
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 13 June 2022
Sec. Family Medicine and Primary Care
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.910623
This article is part of the Research Topic Challenges in Inflammatory Bowel Disease: Current, Future and Unmet Needs View all 13 articles
 Ni Tien1,2†
Ni Tien1,2† Tien-Yuan Wu3,4†
Tien-Yuan Wu3,4† Cheng-Li Lin5
Cheng-Li Lin5 Chia-Jui Wu6
Chia-Jui Wu6 Chung-Y Hsu7
Chung-Y Hsu7 Yi-Jen Fang8,9,10,11,12*
Yi-Jen Fang8,9,10,11,12* Yun-Ping Lim6,13,14*
Yun-Ping Lim6,13,14*Patients with inflammatory bowel disease (IBD) present a higher risk of developing cardiovascular diseases (CVDs) due to chronic inflammation, which plays an essential role in atherogenesis. Hyperlipidemia is another risk factor for CVDs; however, the association between IBD, IBD medications, and hyperlipidemia remains controversial. We conducted a nationwide, population-based, retrospective, cohort study to examine the effect of IBD and IBD medications on the risk of developing hyperlipidemia. The effects of IBD medications on the expression of lipogenesis-related hepatic genes were also evaluated. We obtained data from the Longitudinal Health Insurance Database of Taiwan from patients with new-onset IBD and a comparison cohort of patients without IBD. A Cox proportional hazards regression model was used to analyze the difference in the risk of developing hyperlipidemia between the two cohorts. We also examined the influence of IBD medications on the expression of lipogenesis-related hepatic genes. After adjusting for comorbidities and confounding factors, the case group (N = 14,524) had a higher risk for hyperlipidemia than the control group (N = 14,524) [adjusted hazards ratio (aHR), 2.18]. Patients with IBD that did not receive IBD medications exhibited a significantly higher risk of hyperlipidemia (aHR, 2.20). In those treated with IBD medications, the risk of developing hyperlipidemia was significantly lowered than those without such medications (all aHR ≤ 0.45). Gene expression analysis indicated that IBD medications downregulated the expression of lipogenesis-related genes. Screening blood lipids in IBD patients is needed to explore the specific role and impact of IBD medications in the development of CVD.
Inflammatory bowel diseases (IBDs) have no cure and are characterized by chronic, recurrent exacerbation and intestinal inflammation, resulting in altered gut functions (1). IBDs consist of Crohn's disease (CD) and ulcerative colitis (UC). The fundamental causes of these autoimmune diseases include the interplay between genetic and environmental factors, excluding pathogenic infections (2). IBD diagnoses depend on multiple factors, including clinical, endoscopic, radiological, and histological features, but not infectious etiology (1). In Taiwan, crude CD and UC incidences increased from 0.17 to 0.47 and 0.54 to 0.95 new cases per 100,000 persons, respectively, between 2001 and 2015. Moreover, CD and UC prevalence increased from 0.6 to 3.9 and 2.1 to 12.8 cases per 100,000 persons, respectively, within the same time frame. The male-to-female ratio in the study samples were 2.19 for CD and 1.62 for UC (3–5). Meanwhile, IBD prevalence in the USA is estimated to be 1.1–3 million adults (6). In the Western world, IBD prevalence is approximately 50–200 and 120–200 cases per 100,000 persons for CD and UC, respectively (7). J. Cosnes, C. Gower–Rousseau, P. Seksik, and A. Cortot, “Epidemiology and natural history of inflammatory bowel diseases,” Gastroenterology, vol. 140, no. 6, pp. 1785–1794.e4, 2011. View at: Publisher Site. UC, as a chronic and recurrent intestinal disease, is mainly an autoimmune disease caused by genetic–environmental interactions, rather than colonic colitis caused by general bacterial and viral infections.IBD cases increase annually, and drugs and changes to the environment can improve patient quality of life. Medications remain the main therapeutic strategy for IBD and to relieve inflammation. In addition, surgery can be introduced if medications are unsuccessful or result in serious adverse reactions (1).
