
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 13 September 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.911267
 Eunji Yang1
Eunji Yang1 Sang Ho Park2
Sang Ho Park2 Seoyoung Lee1
Seoyoung Lee1 Donghwan Oh1
Donghwan Oh1 Hoon Young Choi1,3
Hoon Young Choi1,3 Hyeong Cheon Park1,3
Hyeong Cheon Park1,3 Jong Hyun Jhee1*
Jong Hyun Jhee1*Background: High pulse pressure (PP) is associated with increased risk of decline of kidney function. However, little is known about the association between PP and RHF in young adults. This study aimed to evaluate the association between PP and RHF in healthy young adults.
Methods: Data were retrieved from the Korea National Health and Nutrition Examination Survey from 2010 to 2019. A total of 10,365 participants aged 19–39 years with no hypertension and normal kidney function were analyzed. RHF was defined as logarithm transformed estimated glomerular filtration rate (eGFR) with residuals >90th percentile after adjustment for sex, logarithm transformed age, weight, and height. Participants were divided into tertile based on PP levels.
Results: The prevalence of RHF was higher in higher PP tertile group (6.6, 10.5, and 12.7% in T1, T2, and T3; P for trend < 0.001). In multivariable logistic regression analyses, the risk for RHF was increased in higher PP tertiles compared to the lowest tertile [odds ratio (OR), 1.42; 95% confidence interval (CI), 1.19–1.69 in T2; OR, 1.44; 95% CI, 1.20–1.73 in T3]. When PP levels were treated as continuous variable, the risk of RHF was increased 2.36 per 1.0 increase of PP (P < 0.001). In subgroup analyses stratified sex, histories of diabetes or dyslipidemia, and isolated systolic hypertension or isolated diastolic hypertension, there were no significant interactions with PP for the risk for RHF, suggesting that high PP was associated with increased risk of RHF regardless of subgroups. However, the subgroup with BMI showed significant interaction with PP for the risk of RHF, indicating that participants with BMI ≥ 25 kg/m2 were at higher risk of RHF with increasing PP levels than those with BMI < 25 kg/m2 (OR, 1.89; 95% CI, 1.25–2.87 in BMI < 25 kg/m2; OR, 3.16; 95% CI, 1.74–5.73 in BMI ≥ 25 kg/m2; P for interaction = 0.01).
Conclusion: High PP is associated with an increased risk of RHF in healthy young adults and this association is prominent in obese young adults. The assessment of PP and associated RHF may give benefit to early detect the potential risk of CKD development in young adults.
Chronic kidney disease (CKD) is one of the representative health problems and its incidence is increasing worldwide (1, 2). Once kidney failure begins, it is difficult to recover, eventually leads to end-stage kidney disease (ESKD) requiring dialysis or transplantation. Moreover, patients with CKD have an increased risk of cardiovascular complications and death (1, 3). Therefore, early identification and management of risk factors for CKD is crucial. Meanwhile, in recent years, health problems such as hypertension, diabetes, and obesity initiating from young adults are attracting attention (4). Despite the fact that the onset of a health problem at younger age is associated with an increased risk of adverse clinical outcomes, the proportion of young adults who receive medical care is relatively low (5, 6). Given the progressive nature of kidney disease, early identification of the risk factors for CKD in young adults may prevent the development of CKD and reduce the burden of medical expenses.
Renal hyperfiltration (RHF), an abnormally increased status of glomerular filtration rate (GFR), is one of the known risk factors for kidney function deterioration (7–9). In addition, RHF is viewed as a marker of vascular dysfunction and increased arterial stiffness. Physiologically, high protein intake or pregnancy or obesity increase GFR (10–12). High intra-glomerular pressure and shear stress may damage the nephron and lead to detachment of glomerular epithelial cells from the glomerular capillary wall, contributing to the progression of kidney injury (13, 14). Thus, abnormally increased GFR serves as an early sign of kidney disease (3).
Pulse pressure (PP) is derived from the difference between systolic blood pressure (SBP) and diastolic BP (DBP) and reflects the hemodynamic changes of BP and flow. The levels of PP are determined by stroke volume, aortic stiffness, and wave reflection and elevated PP levels represents increased arterial stiffness. Reboldi et al. (14) observed that subjects with RHF were associated with an elevated 24-h PP levels and increased risk of cardiovascular events (CVEs). This study suggested that endothelial dysfunction or alteration in microvascular and macrovascular structures are associated with an increased arterial stiffness, which contributes to occurrence of RHF and adverse cardiovascular outcomes.
Considering the increasing rate of health problems in young adults and the progressive nature of kidney disease, it is crucial to identify risk factors for CKD before the overt disease onset. Thus, we aimed to investigate the predictive role of PP, which is an indicator of increased arterial stiffness, on early detection of RHF among young adults.
Data were retrieved from the Korea National Health and Nutrition Examination Survey (KNHANES 2010–2019). KNHANES is a widespread surveillance system that survey the health and nutrition status of Koreans. Detailed data resource profile about KNHANES was previously reported elsewhere (15). Briefly, KNHANES consists of individual’s health-related performance, quality of life, healthcare utilization, anthropometric measures, biochemical and clinical profiles. It is composed of three component surveys: a health interview, health examination and nutrition survey. The surveys collect detailed information on socioeconomic status, health behaviors, quality of life, healthcare utilization, anthropometric measures, biochemical profiles using fasting blood serum and urine, measures for dental health, vision, hearing and bone density, X-ray test results food intake and dietary behavior (15, 16). For this study, a total of 80,861 participants were screened. The participants were excluded from the study with an age of under 19 or over 40 years, past history of hypertension, eGFR less than 60 ml/min/1.73 m2, and missing data. Finally, 10,365 participants aged 19–39 years with normal kidney function and without hypertension were included for the study analysis. The participants were divided into tertile based on PP levels [the lowest tertile of PP as T1 (n = 3,185), middle tertile of PP as T2 (n = 3,906), and the highest tertile as T3 (n = 3,274)] (Figure 1). All subjects voluntarily participated in the study and provided informed consent. This study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of the Centers for Disease Control and Prevention in Korea (KNHANES 2010-2014 IRB approvals; 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, and 2013-12EXP-03-5C, IRB approval was not required for KNHANES 2015-2017 because this survey was conducted for the purpose of public welfare, KNHANES 2018-2019 IRB approvals; 2018-01-03-P-A and 2018-01-03-C-A).
