- Department of Pulmonology, Medical University of Gdańsk, Gdańsk, Poland
Idiopathic pulmonary fibrosis (IPF) is a progressive, chronic disease of the lungs which is characterized by heavy symptom burden, especially in the last year of life. Despite recently established anti-fibrotic treatment IPF prognosis is one of the worst among interstitial lung diseases. In this review available evidence regarding pharmacological and non-pharmacological management of the main IPF symptoms, dyspnea and cough, is presented.
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most frequent idiopathic interstitial pneumonia and one of the most frequent interstitial lung diseases (ILD) (1). It is a chronic, progressive disease of the lungs which has a distinct pattern, both radiologically and pathologically, of usual interstitial pneumonia (2). Despite recently established anti-fibrotic treatment IPF prognosis is one of the worst among ILDs with a mean survival of 2.5–5.0 years. Its course is characterized by heavy symptom burden especially in the last year of life (3, 4). Symptomatic treatment is an important aspect of palliative care which also addresses spiritual, social and psychological needs. Unfortunately, patients with IPF, as with other chronic lung diseases, are not referred to palliative care centers nearly as often as cancer patients. Furthermore, their symptomatic treatment is withhold partly due to the fear of opioids (5). Planning palliative care for IPF patients can also be hindered by heterogeneous course of the disease—loss of lung function might be gradual, rapid or suddenly accelerates because of a life-threatening acute exacerbation (3). This unpredictability is probably one of the reasons why the majority of IPF patients die in a hospital, subjected to life-prolonging procedures (6, 7). On the other hand, referring the IPF patient to palliative care too early, when the disease is mild, might worsen quality of life in short term, probably due to a worsening of depression or anxiety (8). Nonetheless, there is evidence that palliative care, compared to usual care, might improve respiratory symptoms and quality of life in patients with advanced IPF (9, 10).
The aim of this review is to present current evidence on symptomatic treatment of dyspnea and cough in IPF patients.
Breathlessness—pathophysiology and treatment
Dyspnea in IPF results from both respiratory and circulatory limitations: reduced lung compliance, loss of lung volume, increased dead space ventilation, increased respiratory drive, gas exchange abnormalities and pulmonary hypertension (11). In turn, breathlessness treatment is a multifaceted process involving effective treatment of comorbidities, rehabilitation, pharmacological and oxygen treatment and non-invasive ventilation.
Non-pharmacological treatment
Rehabilitation
Exertional hypoxemia, along with skeletal muscle dysfunction, restrictive ventilatory impairment and cardiovascular limitation, i.e., reduction in stroke volume, are responsible for exercise limitation in ILDs (12, 13). Exercise intolerance, in turn, is associated with reduced quality of life and increased mortality (14).
Cochrane review of 16 studies on pulmonary rehabilitation of ILD patients showed that this intervention can improve dyspnea and health-related quality of life, 6 min walking test (6MWT) distance and cardiopulmonary exercise test parameters, i.e., peak workload, peak oxygen uptake and maximum ventilation. Furthermore, evidence showed that improvements in dyspnea and SGRQ Impact score were sustained at 6–12 months. Sustained improvement affected not only dyspnea but also exercise capacity and health-related quality of life up to 12 months since rehabilitation program (15). Whether increases in 6MWT distance and peak oxygen uptake, both of which are predictors of IPF mortality, translate into prognosis improvement is unknown (16).
The positive effect of pulmonary rehabilitation on ILD patients is probably a result of repetitive chest expansion and stretching of the thoracic muscles what, in turn, translates into improvement of tidal volume. Increase in tidal volume leads then to improvement of peak oxygen uptake (16). Furthermore, it is suggested that rehabilitation improves peripheral oxygen extraction (17).
Rehabilitation of ILD patients is frequently complicated by desaturation which is why clinical supervision is essential for its safety and effectiveness (18). It is advised that supplemental oxygen should be used to maintain spO2 ≥85% during exercise, if needed (18). To no surprise then, most of the studies (18 out of 21) included in the Cochrane review, were conducted in a supervised outpatient setting (15).
There is some conflicting evidence on time of referral to pulmonary rehabilitation for IPF patients. In studies by Kozu et al. and Holland et al. (19, 20) more advanced disease, i.e., lower mMRC score (20), lower forced vital capacity, greater exertional hypoxemia and higher right ventricular systolic pressure (19), predicted smaller improvement of 6MWT distance (20). It is worth underlining that such association was not evident for other than IPF ILD patients (19). On the other hand, study by Ryerson et al. showed that greater baseline 6MWT distance was associated with smaller 6MWT distance gain (21). It is author's view that the above should not discourage trial of pulmonary rehabilitation in advanced IPF patients.
Ambulatory and long term oxygen treatment
Exertional hypoxemia is one of the defining features of ILDs (12). Desaturation during 6MWT alone is an independent mortality and pulmonary hypertension risk factor (22–24). Furthermore, its severity in ILD is reported to be greater than in chronic obstructive pulmonary disease (COPD) (25). Nonetheless, studies failed to show unequivocal results of oxygen treatment in patients without significant hypoxemia at rest. Cochrane review of three crossover randomized controlled trials (RCT), performed in physiology laboratories, on 98 IPF patients altogether, failed to show any effect of short-term supplementary oxygen on exertional dyspnea (26). One of the studies showed increase in endurance time during constant load ergometry (27). None of them titrated oxygen to prevent desaturation, but used pre-determined fixed oxygen flow rate. Another systematic review, by Bell et al., incorporating 9 reports on short-term supplementary oxygen, showed similar results—no significant effect on dyspnea was detected while exercise capacity seemed to improve (28).