IBDs coincide with clotting abnormalities and vascular-related comorbidities, such as deep vein thrombosis, portal vein thrombosis, and ischemic vascular diseases (8). It has been reported that patients with IBD have a venous thromboembolism (VTE) risk 1.7–5.9 times greater than the general population. This has been found to affect 0.55–6.15% of patients with IBD, and the overall prevalence of VTE in IBD subjects was estimated as 1–8% (9). Moreover, VTE-associated mortality is twice as high in patients with IBD than in the general population (10). Further, a meta-analysis demonstrated that IBD is associated with an 18% higher risk of CVD (11), and the risk is higher for females than males [adjusted odds ratio (aOR), 1.28] (8). Additionally, the risk of mesenteric ischemia is increased 3.4-fold, and that of VTE is increased 1.4-fold (12).
The relationship between IBD and hyperlipidemia risk should be evaluated. Moreover, further research is warranted to devise therapeutic modalities to prevent hyperlipidemia and consequently decrease the CVD risk for IBD patients. Preventive or therapeutic strategies can also be developed to identify the pathogenic causes of these complications (9, 11, 13–19). Hyperlipidemia, a well-established CVD risk factor (20), is defined as abnormal lipid levels with total cholesterol (TC) ≥ 200 mg/dL, triglycerides (TGs) ≥ 150 mg/dL, high-density lipoprotein cholesterol (HDL-C) ≤ 40 mg/dL, and low density lipoprotein cholesterol (LDL-C) ≥ 130 mg/dL. Hyperlipidemia can originate from genetics, diet, lifestyle, metabolic disorders, and other diseases (21). Additionally, the degree of hyperlipidemia correlates with CVD severity and can predict prognosis. For example, patients with IBD often have changing blood lipid profiles, similar to those reported in CVD, frequently caused by IBD medications. Thus, risk factor modulation is needed to reduce blood lipid levels and CVD risk (8).
In contrast to lipid levels in hyperlipidemia, patients with IBD have low levels of TC and HDL-C and high levels of LDL-C and TGs (22). The exact mechanism underlying these altered levels is unknown; however, active inflammation and changes to lipid, apolipoprotein, and lipoprotein profiles via altered lipid de novo synthesis and degradation might play a role (22–24). Despite these lipid levels, patients with IBD have an increased risk of CVD (25), resulting in a “lipid paradox”. However, studies showing these results are limited by small sample sizes. Moreover, the relationship between IBD therapeutics and reduced CVD risk remains controversial (26). Thus, we conducted a long-term, retrospective study with a large cohort to evaluate patients with IBD and the effects of their medications.
The mechanisms underlying the increased risk of CVD in patients with IBD are under investigation; however, systemic inflammation might play a role. Different drugs are selectively and broadly used to inhibit inflammation in IBD, controlling the active disease and inhibiting remission. Pharmacological treatment for IBD can be divided into four main classes, (1) aminosalicylates (5-aminosalicylic acid derivatives), (2) corticosteroids, (3) immunosuppressants, and (4) monoclonal antibodies (26). 5-Aminosalicylates are the most widely used and are ideal for IBD with mild to moderate symptoms mainly by blocking prostaglandin and leukotriene production (27). Corticosteroids are also used for reducing inflammation, however, they frequently have side effects dependent on the dose and treatment duration (28). Immunosuppressive and immunoregulatory agents could include the suppression of a specific subgroup of T cells, achieving a therapeutic response after a prolonged period (28). Therefore, these drugs are only useful for long-term control, rather than acute disease. Biologics are groups of monoclonal antibodies for patients with a reduced response to IBD drugs with small molecules. Several anti-tumor necrosis factor (TNF) therapies could inhibit TNF production by macrophages through altered regulatory peptide expression with IBD, which might lead to monocyte apoptosis (28). In addition, biologics can block leukocyte migration by blocking integrin adhesion molecules. Nevertheless, when prolonged treatment results in complications or adverse events, surgery can also be performed.
