
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 29 September 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.920182
This article is part of the Research TopicSoil-Transmitted Helminth Infections from a One Health PerspectiveView all 5 articles
Asthma is a common respiratory disease affecting humans. Helminth parasites, including Toxocara species, have been implicated as predisposing factors of asthma. However, various studies present different findings on asthma-Toxocara association. Herein, we investigated the association of asthma manifestations with Toxocara seropositivity in a case-control setting on 248 participants (147 women and 101 men), with 124 healthy individuals as the control group and 124 patients known to have asthma based on the medical records of asthma clinics of Kerman University of Medical Sciences. Consequently, we presented a scoping review of all previous studies carried out on this topic, summarizing current findings and existing knowledge on this issue. Of 248 participants, 31 (12.5%) were Toxocara-seropositive, of which 19 (15.3%) were in the patient group and 12 (9.7%) in the control group. A significant relationship was found between asthma severity and age in Toxocara-seropositive individuals (P < 0.04). We found no significant relationship between asthma and Toxocara seropositivity. We identified 7,724 related records in three major scientific databases, NCBI PubMed, Scopus, and Google Scholar. The review of the literature showed that there are 80 published articles on asthma-Toxocara relationship with contradictory findings. More than half of the studies were performed in only four countries, namely, Brazil, the Netherlands, the United States, and Iran. The study population in 70% of the studies were children, and few studies investigated asthma-Toxocara association in adults. The most common study designs for investigating the association of asthma and Toxocara seropositivity were cross-sectional (35.0%), case-control (27.5%), and animal experimental (12.5%) studies. This study found no significant relationship between asthma manifestations and toxocariasis in a case-control setting. However, a scoping review of the current literature suggests that further experimental and field longitudinal cohort studies are required to elucidate the nature of asthma-Toxocara interaction in humans.
Asthma, a common inflammatory disease of the respiratory tract, is considered a major public health problem in the adult population as well as a chronic and sometimes life-threatening illness in children, causing negative effects on the socioeconomic status and the quality of life of patients (1). In the past four decades, the prevalence of asthma has increased in all countries. The World Health Organization (WHO) estimated that about 300 million people suffer from the disease worldwide. In addition, the global annual asthma burden is estimated at 250,000 deaths and 15 million disability-adjusted life years (DALYs) (2). Asthma is the most common chronic illness in the pediatric population. In two separate studies, the prevalence of asthma in Iranian adults and children was estimated at 8.9 and 4.4%, respectively (3, 4).
The main etiology of asthma is not completely understood, but there is evidence indicating that asthma is caused by a combination of genetic and environmental factors. Indoor and outdoor allergens, air pollution, cold air, chemical irritants, extreme emotional arousal, and pathogens are among the environmental factors of asthma. However, family history is one major genetic determinant of the disease, with several genes, such as ADAM33, GSTM1, and LTC4S, being implicated (5, 6). Therefore, investigating the nature and significance of risk factors involved in asthma development in adults and children is essential (7).
There is evidence indicating that Toxocara, Anisakis, and Ascaris species are risk factors for asthma in human populations (8). Meta-analysis studies revealed a relationship between the presence of Ascarid geohelminths and asthma (9). Helminthic infection (HI) is capable of stimulating host inflammatory responses against the parasite. Inflammation plays a major role in the pathophysiology of asthma. Inflammatory cells (eosinophils, macrophages, dendritic cells, lymphocytes, etc.) and inflammatory mediators including IL4, IL5, IL9, IL13, IL15, IL16, and IL17A-&F, as well as IgE, are immunologic factors involving in this disease (10). HI in humans is associated with the presence of CD4 T cells, IgE increase, and subsequent production of cytokines, including IL5, IL-4, and IL-13. Some epidemiological and experimental studies suggested that HI, particularly Toxocara spp., promotes the development of asthma (11).
Toxocariasis is one of the neglected tropical diseases with undetermined public health and economic impacts (12). Toxocara canis and Toxocara cati are intestinal nematodes of dogs and cats, which cause human toxocariasis, a cosmopolitan helminthic zoonosis. Human is considered an accidental host that acquires infection by ingestion or inhalation of infective eggs of Toxocara spp. in soil or other contaminated materials (13). Migration of Toxocara larvae into various human body organs can cause many problems, including visceral larva migrans (VLM), ocular larva migrans (OLM) syndrome, and covert toxocariasis (CT) syndrome (14). Associated symptoms of CT in children include cough, pharyngitis, wheezing, pneumonia, and asthma-like symptoms.
According to many studies in the world, the frequency of soil contamination with Toxocara eggs varies in different areas, ranging from 6.6 to 87.1% (15). The rate in Iran has been reported as 21.6% (16, 17). Seroepidemiological studies in different countries indicate the worldwide distribution of toxocariasis. The results of a systematic review in Iran showed a seroprevalence of 15.8% in humans (18).
Many studies examined the association of Toxocara infection with asthma and/or bronchial hyperresponsiveness (BHR) (19, 20). However, conflicting results have been reported from different parts of the world. Several studies indicated a positive correlation between asthma and Toxocara serology, while no significant association has been reported in several other studies (8, 9, 16). The complex interactions of the immune system and hypersensitivity reactions, the hygiene hypothesis, and differences in the genetic makeup and socioeconomic variables can be considered the significant causes of conflicts in the results of studies correlating helminthic infections with autoimmune diseases and asthma (21–25).
In the literature review, the authors noted that most of the studies focused on childhood asthma, and fewer studies have been conducted on the association between asthma and toxocariasis in adult populations (26). Therefore, to elucidate the significance of toxocariasis in the development of asthma and airway allergy, more human and animal studies are required to be conducted on this issue in different geographical areas of the world. In the present study, we investigated the association of allergic asthma manifestations with positive Toxocara serology in southeastern Iran. In addition, we performed a scoping review on previous studies carried out on the association between asthma and toxocariasis, presenting current literature findings and global distribution of knowledge on this issue.
The study protocol was reviewed and approved by the Research Ethics Committees of Kerman University of Medical Sciences (REC code 99000235). The participants in the survey were informed of the main research goals and signed a written consent form before sampling. This research was performed on 248 participants (147 women and 101 men), with 124 healthy individuals as the control group and 124 patients known to have asthma based on the medical records of asthma clinics of Kerman University of Medical Sciences, as well as one private allergy and asthma clinic in the city of Kerman. The participants were clinically evaluated for asthma signs and symptoms by the sub-specialist author (NB) according to the current guidelines on asthma management and prevention (27). The exclusion criteria were allergic conditions other than respiratory allergies and people with occupational lung diseases causing asthma-like pulmonary symptoms. The control group consisted of 124 individuals with no history of asthma and/or allergies, referring to clinical laboratories for routine annual checkups. Participants' data were collected by two investigators through physical examination of the patients and by conducting history and careful interviews with the parents or guardians of the patients. Data were collected by standard pre-coded questionnaires for each participant, including name, age, sex, place of residence, education, and duration of asthma and allergic manifestations (for the patient group). The average age of the participants in the case and control groups was 31.01 and 29.65, respectively (P > 0.05).
According to asthma guidelines, individuals with allergic manifestations were divided into four groups based on the severity of asthma by an asthma sub-specialist (27). The patients were clinically divided into four groups, namely, intermittent, mild persistent, moderate persistent, and severe persistent according to the frequency of symptoms, forced expiratory volume in 1 second (FEV1), and the peak expiratory flow rate (28).
