- 1Department of Dermatology and Venereology, The Sixth Affiliated Hospital of Kunming Medical University, Yuxi, China
- 2Department of Dermatology and Venereology, The Second Affiliated Hospital of Kunming Medical University, Kunming, China
- 3Department of Intense Care Unit, Ziyang Hospital of Traditional Chinese Medicine, Ziyang, China
- 4Department of General Medicine, The Sixth Affiliated Hospital of Kunming Medical University, Yuxi, China
- 5Department of Dermatology and Venereology, The First Affiliated Hospital of Kunming Medical University, Kunming, China
Background: Eczema characterized by itch, sleeplessness, and adverse effects on quality of life is associated with a risk of hematological malignancies. However, there is a controversy pertaining to whether this association implies a greater or lesser risk of hematological cancers. We aimed to explore the link between eczema and hematological malignancies risk.
Methods: We systematically searched PubMed and Embase databases from their inception to February 17, 2022. Two reviewers independently screened articles, extracted data and assessed study quality, respectively. The odds ratios and 95% confidence intervals (CIs) were pooled by using fixed or random-effects models.
Results: 29 studies involving 2,521,574 participants examined the contribution of eczema to hematological malignancies. We found that eczema significantly increased the risk of Hodgkin's lymphoma (1.44; 95% CI, 1.07–1.95), myeloma (1.15; 95% CI, 1.04–1.28), and significantly decreased the risk of lymphocytic leukemia (0.91; 95% CI, 0.84–0.99); however, it is not significantly associated with Non-Hodgkin's lymphoma, and myelocytic leukemia.
Conclusion: Eczema has been shown to be associated with the risk of hematological cancer, this association still needs to be verified in large randomized controlled trials.
Systematic Review Registration: https://inplasy.com/, INPLASY202260097.
Introduction
Hematological malignancies, mainly including lymphoma, leukemia, myeloma, are a common group of highly heterogeneous disorders characterized by uncontrolled proliferation and differentiation of hematopoietic cells (1). In accordance with American Cancer Society, approximately 184,130 new cases and 57,810 deaths of hematological malignancies were estimated in the United States in 2022 (2). Although many researchers have focused on the pathogenesis of hematologic malignancies in recent years and new treatments are available, the long-term survival rate is still unsatisfactory (3–5). Therefore, there is an urgent need to find new specific and non-invasive indicators to evaluate the prognosis of the disease so as to carry out early intervention.
Eczema (atopic dermatitis), one of the most common chronic skin diseases, affecting more than 2.5% of adults and 10% of children (6), may be associated with risk of hematological malignancies (7). However, until now, the role of eczema in the incidence of hematological tumors has been controversial, so this study was designed to explore the relationship between eczema and the risk of hematological cancers.
Methods
Search Strategy
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (8, 9), we performed this study. PubMed and Embase databases were systematically searched till February 17, 2022 to find studies performed on the relationship between eczema and hematological cancers. Electronic search strategy is available in Supplementary Material 1. The bibliographies of these literatures were conducted by meticulous analysis of the references listed in the selected articles. Two reviewers (LJ and ZY) did the screening independently, and resolved the conflict through discussion. Firstly, we screened relevant articles based on the titles and abstracts. Then, all papers passing the initial screening would be reviewed the full text.
Selection Criteria
The inclusion criteria were as follows: all cohort and case-control studies focusing on the relationship between eczema and hematological malignancy; odds ratios (ORs) and 95% confidence intervals (CIs) were provided or calculated. Animal studies, reports with no indication of the association between eczema and cancers, and reviews were excluded. If there is duplication of data in these studies, we selected the study including the largest sample size. The quality of the studies were evaluated according to the Newcastle-Ottawa Scale (NOS) (10). Studies with NOS scores ≥ 7 were considered to be qualified.
Data Analysis
Two teams (LZH and XSY formed one team; ZY, and LJ formed the other) extracted independently all data. When one study included more than one cohort, we pooled the cohorts and considered each cohort as an independent study. For each independent study, we recorded the following information: first author's name, publication year, study region, study design, type of cancer, participants' sex and age, sample size, and adjustment factors.
The ORs and 95% CIs reported in the studies were pooled by meta-analysis. The Cochrane Q and I2 statistics were used to evaluate heterogeneity (11). When P value was <0.10 or the I2 value was >50%, the data were considered heterogeneous, and a random-effects model (12) was applied. Otherwise, a fixed-effects model (13) was used. To further explore the origin of heterogeneity, we performed subgroup analyses by region, study design, and cancer type. To assess the credibility of our results, sensitivity analyses were conducted by excluding each study in turn to estimate the influence of each individual study on the pooled results. Begg's test (14) and Egger's test (15) were used to assess the potential publication bias. STATA software v12.0 (College Station, TX, USA) was used to analyze the data.
Results
Study Selection and Basic Characteristics of the Included Studies
A total of 13,562 studies were retrieved from the PubMed and the Embase databases, and after removing 4,305 duplicates and further excluding 8,971 studies following title and abstract screening and 270 based on the full article, 16 studies remained. However, 13 additional eligible studies were identified following screening of the bibliographies of relevant studies. Finally, 29 studies involving 2,521,574 participants examined the contribution of eczema to hematological malignancies (Figure 1). Details on the characteristics of the studies are summarized in Supplementary Table 1.