However, recently performed RCTs showed different results. A crossover RCT, by Dowman et al., on 11 patients with IPF, showed significant improvement of Borg dyspnea score and endurance time during cycle endurance test with oxygen supplementation where FiO2 (fraction of inspired oxygen) was set at 50%. It is noteworthy that Borg fatigue score did not improve (29). In another crossover RCT, on 20 fibrotic ILD patients, Schaeffer et al. showed that supplemental oxygen with FiO2 equal 60%, increased endurance time, reduced dyspnea and leg discomfort ratings (30). The most recent crossover RCT, AmbOx, the only study yet asserting effect of ambulatory oxygen treatment (AOT) on quality of life of fibrotic ILD patients, reported improvement of total K-BILD (King's Brief ILD questionnaire) scores and its breathlessness, activity and chest symptoms subdomains. However, this subjective improvement did not translate into increase of physical activity measured by biaxial accelerometer. It is worth underlining that each of the 76 AmbOx participants had flow rate of oxygen titrated during screening visit 6MWT to maintain spO2 > 90%. Patients were then instructed to use their lightweight gas cylinders with the set flow during routine activities for 2 × 2 weeks (31). Authors reported that at the end of the trial 33% patients chose to discontinue oxygen treatment delivered via cylinders, however, those who experienced most dyspnea reduction were the most likely to continue. Younger age was also significantly predictive regarding the decision to continue AOT (31).
In light of the above national societies suggest a trial of AOT in patients with significant exertional desaturation, if there is evidence of benefit (32–34). One should take into consideration challenges connected with using the oxygen devices, especially outside home.
National guidelines are more unequivocal when ILD patients develop chronic hypoxemia at rest. Long term oxygen therapy (LTOT) is recommended even though the evidence is lacking (32–34). No RCTs were performed in this indication but three retrospective studies of which two did not include control group and none assessed effect of LTOT on breathlessness (28). Nonetheless, guidelines authors extrapolate evidence on survival benefit from COPD trials (32–34).
High flow nasal cannula
Compared to conventional oxygen therapy high flow nasal cannula (HFNC) provides oxygen at higher FiO2 (up to 100%) and at a higher flow, which matches patient's inspiratory demand and washes out CO2 from pharyngeal dead-space. Reduction of ventilatory dead-space might in turn improve the ventilation-perfusion inequality. High flow, up to 60 l/min, also generates a small amount of positive expiratory pressure. Furthermore, heating and humidifying of the respiratory mixture might reduce the metabolic cost of breathing (35). Laboratory studies show that HFNC might reduce work of breathing not only in healthy volunteers or COPD patients but in IPF patients as well (36, 37).
HFNC effectiveness in treating breathlessness was assessed in a study by Hui et al. on 30 advanced cancer patients, whose dyspnea intensity was ranked ≥3/10 despite supplemental oxygen. Patients received 2-h HFNC and 2-h NIPPV (non-invasive positive pressure ventilation), i.e., BiPAP, in a random sequence, both with FiO2 set at 100%. Dyspnea improved in both treatment arms, with no significant differences between, however, HFNC was better tolerated than BiPAP (38). Similar results were obtained by Koyauchi et al., who retrospectively assessed 84 ILD patients with do-not-intubate order and acute, hypoxic respiratory failure associated with ILD. Fifty-four patients used HFNC, 30—NIPPV. Temporary interruption of the therapy and discontinuation rates were significantly higher in the NIPPV group, whereas oral intake and ability to converse were significantly better in HFNC group. Three-day survival and in-hospital mortality did not differ significantly between the groups (39). Furthermore, HFNC compared to standard oxygen therapy seems to improve endurance time of IPF patients during constant-load exercise testing on cycloergometer in laboratory studies (40–42).
No studies so far have assessed domiciliary HFNC in ILD patients. However, recent two studies by Storgaard et al. in COPD patients with chronic hypoxemia show that HFNC used alongside LTOT might be of added benefit compared to LTOT alone (43, 44). In the first study, a RCT on 200 chronically hypoxemic COPD patients on LTOT, participants in HFNC group were instructed to use HFNC for 8 h daily, mainly during the night, as an add-on to LTOT, for at least 12 months. Seventeen percent of the participants discontinued HFNC. Thirty two percent used HFNC only during the day, 53% used it nightly, whereas the remaining 15% used HFNC both at night and day. Use of HFNC in conjunction with LTOT allowed for reduction of COPD acute exacerbation rate—which was the primary outcome. Furthermore, compared to LTOT alone, patients using HFNC additionally preserved their SGRQ score and 6MWT distance which dropped in the control group and reported significantly reduced mMRC score (43). Qualitative part of the study on 12 patients and 8 relatives showed that patients in the HFNC group found the device easy to use. Moreover, most patients reported that HFNC improved their sleep quality, despite the noise generated by the apparatus. In authors view this improvement was due to airway humidification and reduced work of breathing during sleep. Airway dryness, aggravated by LTOT, was reported by the patients as a significant reason for sleep interruption and awakening. Participants also reported a reduction in cough frequency (44).
