, McGinley B. High flow nasal cannula treatment for obstructive sleep apnea in infants and young children. Pediatr Pulmonol. (2020) 55:2791–8. doi: 10.1002/ppul.25009
74. Hawkins S, Huston S, Campbell K, Halbower A. High-flow, heated, humidified air via nasal cannula treats CPAP-intolerant children with obstructive sleep apnea. J Clin Sleep Med. (2017) 13:981–9. doi: 10.5664/jcsm.6700
75. Joseph L, Goldberg S, Shitrit M, Picard E. High-flow nasal cannula therapy for obstructive sleep apnea in children. J Clin Sleep Med. (2015) 11:1007–10. doi: 10.5664/jcsm.5014
76. Chisti MJ, Salam MA, Smith JH, Ahmed T, Pietroni MAC, Shahunja KM, et al. Bubble continuous positive airway pressure for children with severe pneumonia and hypoxaemia in Bangladesh: an open, randomised controlled trial. Lancet. (2015) 386:1057–65. doi: 10.1016/S0140-6736(15)60249-5
77. Liu C, Cheng WY Li JS, Tang T, Tan PL, Yang L. High-flow nasal cannula vs. continuous positive airway pressure therapy for the treatment of children <2 years with mild to moderate respiratory failure due to pneumonia. Front Pediatr. (2020) 8:590906. doi: 10.3389/fped.2020.590906
78. Hasani A, Chapman TH, McCool D, Smith RE, Dilworth JP, Agnew JE. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. (2008) 5:81–6. doi: 10.1177/1479972307087190
79. Moynihan K, McSharry B, Reed P, Buckley D. Impact of retrieval, distance traveled, and referral center on outcomes in unplanned admissions to a national PICU. Pediatr Crit Care Med. (2016) 17:e34–42. doi: 10.1097/PCC.0000000000000586
80. Miura S, Yamaoka K, Miyata S, Butt W, Smith S. Clinical impact of implementing humidified high-flow nasal cannula on interhospital transport among children admitted to a PICU with respiratory distress: a cohort study. Crit Care. (2021) 25:194. doi: 10.1186/s13054-021-03620-7
81. Kumar J, Hegde R, Maheshwari S, Rao S. Flash pulmonary edema in a post arterial switch operation-high flow oxygen as a treatment modality. Ann Pediatr Cardiol. (2009) 2:175–6. doi: 10.4103/0974-2069.58326
Keywords: high-flow nasal cannula (HFNC), oxygen therapy, children, pediatric, respiratory failure, bronchiolitis, respiratory distress
Citation: Nolasco S, Manti S, Leonardi S, Vancheri C and Spicuzza L (2022) High-Flow Nasal Cannula Oxygen Therapy: Physiological Mechanisms and Clinical Applications in Children. Front. Med. 9:920549. doi: 10.3389/fmed.2022.920549
Received: 14 April 2022; Accepted: 09 May 2022;
Published: 03 June 2022.
Edited by:
Jihad Mallat, Cleveland Clinic Abu Dhabi, United Arab EmiratesReviewed by:
Corrado Pelaia, Magna Græcia University, ItalyCopyright © 2022 Nolasco, Manti, Leonardi, Vancheri and Spicuzza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Santi Nolasco, bm9sYXNjb3NAaG90bWFpbC5pdA==
 Santi Nolasco
Santi Nolasco Sara Manti
Sara Manti Salvatore Leonardi
Salvatore Leonardi Carlo Vancheri
Carlo Vancheri Lucia Spicuzza1
Lucia Spicuzza1