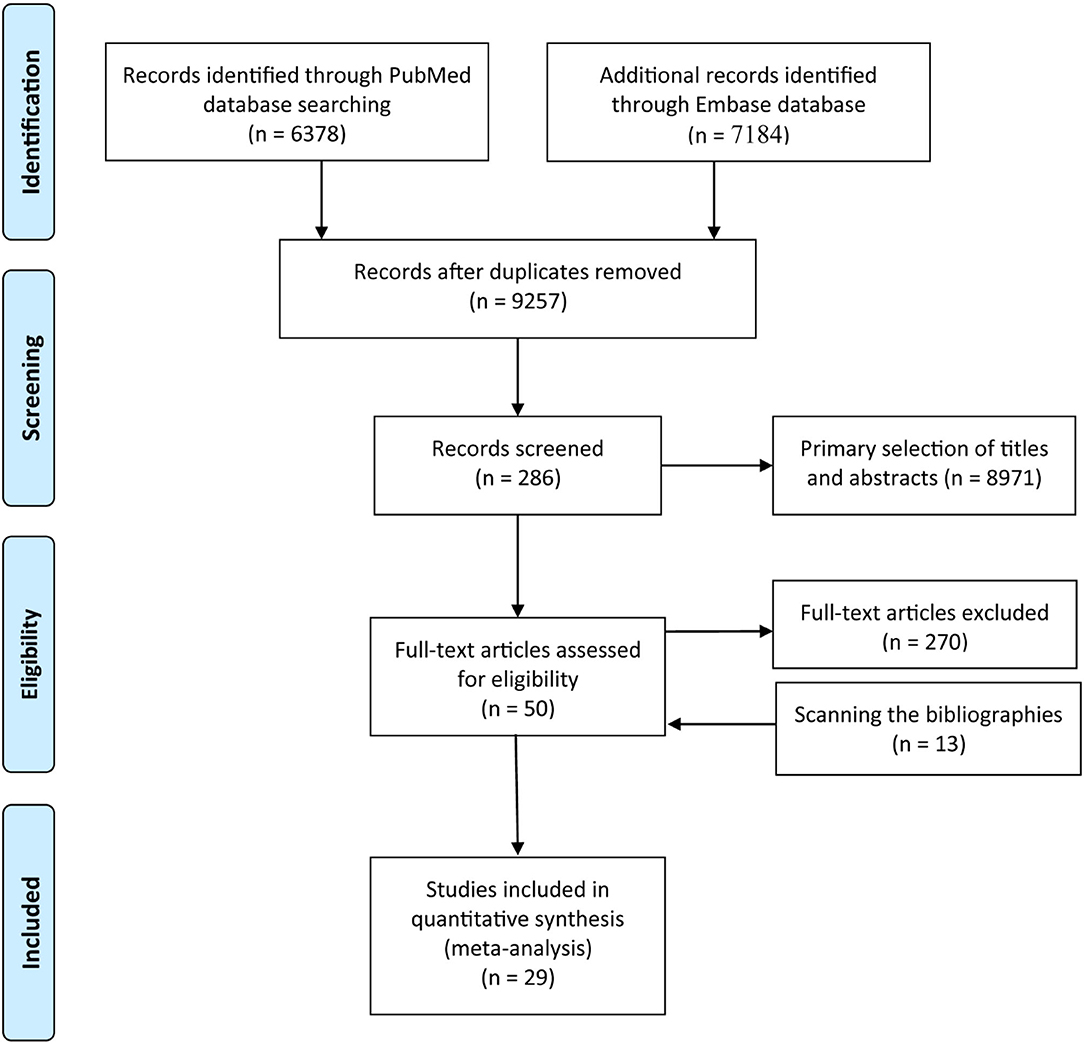
Figure 1. Flow diagram summarizing the pooled analysis phases (i.e., identification, screening, eligibility assessment, and ultimate inclusion).
The Association of Eczema to Non-Hodgkin's Lymphoma
Firstly, the pooled analysis of 17 studies (16–32) indicated that eczema was not significantly associated with an increased risk of Non-Hodgkin's lymphoma (OR, 1.10 [95% CI, 0.99–1.24], P(ES)= 0.087); besides, substantial heterogeneity was observed (Pheterogeneity = 0.000, I2 = 71.3%) (Figure 2A). Sensitivity analysis revealed that after excluding the studies by Bernstein and Ross (18), Fabbro-Peray et al. (21), and Grulich et al. (24). In turn, the overall combined results were altered (Figure 2B), and after excluding the three studies, the resulting data showed that eczema was statistically associated with an increased risk of Non-Hodgkin's lymphoma (OR, 1.19 [95% CI, 1.08–1.32], P(ES) = 0.000; Pheterogeneity = 0.002, I2 = 58.6%) (Figure 2C). Subgroup pooled analyses were performed according to study design, and we found that eczema was statistically associated with an increased risk of Non-Hodgkin's lymphoma in the cohort subgroup (OR, 1.20 [95% CI, 1.07–1.33], P(ES) = 0.001; Pheterogeneity = 0.921, I2 = 0.0%), but not the case-control subgroup (OR, 1.08 [95% CI, 0.95–1.24], P(ES) = 0.252; Pheterogeneity = 0.000, I2 = 75.4%) (Figure 2D). Publication bias tests were performed following Begg's rank correlation and Egger's linear regression tests, which indicated that no publication bias existed among the studies (Begg's: P > |z| = 1.000; Egger's: P = 0.851, [95% CI: −1.568–1.307]) (Figures 2E,F).