Taking the above into consideration, HFNC seems as a viable option for oxygen delivery in IPF patients, although, high quality trials are needed.
Non-invasive ventilation
Respiratory exchange can be supported not only by conventional oxygen therapy or HFNC but also NIPPV. European Respiratory Society (ERS) and American Thoracic Society (ATS) guidelines suggest a trial of NIPPV in breathless patients in the setting of terminal condition (45). Available evidence encompasses two feasibility studies of RCT design in cancer patients (38, 46). Nava et al. randomized 200 end-stage cancer patients with solid tumors and acute respiratory failure to NIPPV (BiPAP) or oxygen. Only patients with PaO2/FiO2 ratio smaller than 250 were enrolled. Evident reversible causes of respiratory failure such as pulmonary edema were an exclusion factor. The study showed that NIPPV was significantly more effective in reducing dyspnea than conventional oxygen therapy but only in hypercapnic patients. Furthermore, 11% of NIPPV patients declined BiPAP due to poor tolerance (46). In a previously mentioned study, by Hui et al., NIPPV resulted in dyspnea reduction in cancer patients in a similar degree to HFNC, however it was significantly worse tolerated than HFNC (38).
The loss of lung compliance in IPF is associated with increased work of breathing and thus dyspnea. Offsetting the inspiratory burden by providing ventilation support could help treat breathlessness in IPF (11, 46). This would be especially true for patients in advanced stages of IPF who may develop hypercapnia—a sign of failing respiratory muscles unable to sustain the imposed load (11, 47). Hypercapnia in IPF could also be a sign of concomitant pleuroparenchymal fibroelastosis (PPFE), characterized by fibrosis involving the visceral pleura and subpleural parenchymal fibroelastosis. The resulting extrapulmonary restriction can in turn produce hypoventilation and hypercapnia (48, 49). Unfortunately, there are no studies which would assess effect of NIPPV on breathlessness in IPF patients. Data on NIPPV in ILD are limited to retrospective studies analyzing its effectiveness in treating acute respiratory failure, especially as means to avoid endotracheal intubation (50).
Dyspnea treatment summary, regarding AOT/LTOT, HFNC, and NIPPV, is presented in Table 1.
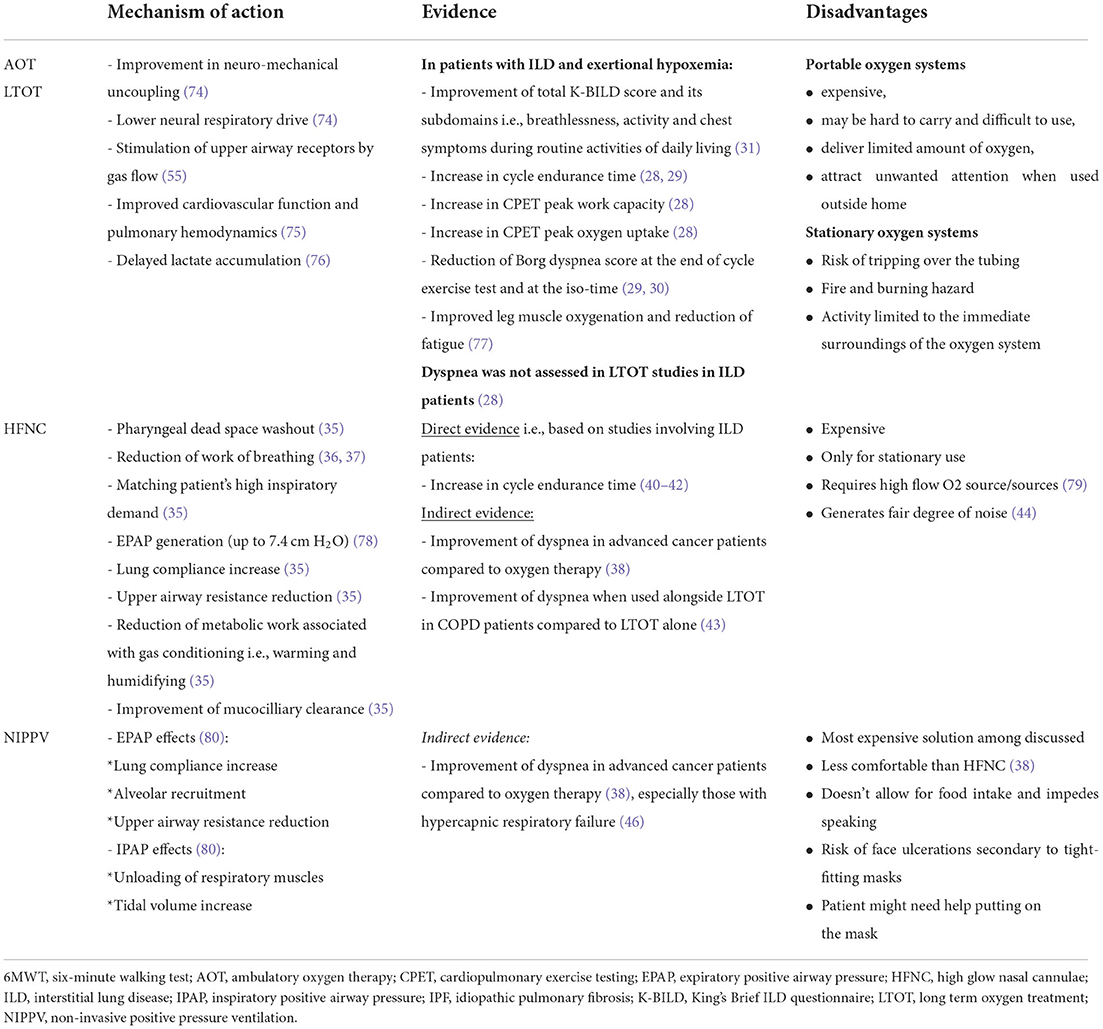
Table 1. Types of oxygen therapy and ventilatory support and their potential in treating breathlessness in IPF patients.