A 5 mL blood sample was collected in a tube without anticoagulant from each participant in the scheme. The serum was separated and stored at −70°C until use. Patient and control samples were serologically evaluated by using a Toxocara canis IgG-ELISA kit (IBL, Hamburg, Germany) with a sensitivity and a specificity of 96.9 and 98.6%, respectively. ELISA was performed according to the manufacturer's instructions. Information from the questionnaire and the result of the serological test were finally entered into MS Excel software.
Data analyses were conducted by SPSS version 22 statistical software. Descriptive statistics were applied to express the data as frequencies and percentages. The main dependent and independent variables include the presence of asthma and Toxocara serology, respectively. For categorical variables, the difference between the groups was tested by using the chi-square test. For continuous variables, Student's t-test or one-way ANOVA was used to examine whether the differences in the mean values of variables between the case and control groups were statistically significant. The p-values < 0.05 were considered statistically significant. Also, the relationship between the prevalence of severity of asthma/allergy and age groups was examined for the studied groups. Further analysis was carried out to map findings of the current literature and the global distribution of knowledge on this topic using MS Excel and ArcGIS software.
We performed a systematic search following the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (29–32). In the current study, principal data sources were obtained from the literature. We searched three major science databases, namely, NCBI PubMed, Scopus, and Google Scholar. Using different combinations of the search terms [“asthma” AND (“toxocara” OR “toxocariasis”)] in title, abstract, or keywords, all the articles published until 8 May 2020 were retrieved and stored in an MS Excel file. Custom search strings were used for each database to reduce the number of irrelevant hits and increase search accuracy. The following inclusion criteria were considered: articles with an English title and articles investigating asthmatic conditions, and Toxocara infection. The articles studying respiratory disorders other than asthma and articles studying other soil-transmitted helminths including Ascaris, Trichuris, and Strongyloides, or hookworm infections were excluded from the study. Also, opinion communications, editorials, perspectives, and letters were excluded. English articles were included in the study. Among non-English articles, only articles with English abstracts were included in the study. If needed, the full texts of non-English articles were translated using Google Translate.
After clearing the original data file, 6,331 of 7,724 studies were selected from. Only articles with the main topic related to the association of asthma and Toxocara infection were included in the study. Titles and abstracts of the studies were checked for relevance to the topic by two persons. Those studies considered relevant by both the researchers were automatically moved to the secondary sorting. A third researcher organized the studies and examined those articles considered relevant by only one of the two researchers. The studies identified as relevant by the third researcher were added to the secondary screening. Following the secondary screening, the full text of the selected documents was examined further to select eligible studies. All relevant studies of the secondary screening process were combined into a single dataset (178 studies) for use in the next stage.
During the next screening stage, the two researchers independently sorted out all the studies related to the initial sorting process using abstracts (if there were no abstracts, sections of results and conclusions were used to determine relevance). In the final sorting process, two researchers independently read the full text of all studies. The studies deemed relevant by the two researchers proceeded to the data extraction process, while studies deemed irrelevant were excluded. Studies with conflicting evaluations were discussed by the three researchers until an agreement was reached. Studies selected from this multi-stage systematic sorting process were included in the data extraction process.
In the final stage, the Excel spreadsheet was filled with data extracted from the eligible studies. For each article, different characteristics, including author name, journal title, year of publication, country, article type, number of citations, age-group, male/female, research methodology (cross-sectional, case-control, cohort, animal experiments, systematic review, and meta-analysis), statistical significance, diagnostic techniques, sample size, and the mean age, were identified.
Serological investigation revealed 31 (12.5%) participants out of 248 as Toxocara-seropositive, of which 19 (15.3%) cases were in the patient group and 12 (9.7%) in the control group. Statistical analysis indicates no significant association between allergic asthma manifestations and Toxocara seropositivity (P = 0.249). Also, there was no significant association between Toxocara serology with different age groups (Table 1). Patients with asthmatic conditions were more likely to be Toxocara-seropositive than the healthy controls (OR = 1.69, 95% CI = 0.78–3.64); however, this was not statistically significant.
A significant relationship was found between the asthma severity and age in Toxocara-seropositive individuals (P < 0.04). It was found that, with increasing age, the severity of asthma manifestations was higher in the patients with positive Toxocara serology (Table 2). Patients with asthma with seronegative findings demonstrated a significant association between education level and severity of allergic manifestations, as the level of education increases, the severity of allergic manifestations decreased (Table 3).
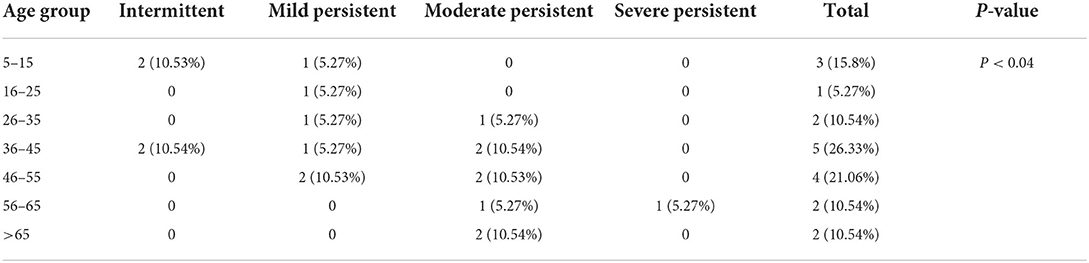
Table 2. Severity of asthma manifestations according to different age groups in individuals with positive Toxocara serology.
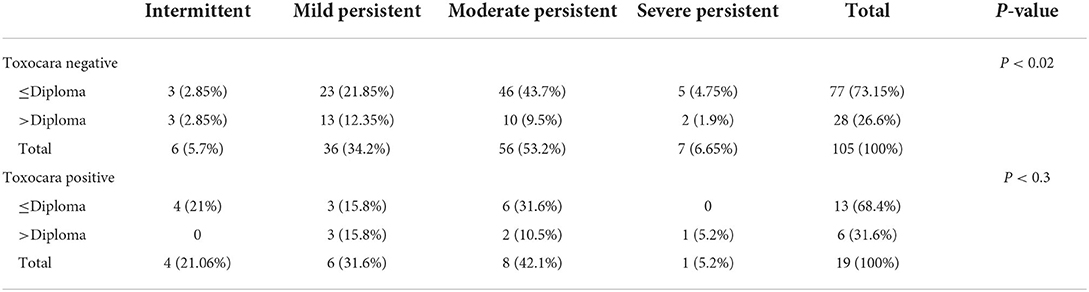
Table 3. Severity of asthma manifestations and education level according to the results of Toxocara serology.
Figure 1 shows the search results of the scoping review, describing the selection process of the records included in this study. In brief, 7,724 records were identified in the three major scientific databases, of which 1,393 studies were duplicate records. A total of 178 studies were selected after removing 6,153 articles not meeting the inclusion criteria according to the titles and/or abstracts. Finally, after reviewing the full texts of the articles, 98 studies did not meet the inclusion criteria, and 80 relevant records were included in the analysis.