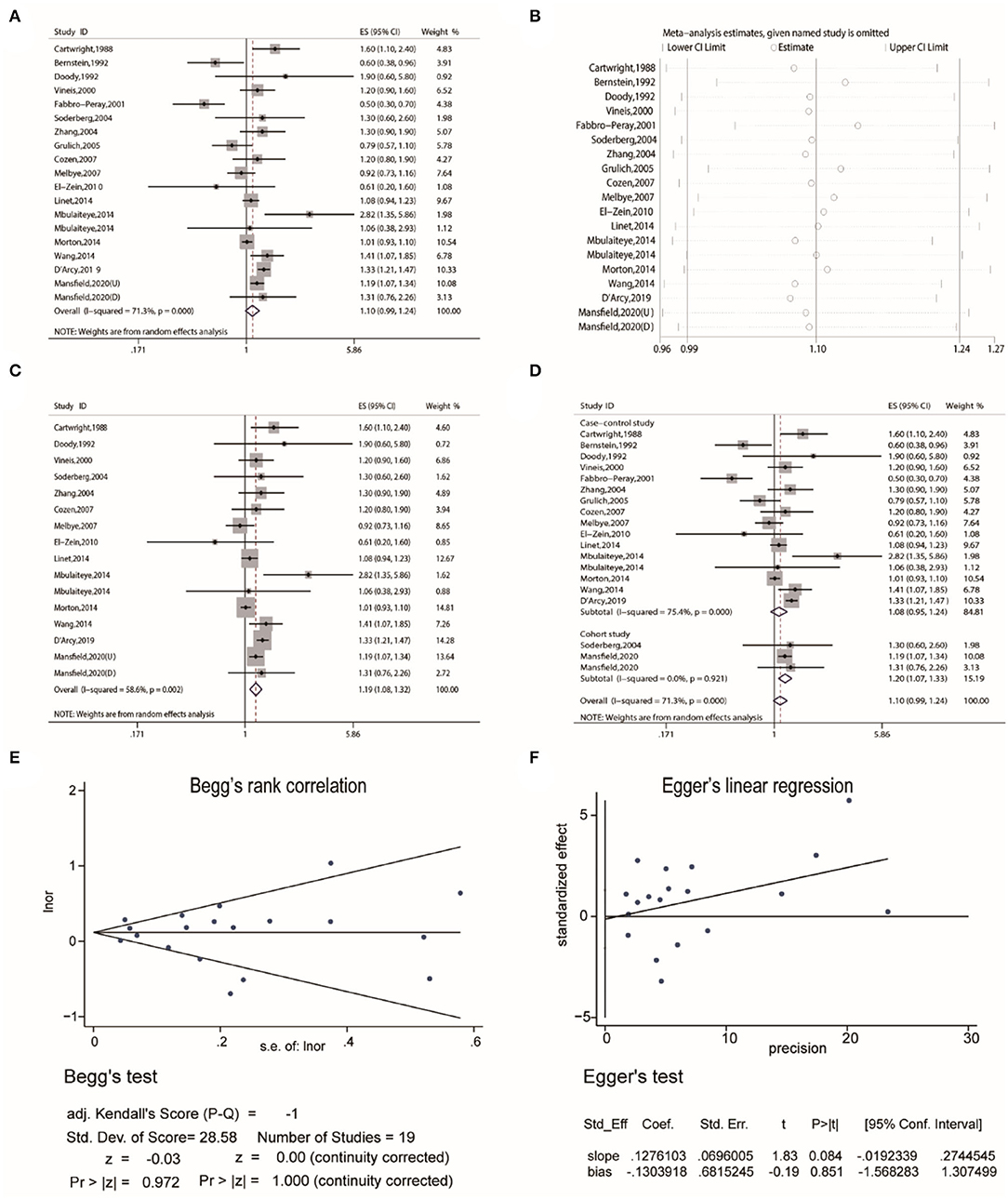
Figure 2. The association between eczema and non-Hodgkin's lymphoma risk. (A) Forest plot of the estimated effects of eczema on non-Hodgkin's lymphoma risk. (B) Sensitivity analysis conducted by recalculating the pooled results of the primary analysis following the exclusion of one study per iteration. (C) Sensitivity analysis conducted by recalculating the pooled results of the primary analysis following the exclusion of the Bernstein et al., Fabbro-Peray et al., and Grulich et al. studies. (D) Subgroup analysis of the estimated effects of eczema on non-Hodgkin's lymphoma risk by study design. (E) Begg's test indicating the lack of publication bias among such studies. (F) Egger's test indicating the lack of publication bias among such studies.
The Association of Eczema to Hodgkin's Lymphoma
Secondly, the pooled analysis of six studies (16, 20, 25, 32, 33) indicated that eczema significantly increased the risk of Hodgkin's lymphoma (OR, 1.44 [95% CI, 1.07–1.95], P(ES) = 0.016; Pheterogeneity = 0.005, I2 = 70.4%) (Figure 3A). Further subgroup analyses performed by study region indicated that eczema significantly increased the risk of Hodgkin's lymphoma in the American subgroup (OR, 2.24 [95% CI, 1.48–3.39], P(ES) = 0.000; Pheterogeneity = 0.289, I2 = 11.0%) (Figure 3B).
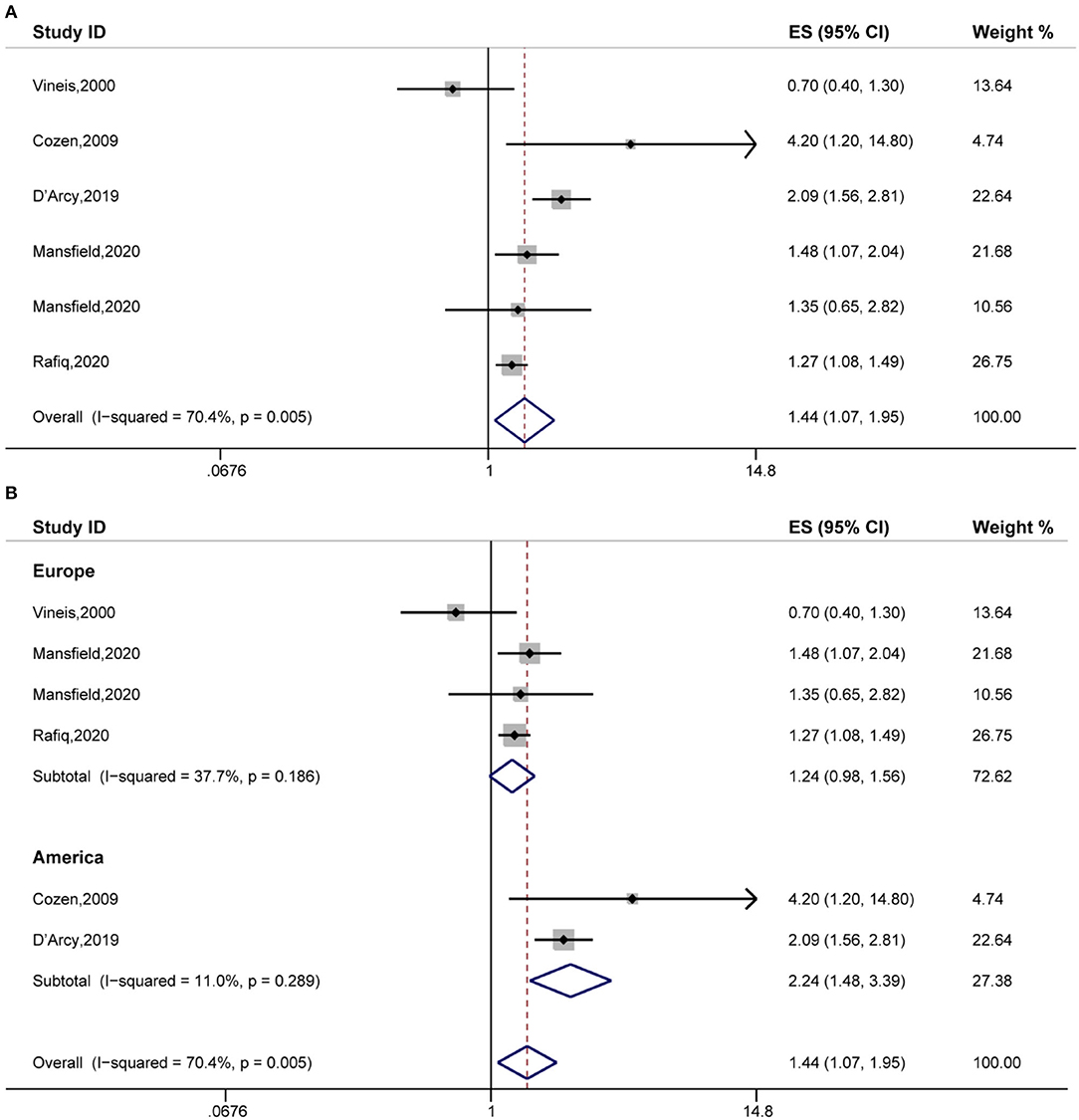
Figure 3. The association between eczema and Hodgkin's lymphoma risk. (A) Forest plot of the estimated effects of eczema on Hodgkin's lymphoma risk. (B) Subgroup analysis of the estimated effects of eczema on Hodgkin's lymphoma risk.
The Association of Eczema to Lymphocytic Leukemia