Pharmacological treatment
Opioids
Pharmacological treatment of dyspnea mainly involves opioids which by acting upon their central and peripheral nervous system receptors can decrease anxiety, modulate central perception of dyspnea and reduce respiratory drive without significant changes in blood gases (51–55). Unfortunately, high quality RCTs of opioid effectiveness in treating ILD-related breathlessness are lacking. Available evidence includes retrospective, population based studies (4), open-label studies with oral opioids (56, 57) and RCTs with nebulized morphine (58–61). Only oral morphine studies showed its effectiveness in treating ILD-related dyspnea. However, one of them employed just a small group of 11 IPF patients (57), whereas the other studied a mixed group with a minority of ILD patients (n = 10; 12%). Nonetheless, guidelines do embrace oral morphine for IPF dyspnea treatment because of its proven effectiveness in chronic lung diseases in general (34). This recommendation is also backed up by data on opioid safety reported not only by retrospective (62) but also prospective studies (63).
Cough
In IPF cough is one of the most frequent symptoms reported by 50–80% of patients (64, 65). Though it is typically described as dry (66), more than half of patients might expectorate sputum (64). Furthermore, cough was found to be an independent predictor of disease progression (64). Chronic cough in IPF can weigh heavily on quality of life by interrupting sleep, limiting speech or by causing significant desaturation, musculoskeletal pain and urinary incontinence. It is to no surprise then that cough might limit social interactions (67).
Mechanism of chronic cough induced by IPF is not precisely understood. It is assumed that increased cough reflex sensitivity, which is in complex interplay with frequent IPF co-morbidities—gastroesophageal reflux disease and obstructive sleep apnea, is involved. This increased sensitivity might be a result of increased traction forces impacting the function of stretch receptors. Other possible mechanisms involve destruction of inhibitory nerves by fibrosis or upregulation of vagal sensory fibers (66, 67). A significant role of stretch receptors in pathogenesis of IPF cough seems to be confirmed by results of the study by Jones et al. (68). Authors found that IPF patients, compared with healthy non-smoking controls, were significantly more susceptible to induction of non-productive cough by mechanical percussion of the chest wall especially when percussor was applied to the posterior lung base (68). As pointed out by van Manen, this correlates with the clinical finding that vibration caused by talking or coughing starts a self-perpetuating cough cycle (67).
Taking the above into consideration, a trial of treatment of IPF cough with neuromodulator i.e., gabapentin, as in idiopathic chronic cough, is recommended (69). Its effectiveness in idiopathic chronic cough was assessed in a RCT on 62 patients, who experienced significant improvement of cough-specific quality of life after 8 weeks of treatment. However, 10 patients, 31% of the gabapentin group, reported side effects with nausea and fatigue as the most common (70). Speech therapy, especially combined with pregabalin is also recommended (66, 69). If the above fails, guidelines suggest a trial of opioids (69). Nonetheless, this recommendation is based only on one RCT, of small-dose, slow-releasing morphine in 27 patients with idiopathic chronic cough (71).
Two other drugs have been trialed in IPF cough and showed efficiency. In a crossover RCT by Horton et al., thalidomide, a potent immunomodulatory drug, significantly improved cough-related quality of life in 20 patients with IPF during 12 weeks of treatment. However, 77% of patients reported side-effects: constipation, dizziness, malaise, anorexia and asymptomatic bradycardia (72). In light of these results thalidomide was not recommended by CHEST Expert Cough Panel experts in treatment of cough in IPF. Small size of the study population, side effects, prescription barriers and cost were among factors influencing this decision (69). In a crossover RCT by Birring et al., a novel formulation of sodium cromoglicate delivered by mesh nebulizer reduced cough frequency but did not improve cough-specific quality of life or cough severity in 24 IPF patients after 2 weeks of treatment (73).
Conclusions
Evidence for different interventions in symptomatic treatment of IPF patients is lacking. Consequently, IPF guidelines often base their recommendations on trials conducted in other chronic lung diseases. High quality trials are needed to verify efficiency of guidelines-compliant pharmacological treatment of cough and breathlessness in IPF patients. Positive results of AOT, NIPPV, and HFNC in treatment of breathlessness in other lung diseases should encourage similar studies in IPF patients as well.
