
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 10 June 2022
Sec. Pulmonary Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.916817
This article is part of the Research Topic Case Reports in Pulmonary Medicine View all 17 articles
Background: Pulmonary actinomycosis (PA), a chronic indolent infection, is a diagnostic challenge. Actinomyces graevenitzii is a relatively rare Actinomyces species isolated from various clinical samples.
Case Presentation: A 47-year-old patient presented with a 3-month history of mucopurulent expectoration and dyspnea and a 3-day history of fever up to 39.0°C. He had dental caries and a history of alcoholism. Computed tomography (CT) images of the chest revealed a consolidation shadow in the right upper and middle lobes, with necrosis containing foci of air. Actinomyces graevenitzii was isolated from bronchoalveolar lavage fluid (BALF) culture and was identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. He received treatment with intravenous piperacillin-sulbactam for 10 days and oral amoxicillin-clavulanate for 7 months. His clinical condition had considerably improved. The consolidation shadow was gradually absorbed.
Conclusion: Early diagnosis and treatment of pulmonary actinomycosis are crucial. Bronchoscopy plays a key role in the diagnostic process, and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS) is an accurate tool for Actinomyces identification.
Actinomycosis is a rare chronic disease caused by the anaerobic Gram-positive bacteria Actinomyces spp, which normally inhabits the human oral cavity, digestive tract and genital tract (1). Due to its non-specific clinical manifestations and imaging characteristics, actinomycosis is easily misdiagnosed or missed. Pulmonary Actinomyces graevenitzii infection is extremely rare. The present case is the only case of pulmonary actinomycosis in our department. In this report, we describe it in detail and review the pertinent literature to improve our understanding of this disease, avoid misdiagnosis, and provide evidence for its clinical diagnosis, treatment, and prognosis.
A 47-year-old man was admitted to the Department of Pulmonary and Critical Care Medicine, Beijing Luhe Hospital, Capital Medical University (Beijing, China) with a 3-month history of mucopurulent expectoration and dyspnea. He was initially diagnosed with bronchitis at a health center in the local town and did not undergo chest radiography and laboratory tests. He received intravenous antibiotics for 14 days, but his symptoms were not relieved. Three days before admission, he started having a fever with a maximum temperature of 39.0°C. He did not report chest pain, hemoptysis, headache, vomiting, and other symptoms. His past medical history was unremarkable with no exposure to chronic diseases, infectious diseases or allergy. The patient was a worker and lived in an urban setting. No data on major epidemic, family history, toxic habits, or occupational exposure was reported. He was married and denied any sexually transmitted infections or drug abuse. He had a 30-year smoking history (20 cigarettes per day on average) and a 20-year history of alcohol intake of a 150 mL daily.
At admission, the patient was febrile with a temperature of 38.9°C, tachycardiac (107 beats/min), and tachypneic (22 breaths/min). His blood pressure (110/80 mmHg) and oxygen saturation (99%, measured by pulse oximetry while breathing ambient air) were normal. Examination of the oral cavity showed poor hygiene. There were wet rales in the right lung. The auscultation of the heart revealed regular rhythm without murmur. The abdomen was tender, and there was no organomegaly. No abnormal findings were found in the neurologic examination.
The laboratory data was as follows: the peripheral blood white cell count was 8,740 cells/μl with 78.40% neutrophils, and 14.10% lymphocytes, and 6.70% monocytes; hemoglobin 110 g/L, the hematocrit 34%, the platelet count 447,000 per cubic millimeter. The C-reactive protein (71.67 mg/L), and erythrocyte sedimentation rate (100 mm/h) were significantly elevated. The PCT was mildly increased (0.13 ng/ml). Biochemical analysis of liver and renal showed normal range except slightly decreased albumin (32.1 g/L). Coagulation profile was normal and the serum tumor markers were negative. The results for possible connective tissue disease, such as antinuclear antibody, extractable nuclear antigen, and antineutrophil cytoplasmic antibody, were also negative. The serum IgM antibodies specific for respiratory pathogens, including Legionella pneumophila antibody, Mycoplasma pneumoniae antibody, Chlamydia pneumoniae antibody, influenza A virus antibody, and influenza B virus antibody, revealed no abnormality. The serum (1,3)-beta-D-glucan test (G test), the galactomannan test (GM test), and the serum TB test (T-SPOT) were all negative. The results of two blood cultures were negative. No pathological findings were detected in repeated sputum smears and cultures. A chest computed tomography (CT) scan showed a consolidation shadow in the upper and middle lobes of right lung with foci of necrosis (Figure 1).