Thirdly, the pooled analysis of 13 studies (19, 20, 22, 26, 32, 34–40) indicated that eczema was significantly associated with a decreased risk of lymphocytic leukemia (OR, 0.91 [95% CI, 0.84–0.99], P(ES) = 0.029; Pheterogeneity = 0.021, I2 = 49.7%) (Figure 4A). Analyses performed in Europe, indicated that eczema significantly decreased the risk of lymphocytic leukemia (OR, 0.79 [95% CI, 0.68–0.91], P(ES) = 0.002; Pheterogeneity = 0.111, I2 = 46.7%) (Figure 4B). Further subgroup analyses performed by specific cancer type indicated that eczema significantly decreased the risk of acute lymphocytic leukemia (OR, 0.76 [95% CI, 0.67–0.88], P(ES) = 0.000; Pheterogeneity = 0.120, I2 = 40.7%) (Figure 4C). Sensitivity analysis revealed that after excluding each study in turn, the overall combined results were not altered (Figure 4D). Publication bias tests were performed following Begg's rank correlation and Egger's linear regression tests, which indicated that no publication bias existed among the studies (Begg's: P > |z| = 0.669; Egger's: P = 0·567, [95% CI:−1.178–2.042]) (Figures 4E,F).
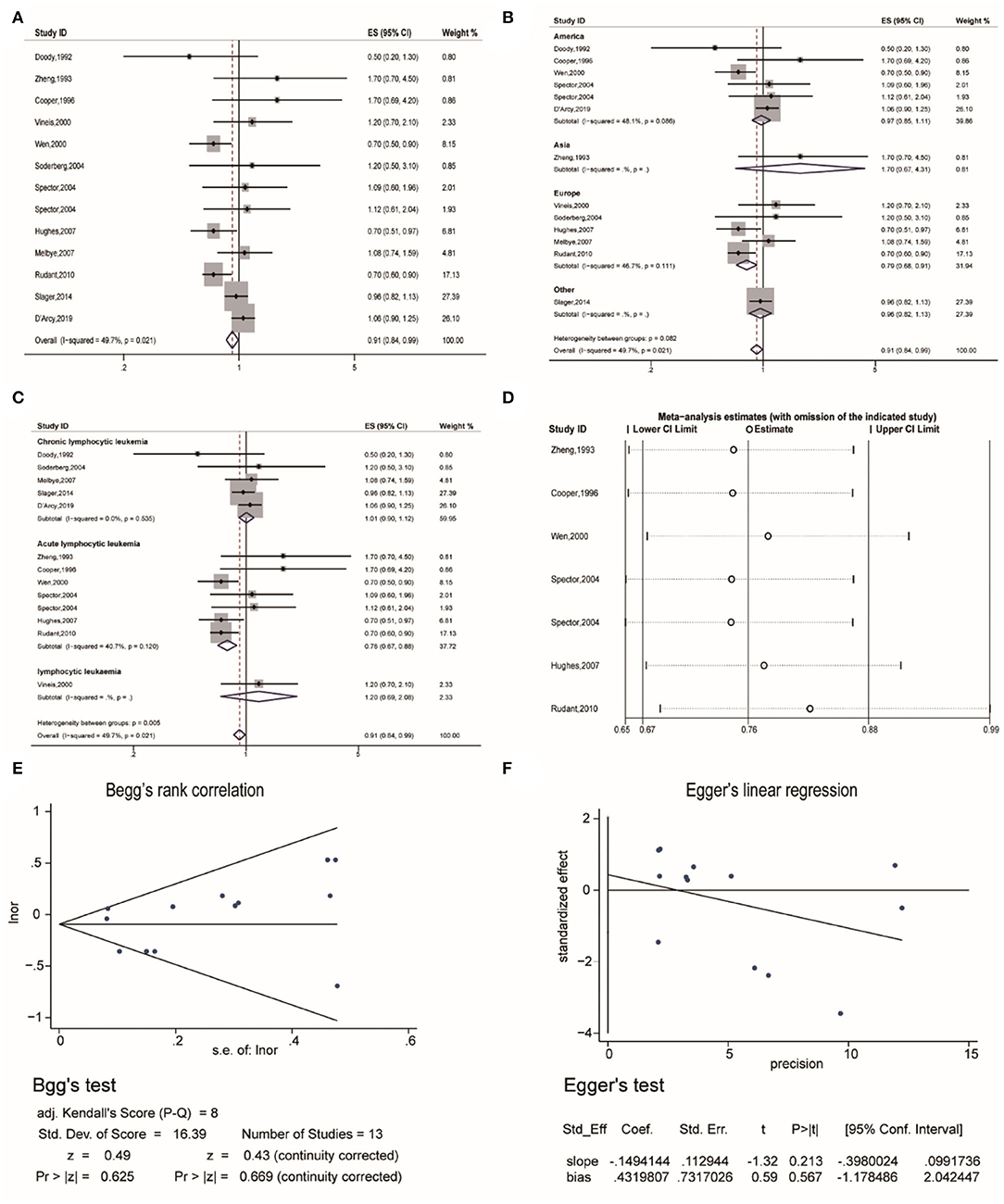
Figure 4. The association between eczema and lymphocytic leukemia risk. (A) Forest plot of the estimated effects of eczema on lymphocytic leukemia risk. (B) Forest plot for the subgroup analysis by region. (C) Forest plot for the subgroup analysis by cancer type. (D) Sensitivity analysis regarding the association between eczema and acute lymphocytic leukemia risk. (E) Begg's test indicating the lack of publication bias among such studies. (F) Egger's test indicating the lack of publication bias among such studies.