The liver is responsible for most processes involved in lipid homeostasis, including lipogenesis and blood lipid balancing. Therefore, any changes in hepatic lipid metabolism might affect the balance and homeostasis of blood lipid levels and could result in the development of non-alcoholic fatty liver disease (NAFLD) (29, 30). Liver X receptor alpha (LXRα), a nuclear receptor activated by ligands and act as transcription factor, is highly and specifically expressed in liver, responsible for lipid metabolism and de novo synthesis and excretion of cholesterol (31). LXRα activation results in the development of steatosis, which is mediated by the hepatic lipogenic pathway, primarily through sterol regulatory element binding protein 1 (SREBP-1c) (32). In addition, hepatic expression of LXRα, SREBP-1c, and their target genes, was found to be significantly upregulated in liver biopsies from NAFLD patients (33). Ultrasonographic monitoring revealed hepatic steatosis in approximately 50% of patients with associated with hypertriglyceridemia (29). Hyperlipidemia is commonly associated with NAFLD and is an independent risk factor of atherogenic dyslipidemia based on various clinical studies (33). Thus, the most important role of LXRα is the maintenance of lipid homeostasis as it regulates the balance of lipid-metabolism genes.
Further studies are warranted to determine how treatments for IBD affect hyperlipidemia risk. Thus, we performed a nationwide, population-based, cohort study to evaluate the risk of hyperlipidemia in patients with IBD from the 2000 to 2012 National Health Insurance Research Database (NHIRD) compared to that in the general population in Taiwan. We also examined the impact of IBD medications on the risk of hyperlipidemia and the expression of lipogenesis-related genes in differentiated HepaRG cells. We show that patients with IBD are more likely to have hyperlipidemia than those without IBD.
We obtained data from the NHIRD. The database covers >99% of the population of 23 million in Taiwan and was constructed using comprehensive inpatient and outpatient health care information, including demographic data, diagnostic codes, and prescription details. The dataset from the NHIRD is a subset of the Longitudinal Health Insurance Database, which comprises data of one million randomly sampled beneficiaries enrolled in the NHI program. The International Classification of Diseases, Clinical Modification (ICD-9-CM, procedure code 555 and 556) was used as the disease diagnostic tool. This study was approved by the Central Regional Research Ethics Committee of China Medical University, Taichung, Taiwan (CMUH-104-REC2-115-R5).
In this population-based cohort study, we established an IBD cohort and a non-IBD cohort of patients enrolled in the database from January 1, 2000 to December 31, 2012 to compare their risk of hyperlipidemia. The index date of the case group (IBD cohort) was defined as the date of the first diagnosis of IBD and that of the control group (as the non-IBD cohort) was a random date during the study period. We excluded patients who had a history of hyperlipidemia before the index date. According to age (5-year intervals), gender, and the index year, the cohorts were frequency matched at a 1:1 ratio. The end date of the follow-up period was the onset of hyperlipidemia, death, or the end of study period (December 31, 2013), whichever came first. The primary outcome of the study was an individual event of hyperlipidemia (ICD-9-CM code 272) is defined as increased serum fasting levels of TC (≥200 mg/dL), LDL-C (≥130 mg/dL), or TG (≥150 mg/dL), and elevations of fasting TC concentration, which may or may not be associated with the elevated TG concentration, and decreased of HDL-C. To avoid subjects being mistakenly diagnosed or mistakenly coded as hyperlipidemia cases, we therefore defined patients with at least two claims for outpatient care and/or one hospitalization visit to ensure the validity of diagnosis. We selected potential confounders based on the previous research for multivariable analysis, including age, sex, and comorbidities of type 2 diabetes mellitus (T2DM) (ICD-9-CM 250), obesity (ICD-9-CM 278), coronary artery disease (CAD) (ICD-9-CM 410–414), hypertension (ICD-9-CM 401–405), and chronic kidney disease (CKD) (ICD-9-CM 585, 586). Further analysis was performed to investigate the effect of IBD treatment available in Taiwan on the risk of hyperlipidemia compared to non-IBD controls, IBD without medical treatment, and IBD without surgical treatment.