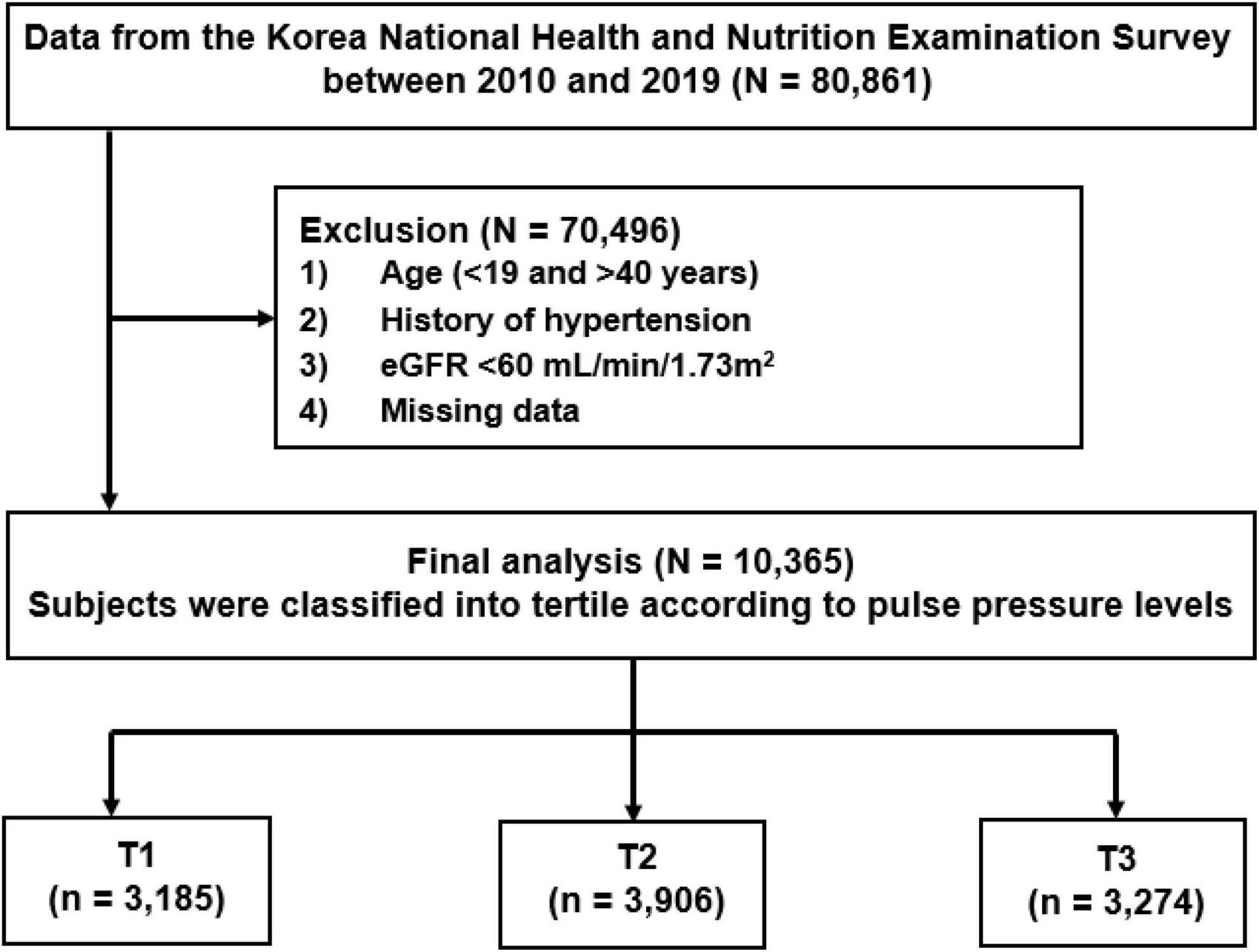
Figure 1. Study T1-3 represents tertile according to pulse pressure levels. T1 is the lowest and T3 is the highest pulse pressure. eGFR, estimated glomerular filtration rate.
Demographic and socioeconomic data, including age, sex, levels of education and income, smoking status, alcohol intake, and medical histories. Education status was classified as low level for those who graduated from elementary school to middle school and as high level for those who graduated from high school to graduated university. Households with an average monthly income of less than 2 million won were classified as low income level, and those with more than 2 million won were classified as high income level. For the past medical histories, diabetes, dyslipidemia, and cardiovascular disease (CVD) were defined as a medical diagnosis. Anthropometric indices including height, weight, SBP, DBP were examined by skilled study workers. BPs were measured by special investigation nurse in the Korea Centers for Disease Control and Prevention. Participants sat in a comfortable position after they had rested for at least 5 min and had not smoked within 30 min of the measurements. BP was measured on three consecutive occasions in a relaxed environment. The mean of the second and the third measurements was adopted for the data analysis (17). PP was calculated by the difference between SBP and DBP. Blood and urine samples were analyzed for creatinine, hemoglobin, fasting plasma glucose, HbA1c, Total cholesterol, and low-density lipoprotein-cholesterol (LDL-C). Proteinuria was measured by the dipstick urine analysis method using Urisys 2400 in 2010–2018 (Roche, Germany) and UC-3500 in 2019 (Sysmex/Japan). The dipstick urine test results were described as negative, trace, 1+, 2+, 3+, or 4+. Proteinuria was defined as more than trace levels. Serum creatinine level was measured by the Jaffe rate-blanked and compensated method using Hitachi Automatic Analyzer 7600-210 in 2019–2018 (Hitache, Japan) and by the Kinetic colorimetric assay method using Cobas in 2019 (Roche, Germany). The estimated glomerular filtration rate (eGFR) was calculated by the CKD-Epidemiology Collaboration (CKD-EPI) equation (18).