The Association of Eczema to Myelocytic Leukemia
Fourthly, the pooled analysis of eight studies (19, 20, 32, 34, 35, 38, 39, 41) indicated that eczema was not significantly associated with an increased risk of myelocytic leukemia (OR, 1.04 [95% CI, 0.90–1.19], P(ES) = 0.616; Pheterogeneity = 0.633, I2 = 0.0%) (Figure 5A), and the sensitivity analysis revealed that excluding each study in turn did not alter the overall combined results (Figure 5B).
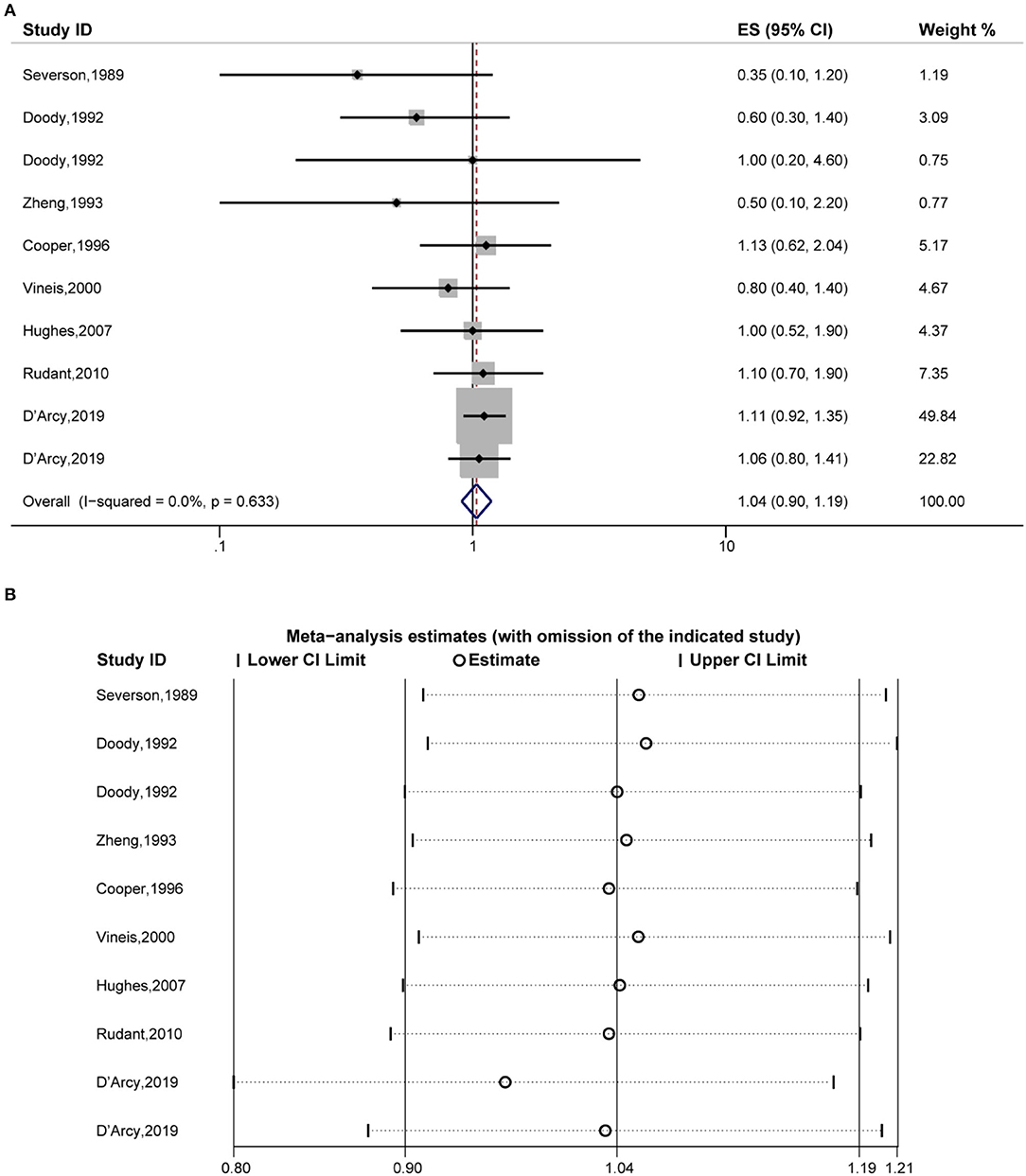
Figure 5. Forest plot of the estimated effects of eczema on myelocytic leukemia risk and the sensitivity analysis. (A) Forest plot of eczema on myelocytic leukemia risk. (B) Sensitivity analysis was conducted by recalculating the pooled results of the primary analysis following the exclusion of one study per iteration.