Author contributions
PJ wrote the first draft of the manuscript. All authors contributed to conception of the review. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. (2013) 188:733–48. doi: 10.1164/rccm.201308-1483ST
2. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. (2018) 198:e44–68. doi: 10.1164/rccm.201807-1255ST
3. Fujimoto H, Kobayashi T, Azuma A. Idiopathic pulmonary fibrosis: treatment and prognosis. Clin Med Insights Circ Respir Pulm Med. (2015) 9:179–85. doi: 10.4137/CCRPM.S23321
4. Bajwah S, Higginson IJ, Ross JR, Wells AU, Birring SS, Patel A, et al. Specialist palliative care is more than drugs: a retrospective study of ILD patients. Lung. (2012) 190:215–20. doi: 10.1007/s00408-011-9355-7
5. Brown CE, Jecker NS, Curtis JR. Inadequate palliative care in chronic lung disease an issue of health care inequality. Ann Am Thorac Soc. (2016) 13:311–6. doi: 10.1513/AnnalsATS.201510-666PS
6. Lindell KO, Liang Z, Hoffman LA, Rosenzweig MQ, Saul MI, Pilewski JM, et al. Palliative care and location of death in decedents with idiopathic pulmonary fibrosis. Chest. (2015) 147:423–9. doi: 10.1378/chest.14-1127
7. Rajala K, Lehto JT, Saarinen M, Sutinen E, Saarto T, Myllärniemi M. End-of-life care of patients with idiopathic pulmonary fibrosis. BMC Palliat Care. (2016) 15:85. doi: 10.1186/s12904-016-0158-8
8. Janssen K, Rosielle D, Wang Q, Kim HJ. The impact of palliative care on quality of life, anxiety, and depression in idiopathic pulmonary fibrosis: a randomized controlled pilot study. Respir Res. (2020) 21:1–9. doi: 10.1186/s12931-019-1266-9
9. Higginson IJ, Bausewein C, Reilly CC, Gao W, Gysels M, Dzingina M, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med. (2014) 2:979–87. doi: 10.1016/S2213-2600(14)70226-7
10. Bajwah S, Ross JR, Wells AU, Mohammed K, Oyebode C, Birring SS, et al. Palliative care for patients with advanced fibrotic lung disease: a randomised controlled phase II and feasibility trial of a community case conference intervention. Thorax. (2015) 70:830–9. doi: 10.1136/thoraxjnl-2014-206583
11. Plantier L, Cazes A, Dinh-Xuan A-T, Bancal C, Marchand-Adam S, Crestani B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur Respir Rev. (2018) 27:170062. doi: 10.1183/16000617.0062-2017
12. Molgat-Seon Y, Schaeffer MR, Ryerson CJ, Guenette JA. Cardiopulmonary exercise testing in patients with interstitial lung disease. Front Physiol. (2020) 11:832. doi: 10.3389/fphys.2020.00832
13. Panagiotou M, Polychronopoulos V, Strange C. Respiratory and lower limb muscle function in interstitial lung disease. Chron Respir Dis. (2016) 13:162–72. doi: 10.1177/1479972315626014
14. Spruit MA, Singh SJ, Garvey C, Zu Wallack R, Nici L, Rochester C, et al. An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. (2013) 188:e13–64. doi: 10.1164/rccm.201309-1634ST
15. Dowman L, Hill CJ, May A, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. (2021) 2:CD006322. doi: 10.1002/14651858.CD006322.pub4
16. Vainshelboim B. Exercise training in idiopathic pulmonary fibrosis: is it of benefit? Breathe. (2016) 12:130–8. doi: 10.1183/20734735.006916
17. Keyser RE, Woolstenhulme JG, Chin LMK, Nathan SD, Weir NA, Connors G, et al. Cardiorespiratory function before and after aerobic exercise training in patients with interstitial lung disease. J Cardiopulm Rehabil Prev. (2015) 35:47–55. doi: 10.1097/HCR.0000000000000083
18. Holland Simone Dal Spruit, Martijn A. AEC, editor. Pulmonary Rehabilitation. European Respiratory Society (2021). doi: 10.1183/2312508X.erm9321
19. Holland AE, Hill CJ, Glaspole I, Goh N, McDonald CF. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med. (2012) 106:429–35. doi: 10.1016/j.rmed.2011.11.014
20. Kozu R, Jenkins S, Senjyu H. Effect of disability level on response to pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology. (2011) 16:1196–202. doi: 10.1111/j.1440-1843.2011.02029.x
21. Ryerson CJ, Cayou C, Topp F, Hilling L, Camp PG, Wilcox PG, et al. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: a prospective cohort study. Respir Med. (2014) 108:203–10. doi: 10.1016/j.rmed.2013.11.016
22. Papakosta D, Pitsiou G, Daniil Z, Dimadi M, Stagaki E, Rapti A, et al. Prevalence of pulmonary hypertension in patients with idiopathic pulmonary fibrosis: correlation with physiological parameters. Lung. (2011) 189:391–9. doi: 10.1007/s00408-011-9304-5