All chemicals were purchased from Sigma-Aldrich (St. Louis, Missouri, USA) and were of the highest-purity grade available. Chemicals were dissolved in dimethyl sulfoxide (DMSO) at appropriate concentrations before use. Human hepatoma HepaRGTM cells were purchased from Thermo Fisher Scientific (Waltham, Massachusetts, USA). Frozen cells were thawed and maintained in Williams' E medium (Sigma-Aldrich) supplemented with 10% Fetal CloneTM II serum (HycloneTM, GE Healthcare, Chicago, Illinois, USA), 1 × L-glutamine, 5 μg/mL human insulin, and 50 μM hydrocortisone hemisuccinate without antibiotics for 2 weeks. Next, the medium was replaced with the same medium plus 2% DMSO for two additional weeks to induce differentiated hepatocyte-like properties. Cells were cultured in a humidified atmosphere of 5% CO2 at 37°C. Cell viability was assessed using p-nitrophenylphosphate in an acid phosphatase assay (ACP), as previously reported (34).
To evaluate the effects of IBD medications (corticosteroids, immunomodulators, and aminosalicylates) on hepatic lipogenesis-related gene expression, mRNA levels were measured. Total RNA was extracted from differentiated HepaRG cells under various treatment conditions using a Direct-zolTM RNA MiniPrep kit (ZYMO Research, Irvine, CA, USA) according to the manufacturer's protocol. The quantity and purity of RNA were confirmed by calculating the ratio of the absorbance at 260 nm to the absorbance at 280 nm. Total RNA (1 μg) was subjected to synthesis of first-strand cDNA using a MultiScribeTM reverse transcriptase kit (ThermoFisher Scientific). Expression of SREBP-1c, SCD, FAS, adenosine 5′-triphosphate citrate lyase (ACLY), ACC, LXRα, and β-actin was analyzed by qRT-PCR using Luminaris Color HiGreen qPCR master mix (ThermoFisher Scientific) in the StepOnePlusTM Real-Time PCR System following the standard procedure. Each pair of specific primers used for RT-PCR analysis is listed in Table 1. The amount of target cDNA in each sample was calculated by determining a fractional PCR threshold cycle number (Ct value). The relative mRNA levels were normalized to those of β-actin, and the target cDNA expression was calculated as follows: 2−(Cttargetgene−Ctβ-actin). Data are presented as fold-change compared to the control group.
We evaluated the frequency and percentage of each categorical variable and the mean and standard deviation (SD) of each continuous variable. A chi-squared test was used to examine the differences of the demographic categorical variables between the IBD and non-IBD cohorts. Student's t-test was used to measure the association of continuous demographic variables between the two cohorts. To address the concern of constant proportionality, we examined the proportional hazard model assumption using a test of scaled Schoenfeld residuals. Results showed that there was no significant relationship between Schoenfeld residuals for IBD and follow-up time (p-value = 0.68) in the model evaluating the hyperlipidemia risk. Stratified Cox models were used to estimate the risk of hyperlipidemia by sex, age, and comorbidity between the two cohorts. The aHR was obtained for age, sex, and comorbidities of T2DM, obesity, CAD, hypertension, and CKD disease through multivariable analysis. The Kaplan–Meier method was applied to estimate cumulative incidence curves of hyperlipidemia in both cohorts, with significance based on the log-rank test. Analyses were performed in SAS software, version 9.4, and survival curves were drawn using R software.
For in vitro studies, data obtained from separate measurements were reported as the mean ± standard error (SE). The P-value for each experimental comparison was determined using analysis of variance, followed by the least significant difference test for multiple comparisons. All P-values were determined relative to the control group, as indicated in the figures. All statistical analyses were performed using SPSS for Windows, version 20.0 (IBM SPSS, Armonk, NY, USA). P < 0.05 was considered statistically significant.