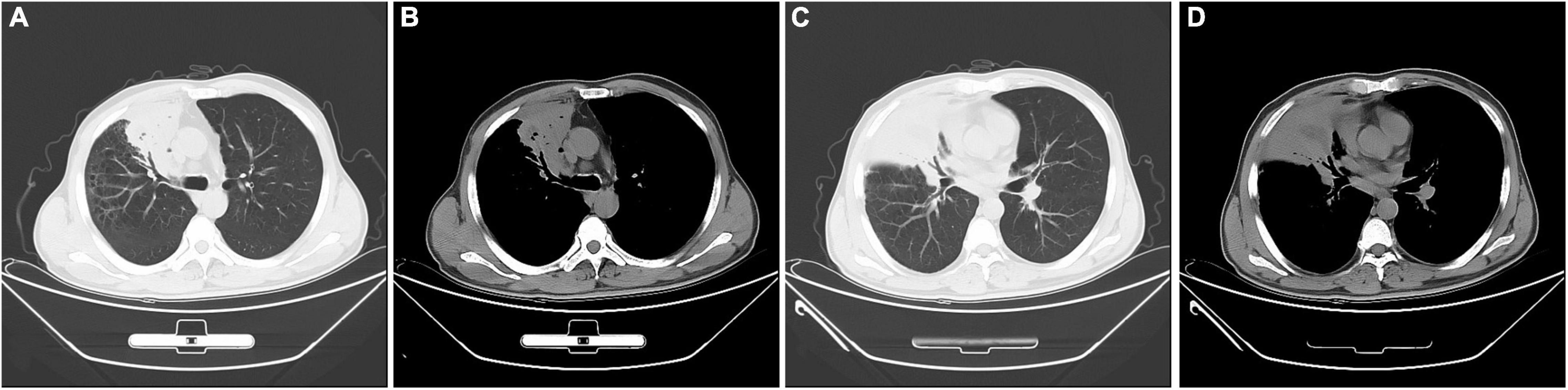
Figure 1. Chest computed tomography images at admission. A consolidation shadow in the right upper and middle lobes, with necrosis containing foci of air. (A,C) lung window; (B,D) mediastinal window.
Initially, We considered pneumonia as a possible diagnosis, which needed to be identified with lung cancer or pulmonary tuberculosis, and Antibiotic therapy with intravenous piperacillin-sulbactam was initiated. To make a definitive diagnosis, we performed a flexible bronchoscopy (Figures 2A–C), revealing purulent secretions in the medial and lateral segments of the right middle lobe (RML), drastically obstructing the lumen. After suction, the purulent secretions of the medial segment were reduced and the lateral segment was unobstructed. Then, bronchial brushing (BB), bronchial biopsy, and bronchoalveolar lavage were performed in the medial segment of the RML. Bronchial mucosal biopsy and BB specimens revealed only non-specific inflammation of the mucosa, and no pathogens were observed in the bronchoalveolar lavage fluid (BALF) through routine specimen smear and culture. On the third day after admission, the patient had no fever and continued to receive intravenous piperacillin-sulbactam therapy. To reduce secretions and search pathogens, we performed a second flexible bronchoscopy (Figures 2D–F), showing purulent secretions in the medial segment of the RML and the anterior segment of the right upper lobe (RUL). The BALF sample of the second time was sent for microbiological analysis. We took an appropriate amount of BALF samples for Gram staining, which is blue-purple (Figure 3). The BALF specimen was vortexed and shaken for 30–60 s. We used a sterile tool to spread a 10 μL calibration sample on the surface of the blood plate. The inoculated blood plate was incubated at 35°C for 48 h in a carbon dioxide incubator (5–10% CO2). The colony growing on the culture medium was smeared on a target plate. After drying, it was covered using a 1 μL Bruker matrix solution. When it was dry, the target plate was loaded into the machine: Microflex LT (Bruker Daltonics, Bremen, Germany). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS) for the identification of pathogens was performed, and Actinomyces graevenitzii was identified from the BALF. These results indicated that the pulmonary lesion of our patient was caused by A. graevenitzii. After administration of antibiotic treatment with piperacillin-sulbactam for ten days, the clinical condition of the patient improved obviously, and the CRP dropped to 29.34 mg/L, though the pulmonary lesions on the chest CT scan (Figures 4A–D) were not significantly absorbed. The patient was discharged with oral amoxicillin-clavulanate. A timeline with all relevant data from this clinical case is available in Figure 5. At the 7-month follow-up, the clinical condition of the patient was getting better and the chest CT scans (Figure 4) revealed that the consolidation shadow of the right lung was gradually absorbed.