The study endpoint was RHF which was defined by using the previously reported method with modification (19). In detail, residuals were calculated from linear regression analysis with logarithm transformed eGFR as dependent variable and logarithm-transformed age, sex, weight, and height as independent variables. RHF was defined with residuals of >90th percentile from the model.
All statistical analyses were performed using IBM SPSS software for Windows version 25.0 (IBM Corporation, Chicago, IL, United States). Continuous variables were expressed as mean ± standard deviation and categorical variables as absolute numbers with percentages. All data were tested for normality before the statistical analysis. Baseline characteristics of tertiles classified by PP levels were compared by using analysis of variance test for continuous variables, and the chi-squared test or Fisher’s exact test for categorical variables. Pearson’s correlation analysis was performed to identify associated factors with PP levels. To evaluate the association between PP and RHF, multivariable logistic regression analysis was performed with adjustment for covariates such as age, sex, body mass index (BMI), income and education status, alcohol and smoking status, history of diabetes, hemoglobin, total cholesterol, and proteinuria. Additionally, Receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff values of PP and corresponding SBP and DBP levels for the risk of RHF. Subgroup analysis was performed stratified by sex, BMI (<25 or ≥25 kg/m2), histories of diabetes or dyslipidemia, and isolated systolic hypertension (ISH) or isolated diastolic hypertension (IDH). Based on the 2017 ACC/AHA criteria, ISH was defined as SBP ≥ 130mmHg and DBP < 80mmHg and IDH was defined as SBP < 130mmHg and DBP ≥ 80mmHg (20). P for interactions for the risk of RHF were assessed between each subgroup and PP levels. Two-sided P value < 0.05 were considered statistically significant.
A total of 10,365 participants were analyzed for this study (Figure 1). The baseline characteristics according to tertile of PP are shown in Table 1. The mean age of study participants were 30.1 ± 6.1 years and 5,716 (55.1%) were female. The mean levels of eGFR in study participants were 110.3 ± 23.6 ml/min/1.73m2 and that of PP were 36.6 ± 7.5mmHg. The mean levels of PP were 28.6 ± 3.1, 35.9 ± 2.0, 45.2 ± 5.4 mmHg in T1, T2, and T3, respectively. Participants in higher tertiles were more likely to be young and male. Participants in higher tertiles had higher levels of BMI, were more frequent smoker, had lower education status and less income, had higher SBP and lower DBP, had more frequent past histories of dyslipidemia or CVDs compared to the lowest tertile. In laboratory tests, participants in higher tertiles showed higher levels of eGFR, hemoglobin, fasting plasma glucose, HbA1c and lower levels of total cholesterol and LDL-C than the lowest tertile. As previous studies reported that PP levels are higher in male than female in young adults, we further evaluated the differences in baseline characteristics according to the PP levels between male and female (Supplementary Tables 1, 2) (21, 22). The mean levels of PP were higher in male compared with female in this study (38.6 ± 8.0 mmHg in male and 35.0 ± 6.7 mmHg in female, P < 0.001). The male participants in higher PP tertiles were more frequent smoker. However, female participants did not show differences in smoking status according to tertiles of PP. In laboratory test, male participants with higher tertiles of PP showed no difference in hemoglobin levels, whereas female participants with higher tertile showed low levels of hemoglobin. In addition, male participants with higher tertile showed lower levels of total cholesterol, whereas female did not.
Next, we performed Pearson‘s correlation analysis to evaluate the modifiable factors which are associated with high PP levels (Table 2). Male sex (r = 0.24, P < 0.001), BMI (r = 0.19, P < 0.001), smoking (r = 0.09, P < 0.001), SBP (r = 0.61, P < 0.001), eGFR (r = 0.07, P < 0.001), hemoglobin (r = 0.15, P < 0.001), and fasting plasma glucose (r = 0.04, P < 0.001) levels were positively associated with PP levels. In contrast, age (r = –0.17, P < 0.001), DBP (r = –0.17, P < 0.001) and total cholesterol (r = –0.04, P < 0.001) levels were negatively associated with PP levels.
The prevalence of RHF was significantly higher in higher tertiles (6.6, 10.5, and 12.7% in T1, T2, and T3, respectively; P for trend < 0.001). To examine the association between PP and the risk for RHF, logistic regression analyses were performed. In unadjusted model, higher tertiles showed increased risks for RHF compared to lowest tertile [odds ratio (OR), 1.65; 95% confidence interval (CI), 1.39–1.96; P < 0.001 in T2; OR, 2.05; 95% CI, 1.73–2.44; P < 0.001 in T3). After adjustment for clinical variables including age, sex, BMI, education and income status, alcohol and smoking status, history of diabetes, levels of hemoglobin, total cholesterol, and proteinuria, the increased risks of RHF were observed in higher tertiles compared to the lowest tertile (OR, 1.42; 95% CI 1.19–1.69; P < 0.001 in T2; OR, 1.44; 95% CI, 1.20–1.73; P < 0.001 in T3). These associations were consistently observed when the PP levels were treated as continuous variable that increasing PP levels (per 1.0 increase of log-transformed values) were associated with 2.36-folded increased risk of RHF in multivariable logistic regression model (OR, 2.36; 95% CI, 1.67–3.32; P < 0.001) (Table 3 and Supplementary Table 3).
We additionally performed ROC curve analysis to determine the cutoff values of PP and corresponding SBP and DBP levels for the risk of RHF (Figure 2). The area under the ROC curve was 0.700 (95% CI, 0.684–0.716, P < 0.001) and the sensitivity and specificity of the curve were 74.6 and 56.6%, respectively. The cutoff value of PP levels, which increases the risk of RHF, was 30 mmHg, and the corresponding SBP and DBP levels were 113 and 83 mmHg, respectively.