Table 2 shows the baseline characteristics of the study population from 2000 to 2013. After propensity score matching, and calculating the real-world database power as 0.9996, this study included 14,524 patients with and without IBD, respectively. Among the types of IBD, 1,362 (9.38%) and 13,162 (90.6%) patients had UC and CD, respectively. The mean ages for the IBD and non-IBD cohorts were 44.3 (SD = 16.4) and 43.9 (SD = 16.7) years, respectively. No significant differences were observed between the two cohorts in sex, age, or comorbidity of CKD. Patients with IBD were at higher risk of T2DM, obesity, CAD, and hypertension. Regarding treatment for patients with IBD, 68.6%, 0.97%, 3.60%, 0.03%, and 1.46% of patients received corticosteroids, immunomodulators, aminosalicylates, monoclonal antibodies, and surgical treatment, respectively. As shown in Figure 1, the Kaplan–Meier plots revealed that the cumulative incidence curve of hyperlipidemia showed a significantly higher risk in patients with IBD than those without.
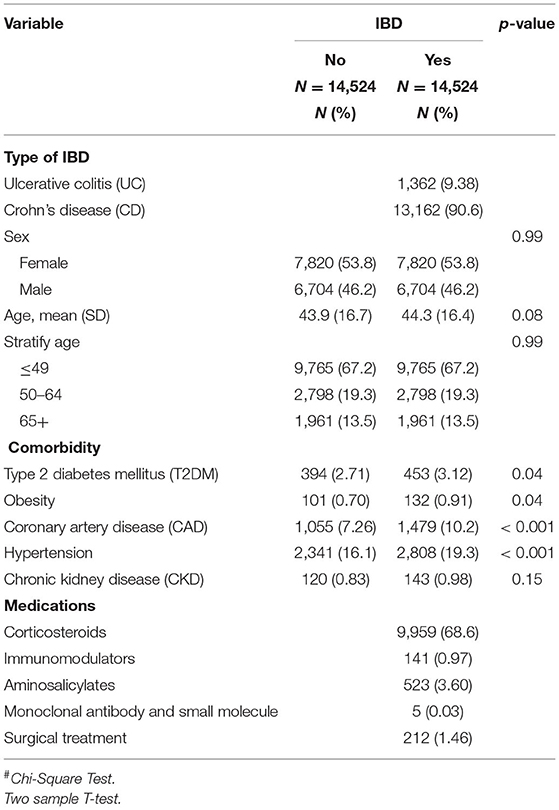
Table 2. Demographic characteristics, comorbidities, and medications in patient with and without inflammatory bowel disease (IBD).
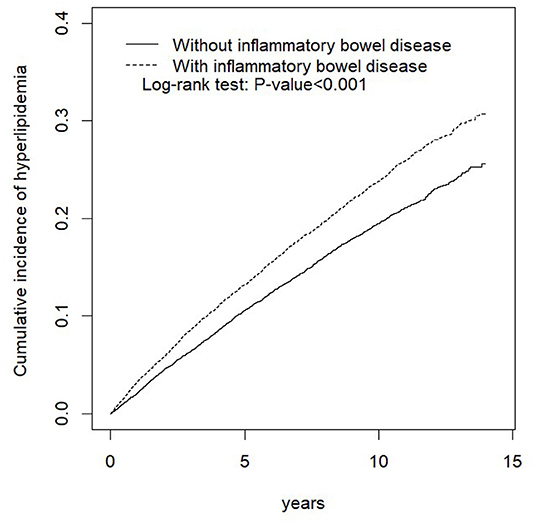
Figure 1. Cumulative incidence of hyperlipidemia compared between the cohort with and without inflammatory bowel disease (IBD) using the Kaplan–Meier method.