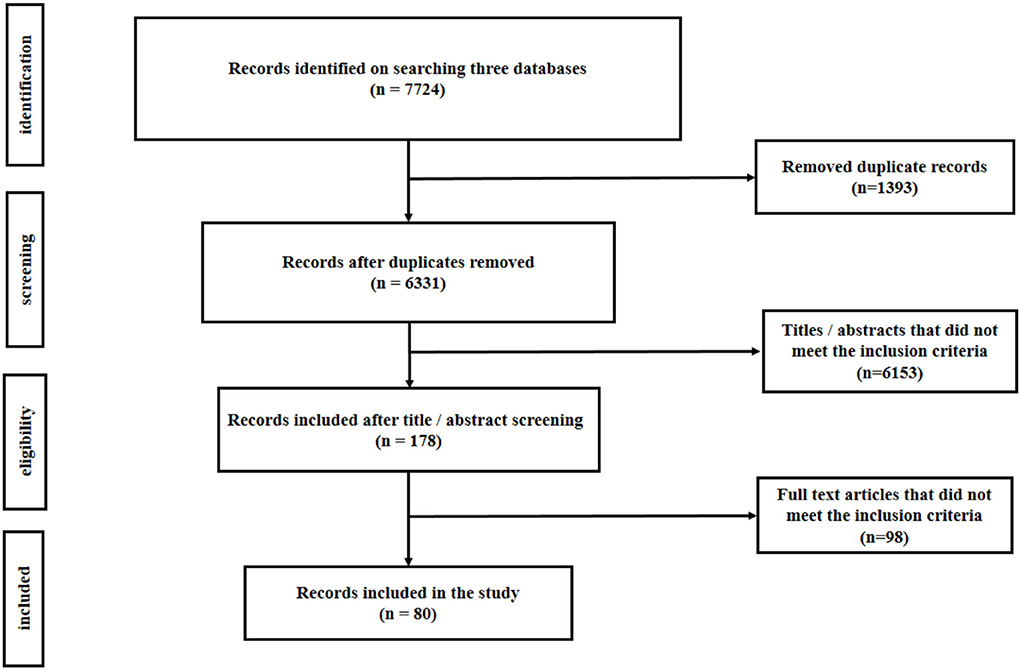
Figure 1. Flowchart showing the number of records retrieved at different stages of literature search.
Figure 2 shows the distribution of asthma-Toxocara articles published according to different geographical locations. Geographically, more than half of the studies were performed in only four countries, namely, Brazil, the Netherlands, the United States, and Iran.
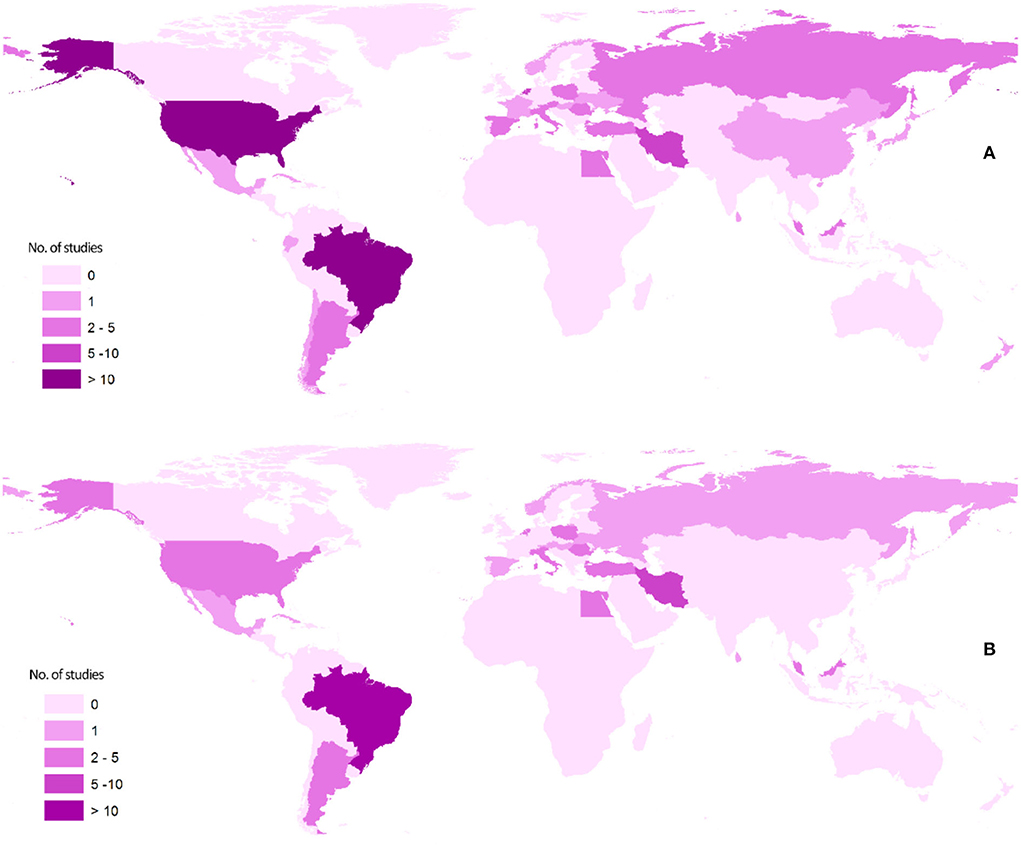
Figure 2. Maps showing the geographical distribution of the literature published on the association of asthma and Toxocara serology: (A) articles of all types and (B) analytical articles, that is, cross-sectional, case-control, and cohort.
The most common study designs for investigating the association of asthma and Toxocara seropositivity were cross-sectional (28/80, 35.0%), case-control (22/80, 27.5%), and animal experimental (10/80, 12.5%) studies. Other types of study designs included cohort studies, case reports, and reviews (Figure 3). It is noteworthy that the study population in 70% of the analytical articles (cross-sectional, case-control, and cohort) was children (Table 4).
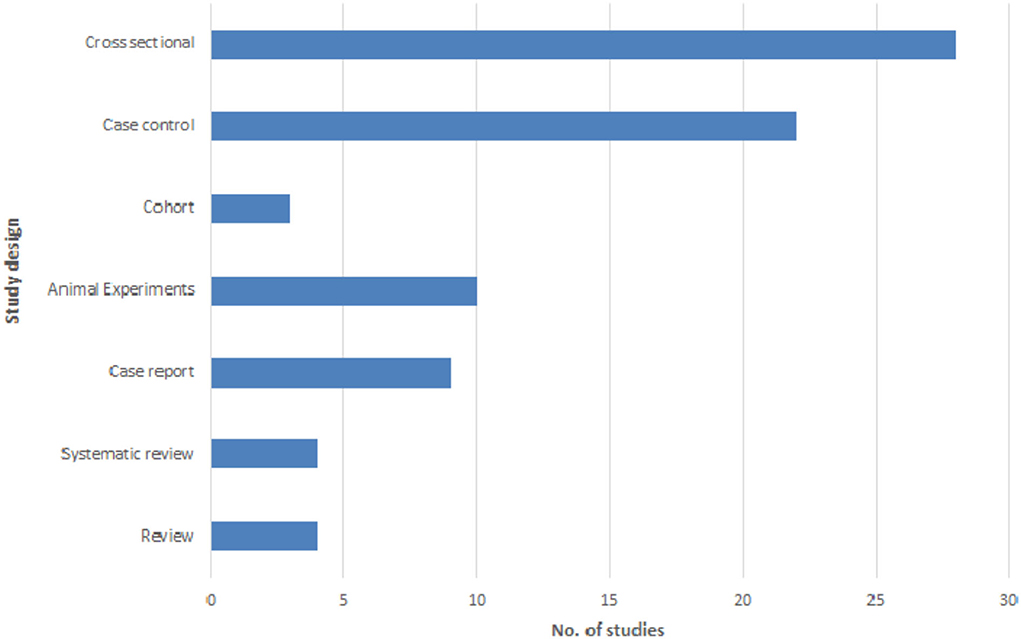
Figure 3. Frequency distribution of the studies investigating the asthma-Toxocara association according to different study designs.