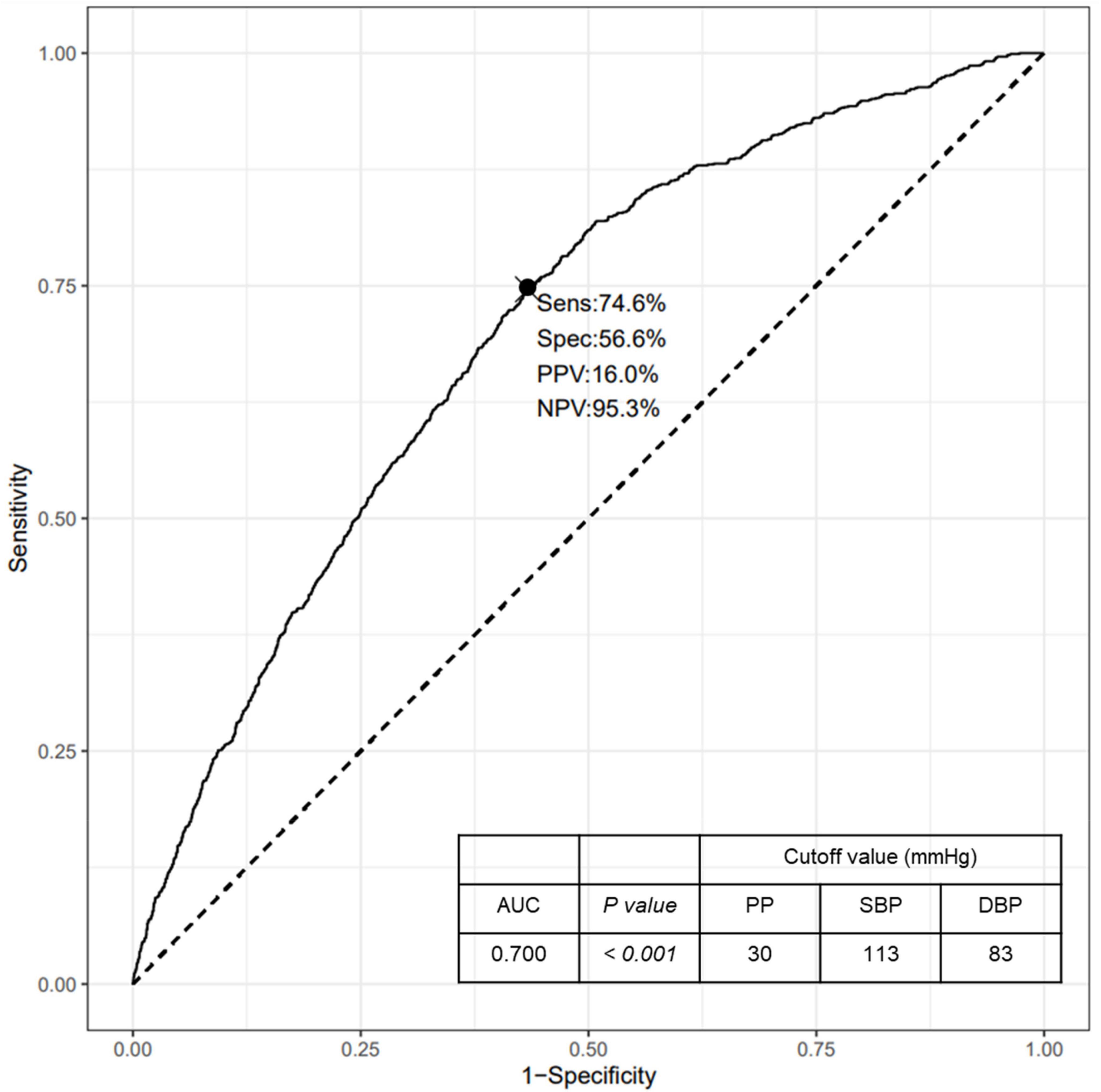
Figure 2. ROC curve for the risk of RHF according to the PP levels. ROC, Receiver operating characteristic; RHF, renal hyperfiltration; PP, pulse pressure; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; SBP, systolic blood pressure; DBP, diastolic blood pressure.
To further confirm the association between PP levels and the risk of RHF, subgroup analyses stratified with sex (female vs. male), BMI (<25 vs. ≥25kg/m2), histories of diabetes or dyslipidemia (no vs. yes), and ISH or IDH (no vs. yes) were performed (Figure 3 and Supplementary Table 4). There were no significant interactions between subgroups including sex, histories of diabetes or dyslipidemia, and ISH or IDH and the PP levels for the risk of RHF, suggesting that the association of increased risk for RHF with high PP levels was regardless of sex, histories of diabetes or dyslipidemia, and ISH or IDH. However, the subgroup with BMI showed significant interaction with the PP levels for the risk of RHF, indicating that participants with BMI higher than 25 kg/m2 showed an increased risk of RHF with increasing PP levels than those with BMI lower than 25 kg/m2 [OR (per 1.0 increase in log-transformed PP), 1.89; 95% CI, 1.25–2.87 in BMI < 25 kg/m2; OR (per 1.0 increase in log-transformed PP), 3.16; 95% CI, 1.74–5.73 in BMI ≥ 25 kg/m2; P for interaction = 0.01).