Table 3 displays the stratified analyses of the risk of hyperlipidemia by sex, age, and comorbidity between the two cohorts. Compared with the control cohort, the IBD cohort faced a 2.18-fold higher risk of hyperlipidemia (95% CI, 2.03–2.34). Compared with that in the control cohort, UC resulted in a 2.60-fold increased hyperlipidemia risk (95% CI, 2.06–3.28), and the CD group was associated with a 2.15-fold increased hyperlipidemia risk (95% CI, 1.99–2.32). Moreover, females had a 2.10-fold higher risk of hyperlipidemia in the IBD cohort after controlling for other factors (95% CI, 1.90–2.32), while the risk for males in the IBD cohort was 2.25-fold (95% CI, 2.03–2.49). Among patients aged ≤ 49 years, the IBD cohort had a 2.00-fold higher risk of hyperlipidemia than the non-IBD cohort (95% CI, 1.81–2.21). Similarly, those aged 50–64 years had a 2.10-fold higher risk of hyperlipidemia in the IBD cohort (95% CI, 1.86–2.37). Additionally, among those aged ≥65 years, the IBD cohort had a 2.78-fold higher risk of hyperlipidemia (95% CI, 2.34–3.31). Moreover, among patients without comorbidities, the IBD cohort had a 2.06-fold higher risk of hyperlipidemia compared to the non-IBD cohort. Meanwhile, patients with comorbidities in the IBD cohort had a 2.25-fold higher risk of hyperlipidemia than those in the non-IBD cohort.
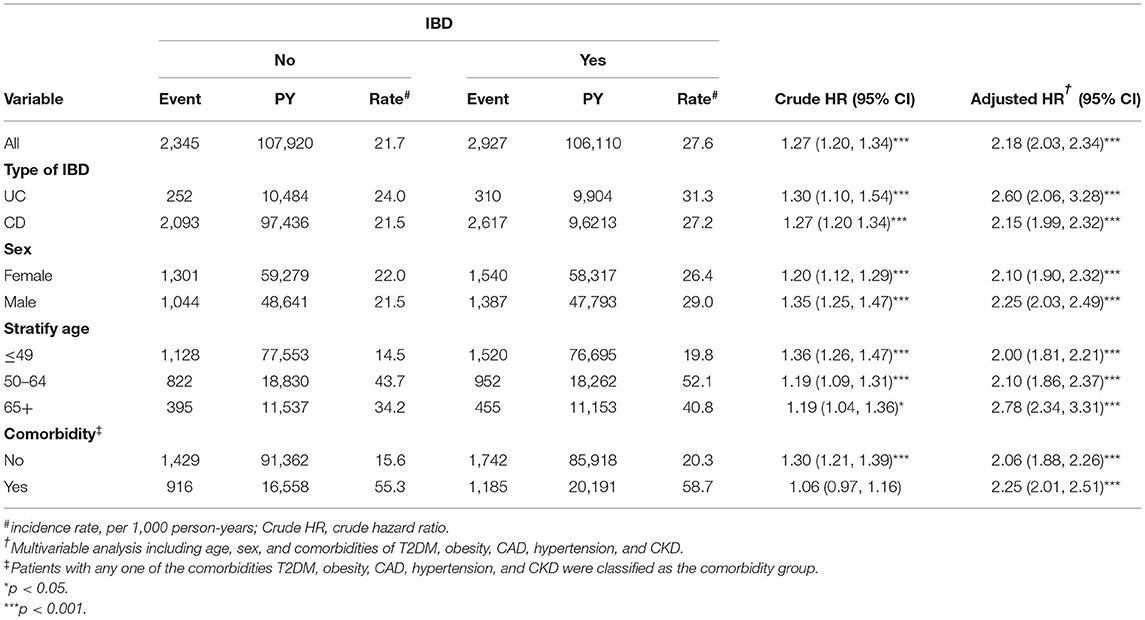
Table 3. Comparison of incidence (CI) and hazard ratio (HR) of hyperlipidemia stratified by sex, age, and comorbidities between with and without inflammatory bowel disease (IBD).