Figure 2. A series of bronchoscopy images. The first bronchoscopy images (A–C), the secondary bronchoscopy images (D–F). (A) The medial and lateral segments of the RML were blocked by purulent yellow secretions. (B) The medial subsegment of the RML was completely obstructed by an endobronchial white necrotized mass. (C) The media subsegment of the RML became unobstructed after suction. (D) The medial and lateral segments of the RML were blocked by purulent yellow secretions. (E) The medial subsegment of the RML was completely obstructed by a an endobronchial white necrotized mass. (F) The media subsegment of the RML became unobstructed after suction. RML: Right middle lobe.
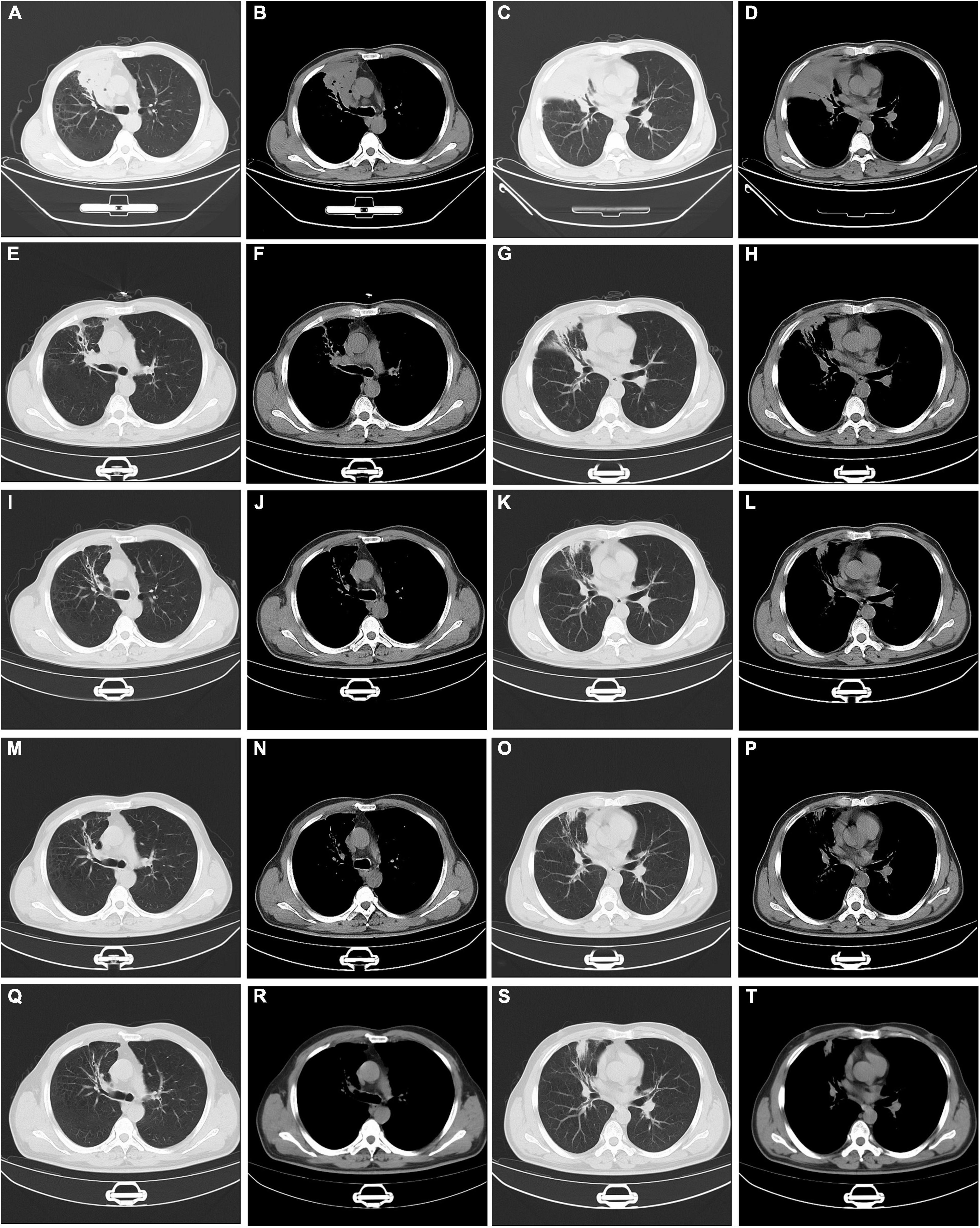
Figure 4. Serial changes on chest computed tomography findings. Chest CT at discharge (A–D). Chest CT at one month’s follow-up (E–H). Chest CT at three months’ follow-up (I–L). Chest CT at five months’ follow-up (M–P). Chest CT at seven months’ follow-up (Q–T). CT: computed tomography.
Nowdays, more and more pulmonary actinomycosis is identified, through its clinical manifestations and imaging characteristics lack specificity. However, to date, there are only few reports of pulmonary Actinomyces graevenitzii infection. In the present study, we described a case of pulmonary Actinomyces graevenitzii infection diagnosed by microbiological identification through matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS). After treatment with targeted antibiotic, the clinical manifestations and imaging presentations of the patient gradually improved.