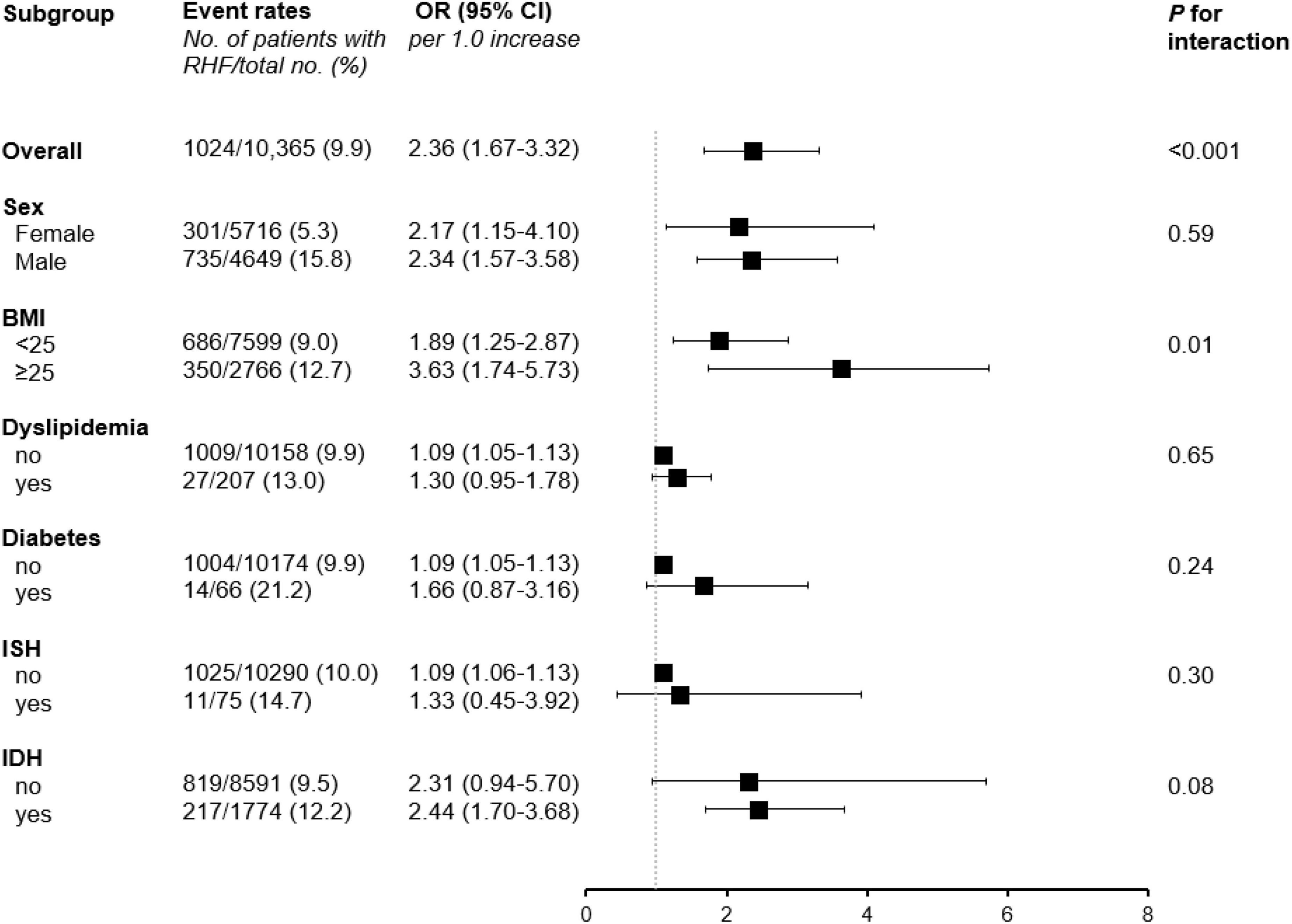
Figure 3. Subgroup analyses stratified by sex, BMI, histories of diabetes or dyslipidemia, and ISH or IDH. ORs were calculated per 1.0 increase of log-transformed PP levels after adjustment for age, sex, BMI, income, education alcohol and smoking status, history of diabetes, hemoglobin, total cholesterol, and proteinuria. RHF, renal hyperfiltration; OR, odds ratio; CI, confidence interval; BMI, body mass index; ISH, isolated systolic hypertension; IDH, isolated diastolic hypertension.
The results of the present study using large cohort data of young Korean adults showed that elevated PP was associated with an increased risk of RHF in young adults with normal kidney function. In particular, significant interaction between obesity and PP levels for the risk of RHF was found that obese young adults with BMI larger than 25 kg/m2 were more prone to the risk of RHF than those with BMI less than 25 kg/m2. These findings suggest that the identification of elevated PP levels in young adults may play a role in early identification of RHF, one of the risk factors for future deterioration of kidney function.
RHF is known to precede progressive kidney injury. RHF refers to the state in which GFR is abnormally increased in the kidney, which cause chronic decline of kidney function (23). Considering the progressive nature of kidney disease, early identification and management of risk factors are crucial to prevent further progression to ESKD needing chronic dialysis or kidney transplantation (24–26). Previous studies have demonstrated that RHF is associated with hypertension, diabetes, obesity, and smoking (27, 28). However, the association between elevated PP levels and the RHF risk is poorly elucidated. In this study, elevated PP levels were associated with an increased risk for RHF. PP, calculated by the difference between SBP and DBP, serves as an indicator of hemodynamic changes in BP and indicates vascular dysfunction including RHF (29, 30). Mechanistically, PP is caused by the close interaction of blood ejected from left ventricle with aorta or large arteries (29). When the elasticity of the aorta or large arteries decreases, the stiffness of those arteries conversely increases with increasing PP levels (31). The increase in PP contributes to vascular endothelial damage, which leads to mechanical fatigue and deepens vascular stiffness. This process viciously worsens central wave reflection and increases the PP levels again (32, 33). The increased PP with increased arterial stiffness derived from the vicious cycle affect the renal vasculature, consequently impairing renal hemodynamics (33). Initially, elevated PP results in glomerular hyperfiltration. However, chronic and steady increase in pressure and arterial stiffness eventually leads to ischemic and fibrotic changes in glomerulus. These changes further lead to loss of renal autoregulation with glomerular hypertrophy and sclerosis, ultimately contributing to nephrosclerosis and decline of kidney function (7, 34).