23. Lama VN, Flaherty KR, Toews GB, Colby T V., Travis WD, Long Q, et al. Prognostic value of desaturation during a 6 minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. (2003) 168:1084–90. doi: 10.1164/rccm.200302-219OC
24. Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med. (2006) 100:1734–41. doi: 10.1016/j.rmed.2006.02.004
25. Du Plessis JP, Fernandes S, Jamal R, Camp P, Johannson K, Schaeffer M, et al. Exertional hypoxemia is more severe in fibrotic interstitial lung disease than in COPD. Respirology. (2018) 23:392–8. doi: 10.1111/resp.13226
26. Sharp C, Adamali H, Millar AB. Ambulatory and short-burst oxygen for interstitial lung disease. Cochrane Database Syst Rev. (2016) 7:CD011716. doi: 10.1002/14651858.CD011716.pub2
27. Arizono S, Taniguchi H, Sakamoto K, Kondoh Y, Kimura T, Kataoka K, et al. Benefits of supplemental oxygen on exercise capacity in IPF patients with exercise-induced hypoxemia. Eur Respir J. (2015) 46:OA4971. doi: 10.1183/13993003.congress-2015.OA4971
28. Bell EC, Cox NS, Goh N, Glaspole I, Westall GP, Watson A, et al. Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev. (2017) 26:160080. doi: 10.1183/16000617.0080-2016
29. Dowman LM, McDonald CF, Bozinovski S, Vlahos R, Gillies R, Pouniotis D, et al. Greater endurance capacity and improved dyspnoea with acute oxygen supplementation in idiopathic pulmonary fibrosis patients without resting hypoxaemia. Respirology. (2017) 22:957–64. doi: 10.1111/resp.13002
30. Schaeffer MR, Ryerson CJ, Ramsook AH, Molgat-Seon Y, Wilkie SS, Dhillon SS, et al. Effects of hyperoxia on dyspnoea and exercise endurance in fibrotic interstitial lung disease. Eur Respir J. (2017) 49:1602494. doi: 10.1183/13993003.02494-2016
31. Visca D, Mori L, Tsipouri V, Fleming S, Firouzi A, Bonini M, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med. (2018) 6:759–70. doi: 10.1016/S2213-2600(18)30289-3
32. Hardinge M, Annandale J, Bourne S, Cooper B, Evans A, Freeman D, et al. British Thoracic Society guidelines for home oxygen use in adults: accredited by NICE. Thorax. (2015) 70:i1–43. doi: 10.1136/thoraxjnl-2015-206865
33. Jacobs SS, Krishnan JA, Lederer DJ, Ghazipura M, Hossain T, Tan AYM, et al. Home oxygen therapy for adults with chronic lung disease an official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. (2020) 202:E121–41. doi: 10.1164/rccm.202009-3608ST
34. Piotrowski W, Bestry I, Białas A, Boros P, Grzanka P, Jassem E, et al. Guidelines of the Polish Respiratory Society for diagnosis and treatment of idiopathic pulmonary fibrosis. Adv Respir Med. (2020) 88:42–94. doi: 10.5603/ARM.2020.0081
35. Ischaki E, Pantazopoulos I, Zakynthinos S. Nasal high flow therapy: a novel treatment rather than a more expensive oxygen device. Eur Respir Rev. (2017) 26:170028. doi: 10.1183/16000617.0028-2017
36. Bräunlich J, Beyer D, Mai D, Hammerschmidt S, Seyfarth HJ, Wirtz H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. (2013) 85:319–25. doi: 10.1159/000342027
37. Mündel T, Feng S, Tatkov S, Schneider H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol. (2013) 114:1058–65. doi: 10.1152/japplphysiol.01308.2012
38. Hui D, Morgado M, Chisholm G, Withers L, Nguyen Q, Finch C, et al. High-flow oxygen and bilevel positive airway pressure for persistent dyspnea in patients with advanced cancer: a phase II randomized trial. J Pain Symptom Manage. (2013) 46:463–73. doi: 10.1016/j.jpainsymman.2012.10.284
39. Koyauchi T, Hasegawa H, Kanata K, Kakutani T, Amano Y, Ozawa Y, et al. Efficacy and tolerability of high-flow nasal cannula oxygen therapy for hypoxemic respiratory failure in patients with interstitial lung disease with do-not-intubate orders: a retrospective single-center study. Respiration. (2018) 96:323–9. doi: 10.1159/000489890
40. Badenes-Bonet D, Cejudo P, Rodó-Pin A, Martín-Ontiyuelo C, Chalela R, Rodríguez-Portal JA, et al. Impact of high-flow oxygen therapy during exercise in idiopathic pulmonary fibrosis: a pilot crossover clinical trial. BMC Pulm Med. (2021) 21:355. doi: 10.1186/s12890-021-01727-9
41. Harada J, Nagata K, Morimoto T, Iwata K, Matsunashi A, Sato Y, et al. Effect of high-flow nasal cannula oxygen therapy on exercise tolerance in patients with idiopathic pulmonary fibrosis: a randomized crossover trial. Respirology. (2022) 27:144–51. doi: 10.1111/resp.14176
42. Al Chikhanie Y, Veale D, Verges S, Hérengt F. The effect of heated humidified nasal high flow oxygen supply on exercise tolerance in patients with interstitial lung disease: a pilot study. Respir Med. (2021) 186:106523. doi: 10.1016/j.rmed.2021.106523