Actinomycosis is a slowly progressing granulomatous disease caused by Actinomyces species. Actinomyces is an anaerobic gram-positive bacterium, belonging to the human commensal flora of the oropharynx, gastrointestinal tract, and urogenital tract. Some species of Actinomyces have already been described, including Actinomyces israelii (2, 3), Actinomyces odontolyticus (4), Actinomyces viscocus (5), Actinomyces meyeri (6), Actinomyces gerencseriae (7), Actinomyces naeslundii (8), and Actinomyces graevenitzii (9–16). Among them, A. israelii is the most prevalent species isolated in human infections (9, 17–19). However, in our case, A. graevenitzii was identified as the pathogenic bacteria isolated from bronchoalveolar lavage fluid. A. graevenitzii was first described in 1997 by Ramos et al. (20) in human clinical specimens (three respiratory and one bone samples). Being a catalase-negative, facultatively anaerobic, gram-positive, rod-shaped organism, A. graevenitzii is isolated almost exclusively from oral or respiratory sites and may have a unique ability to cause clinical actinomycosis (21). Although the clinical prevalence and pathogenic potential of A. graevenitzii is little known, the frequency of isolation from clinical specimens is increasing. We performed a PubMed search with the term “Actinomyces graevenitzii” or “pulmonary Actinomyces graevenitzii infection,” and found that seven case reports in patients with pulmonary Actinomyces graevenitzii infection had been published (1, 6, 10–15). We reviewed the eight cases involving pulmonary actinomycosis in patients with A. graevenitzii infection, including our patient. The clinical features of the cases are shown in Table 1.