The Association of Eczema to Myeloma
Fifthly, the pooled analysis of six studies (16, 19, 20, 22, 32, 42) indicated that eczema was significantly associated with an increased risk of myeloma (OR, 1.15 [95% CI, 1.04–1.28], P(ES) = 0.008; Pheterogeneity = 0.226, I2 = 26.5%) (Figure 6A). Further analyses performed in America indicated that eczema significantly increased the risk of myeloma (OR, 1.23 [95% CI, 1.07–1.41], P(ES) = 0.004; Pheterogeneity = 0.295, I2 = 18.0%) (Figure 6B).

Figure 6. Forest plot of the estimated effects of eczema on myeloma risk. (A) Forest plot. (B) Forest plot for the subgroup analysis by region.
Discussion
Eczema characterized by itch, sleeplessness, and adverse effects on quality of life is associated with a risk of several diseases (43). A meta-analysis found that eczema was associated with increased risk of various cardiovascular diseases in cohort studies (44). A recent study also suggested that eczema is associated with an increased risk of developing depression and anxiety (45). But at present, the relationship between eczema and hematological tumors is still controversial, and our study aims to resolve this issue.
Our study indicated that the relationship between eczema and the risk of various types of hematological tumors was different. Firstly, our study showed no statistical relationship between eczema and an increased risk of non-Hodgkin's lymphoma, but the new results after excluding three articles with visible bias in sensitivity analysis showed that eczema was associated with an increased risk of non-Hodgkin's lymphoma, which was consistent with the findings of the cohort subgroup. In addition, eczema was significantly associated with the increased risk of Hodgkin's lymphoma and myeloma. Eczema was likely to the increased risk of myelocytic leukemia without statistical significance. On the contrary, eczema was statistically related with the decreased risk of lymphocytic leukemia. This difference is possibly due to the different risk factor spectrum of non-Hodgkin's lymphoma, Hodgkin's lymphoma and other hematological tumors (46). Of course, the existence of confounding bias and different treatment detections (especially immunomodulatory systemic therapeutics) may also be important reasons (16). What's more, T-cell NHL presenting in an indolent manner is always misdiagnosed as a non-neoplastic skin condition, and this diagnostic confusion could slightly attenuate the link between eczema and NHL risk (32).
Further subgroup analyses suggested that different study regions affect the association between eczema and hematological malignancies. Eczema was statistically associated with the increased risk of Hodgkin's lymphoma and myeloma in the American subgroup, but this link would be substantially attenuated in the European subgroup. This result was resulted from differences in race and environmental factors (47, 48).
In addition, eczema was significantly associated with the decreased risk of acute lymphocytic leukemia, but it was not statistically associated with the risk of chronic lymphocytic leukemia. This may be because some treatments for eczema (Glucocorticoids, biological inhibitors, etc.) may have a certain preventive effect on acute lymphoid leukemia (49), but this effect is not obvious for chronic lymphoid leukemia. Meanwhile, patients with eczema often receive phototherapy, which may affect their risk of cancer because of its therapeutic effect on cancer (50).
Although our study addresses the shortcomings of a single study with a small sample size and a non-universal population investigated, there are still many limitations. Firstly, there is a different degree of heterogeneity in some of our results, which affects the reliability of our results, but we performed subgroup analyses to look for sources of heterogeneity; In addition, although most of the studies we included had adjusted for confounding factors, the confounding factors adjusted were different.
Conclusion
This study demonstrated that eczema significantly increases the risk of Hodgkin's lymphoma, myeloma, and significantly decreases the risk of lymphocytic leukemia. Although eczema has been shown to be associated with the risk of many hematological malignancies, this association still needs to be verified in large randomized controlled trials. At the same time, the mechanism of eczema leading to cancer is needed to explore.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
Material preparation, data collection, and analysis were performed by ZL, SX, JL, HJ, YT, and YZ. The first draft of the manuscript was written by ZL, JL, and HJ. All authors contributed to the study conception, design, commented on previous versions of the manuscript, and approved the final manuscript.