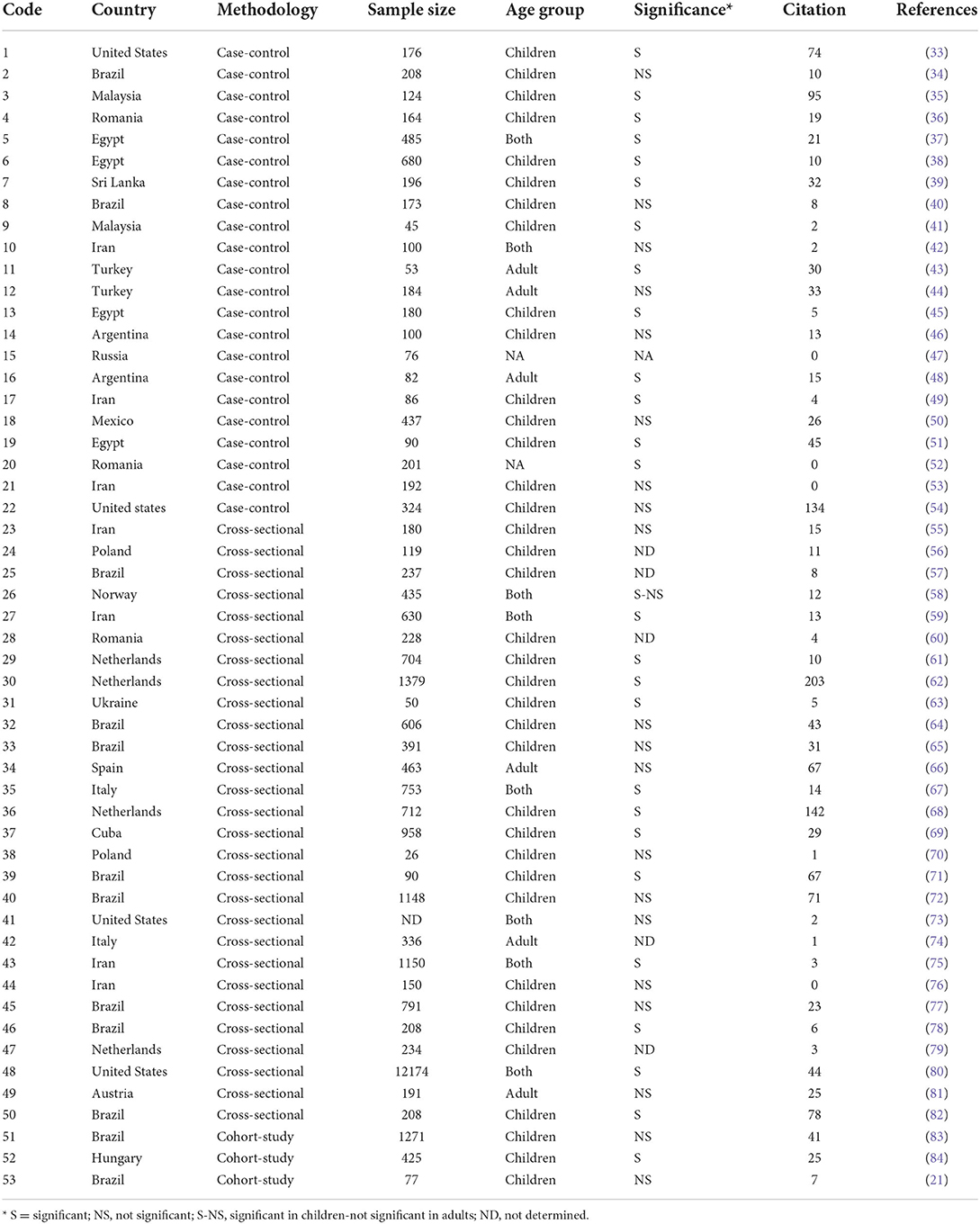
Table 4. Summary of the analytical studies published on the asthma-Toxocara association according to different article features.
Figure 4 shows the time trends of asthma-Toxocara studies from 1972 to 2019. During the past three decades, these studies have been increasingly followed by different research workers around the world, and on average, 2.5 articles on this topic were published annually. The most frequently cited articles were reviews, systematic reviews, and cross-sectional studies, with 74.5, 45.5, and 33.3 citations per article, respectively.
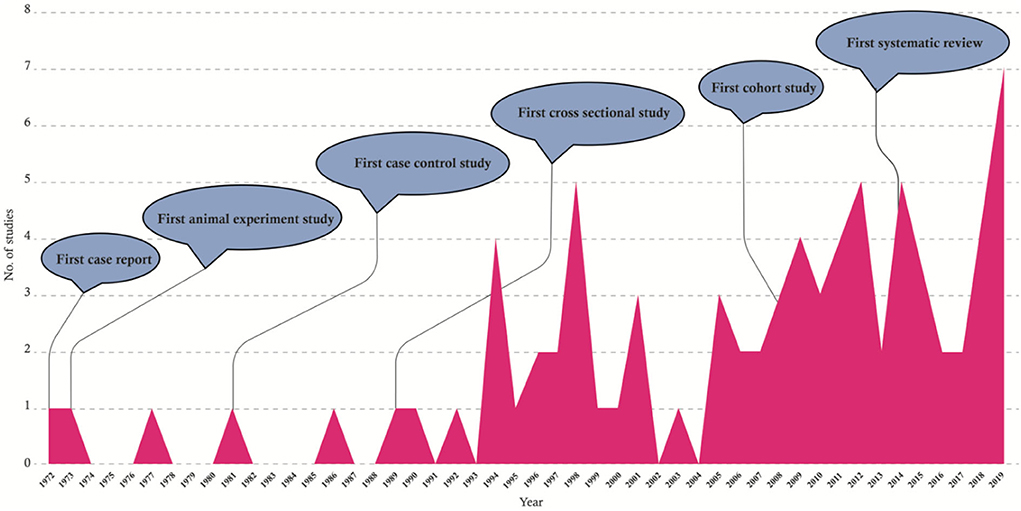
Figure 4. Graphical presentation of the time trends of the studies published on the asthma-Toxocara association from 1972 to 2019.
The main purpose of our study was to investigate the probable association between asthma and Toxocara infection through a case-control study and a subsequent scoping review of the current literature examining the published evidence of the association between asthma and Toxocara seropositivity. Asthma is a chronic disease affecting children and adults worldwide, and a variety of environmental and genetic factors have been implicated in the development of the disease. House dust mite exposure, upper respiratory viral infections, and air pollution are among the probable environmental factors affecting asthma. Helminth infections are considered among the environmental factors associated with the development of asthma. In our study, although the likelihood of being Toxocara-seropositive in patients with asthmatic conditions was roughly more than that of the healthy controls, this presents no statistically significant association between Toxocara serology and asthma symptoms in this case-control setting. There are conflicting findings in the literature regarding the relationship between helminths and asthma. Several uncontrollable confounding factors can be implicated in this kind of study, including differences in diet and the genetic structure of the populations in various studies, other concurrent infections, and environmental pollutants. Also, different species of helminths demonstrated different associations with asthma and other allergic diseases. Some studies showed that anti-helminthic drugs for Ascaris lumbricoides are capable of reducing the symptoms of asthma (85, 86). There is evidence indicating that helminths can cause asthma and allergic manifestations in humans (8, 87, 88). Robust longitudinal cohorts are needed to address the correlation between helminth infections and the development of asthma and airway allergy and atopy.