Table 4 presents the incidence, as well as the crude and aHR of hyperlipidemia among IBD patients with and without treatment compared to those without IBD. Compared to the control cohort, the IBD cohort without treatment had a 2.20-fold higher risk of hyperlipidemia (95% CI, 2.05–2.36). Stratification of each type of IBD treatment suggested that immunomodulators, aminosalicylates, and corticosteroids could significantly decrease the risk of hyperlipidemia (aHR, 0.29; 95% CI, 0.18–0.49, aHR, 0.43; 95% CI, 0.34–0.55, aHR, 0.45; 95% CI, 0.42–0.49, respectively) compared to that of patients with IBD without treatment. Further stratification according to surgical treatment for IBD showed that surgical treatment could reduce the risk of hyperlipidemia (aHR, 0.32; 95% CI, 0.20–0.51).
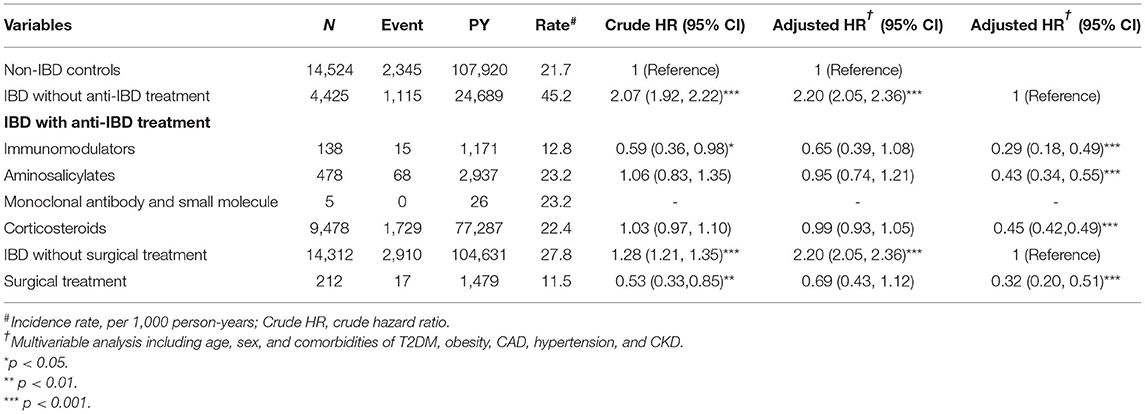
Table 4. Incidence, crude, and adjusted hazard ratio (aHR) of hyperlipidemia compared among inflammatory bowel disease (IBD) patients with and without IBD treatment compared to non-IBD controls.
We assessed the influence of corticosteroids (budesonide, prednisolone, methylprednisolone, and hydrocortisone), immunomodulators (azathioprine, methotrexate, cyclosporine, tacrolimus, and 6-mercaptopurine), and aminosalicylates (sulfasalazine, balsalazide, and 5-aminosalicylate) on hepatic gene expression. The concentrations were adjusted according to the maximum plasma or serum drug concentrations as they exhibit hepatotoxicity (35–47). We tested the toxicity of the drugs in a cell viability assay using human hepatoma HepaRG cells. The concentrations of corticosteroids were 0.01, 1.13, 0.72, and 1.42 μM for budesonide, prednisolone, methylprednisolone, and hydrocortisone, respectively; those of immunomodulators were 0.15, 1.77, 1.91, 0.03, and 0.77 μM for azathioprine, methotrexate, cyclosporine, tacrolimus, and 6-mercaptopurine, respectively; and those of aminosalicylates were 61.5, 1.40, and 60.1 μM for sulfasalazine, balsalazide, and 5-aminosalicylate, respectively.