Funding
We would like to thank the National Natural Science Foundation of China (grant numbers 81760136 and 31960136), the Special and Joint Program of Yunnan Provincial Science and Technology Department and Kunming Medical University (202001AY070001-097), the Yunnan health training project of high level talents, and the Scientific Research Fund project of Yunnan Education Department (2021J0304 and 2019J1308) for supporting our work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.912136/full#supplementary-material
References
1. Zhao Y, Peng H. The Role of N(6)-Methyladenosine (M(6)a) Methylation Modifications in Hematological Malignancies. Cancers. (2022) 14:332. doi: 10.3390/cancers14020332
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
3. Mehrpouri M, Pourbagheri-Sigaroodi A, Bashash D. The contributory roles of histone deacetylases (Hdacs) in hematopoiesis regulation and possibilities for pharmacologic interventions in hematologic malignancies. Int Immunopharmacol. (2021) 100:108114. doi: 10.1016/j.intimp.2021.108114
4. Infectiology Group CSoH Chinese Medical Association; Lymphocytic Disease Group Chinese Chinese Society of Hematology Chinese Medical Association; Anti-lymphoma Alliance Chinese Chinese Society of Clinical Oncology (CSCO). Chinese Expert Consensus on Prevention and Treatment of Immunotherapeutic and Molecular Targeted Agents-Related Infections in Patients with Hematological Malignancies (2021 Version). Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi. (2021) 42:717–27. doi: 10.3760/cma.j.issn.0253-2727.2021.09.002
5. Zhang X, Zhong L, Zou Z, Liang G, Tang Z, Li K, et al. Clinical and Prognostic Pan-Cancer Analysis of N6-Methyladenosine Regulators in Two Types of Hematological Malignancies: A Retrospective Study Based on Tcga and Gtex Databases. Front Oncol. (2021) 11:623170. doi: 10.3389/fonc.2021.623170
6. Luschkova D, Zeiser K, Ludwig A, Traidl-Hoffmann C. Atopic Eczema Is an Environmental Disease. Allergologie select. (2021) 5:244–50. doi: 10.5414/ALX02258E
7. Fereidouni M, Ferns GA, Bahrami A. Current status and perspectives regarding the association between allergic disorders and cancer. IUBMB Life. (2020) 72:1322–39. doi: 10.1002/iub.2285
8. Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (Strobe): Explanation and Elaboration. PLoS Med. (2007) 4:e297. doi: 10.1097/EDE.0b013e3181577511
9. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-Analysis of Observational Studies in Epidemiology: a Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (Moose) Group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
10. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
11. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
12. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
13. Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. (1955) 19:251–3. doi: 10.1111/j.1469-1809.1955.tb01348.x
14. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
16. Mansfield KE, Schmidt SAJ, Darvalics B, Mulick A, Abuabara K, Wong AYS, et al. Association between Atopic Eczema and Cancer in England and Denmark. JAMA Dermatol. (2020) 156:1086–97. doi: 10.1001/jamadermatol.2020.1948
17. Cartwright RA, McKinney PA, O'Brien C, Richards ID, Roberts B, Lauder I, et al. Non-Hodgkin's lymphoma: case control epidemiological study in yorkshire. Leuk Res. (1988) 12:81–8. doi: 10.1016/S0145-2126(98)80012-X
18. Bernstein L, Ross RK. Prior medication use and health history as risk factors for Non-Hodgkin's lymphoma: preliminary results from a case-control study in Los Angeles County. Cancer Res. (1992) 52(19 Suppl):5510s–5s.
19. Doody MM, Linet MS, Glass AG, Friedman GD, Pottern LM, Boice JD Jr, et al. Leukemia, lymphoma, and multiple myeloma following selected medical conditions. Cancer Causes Control. (1992) 3:449–56. doi: 10.1007/BF00051358
20. Vineis P, Crosignani P, Sacerdote C, Fontana A, Masala G, Miligi L, et al. Haematopoietic cancer and medical history: a multicentre case control study. J Epidemiol Community Health. (2000) 54:431–6. doi: 10.1136/jech.54.6.431
21. Fabbro-Peray P, Daures JP, Rossi JF. Environmental risk factors for Non-Hodgkin's lymphoma: a population-based case-control study in Languedoc-Roussillon, France. Cancer Causes Control. (2001) 12:201–12. doi: 10.1023/A:1011274922701
22. Söderberg KC, Hagmar L, Schwartzbaum J, Feychting M. Allergic conditions and risk of hematological malignancies in adults: a cohort study. BMC Public Health. (2004) 4:51. doi: 10.1186/1471-2458-4-51
23. Zhang Y, Holford TR, Leaderer B, Zahm SH, Boyle P, Morton LM, et al. Prior medical conditions and medication use and risk of non-hodgkin lymphoma in connecticut United States Women. Cancer Causes Control. (2004) 15:419–28. doi: 10.1023/B:CACO.0000027506.55846.5d
24. Grulich AE, Vajdic CM, Kaldor JM, Hughes AM, Kricker A, Fritschi L, et al. Birth order, atopy, and risk of Non-Hodgkin lymphoma. J Natl Cancer Inst. (2005) 97:587–94. doi: 10.1093/jnci/dji098
25. Cozen W, Cerhan JR, Martinez-Maza O, Ward MH, Linet M, Colt JS, et al. The effect of atopy, childhood crowding, and other immune-related factors on Non-Hodgkin lymphoma risk. Cancer Causes Control. (2007) 18:821–31. doi: 10.1007/s10552-007-9025-5
26. Melbye M, Smedby KE, Lehtinen T, Rostgaard K, Glimelius B, Munksgaard L, et al. Atopy and risk of Non-Hodgkin lymphoma. J Natl Cancer Inst. (2007) 99:158–66. doi: 10.1093/jnci/djk019
27. El-Zein M, Parent ME, Kâ K, Siemiatycki J, St-Pierre Y, Rousseau MC. History of asthma or eczema and cancer risk among men: a population-based case-control study in montreal, Quebec, Canada. Ann Allergy Asthma Immunol. (2010) 104:378–84. doi: 10.1016/j.anai.2010.03.003
28. Linet MS, Vajdic CM, Morton LM, De Roos AJ, Skibola CF, Boffetta P, et al. Medical history, lifestyle, family history, and occupational risk factors for follicular lymphoma: the interlymph Non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. (2014) 2014:26–40. doi: 10.1093/jncimonographs/lgu006
29. Mbulaiteye SM, Morton LM, Sampson JN, Chang ET, Costas L, de Sanjosé S, et al. Medical history, lifestyle, family history, and occupational risk factors for sporadic burkitt lymphoma/leukemia: the interlymph Non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. (2014) 2014:106–14. doi: 10.1093/jncimonographs/lgu003
30. Morton LM, Slager SL, Cerhan JR, Wang SS, Vajdic CM, Skibola CF, et al. Etiologic heterogeneity among non-hodgkin lymphoma subtypes: the interlymph Non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. (2014) 2014:130–44. doi: 10.1093/jncimonographs/lgu013
31. Wang SS, Flowers CR, Kadin ME, Chang ET, Hughes AM, Ansell SM, et al. Medical history, lifestyle, family history, and occupational risk factors for peripheral T-Cell lymphomas: the interlymph Non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. (2014) 2014:66–75. doi: 10.1093/jncimonographs/lgu012
32. D'Arcy M, Rivera DR, Grothen A, Engels EA. Allergies and the subsequent risk of cancer among elderly adults in the United States. Cancer Epidemiol Biomarkers Prev. (2019) 28:741–50. doi: 10.1158/1055-9965.EPI-18-0887
33. Rafiq M, Hayward A, Warren-Gash C, Denaxas S, Gonzalez-Izquierdo A, Lyratzopoulos G, et al. Allergic disease, corticosteroid use, and risk of hodgkin lymphoma: a united kingdom nationwide case-control study. J Allergy Clin Immunol. (2020) 145:868–76. doi: 10.1016/j.jaci.2019.10.033
34. Zheng W, Linet MS, Shu XO, Pan RP, Gao YT, Fraumeni JF Jr. Prior medical conditions and the risk of adult leukemia in Shanghai, People's Republic of China. Cancer Causes Control. (1993) 4:361–8. doi: 10.1007/BF00051339
35. Cooper GS, Kamel F, Sandler DP, Davey FR, Bloomfield CD. Risk of adult acute leukemia in relation to prior immune-related conditions. Cancer Epidemiol Biomarkers Prev. (1996) 5:867–72.