The pathogenesis of actinomycosis remains unclear, however some factors probably promote the disease (1). Pulmonary actinomycosis results mainly from aspiration of oropharyngeal or gastrointestinal secretions, and poor oral hygiene (22), pre-existing dental disease (4, 23), and alcohol abuse are the important predisposing factors for actinomycosis. Other risk factors include chronic lung diseases, such as emphysema, chronic bronchitis, and bronchiectasis (1). When local mucosal tissue is damaged, infection occurs in the form of continuous growth through the anatomical barrier, leading to the formation of abscesses and fistulas (17). In the eight cases, our case had dental caries, one case had periodontitis (15), and another case had septic mouth with several teeth missing (14). In our case, the patient also had a drinking habit. Actinomycosis is frequently associated with immunocompromised states. Cohen R D et al. described a case of pulmonary actinomycosis in association with infliximab treatment for Crohn’s disease (16). One case of disseminated coinfection with A. graevenitzii and Mycobacterium tuberculosis has been reported (10). Besides those, it can also affect healthy people (4). S Gliga et al. reported a healthy young man of pulmonary A. graevenitzii infection (13).
The clinical symptoms of pulmonary actinomycosis are often non-specific, including fever, cough, dyspnea, or chest pain (24). Therefore, early diagnosis of the slowly progressing actinomycosis is difficult. In eight cases, patients were required be distinguished from tuberculosis, cancer, pulmonary coccidioidomycosis, or atypical pneumonia.
Radiological findings of pulmonary actinomycosis are also non-specific, including mass (23), nodules, patchy infiltrates, segmental air-space consolidation, and cavitation. It’s often confused with malignancy or tuberculosis. A characteristic CT finding is a central low density within the parenchymal consolidation and adjacent pleural thickening (25, 26). Initially, the disease is present as a small, poorly defined nodule in the peripheral lung, with or without an interlobular septal thickening. Gradually, the nodule develops into a segmental air-space consolidation or mass. With the slow progression of infection, a cavity forms in central areas of low attenuation. CT enhanced images may show rim-like peripheral enhancement and multiple central low-attenuation areas. Further progression of the disease may involve the pleura, the chest wall, or the neighboring pulmonary lobes (25–28). In the eight cases, four cases showed nodules, three cases showed air-space consolidation. And five of eight cases had a cavity formation.
Bacterial cultures and histopathological features of biopsy specimens are the cornerstone of diagnosis. However, it is challenging to confirm the presence of bacterial by culture due to antibiotic treatment, concomitant organisms growth, or inadequate conditions (1). Therefore, this requires clear communication of the suspicion of actinomycosis with the microbiology laboratory. With the development of techniques, more bacterial identification methods are increasingly used, including 16S rRNA gene sequence analysis, next-generation sequencing (29) or MALDI-TOF/MS. In our case, the patient was diagnosed by bronchoalveolar lavage fluid culture using mass spectrometry, with a consolidation with central low density on chest CT. In addition, PCR analysis and 16S rRNA gene sequencing analysis are used to identify pathogens in four cases (10–12, 15). For pathology, the main non-surgical diagnostic methods are ultrasound or CT-guided percutaneous lung biopsy and transbronchial biopsy, with a high positive rate. The identification of actinomycete hyphae and sulfur particles in biopsy samples is the gold standard for diagnosis. In the eight cases, pulmonary A. graevenitzii infection was confirmed via sulfur granules or Actinomyces species detected in sputum (10), bronchoalveolar lavage (BAL) (12, 13, 15, 16), video-assisted thoracoscopic lung biopsy (11, 12), or transbronchial needle aspiration biopsy of lymph node guided by endoscopic ultrasonography (14).
A long term beta-lactam antibiotic therapy is needed for patiens with pulmonary actinomycosis. The intravenous administration of penicillin G is recommended for 2 to 6 weeks, followed by oral administration of penicillin V or amoxicillin for 6 to 12 months (1). It may be necessary to perform surgical management to drain voluminous abscesses, marsupialize chronic sinus tracts, and excise fibrotic lesions. All the eight cases were treated with antibiotics, mainly beta-lactams, and the duration of treatment varied from 5 weeks to 7 months with one case not mentioned. Of note, our case was successfully treated for 7 months and was being followed up closely. Pleasingly, his clinical status and imaging presentations were getting better.