43. Storgaard LH, Hockey HU, Laursen BS, Weinreich UM. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis. (2018) 13:1195–205. doi: 10.2147/COPD.S159666
44. Storgaard LH, Weinreich UM, Laursen BS. COPD Patients' experience of long-term domestic oxygen-enriched nasal high flow treatment: a qualitative study. COPD J Chronic Obstr Pulm Dis. (2020) 17:175–83. doi: 10.1080/15412555.2020.1736998
45. Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. (2017) 50:1602426. doi: 10.1183/13993003.02426-2016
46. Nava S, Ferrer M, Esquinas A, Scala R, Groff P, Cosentini R, et al. Palliative use of non-invasive ventilation in end-of-life patients with solid tumours: a randomised feasibility trial. Lancet Oncol. (2013) 14:219–27. doi: 10.1016/S1470-2045(13)70009-3
47. Bennett D, Fossi A, Bargagli E, Refini RM, Pieroni M, Luzzi L, et al. Mortality on the waiting list for lung transplantation in patients with idiopathic pulmonary fibrosis: a single-centre experience. Lung. (2015) 193:677–81. doi: 10.1007/s00408-015-9767-x
48. Chua F, Desai SR, Nicholson AG, Devaraj A, Renzoni E, Rice A, et al. Pleuroparenchymal fibroelastosis a review of clinical, radiological, and pathological characteristics. Ann Am Thorac Soc. (2019) 16:1351–9. doi: 10.1513/AnnalsATS.201902-181CME
49. Tanizawa K, Handa T, Kubo T, Chen-Yoshikawa TF, Aoyama A, Motoyama H, et al. Clinical significance of radiological pleuroparenchymal fibroelastosis pattern in interstitial lung disease patients registered for lung transplantation: a retrospective cohort study. Respir Res. (2018) 19:162. doi: 10.1186/s12931-018-0860-6
50. Faverio P, De Giacomi F, Sardella L, Fiorentino G, Carone M, Salerno F, et al. Management of acute respiratory failure in interstitial lung diseases: overview and clinical insights. BMC Pulm Med. (2018) 18:70. doi: 10.1186/s12890-018-0643-3
51. Mahler DA. Opioids for refractory dyspnea. Expert Rev Respir Med. (2013) 7:123–35. doi: 10.1586/ers.13.5
52. Krajnik M, Jassem E, Sobanski P. Opioid receptor bronchial tree: current science. Curr Opin Support Palliat Care. (2014) 8:191–9. doi: 10.1097/SPC.0000000000000072
53. Ekström M, Nilsson F, Abernethy AA, Currow DC. Effects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease. A systematic review. Ann Am Thorac Soc. (2015) 12:1079–92. doi: 10.1513/AnnalsATS.201501-034OC
54. Jennings A-L, Davies AN, Higgins JPT, Gibbs JSR, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. (2002) 57:939–44. doi: 10.1136/thorax.57.11.939
55. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. (2012) 185:435–52. doi: 10.1164/rccm.201111-2042ST
56. Currow DC, McDonald C, Oaten S, Kenny B, Allcroft P, Frith P, et al. Once-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J Pain Symptom Manage. (2011) 42:388–99. doi: 10.1016/j.jpainsymman.2010.11.021
57. Allen S, Raut S, Woollard J, Vassallo M. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat Med. (2005) 19:128–30. doi: 10.1191/0269216305pm998oa
58. Harris-Eze AO, Sridhar G, Clemens RE, Zintel TA, Gallagher CG, Marciniuk DD. Low-dose nebulized morphine does not improve exercise in interstitial lung disease. Am J Respir Crit Care Med. (1995) 152:1940–5. doi: 10.1164/ajrccm.152.6.8520759
59. Leung R, Hill P, Burdon J. Effect of inhaled morphine on the development of breathlessness during exercise in patients with chronic lung disease. Thorax. (1996) 51:596–600. doi: 10.1136/thx.51.6.596
60. Noseda A, Carpiaux J, Markstein C, Meyvaert A, de Maertelaer V. Disabling dyspnoea in patients with advanced disease: lack of effect of nebulized morphine. Eur Respir J. (1997) 10:1079–83. doi: 10.1183/09031936.97.10051079
61. Young IH, Daviskas E, Keena VA. Effect of low dose nebulised morphine on exercise endurance in patients with chronic lung disease. Thorax. (1989) 44:387–90. doi: 10.1136/thx.44.5.387
62. Freeman N, Le LW, Singer LG, Colman R, Zimmermann C, Wentlandt K. Impact of a transplant palliative care clinic on symptoms for patients awaiting lung transplantation. J Hear Lung Transplant. (2016) 35:1037–9. doi: 10.1016/j.healun.2016.05.006
63. Bajwah S, Davies JM, Tanash H, Currow DC, Oluyase AO, Ekström M. Safety of benzodiazepines and opioids in interstitial lung disease: a national prospective study. Eur Respir J. (2018) 52:OA3822. doi: 10.1183/13993003.congress-2018.OA3822