In this study, participants with high PP levels showed the increased risk of RHF as well as higher proportion of obesity and smoking status, which are known risk factors for CKD. Furthermore, when Pearson’s correlation analysis was performed to evaluate the modifiable factors with high PP levels, male sex, BMI, smoking, and fasting plasma glucose levels showed the positive association with PP levels, whereas age and total cholesterol levels showed the negative association with PP levels. As noted in previous reports, obesity, smoking, and hyperglycemia are associated with elevated PP levels and serve as risk factors for arterial stiffness in young adults similar to older generation (35–39). Thus, modifying these factors might give benefit to overcome deleterious effects of high PP levels in young adults. Meanwhile, younger age and male sex are known to associate with high PP levels in the younger population. Younger men are commonly associated with spurious systolic hypertension or hyperkinetic status which contribute to high PP levels (21, 40). In addition, unlike elderly patients, elevated cholesterol levels without overt atheroma are associated with reduced arterial stiffness in the younger population (41–43). Clear mechanism between these factors and elevated PP levels is unclear and needs to be elucidated in young adults. Nevertheless, early identification of modifiable factors associated with high PP levels, such as obesity, smoking, or hyperglycemia, may have clinical implications for preventing RHF and future kidney dysfunction.
Another interesting finding of the present study is that obese young adults with BMI larger than 25 kg/m2 showed more increased risk of RHF compared to those with BMI less than 25 kg/m2. RHF is one of the well-known major alterations resulting from obesity-related glomerulopathy (44). The glomerulus enlarges in response to obesity-related changes including RHF and increased renal plasma flow. Although most obese patients have stable or slowly progressive proteinuria, up to one-third develop nephrosclerosis and progressive kidney failure or ESKD (45). Furthermore, obesity is also associated with arterial stiffness or elevated PP even in child to young adults similar to older generation (46, 47). Taken together, even in healthy young adults with no history of hypertension and without vascular damage, concurrent obesity and elevated PP may further accelerate RHF and lead to more rapid and severe progression of renal hemodynamic changes. Resultantly, obese young adults with high PP may face irreversible nephrosclerotic changes, kidney failure, and initiation of dialysis at an early age. Therefore, the findings of this study suggest that young adults, particularly those with obesity, need monitoring of PP levels and associated RHF to prevent future development of kidney disease.
Given that high PP levels or ISH are typical in male than female in young adults, there may exist sex-disparity in the association between high PP levels and RHF. In this study, the mean levels of PP were higher in male compared with female. However, subgroup analysis stratified by sex showed no significant interaction between sex and PP levels for RHF suggesting that the association of increased risk for RHF with high PP levels was regardless of sex. Previous studies reported that high PP levels or ISH are typical in male than female in young adults (48). Spurious systolic hypertension or sympathetic overactivity observed in healthy young men are suggested as a mechanism for high PP levels (21, 22, 40, 49, 50). However, as noted in the previous studies, high PP levels in young men were a result of high arterial distensibility or hyperkinetic state and were considered as benign condition. It is unclear whether high PP levels in young men contribute to adverse effects on vascular physiology. However, as shown in the present study, the RHF associated with high PP levels may not be affected by sex differences. Further in-depth studies are needed to elucidate the sex-specific effects of PP on renal vascular physiology in young adults.
This study had several limitations. First, direct measurement of GFR was not performed in this study. The gold standard methods to assess GFR include direct measurement of renal clearance of endogenous or exogenous substances (51, 52). However, the KNHANES data was designed for the purpose of national health examination and direct measurement of renal clearance was not included. Second, the definition of RHF is not generally defined in children to young adults. Some of studies used cutoff criteria with directly measured GFR or eGFR to define RHF (3, 53). In this study, multivariable linear regression methods calculating RHF adjusted for logarithm-transformed age, sex, weight and height were used to reduce the possible confoundings. Further studies are needed to validate the definition of RHF in young adults. Third, the interpretation of subgroup analyses stratified by histories of diabetes or dyslipidemia and ISH or IDH are limited. The numbers of participants with diabetes or dyslipidemia and ISH or IDH were small as this study was consisted of relatively healthy young adults aged 19 to 39 years. Further study with large number of subjects is warranted to confirm the subgroup effects between PP and RHF in young adults. Finally, due to the observational nature of this study, the causal relationship between high PP and the risk of RHF cannot be demonstrated. Further longitudinally design studies should be performed to affirm the present study findings.
In conclusion, high PP is associated with an increased risk of RHF in young adults with no history of hypertension and with normal kidney function. In particular, obese young adults with elevated PP levels may show more increased risk of RHF. Considering that early identification and management of kidney injury starting from the young adults may prevent progression of kidney disease, assessment of PP and associated RHF may give benefit to early detect the potential risk of CKD development in young adults.
The datasets presented in this study can be found in online repository. The names of the repository and accession numbers can be found at: https://knhanes.kdca.go.kr/knhanes/sub03/sub03_01.do.
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of the Centers for Disease Control and Prevention in Korea. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JHJ contributed to the research idea and study design. EJY was responsible for data acquisition. EJY, SHP, SYL, and DHO contributed to the data analysis/interpretation. EJY and DHO performed the statistical analysis. HYC and HCP were responsible for supervision or mentorship. JHJ was the guarantor. All authors contributed to the important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
This study was supported by the Young Investigator Research Grant from the KOREAN NEPHROLOGY RESEARCH FOUNDATION (KSN 2021). The funding source had no role in the conception of the study or the collection, analysis, and interpretation of the data; writing of the manuscript; or the decision to submit for publication.
The research data used in this study were obtained from the Korea National Health and Nutrition Examination Survey (KNHANES V, VI, VII, and VIII) from 2010 to 2019, Republic of Korea.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.911267/full#supplementary-material
Supplementary Table 1 | Baseline characteristics in male (n = 4,649).
Supplementary Table 2 | Baseline characteristics in female (n = 5,716).
Supplementary Table 3 | The regression coefficients for the risk of RHF.
Supplementary Table 4 | Subgroup analysis stratified by sex, BMI, histories of diabetes or dyslipidemia, and ISH or IDH.