In conclusion, our case and literature review indicated that pulmonary A. graevenitzii infection was a rare disease with non-specific clinical characteristics. The diagnosis is mainly based on pathogen identification and histopathology. We need to consider the possibility of pulmonary actinomycosis when investigating a consolidation shadow with necrosis or trans-fissural extension. Bronchoscopy plays a key role in the non-invasive diagnostic procedure, providing specimens to make the final diagnosis. In addition, matrix- assisted laser desorption/ionization time-of-flight mass spectrometry is an accurate tool for Actinomyces identification.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YY and ZH: concept and writing of the manuscript. DP: interpretation of the sources and patient acquisition. ZX: image example. JW and SZ: supervision and concept. All authors read and approved the final draft, contributed to the article, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Valour F, Sénéchal A, Dupieux C, Karsenty J, Lustig S, Breton P, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. (2014) 7:183–97. doi: 10.2147/IDR.S39601
2. Tabarsi P, Yousefi S, Jabbehdari S, Marjani M, Baghaei P. Pulmonary actinomycosis in a patient with AIDS/HCV. J Clin Diagn Res. (2017) 11:OD15–7. doi: 10.7860/JCDR/2017/27593.10092
4. Crisafulli E, Bernardinello N, Alfieri V, Pellegrino F, Lazzari C, Gnetti L, et al. A pulmonary infection by Actinomyces odontolyticus and Veillonella atypica in an immunocompetent patient with dental caries. Respirol Case Rep. (2019) 7:e00493. doi: 10.1002/rcr2.493
5. Mosimann J, Hany A, Kayser FH. Pulmonary Actinomyces-viscosus infection. Schweiz Med Wochenschr. (1979) 109:720–2.
6. Shimoda M, Yoshiyama T, Okumura M, Tanaka Y, Morimoto K, Kokutou H, et al. Actinomyces meyeri pleural infection that was difficult to treat due to delayed culture: a case report and literature review of 28 cases. Respir Med Case Rep. (2021) 34:101530. doi: 10.1016/j.rmcr.2021.101530
7. Llamas-Velasco M, Domínguez I, Ovejero E, Pérez-Gala S, García-Diez A. Empyema necessitatis revisited. Eur J Dermatol. (2010) 20:115–9.
8. Supriya BG, Harisree S, Savio J, Ramachandran P. Actinomyces naeslundii causing pulmonary endobronchial Actinomycosis - A case report. Indian J Pathol Microbiol. (2019) 62:326–8. doi: 10.4103/IJPM.IJPM_706_17
9. Kononen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. (2015) 28:419–42. doi: 10.1128/CMR.00100-14
10. Tietz A, Aldridge KE, Figueroa JE. Disseminated coinfection with Actinomyces graevenitzii and Mycobacterium tuberculosis: case report and review of the literature. J Clin Microbiol. (2005) 43:3017–22. doi: 10.1128/JCM.43.6.3017-3022.2005
11. Fujita Y, Iikura M, Horio Y, Ohkusu K, Kobayashi N. Pulmonary Actinomyces graevenitzii infection presenting as organizing pneumonia diagnosed by PCR analysis. J Med Microbiol. (2012) 61:1156–8. doi: 10.1099/jmm.0.040394-0
12. Nagaoka K, Izumikawa K, Yamamoto Y, Yanagihara K, Ohkusu K, Kohno S. Multiple lung abscesses caused by Actinomyces graevenitzii mimicking acute pulmonary coccidioidomycosis. J Clin Microbiol. (2012) 50:3125–8. doi: 10.1128/JCM.00761-12
13. Gliga S, Devaux M, Gosset Woimant M, Mompoint D, Perronne C, Davido B. Actinomyces graevenitzii pulmonary abscess mimicking tuberculosis in a healthy young man. Can Respir J. (2014) 21:75–7. doi: 10.1155/2014/841480