The literature presents conflicting results regarding the association between asthma and toxocariasis. In our review of all case-control studies published to date, eight studies reported a non-significant and 13 studies reported a significant relationship between Toxocara infection and asthma; however, recent meta-analyses indicated a probable relationship between asthma and Toxocara serology (16, 26). Among three case-control studies conducted in different regions in Iran, two studies in the northern provinces of Alborz and Khorasan suggested no significant association between asthma and Toxocara serology; however, one study conducted in Isfahan, central Iran, indicated a significant association (42, 49, 53). Studies in Malaysia reported a significant relationship of allergic asthma manifestations with Toxocara seropositivity (35, 41). These studies were performed on children, while our study was conducted on all age-groups. This is particularly supported by the interesting study of Jogi et al. (58) in which the association between Toxocara serology and asthma was found significant in children, while no significant association was found in adults. It should be noted that 15 out of 22 published case-control studies were conducted on childhood asthma, and more studies are required for the adult population.
Several cross-sectional studies were conducted to describe the serum levels of anti-Toxocara antibodies in asthmatic individuals, showing different results in different geographical areas (Table 4). For instance, between 2016 and 2020, four studies were conducted on this topic in Iran, in which no significant asthma-Toxocara relationships were found in two studies, while the remaining studies indicate significant results (55, 59, 75, 76). The same is true in Brazil, where the data indicating significant and non-significant relationships were documented in three and four studies, respectively (57, 64, 65, 71, 72, 77, 78, 82).
Accidental ingestion of Toxocara spp. eggs leads to several syndromes in humans, and the ability of the helminth to cause VLM, OLM, neurological and cutaneous manifestations, and allergies is of interest to medical specialists. The spectrum of the disease manifestations from an asymptomatic infection to severe organ damage is related to the site of larval migration, parasite load, and inflammatory response in humans (13). The association of tissue helminth infections with peripheral blood eosinophilia, the presence of cytokines including IL-10 and IL-4, and elevated IgE levels are hypothetical pieces of evidence for the relationship between HI and asthma. Toxocara infection modulates the immune response to Th2 and the secretion of IL4, IL5, and IL13, which could theoretically be associated with asthma (89). However, protective effects of helminth infections in asthma symptom development have been documented in some studies. A nonsignificant increase in asthma risk has been reported by Leonardi-Bee et al. (9) in individuals infected with any helminth parasites. Nonetheless, they found that infection with Ascaris lumbricoides was significantly related to asthma, while hookworm infection was associated with significantly reduced risk of asthma. Toxocara infection in BALB/c mice models has shown respiratory morbidity and eosinophilic inflammation (90); however, more human and animal investigations are required to clarify mechanisms involved in airway hypersensitivity (83, 91).
According to a recent systematic review and meta-analysis, the global seroprevalence of Toxocara has been estimated to be 19.0% (92). In Iran, Toxocara seropositivity in humans has been reported as 1.7–33.7%, and the prevalence in dogs and cats has been estimated at 24.2 and 32.6%, respectively (93). The environmental contamination with eggs ranges from 1.7 to 63.3% in public parks and from 3.2 to 16.0% in vegetables (94).
In the present study, there were no significant relationships between age, sex, and literacy levels of the participants and Toxocara seropositivity, which was quite similar to the findings of the studies conducted in Malaysia, Nigeria, Turkey, and the United States (44, 54, 95). Nonetheless, Buijs et al. (62) indicated that Toxocara seropositivity was significantly lower in children younger than 10 years, which could explain the differences in the results (62). In previous studies, the association of age with the severity of allergic manifestations in asthma patients with positive Toxocara serology was not evaluated. In the present study, the severity of asthma manifestations increased with age among seropositive individuals (Table 2). Also, no significant relationship was found between the education level and severity of allergic manifestations in asthma among patients with positive Toxocara serology (Table 3). It is worth noting that a significant relationship between education level and the severity of allergic manifestations was observed among asthma patients with negative Toxocara serology.
Studies on the association between asthma and Toxocara seropositivity have been conducting for nearly half a century. The probable association of asthma with toxocariasis was investigated for the first time by Brown in 1972 who evaluated “Toxocaral antibody in nine asthmatic patients” (96). Subsequently, several animal experimental, case-control, and cross sectional studies were conducted until the new millennium. Nonetheless, most of the studies (65%) have been published in the last 15 years. In recent years, at least two studies have been conducted each year in this regard. Therefore, different types and levels of evidence are still lacking, and more focused animal investigations and extensive case-control and cohort studies are required to elucidate the role of Toxocara infection in the development of asthma in human.
More than half of the studies were performed in mainly four countries. As seen in Figure 2, most of the analytical studies are geographically restricted to America, Europe, and the Middle East. There is a large gap in knowledge on this topic in Africa and Asia, since very few studies were conducted in these regions, where many zoonotic helminths including Toxocara spp. are endemic.
Since 2010, conflicting results have been published regarding the association between asthma symptoms and Toxocara seropositivity. Interestingly, an equal number of articles proposed significant (14 records) and non-significant (14 records) associations between asthma and toxocariasis. Among 80 studies investigating the association of asthma with Toxocara seropositivity, 35 studies (43.8%) were significant, and 23 studies (28.7%) were not significant. In the remaining studies (mostly case reports, reviews, and cross-sectional studies), the significance values were not reported and/or not applicable (see the Supplementary file).
It is interesting to note that only a few studies investigated the association of asthma with Toxocara seropositivity in adults. The review of the literature revealed that 70% of the studies were exclusively conducted on children, and only 11.3 and 15.1% of the studies were performed in adults and all age groups, respectively. More studies are required on the probable role of toxocariasis in asthma development in the adult population. In addition, in-depth age-specific analyses should be performed on existing datasets to clarify the contribution of age in asthma-Toxocara interaction.
The most frequently cited articles were reviews, systematic reviews, and cross-sectional studies with 74.5, 45.5, and 33.3 citations per article, respectively. The three most cited articles were published in the Current Opinion in Allergy and Clinical Immunology (a review with 244 citations), European Respiratory Journal (a cross-sectional study with 203 citations), and American Journal of Epidemiology (a cross-sectional study with 142 citations). The average number of citations per article was 31.2. The total sample size of the cohort, case-control, and cross-sectional studies was 36,537, with an average sample size of 198, 591, and 1,086 subjects, respectively.
Despite a multitude of studies conducted on the probable role of Toxocara infection on asthma development in different human communities, the level of evidence is not sufficient, particularly in adult populations, and further detailed animal investigations and extensive case-control and cohort studies as well as cellular–molecular explorations are required to elucidate the nature of asthma-Toxocara interaction in humans.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Research Ethics Review Committee of Kerman University of Medical Sciences, Approval code: IR.KMU.REC.1399.285. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
NB and MF: conceptualization and study design. AL and MB: data curation. NB, MB, and MF: data analysis and data validation. MF: funding acquisition. AL and MF: laboratory experiments. MB and MF: writing—original draft preparation. NB, AL, MB, and MF: revising and final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Iranian Management and Planning Organization and Vice-Chancellor for Research, Kerman University of Medical Sciences (grant no. T.83/04).
Special thanks to Dr. Hashem Khanbabaei and Mr. Saeid Nasibi for their technical assistance in preparing the figures.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.920182/full#supplementary-material
1. Holgate ST, Arshad HS, Roberts GC, Howarth PH, Thurner P, Davies DE, et al. new look at the pathogenesis of asthma. Clin Sci. (2010) 118:439–50. doi: 10.1042/CS20090474
2. Cruz AA. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach. Geneva: World Health Organization. (2007).
3. Fazlollahi MR, Najmi M, Fallahnezhad M, Sabetkish N, Kazemnejad A, Bidad K, et al. The prevalence of asthma in Iranian adults: the first national survey and the most recent updates. Clin Respir J. (2018) 12:1872–81. doi: 10.1111/crj.12750
4. Hassanzadeh J, Mohammadbeigi A, Mousavizadeh A, Akbari M. Asthma prevalence in Iranian guidance school children, a descriptive meta-analysis. J Res Med Sci. (2012) 17:17–21.
5. Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy. (2011) 41:1059–71. doi: 10.1111/j.1365-2222.2011.03776.x
6. Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. (2006) 7:95–100. doi: 10.1038/sj.gene.6364284
7. Martinez FD. Genes, environments, development and asthma: a reappraisal. Eur Respir J. (2007) 29:179–84. doi: 10.1183/09031936.00087906
8. Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. (2009) 9:29. doi: 10.1097/ACI.0b013e32831f44a6
9. Leonardi-Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med. (2006) 174:514–23. doi: 10.1164/rccm.200603-331OC
10. Wong C, Ho C, Ko F. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin ExpImmunol. (2001) 125:177–83. doi: 10.1046/j.1365-2249.2001.01602.x
11. Magnaval J-F, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. (2001) 39:1. doi: 10.3347/kjp.2001.39.1.1
12. Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. (2009) 25:182–8. doi: 10.1016/j.pt.2009.01.006
13. Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. (2010) 104:3–23. doi: 10.1179/136485910X12607012373957
14. Lee RM, Moore LB, Bottazzi ME, Hotez PJ. Toxocariasis in North America: a systematic review. PLoS Negl Trop Dis. (2014) 8:e3116. doi: 10.1371/journal.pntd.0003116
15. Fakhri Y, Gasser RB, Rostami A, Fan CK, Ghasemi SM, Javanian M, et al. Toxocara eggs in public places worldwide: a systematic review and meta-analysis. Environ Pollut. (2018) 242:1467–75. doi: 10.1016/j.envpol.2018.07.087
16. Li L, Gao W, Yang X, Wu D, Bi H, Zhang S, et al. Asthma and toxocariasis. Ann Allergy Asthma Immunol. (2014) 113:187–92. doi: 10.1016/j.anai.2014.05.016
17. Maleki B, Khorshidi A, Gorgipour M, Mirzapour A, Majidiani H, Foroutan M. Prevalence of Toxocara spp. eggs in soil of public areas in Iran: a systematic review and meta-analysis. Alexand J Med. (2018) 54:97–101. doi: 10.1016/j.ajme.2017.06.001
18. Abdi J, Darabi M, Sayehmiri KK. Epidemiological situation of toxocariasis in Iran: meta-analysis and systematic review. Pak J Biol Sci. (2012) 15:1052–5. doi: 10.3923/pjbs.2012.1052.1055
19. Mims JW. Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol. (2015) 5:S2–6. doi: 10.1002/alr.21609
20. Magnaval JF, Fillaux J, Cassaing S, Valentin A, Iriart X, Berry A. Human toxocariasis and atopy. Parasite. (2020) 27:32. doi: 10.1051/parasite/2020029
21. Fialho PMMPMM, Correa CRSCRS, Lescano SZSZ. Asthma and seroconversion from Toxocara spp. infection: which comes first? Biomed Res Int. (2018) 2018:4280792. doi: 10.1155/2018/4280792
22. Arrais M, Maricoto T, Nwaru BI, Cooper PJ, Gama JMR, Brito M, et al. Helminth infections and allergic diseases: systematic review and meta-analysis of the global literature. J Allergy Clin Immunol. (2022) 149:1–14. doi: 10.1016/j.jaci.2021.12.777
23. Arrais M, Maricoto T, Cooper P, Gama JM, Nwaru BI, Brito M, et al. Helminth infections, atopy, asthma and allergic diseases: protocol for a systematic review of observational studies worldwide. BMJ Open. (2020) 10:e038085. doi: 10.1136/bmjopen-2020-038085
24. Feary J, Britton J, Leonardi-Bee J. Atopy and current intestinal parasite infection: a systematic review and meta-analysis. Allergy. (2011) 66:569–78. doi: 10.1111/j.1398-9995.2010.02512.x
25. Mohammadzadeh I, Riahi SM, Saber V, Darvish S, Amrovani M, Arefkhah N, et al. The relationship between Toxocara species seropositivity and allergic skin disorders: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. (2018) 112:529–37. doi: 10.1093/trstmh/try094
26. Aghaei S, Riahi SM, Rostami A, Mohammadzadeh I, Javanian M, Tohidi E, et al. Toxocara spp. infection and risk of childhood asthma: a systematic review and meta-analysis. Acta Trop. (2018) 182:298–304. doi: 10.1016/j.actatropica.2018.03.022
27. GINA. Global strategy for global: strategy for asthma management and prevention. Glob Initiat Asthma. (2019) 45:1–199. Available online at: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf
28. Yawn BP. Factors accounting for asthma variability: achieving optimal symptom control for individual patients. Prim Care Respir J. (2008) 17:138–47. doi: 10.3132/pcrj.2008.00004
29. Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, Mcewen SA, et al. scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. (2014) 5:371–85. doi: 10.1002/jrsm.1123
30. Armstrong R, Hall BJ, Doyle J, Waters E. “Scoping the scope” of a cochrane review. J Public Health. (2011) 33:147–50. doi: 10.1093/pubmed/fdr015
31. Shankardass K, Solar O, Murphy K, Greaves L, O'Campo P. A scoping review of intersectoral action for health equity involving governments. Int J Public Health. (2012) 57:25–33. doi: 10.1007/s00038-011-0302-4
32. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. (2010) 5:1–9. doi: 10.1186/1748-5908-5-69
33. Desowitz RS, Rudoy R, Barnwell JW. Antibodies to canine helminth parasites in asthmatic and nonasthmatic children. Int Arch Allergy Immunol. (1981) 65:361–6.
34. Cadore PS, Zhang L, Lemos LL, Lorenzi C, Telmo PL, dos Santos PC, et al. Toxocariasis and childhood asthma: a case-control study. J Asthma. (2016) 53:601–6. doi: 10.3109/02770903.2015.1064951
35. Chan PW, Anuar AK, Fong MY, Debruyne JA, Ibrahim J. Toxocara seroprevalence and childhood asthma among Malaysian children. Pediatr Int. (2001) 43:350–3. doi: 10.1046/j.1442-200X.2001.01421.x
36. Cobzaru R-G, Rîpă C, Leon MM, Luca MC, Ivan A, Luca M. Correlation between asthma and Toxocara canis infection. Rev Med Chir Soc Med Nat Iasi. (2012) 116:727–30.
37. Elshazly AM, Attia G, El-Ghareeb AS, Belal US. Clinical varieties of Toxocariasis canis in children's hospital, Mansoura University: is it an underestimated problem? J Egypt Soc Parasitol. (2011) 41:263–74.
38. El-Shazly AM, Abdel Baset SM, Kamal A, Mohammed KA, Sakrs TI, Hammad SM. Seroprevalence of human toxocariasis (visceral larva migrans). J Egy Soc Parasitol. (2009) 39:731–44.
39. Fernando D, Wickramasinghe P, Kapilananda G, Dewasurendra RL, Amarasooriya M, Dayaratne A. Toxocara seropositivity in Sri Lankan children with asthma. Pediatr Int. (2009) 51:241–5. doi: 10.1111/j.1442-200X.2008.02687.x
40. Grama DF, Lescano SZ, Pereira Mota KC, dos Anjos Pultz B, Miranda JS, Silva Segundo GR, et al. Seroprevalence of Toxocara spp. in children with atopy. Trans R Soc Trop Med Hyg. (2014) 108:797–803. doi: 10.1093/trstmh/tru165
41. Hakim L. Correction: prevalence of Toxocara canis antibody among children with bronchial asthma in Klang Hospital, Malaysia. Trans R Soc Trop Med Hyg. (1997) 91:728.