1. Geng TT, Talaei M, Jafar TH, Yuan JM, Koh WP. Pulse pressure and the risk of end-stage renal disease among Chinese adults in Singapore: the Singapore Chinese health study. J Am Heart Assoc. (2019) 8:e013282. doi: 10.1161/jaha.119.013282
2. Dupuis ME, Nadeau-Fredette AC, Madore F, Agharazii M, Goupil R. Association of glomerular hyperfiltration and cardiovascular risk in middle-aged healthy individuals. JAMA Netw Open. (2020) 3:e202377. doi: 10.1001/jamanetworkopen.2020.2377
3. Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ. Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol. (2015) 26:1426–33. doi: 10.1681/asn.2014010115
4. Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. (2000) 342:1478–83. doi: 10.1056/nejm200005183422003
5. Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. (2020) 75:2921–30. doi: 10.1016/j.jacc.2020.04.038
6. Kim HC, Cho SMJ, Lee H, Lee H-H, Baek J, Heo JE, et al. Korea hypertension fact sheet 2020: analysis of nationwide population-based data. Clin Hypertens. (2021) 27:8. doi: 10.1186/s40885-021-00166-2
7. Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M. Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron. (2019) 143:38–42. doi: 10.1159/000499486
8. Premaratne E, Verma S, Ekinci EI, Theverkalam G, Jerums G, MacIsaac RJ. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab. (2015) 41:5–17. doi: 10.1016/j.diabet.2014.10.003
9. Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. (2012) 8:293–300. doi: 10.1038/nrneph.2012.19
10. Jhee JH, Kee YK, Park S, Kim H, Park JT, Han SH, et al. High-protein diet with renal hyperfiltration is associated with rapid decline rate of renal function: a community-based prospective cohort study. Nephrol Dial Transplant. (2020) 35:98–106. doi: 10.1093/ndt/gfz115
11. Silva Junior GB, Bentes AC, Daher EF, Matos SM. Obesity and kidney disease. J Bras Nefrol. (2017) 39:65–9. doi: 10.5935/0101-2800.20170011
12. Dunlop W, Davison JM. Renal haemodynamics and tubular function in human pregnancy. Baillieres Clin Obstet Gynaecol. (1987) 1:769–87. doi: 10.1016/s0950-3552(87)80034-2
13. Yoo KD, Yoon HJ, Hwang SS, Heo NJ, Chin HJ, Yang SH, et al. Different association between renal hyperfiltration and mortality by sex. Nephrology. (2017) 22:804–10. doi: 10.1111/nep.12857
14. Reboldi G, Verdecchia P, Fiorucci G, Beilin LJ, Eguchi K, Imai Y, et al. Glomerular hyperfiltration is a predictor of adverse cardiovascular outcomes. Kidney Int. (2018) 93:195–203. doi: 10.1016/j.kint.2017.07.013
15. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea national health and nutrition examination survey (KNHANES). Int J Epidemiol. (2014) 43:69–77. doi: 10.1093/ije/dyt228
16. Ryu KS, Kang HYJ, Lee SW, Park HW, You NY, Kim JH, et al. Screening model for estimating undiagnosed diabetes among people with a family history of diabetes mellitus: a KNHANES-based study. Int J Environ Res Public Health. (2020) 17:8903. doi: 10.3390/ijerph17238903
17. Cho SMJ, Lee H, Pyun WB, Kim HC. Differential control rate of systolic and diastolic blood pressure among Korean adults with hypertension: the sixth Korean national health and nutrition examination survey, 2013-2015 (KNHANES VI). Korean Circ J. (2019) 49:1035–48. doi: 10.4070/kcj.2019.0049
18. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
19. Oh SW, Yang JH, Kim MG, Cho WY, Jo SK. Renal hyperfiltration as a risk factor for chronic kidney disease: a health checkup cohort study. PLoS One. (2020) 15:e0238177. doi: 10.1371/journal.pone.0238177
20. Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2018) 71:e127–248. doi: 10.1016/j.jacc.2017.11.006
21. Mahmud A, Feely J. Spurious systolic hypertension of youth: fit young men with elastic arteries. Am J Hypertens. (2003) 16:229–32. doi: 10.1016/s0895-7061(02)03255-7
22. Saladini F, Dorigatti F, Santonastaso M, Mos L, Ragazzo F, Bortolazzi A, et al. Natural history of hypertension subtypes in young and middle-age adults. Am J Hypertens. (2009) 22:531–7. doi: 10.1038/ajh.2009.21
23. Li J, Huang JY, Lo K, Zhang B, Huang YQ, Feng YQ. Association of pulse pressure with all-cause mortality in young adults. Postgrad Med J. (2020) 96:461–6. doi: 10.1136/postgradmedj-2019-137070
24. Nagib SN, Abdelwahab S, Amin GEE, Allam MF. Screening and early detection of chronic kidney disease at primary healthcare. Clin Exp Hypertens. (2021) 43:416–8. doi: 10.1080/10641963.2021.1896726
25. Park S, Lee S, Kim Y, Lee Y, Kang MW, Kim K, et al. Causal effect of alcohol use on the risk of end-stage kidney disease and related comorbidities: a Mendelian randomization study. Kidney Res Clin Pract. (2021) 40:282–93. doi: 10.23876/j.krcp.20.186
26. Iseki K. Nutrition and quality of life in chronic kidney disease patients: a practical approach for salt restriction. Kidney Res Clin Pract. (2022). [Epub ahead of print]. doi: 10.23876/j.krcp.21.203
27. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. (2017) 12:2032–45. doi: 10.2215/cjn.11491116
28. Mickelsson M, Söderström E, Stefansson K, Andersson J, Söderberg S, Hultdin J. Smoking tobacco is associated with renal hyperfiltration. Scand J Clin Lab Invest. (2021) 81:622–8. doi: 10.1080/00365513.2021.1989713
29. Safar ME, Plante GE, Mimran A. Arterial stiffness, pulse pressure, and the kidney. Am J Hypertens. (2015) 28:561–9. doi: 10.1093/ajh/hpu206