14. Caballero Vázquez A, Cruz Rueda JJ, Ceballos Gutierrez JA. Diagnosis of actinomyces graevenitzii lung infection using linear EBUS. Arch Bronconeumol. (2017) 53:351–2. doi: 10.1016/j.arbres.2016.10.014
15. Himeji D, Hara S, Kawaguchi T, Tanaka GI. Pulmonary actinomyces graevenitzii infection diagnosed by bronchoscopy using endobronchial ultrasonography with a guide sheath. Intern Med. (2018) 57:2547–51. doi: 10.2169/internalmedicine.9799-17
16. Cohen RD, Bowie WR, Enns R, Flint J, Fitzgerald JM. Pulmonary actinomycosis complicating infliximab therapy for Crohn’s disease. Thorax. (2007) 62:1013–4. doi: 10.1136/thx.2006.075150
18. Pulverer G, Schutt-Gerowitt H, Schaal K. Human cervicofacial actinomycoses: microbiological data for 1997 cases. Clin Infect Dis. (2003) 37:490–7. doi: 10.1086/376621
19. Martinez-Giron R, Pantanowitz L. Pulmonary actinomycosis: cytomorphological features. Monaldi Arch Chest Dis. (2021) 92:1641. doi: 10.4081/monaldi.2021.1641
20. Ramos CP, Falsen E, Alvarez N, Akervall E, Sjödén B, Collins MD. Actinomyces graevenitzii sp. nov., isolated from human clinical specimens. Int J Syst Bacteriol. (1997) 47:885–8. doi: 10.1099/00207713-47-3-885
21. Hall V. Actinomyces–gathering evidence of human colonization and infection. Anaerobe. (2008) 14:1–7. doi: 10.1016/j.anaerobe.2007.12.001
22. Grzywa-Celińska A, Emeryk-Maksymiuk J, Szmygin-Milanowska K, Czekajska-Chehab E, Milanowski J. Pulmonary actinomycosis - the great imitator. Ann Agric Environ Med. (2017) 25:211–2. doi: 10.26444/aaem/75652
23. Ito T, Yoshida T, Sakuraba M, Tanaka A, Hamada K, Ito A. Insufficient initial treatment but good recovery after diagnosis of pulmonary actinomycosis. Oxf Med Case Rep. (2019) 2019:510–2. doi: 10.1093/omcr/omz123
24. Bonnefond S, Catroux M, Melenotte C, Karkowski L, Rolland L, Trouillier S, et al. Clinical features of actinomycosis: a retrospective, multicenter study of 28 cases of miscellaneous presentations. Medicine. (2016) 95:e3923. doi: 10.1097/MD.0000000000003923
25. Cheon JE, Im JG, Kim MY, Lee JS, Choi GM, Yeon KM. Thoracic actinomycosis: CT findings. Radiology. (1998) 209:229–33.
26. Han JY, Lee KN, Lee JK, Kim YH, Choi SJ, Jeong YJ, et al. An overview of thoracic actinomycosis: CT features. Insights Imaging. (2013) 4:245–52. doi: 10.1007/s13244-012-0205-9
27. Kim TS, Han J, Koh WJ, Choi JC, Chung MJ, Lee JH, et al. Thoracic actinomycosis: CT features with histopathologic correlation. AJR Am J Roentgenol. (2006) 186:225–31. doi: 10.2214/AJR.04.1749
28. Łyżwa E, Siemion-Szcześniak I, Sobiecka M, Kacprzak A, Winiarska A, Szołkowska M, et al. Pulmonary actinomycosis complicated by fistula of the chest wall. Adv Respir Med. (2021) 89:532–7. doi: 10.5603/ARM.a2021.0071
Keywords: pulmonary actinomycosis (PA), Actinomyces graevenitzii, bronchoscopy, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, consolidation
Citation: Yuan Y, Hou Z, Peng D, Xing Z, Wang J and Zhang S (2022) Pulmonary Actinomyces graevenitzii Infection: Case Report and Review of the Literature. Front. Med. 9:916817. doi: 10.3389/fmed.2022.916817
Received: 10 April 2022; Accepted: 24 May 2022;
Published: 10 June 2022.
Edited by:
Shu-Min Lin, Linkou Chang Gung Memorial Hospital, TaiwanReviewed by:
Mohamed Yassin, University of Pittsburgh, United StatesCopyright © 2022 Yuan, Hou, Peng, Xing, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuai Zhang, emhhbmdzaHVhaTE5NzRAY2NtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.