42. Khozime A, Mirsadraee M, Borji H. Toxocara sero-prevalence and its relationship with allergic asthma in asthmatic patients in north-eastern Iran. J Helminthol. (2019) 93:677–80. doi: 10.1017/S0022149X1800086X
43. Kuk S, Ozel E, Oguzturk H, Kirkil G, Kaplan M. Seroprevalence of Toxocara antibodies in patients with adult asthma. South Med J. (2006) 99:719–23. doi: 10.1097/01.smj.0000223949.11527.48
44. Kustimur S, Dogruman AF, Oguzulgen K, Bakir H, Maral I, Turktas H, et al. Toxocara seroprevalence in adults with bronchial asthma. Trans R Soc Trop Med Hyg. (2007) 101:270–4. doi: 10.1016/j.trstmh.2006.08.013
45. Labeeb El-Tantawy N, Ahmed El-Nahas H, Mohamed El-Assmy M, Ahmed AL-Gazoui R. Clinico-seroepidemiological evaluation of toxocariasis in asthmatic pediatric children in mansoura city in Egypt. Arch Clin Microbiol. (2013) 4:4195–201. doi: 10.3823/271
46. López LM, Bojanich MV, Jacobacci JM, Sercic C, Michelini A, Alonso JM. Toxocara canis and bronchial asthma. Medicina. (2010) 70:75–8.
47. Mazmanyan MV, Chervinskaya TA, Tumolskaya NI, Shustova VI, Poletaeva OG. Excretory-secretory antigen Toxocara canis as a sensitization factor in bronchial asthma. Immunologiya. (1998) 3:40–4.
48. Minvielle MC, Niedfeld G, Ciarmela MLAF, Ghiani H, Basualdo JA. Asthma and covert toxocariasis. Medicina. (1999) 59:243–8.
49. Momen T, Esmaeil N, Reisi M. Seroprevalence of ToxocaraCanis in asthmatic children and its relation to the severity of diseases: a case-control study. Med Arch. (2018) 72:174–7. doi: 10.5455/medarh.2018.72.174-177
50. Muñoz-Guzmán MA, del Río-Navarro BE, Valdivia-Anda G, Alba-Hurtado F. The increase in seroprevalence to Toxocara canis in asthmatic children is related to cross-reaction with Ascaris suum antigens. Allergol Immunopathol. (2010) 38:115–21. doi: 10.1016/j.aller.2009.09.007
51. Oteifa NM, Moustafa MA, Elgozamy BM. Toxocariasis as a possible cause of allergic diseases in children. J Egypt Soc Parasitol. (1998) 28:365–72.
52. Popescu A, Greblescu R. Toxocara infection in patients with asthma and benefits of antihelmintic therapy. Allergy Eur J Allergy Clin Immunol. (2014) 69:170–1.
53. Sadri H, Gharavi MJ, Arjmand R, Zibaei M, Elahimehr N, Shaker Y. Toxocara infection in asthmatic children: a case-control study in Karaj district, Iran. Arch Pediatr Infect Dis. (2019) 7:e82370. doi: 10.5812/pedinfect.82370
54. Sharghi N, Schantz PM, Caramico L, Ballas K, Teague BA, Hotez PJ. Environmental exposure to Toxocara as a possible risk factor for asthma: a clinic-based case-control study. Clin Infect Dis. (2001) 32:e111–6. doi: 10.1086/319593
55. Mosayebi M, Moini L, Hajihossein R, Didehdar M, Eslamirad Z. Detection of specific antibody reactivity to toxocara larval excretory-secretory antigens in asthmatic patients (5–15 years). Open Microbiol J. (2016) 10:162–7. doi: 10.2174/1874285801610010162
56. Mazur-Melewska K, Jończyk-Potoczna K, Kemnitz P, Mania A, Figlerowicz M, Słuzewski W. Pulmonary presentation of Toxocara sp. infection in children. Pneumonol Alergol Pol. (2015) 83:250–5. doi: 10.5603/PiAP.a2015.0043
57. Pastorino AC, Accioly AP, Lanzellotti R, Camargo MC, Jacob CM, Grumach AS. Asthma-clinical and epidemiological aspects of 237 outpatients in a specialized pediatric unit. J Pediatr (Rio J). (1998) 74:49–58.
58. Jõgi NO, Svanes C, Siiak SP, Logan E, Holloway JW, Igland J, et al. Zoonotic helminth exposure and risk of allergic diseases: a study of two generations in Norway. Clin Exp Allergy. (2018) 48:66–77. doi: 10.1111/cea.13055
59. Aghamolaie S, Seyyedtabaei SJ, Behniafar H, Foroutan M, Saber V, Hanifehpur H, et al. Seroepidemiology, modifiable risk factors and clinical symptoms of Toxocara spp. infection in northern Iran. Trans R Soc Trop Med Hyg. (2019) 113:116–22. doi: 10.1093/trstmh/try118
60. Bahnea RG, Ivan A, Cardei E, Luca MC, Stoica O, Luca M. Retrospective clinical and laboratory study of the toxocariasis cases hospitalised between 2005 and 2008. Rev Med Chir Soc Med Nat Iasi. (2008) 112:938–41.
61. Buijs J, van Knapen F. Toxocara infection in children and the relation with allergic manifestations. Vet Q. (1994) 16:13–4.
62. Buijs J, Borsboom G, Renting M, Hilgersom WJ, van Wieringen JC, Jansen G, et al. Relationship between allergic manifestations and Toxocara seropositivity: a cross-sectional study among elementary school children. Eur Respir J. (1997) 10:1467–75.
63. Dralova A, Usachova E. Some clinical and cytokine features of the clinical course of recurrent respiratory system diseases in children with the toxocariasis invasion. Georgian Med News. (2015) 249:62–7.
64. Ferreira MU, Rubinsky-Elefant G, de Castro TG, Hoffmann ÉHE, da Silva-Nunes M, Cardoso MA, et al. Bottle feeding and exposure to toxocara as risk factors for wheezing illness among under-five Amazonian children: a population-based cross-sectional study. J Trop Pediatr. (2007) 53:119–24. doi: 10.1093/tropej/fml083
65. Fragoso RP, Monteiro MBM, Lemos EM, Pereira FEL. Anti-Toxocara antibodies detected in children attending elementary school in Vitoria, State of Espírito Santo, Brazil: prevalence and associated factors. Rev Soc Bras Med Trop. (2011) 44:461–6. doi: 10.1590/S0037-86822011000400012
66. Gonzalez-Quintela A, Gude F, Campos J, Garea MT, Romero PA, Rey J, et al. Toxocara infection seroprevalence and its relationship with atopic features in a general adult population. Int Arch Allergy Immunol. (2006) 139:317–24. doi: 10.1159/000091603
67. Incorvaia C. Qualizza, Grande, Allegra L. Seroprevalence of IgG anti-Toxocara species antibodies in a population of patients with suspected allergy. Int J Gen Med. (2011) 4:783–7. doi: 10.2147/IJGM.S24324
68. Buijs J, Borsboom G, van Gemund JJ, Hazebroek A, van Dongen PAM, van Knapen F, et al. Toxocara seroprevalence in 5-year-old elementary schoolchildren: relation with allergic asthma. Am J Epidemiol. (1994) 140:839–47.