30. Owens EA, Jie L, Reyes BAS, Van Bockstaele EJ, Osei-Owusu P. Elastin insufficiency causes hypertension, structural defects and abnormal remodeling of renal vascular signaling. Kidney Int. (2017) 92:1100–18. doi: 10.1016/j.kint.2017.04.044
31. Zhang Y, Lacolley P, Protogerou AD, Safar ME. Arterial stiffness in hypertension and function of large arteries. Am J Hypertens. (2020) 33:291–6. doi: 10.1093/ajh/hpz193
32. Battistoni A, Michielon A, Marino G, Savoia C. Vascular aging and central aortic blood pressure: from pathophysiology to treatment. High Blood Press Cardiovasc Prev. (2020) 27:299–308. doi: 10.1007/s40292-020-00395-w
33. Safar ME, Nilsson PM, Blacher J, Mimran A. Pulse pressure, arterial stiffness, and end-organ damage. Curr Hypertens Rep. (2012) 14:339–44. doi: 10.1007/s11906-012-0272-9
34. Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin Nephrol. (2003) 23:194–9. doi: 10.1053/anep.2003.50017
35. DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. (2014) 10:364–76. doi: 10.1038/nrendo.2014.44
36. Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension. (2003) 41:183–7. doi: 10.1161/01.hyp.0000047464.66901.60
37. Taimour S, Gottsäter A, Jujic A, Nilsson PM. Hyperglycemia and arterial stiffness across two generations. J Hypertens. (2021) 39:471–5. doi: 10.1097/hjh.0000000000002677
38. Cooper JN, Buchanich JM, Youk A, Brooks MM, Barinas-Mitchell E, Conroy MB, et al. Reductions in arterial stiffness with weight loss in overweight and obese young adults: potential mechanisms. Atherosclerosis. (2012) 223:485–90. doi: 10.1016/j.atherosclerosis.2012.05.022
39. Kappus RM, Fahs CA, Smith D, Horn GP, Agiovlasitis S, Rossow L, et al. Obesity and overweight associated with increased carotid diameter and decreased arterial function in young otherwise healthy men. Am J Hypertens. (2014) 27:628–34. doi: 10.1093/ajh/hpt152
40. Julius S, Krause L, Schork NJ, Mejia AD, Jones KA, van de Ven C, et al. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J Hypertens. (1991) 9:77–84. doi: 10.1097/00004872-199101000-00012
41. Dart AM, Kingwell BA. Pulse pressure–a review of mechanisms and clinical relevance. J Am Coll Cardiol. (2001) 37:975–84. doi: 10.1016/s0735-1097(01)01108-1
42. Kupari M, Hekali P, Keto P, Poutanen VP, Tikkanen MJ, Standerstkjöld-Nordenstam CG. Relation of aortic stiffness to factors modifying the risk of atherosclerosis in healthy people. Arterioscler Thromb. (1994) 14:386–94. doi: 10.1161/01.atv.14.3.386
43. Lehmann ED, Watts GF, Gosling RG. Aortic distensibility and hypercholesterolaemia. Lancet. (1992) 340:1171–2. doi: 10.1016/0140-6736(92)93210-e
44. Tsuboi N, Okabayashi Y. The renal pathology of obesity: structure-function correlations. Semin Nephrol. (2021) 41:296–306. doi: 10.1016/j.semnephrol.2021.06.002
45. D’Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. (2016) 12:453–71. doi: 10.1038/nrneph.2016.75
46. Kwagyan J, Tabe CE, Xu S, Maqbool AR, Gordeuk VR, Randall OS. The impact of body mass index on pulse pressure in obesity. J Hypertens. (2005) 23:619–24. doi: 10.1097/01.hjh.0000160220.71350.5f
47. Zachariah JP, Graham DA, de Ferranti SD, Vasan RS, Newburger JW, Mitchell GF. Temporal trends in pulse pressure and mean arterial pressure during the rise of pediatric obesity in US children. J Am Heart Assoc. (2014) 3:e000725. doi: 10.1161/jaha.113.000725
48. Saladini F, Fania C, Mos L, Mazzer A, Casiglia E, Palatini P. Office pulse pressure is a predictor of favorable outcome in young- to middle-aged subjects with stage 1 hypertension. Hypertension. (2017) 70:537–42. doi: 10.1161/hypertensionaha.117.09516
49. O’Rourke MF, Vlachopoulos C, Graham RM. Spurious systolic hypertension in youth. Vasc Med. (2000) 5:141–5. doi: 10.1177/1358836x0000500303
50. Hulsen HT, Nijdam ME, Bos WJ, Uiterwaal CS, Oren A, Grobbee DE, et al. Spurious systolic hypertension in young adults; prevalence of high brachial systolic blood pressure and low central pressure and its determinants. J Hypertens. (2006) 24:1027–32. doi: 10.1097/01.hjh.0000226191.36558.9c
51. Rowe C, Sitch AJ, Barratt J, Brettell EA, Cockwell P, Dalton RN, et al. Biological variation of measured and estimated glomerular filtration rate in patients with chronic kidney disease. Kidney Int. (2019) 96:429–35. doi: 10.1016/j.kint.2019.02.021
52. Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. (2009) 20:2305–13. doi: 10.1681/asn.2009020171
Keywords: pulse pressure, renal hyperfiltration, estimated glomerular filteration rate, young adult, kidney function
Citation: Yang E, Park SH, Lee S, Oh D, Choi HY, Park HC and Jhee JH (2022) Pulse pressure and the risk of renal hyperfiltration in young adults: Results from Korea National Health and Nutrition Examination Survey (2010–2019). Front. Med. 9:911267. doi: 10.3389/fmed.2022.911267
Received: 02 April 2022; Accepted: 08 August 2022;
Published: 13 September 2022.
Edited by:
Chia-Ter Chao, National Taiwan University Hospital, TaiwanReviewed by:
Hyoungnae Kim, Soonchunhyang University Hospital Seoul, South KoreaCopyright © 2022 Yang, Park, Lee, Oh, Choi, Park and Jhee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jong Hyun Jhee, ampobG92ZTc3QHl1aHMuYWM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.