64. Ryerson CJ, Abbritti M, Ley B, Elicker BM, Jones KD, Collard HR. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology. (2011) 16:969–75. doi: 10.1111/j.1440-1843.2011.01996.x
65. Guenther A, Krauss E, Tello S, Wagner J, Paul B, Kuhn S, et al. The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res. (2018) 19:141. doi: 10.1186/s12931-018-0845-5
66. Wakwaya Y, Ramdurai D, Swigris JJ. Managing cough in idiopathic pulmonary fibrosis. Chest. (2021) 160:1774–82. doi: 10.1016/j.chest.2021.05.071
67. Van Manen MJG, Birring SS, Vancheri C, Cottin V, Renzoni EA, Russell AM, et al. Cough in idiopathic pulmonary fibrosis. Eur Respir Rev. (2016) 25:278–86. doi: 10.1183/16000617.0090-2015
68. Jones RM, Hope-Gill BD, Eccles R, Harrison NK. Mechanical induction of cough in idiopathic pulmonary fibrosis. In: American Thoracic Society International Conference Abstracts. New Orleans: American Thoracic Society (2010). p. A5553. doi: 10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A5553
69. Birring SS, Kavanagh JE, Irwin RS, Keogh KA, Lim KG, Ryu JH, et al. Treatment of interstitial lung disease associated cough: CHEST guideline and expert panel report. Chest. (2018) 154:904–17. doi: 10.1016/j.chest.2018.06.038
70. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. (2012) 380:1583–9. doi: 10.1016/S0140-6736(12)60776-4
71. Morice AH, Menon MS, Mulrennan SA, Everett CF, Wright C, Jackson J, et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med. (2007) 175:312–5. doi: 10.1164/rccm.200607-892OC
72. Horton MR, Santopietro V, Mathew L, Horton KM, Polito AJ, Liu MC, et al. Thalidomide for the treatment of cough in idiopathic pulmonary fibrosis: a randomized trial. Ann Intern Med. (2012) 157:398–406. doi: 10.7326/0003-4819-157-6-201209180-00003
73. Birring SS, Wijsenbeek MS, Agrawal S, van den Berg JWK, Stone H, Maher TM, et al. A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: a randomised, double-blind, proof-of-concept, phase 2 trial. Lancet Respir Med. (2017) 5:806–15. doi: 10.1016/S2213-2600(17)30310-7
74. Schaeffer MR, Ryerson CJ, Ramsook AH, Molgat-Seon Y, Wilkie SS, Dhillon SS, et al. Neurophysiological mechanisms of exertional dyspnoea in fibrotic interstitial lung disease. Eur Respir J. (2018) 51:1701726. doi: 10.1183/13993003.01726-2017
75. Harris-Eze AO, Sridhar G, Clemens RE, Gallagher CG, Marciniuk DD. Oxygen improves maximal exercise performance in interstitial lung disease. Am J Respir Crit Care Med. (1994) 150:1616–22. doi: 10.1164/ajrccm.150.6.7952624
76. O'Donnell DE, Milne KM, James MD, de Torres JP, Neder JA. Dyspnea in COPD: new mechanistic insights and management implications. Adv Ther. (2020) 37:41. doi: 10.1007/s12325-019-01128-9
77. Marillier M, Bernard AC, Verges S, Moran-Mendoza O, O'Donnell DE, Neder JA. Oxygen supplementation during exercise improves leg muscle fatigue in chronic fibrotic interstitial lung disease. Thorax. (2021) 76:672–80. doi: 10.1136/thoraxjnl-2020-215135
78. Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. (2007) 20:126–31. doi: 10.1016/j.aucc.2007.08.001
79. Goda K, Kenzaka T, Kuriyama K, Hoshijima M, Akita H. End-of-life home care of an interstitial pneumonia patient supported by high-flow nasal cannula therapy: a case report. World J Clin Cases. (2020) 8:4853. doi: 10.12998/wjcc.v8.i20.4853
80. Simonds AK, editor. ERS Practical Handbook of Noninvasive Ventilation. Vol. 11. European Respiratory Society (2015). Available online at: https://books.ersjournals.com/content/ers-practical-handbook-of-noninvasive-ventilation.tab-info
Keywords: breathlessness, dyspnea, cough, idiopathic pulmonary fibrosis (IPF), non-invasive ventilation (NIV), high flow nasal cannula (HFNC), ambulatory oxygen therapy (AOT), non-invasive positive pressure ventilation (NIPPV)
Citation: Janowiak P, Szymanowska-Narloch A and Siemińska A (2022) IPF Respiratory Symptoms Management — Current Evidence. Front. Med. 9:917973. doi: 10.3389/fmed.2022.917973
Received: 11 April 2022; Accepted: 11 July 2022;
Published: 28 July 2022.
Edited by:
Jin Woo Song, Asan Medical Center, South KoreaReviewed by:
Leticia Kawano Dourado, HCor Research Institute, BrazilCopyright © 2022 Janowiak, Szymanowska-Narloch and Siemińska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piotr Janowiak, cGlvdHIuamFub3dpYWtAZ3VtZWQuZWR1LnBs
 Piotr Janowiak
Piotr Janowiak Amelia Szymanowska-Narloch
Amelia Szymanowska-Narloch