69. Kanobana K, Vereecken K, Junco Diaz R, Sariego I, Rojas L, Bonet Gorbea M, et al. Toxocara seropositivity, atopy and asthma: a study in Cuban schoolchildren. Trop Med Int Heal. (2013) 18:403–6. doi: 10.1111/tmi.12073
70. Karney A, Mordasewicz-Goliszewska M, Dobosz S, Krynicka-Czech B, Kowalewska-Kantecka B, Marczyńska M. Clinical manifestation of toxocariasis in children of 1-day hospitalization ward institute of mother and child in 2008–2009. Pediatr Pol. (2010) 85:576–81. doi: 10.1016/S0031-3939(10)70557-6
71. Manini MP, Marchioro AA, Colli CM, Nishi L, Falavigna-Guilherme AL. Association between contamination of public squares and seropositivity for Toxocara spp. in children. Vet Parasitol. (2012) 188:48–52. doi: 10.1016/j.vetpar.2012.03.011
72. Mendonça LR, Veiga RV, Dattoli VCC, Figueiredo CA, Fiaccone R, Santos J, et al. Toxocara seropositivity, atopy and wheezing in children living in poor neighbourhoods in urban latin American. PLoS Negl Trop Dis. (2012) 6:1–9. doi: 10.1371/journal.pntd.0001886
73. Ogundipe F, Hennis ECA, Mehari A, Gillum RF. Toxocara species exposure, symptoms of asthma, and fractional exhaled nitric oxide in the US population. Ann Allergy Asthma Immunol. (2017) 119:569–70. doi: 10.1016/j.anai.2017.09.063
74. Qualizza R, Incorvaia C, Ballerini R, Grande R. Chronic asthma and neglected Toxocara infection. World Allergy Organ J. (2012) 5:S202. doi: 10.1097/01.WOX.0000411700.04936.16
75. Rezaiemanesh MRMR, Afzalaghaee M, Hamidi S, Eshaghzadeh A, Paydar M, Hejazi SHSH. Prevalence of toxocariasis and its related risk factors in humans, dogs and cats in northeastern Iran: a population-based study. Trans R Soc Trop Med Hyg. (2019) 113:399–409. doi: 10.1093/trstmh/trz011
76. Shamsian SASA, Sayedi SJSJ, Zibaei M, Vaghei S, Moghaddas E. Frequency of toxocariasis among asthmatic children in northeastern Iran. Arch Clin Infect Dis. (2019) 14:e82967. doi: 10.5812/archcid.82967
77. Silva MB, Amor ALM, Santos LN, Galvão AA, Oviedo Vera AV, Silva ES, et al. Risk factors for Toxocara spp. seroprevalence and its association with atopy and asthma phenotypes in school-age children in a small town and semi-rural areas of Northeast Brazil. Acta Trop. (2017) 174:158–64. doi: 10.1016/j.actatropica.2016.04.005
78. Tonelli E. Toxocariasis and asthma: a relevant association. J Pediatr (Rio J). (2005) 81:95–6. doi: 10.2223/1312
79. van Dongen PA, Buijs J, van Gemund JJ, van den Bergh JP, Bozon IJ. How innocent is Toxocara? Ned Tijdschr Geneeskd. (1989) 133:1789.
80. Walsh MG. Toxocara infection and diminished lung function in a nationally representative sample from the United States population. Int J Parasitol. (2011) 41:243–7. doi: 10.1016/j.ijpara.2010.09.006
81. Zacharasiewicz A, Auer H, Brath H, Stohlhofer B, Frank W, Aspöck H, et al. Toxocara and bronchial hyperreactivity: results of a seroprevalence study. Wien Klin Wochenschr. (2000) 112:922–6.
82. Figueiredo SDP, Taddei JAAC, Menezes JJC, Novo NF, Silva EOM, Cristóvão HLG, et al. Clinical-epidemiological study of toxocariasis in a pediatric population. J Pediatr (Rio J). (2005) 81:126–32. doi: 10.2223/JPED.1317
83. Alcantara-Neves NM, Britto GDSG, Veiga RV, Figueiredo CA, Fiaccone RL, Da Conceição JS, et al. Effects of helminth co-infections on atopy, asthma and cytokine production in children living in a poor urban area in Latin America. BMC Res Notes. (2014) 7:1–11. doi: 10.1186/1756-0500-7-817
84. Bede O, Szénási Z, Danka J, Gyurkovits K, Nagy D. Toxocariasis associated with chronic cough in childhood: a longitudinal study in Hungary. J Helminthol. (2008) 82:357–63. doi: 10.1017/S0022149X0804827X
85. Lynch NR, Hagel I, Perez M, Di Prisco MC, Lopez R, Alvarez N. Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J Allergy Clin Immunol. (1993) 92:404–11.
86. Lynch NR, Palenque M, Hagel I, Diprisco MC. Clinical improvement of asthma after anthelminthic treatment in a tropical situation. Am J Respir Crit Care Med. (1997) 156:50–4.
87. Flohr C, Quinnell RJ, Britton J. Do helminth parasites protect against atopy and allergic disease? Clin Exp Allergy. (2009) 39:20–32. doi: 10.1111/j.1365-2222.2008.03134.x
88. Carvalho EM, Bastos LS, Araujo MI. Worms and allergy. Parasite Immunol. (2006) 28:525–34. doi: 10.1111/j.1365-3024.2006.00894.x
89. Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. (2002) 17:7–17. doi: 10.1016/S1074-7613(02)00332-1
90. Buijs J, Egbers MW, Lokhorst WH, Savelkoul HF, Nijkamp FP. Toxocara-induced eosinophilic inflammation. Airway function and effect of anti-IL-5. Am J Respir Crit Care Med. (1995) 151:873–8.
91. Holt PG, Yabuhara A, Prescott S, Venaille T, Macaubas C, Holt BJ, et al. Allergen recognition in the origin of asthma. In: Ciba Foundation Symposium. Hoboken: Wiley (1997). p. 35–55.
92. Rostami A, Riahi SM, Holland CV, Taghipour A, Khalili-Fomeshi M, Fakhri Y, et al. Seroprevalence estimates for toxocariasis in people worldwide: a systematic review and meta-analysis. PLoS Negl Trop Dis. (2019) 13:e0007809. doi: 10.1371/journal.pntd.0007809
93. Eslahi AV, Badri M, Khorshidi A, Majidiani H, Hooshmand E, Hosseini H, et al. Prevalence of Toxocara and Toxascaris infection among human and animals in Iran with meta-analysis approach. BMC Infect Dis. (2020) 20:1–17. doi: 10.1186/s12879-020-4759-8
94. Zibaei M, Sadjjadi SM. Trend of toxocariasis in Iran: a review on human and animal dimensions. Iran J Vet Res. (2017) 18:233–42.
95. Ajayi OO, Duhlinska DD, Agwale SM, Njoku M. Frequency of human toxocariasis in Jos, Plateau State, Nigeria. Mem Inst Oswaldo Cruz. (2000) 95:147–9. doi: 10.1590/S0074-02762000000200002
Keywords: pediatric asthma, toxocariasis, scoping review, publication trend, soil-transmitted helminths, adulthood asthma
Citation: Bazargan N, Lari AN, Borhani M and Fasihi Harandi M (2022) Allergic asthma manifestations in human and seropositivity to Toxocara, a soil-transmitted helminth of carnivores: A case-control study and scoping review of the literature. Front. Med. 9:920182. doi: 10.3389/fmed.2022.920182
Received: 14 April 2022; Accepted: 05 September 2022;
Published: 29 September 2022.
Edited by:
Serena Cavallero, Sapienza University of Rome, ItalyReviewed by:
Alessia Libera Gazzonis, University of Milan, ItalyCopyright © 2022 Bazargan, Lari, Borhani and Fasihi Harandi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Majid Fasihi Harandi, ZmFzaWhpQGttdS5hYy5pcg==; bWFqaWQuZmFzaWhpQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.