36. Wen W, Shu XO, Linet MS, Neglia JP, Potter JD, Trigg ME, et al. Allergic disorders and the risk of childhood acute lymphoblastic leukemia (United States). Cancer Causes and Control. (2000) 11:303–7. doi: 10.1023/A:1008958724739
37. Spector L, Groves F, DeStefano F, Liff J, Klein M, Mullooly J, et al. Medically recorded allergies and the risk of childhood acute lymphoblastic leukaemia. Eur J Cancer. (2004) 40:579–84. doi: 10.1016/j.ejca.2003.08.024
38. Hughes AM, Lightfoot T, Simpson J, Ansell P, McKinney PA, Kinsey SE, et al. Allergy and Risk of Childhood Leukaemia: Results from the Ukccs. Int J Cancer. (2007) 121:819–24. doi: 10.1002/ijc.22702
39. Rudant J, Orsi L, Menegaux F, Petit A, Baruchel A, Bertrand Y, et al. Childhood acute leukemia, early common infections, and allergy: the escale study. Am J Epidemiol. (2010) 172:1015–27. doi: 10.1093/aje/kwq233
40. Slager SL, Benavente Y, Blair A, Vermeulen R, Cerhan JR, Costantini AS, et al. Medical history, lifestyle, family history, and occupational risk factors for chronic lymphocytic leukemia/small lymphocytic lymphoma: the interlymph Non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. (2014) 2014:41–51. doi: 10.1093/jncimonographs/lgu001
41. Severson RK, Davis S, Thomas DB, Stevens RG, Heuser L, Sever LE. Acute myelocytic leukemia and prior allergies. J Clin Epidemiol. (1989) 42:995–1001. doi: 10.1016/0895-4356(89)90165-0
42. Lewis DR, Pottern LM, Brown LM, Silverman DT, Hayes RB, Schoenberg JB, et al. Multiple myeloma among blacks and whites in the united states: the role of chronic antigenic stimulation. Cancer Causes Control. (1994) 5:529–39. doi: 10.1007/BF01831381
43. Silverwood RJ, Mansfield KE, Mulick A, Wong AYS, Schmidt SAJ, Roberts A, et al. Atopic eczema in adulthood and mortality: Uk population-based cohort study, 1998-2016. J Allergy Clin Immunol. (2021) 147:1753–63. doi: 10.1016/j.jaci.2020.12.001
44. Ascott A, Mulick A, Yu AM, Prieto-Merino D, Schmidt M, Abuabara K, et al. Atopic Eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol. (2019) 143:1821–9. doi: 10.1016/j.jaci.2018.11.030
45. Long Q, Jin H, You X, Liu Y, Teng Z, Chen Y, et al. Eczema is a shared risk factor for anxiety and depression: a meta-analysis and systematic review. PLoS ONE. (2022) 17:e0263334. doi: 10.1371/journal.pone.0263334
46. Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. (2011) 103:1827–39. doi: 10.1093/jnci/djr483
47. Clarke CA, Glaser SL, Gomez SL, Wang SS, Keegan TH, Yang J, et al. Lymphoid Malignancies in US Asians: Incidence Rate Differences by Birthplace and Acculturation Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. (2011) 20:1064–77. doi: 10.1158/1055-9965.EPI-11-0038
48. Wu SJ, Huang SY, Lin CT, Lin Y Jr, Chang CJ, Tien HF. The incidence of chronic lymphocytic leukemia in Taiwan, 1986-2005: a distinct increasing trend with birth-cohort effect. Blood. (2010) 116:4430–5. doi: 10.1182/blood-2010-05-285221
49. Jabbour E, Kantarjian H. Management of older patients with acute lymphocytic leukemia - novel treatment strategies. Clin Lymphoma Myeloma Leuk. (2020) 20(Suppl 1):S30–S1. doi: 10.1016/S2152-2650(20)30452-3
Keywords: eczema, hematological malignancies, Hodgkin's lymphoma, lymphocytic leukemia, myelocytic leukemia, risk
Citation: Liang Z, Liu J, Jin H, Teng Y, Xu S, Yan W and Zhu Y (2022) Potential Correlation Between Eczema and Hematological Malignancies Risk: A Systematic Review and Meta-Analysis. Front. Med. 9:912136. doi: 10.3389/fmed.2022.912136
Received: 04 April 2022; Accepted: 30 May 2022;
Published: 29 June 2022.
Edited by:
Giovanni Damiani, University of Milan, ItalyReviewed by:
Irina Khamaganova, Pirogov Russian National Research Medical University, RussiaLaura Cristina Gironi, Azienda Ospedaliero Universitaria Maggiore della Carità, Italy
Copyright © 2022 Liang, Liu, Jin, Teng, Xu, Yan and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Zhu, emh1eXVuODM3N0AxNjMuY29t; b3JjaWQub3JnLzAwMDAtMDAwMy0yNjkxLTYyMjA=
†These authors have contributed equally to this work and share first authorship
 Zuohui Liang1†
Zuohui Liang1† Hongxia Jin
Hongxia Jin Yirong Teng
Yirong Teng Shuangyan Xu
Shuangyan Xu Yun Zhu
Yun Zhu