The cells were treated with IBD medications for 48 h, and the ACP assay was used to assess the cell viability. Our results indicated that the cell viability from each drug remained >95% compared with the control group (Figure 2). Moreover, the expression of lipogenesis-related hepatic genes, such as SREBP-1c, SCD, FAS, ACLY, ACC, and LXRα were assessed using RT-PCR in differentiated HepaRG cells treated with IBD medications for 48 h. The total RNA was extracted, and gene expression was analyzed. SREBP-1c, a transcription factor that regulates lipid homeostasis by modulating the expression of a series of target lipogenic genes (33), is mainly distributed in the liver and participates in hepatic fatty acid synthesis by upregulating the expression of downstream genes. T0901317, a synthetic LXRα agonist, significantly induced the expression of these target genes. However, the expression levels of SREBP-1c, SCD, FAS, ACLY, ACC, and LXRα were significantly lower in most treatment groups than the untreated groups (Figures 3A–F). IBD medications reduce the risk of hyperlipidemia and partially reduce the expression of hepatic genes involved in lipogenesis, resulting in improved blood lipid profiles.
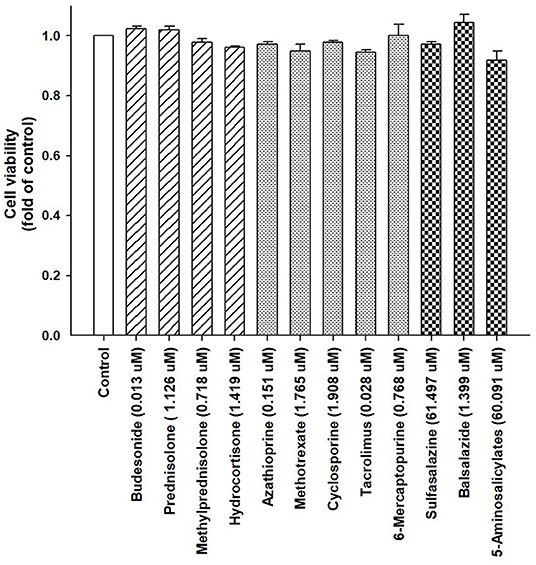
Figure 2. Viability of HepaRG cells following exposure to IBD medications. HepaRG cells were exposed to corticosteroids (budesonide, prednisolone, methylprednisolone, and hydrocortisone), immunomodulators (azathioprine, methotrexate, cyclosporine, tacrolimus, and 6-mercaptopurine), and aminosalicylates (sulfasalazine, balsalazide, and 5-aminosalicylate) for 48 h. Cell viability was monitored by cellular acid phosphatase activity using p-nitrophenylphosphate as a substrate. The data are shown as the mean ± SE (error bars) (n = 4).

Figure 3. Expression of hepatic lipid metabolism-related genes following treatment with T0901317 and IBD medications. Differentiated HepaRG cells were treated for 48 h with T0901317 (10 μM), corticosteroids (budesonide, prednisolone, methylprednisolone, and hydrocortisone), immunomodulators (azathioprine, methotrexate, cyclosporine, tacrolimus, and 6-mercaptopurine), and aminosalicylates (sulfasalazine, balsalazide, and 5-aminosalicylate). Following treatment, mRNA was extracted and the expression levels of (A) SREBP-1c; (B) SCD; (C) FAS; (D) ACLY; (E) ACC; and (F) LXRα were analyzed by qRT-PCR. Values were normalized to the expression of β-actin, with the levels of DMSO-treated cells set at 1. Results are expressed as the mean ± SE (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001 compared with cells treated with DMSO.
To the best of our knowledge, this was the first study to report the correlation of IBD medications and risk of hyperlipidemia in patients with IBD, particularly in Asian populations. This study assessed 23 million people living in Taiwan using the NHIRD (47). The results show that the risk of hyperlipidemia was 2.18 times higher in patients with IBD than the general population after adjusting for confounding factors and comorbidities (P < 0.001). Moreover, this exclusive, nationwide population-based cohort study presented a comprehensive and complete assessment of an Asian population and discovered a significant association between IBD, IBD medications, and hyperlipidemia. We also found that without IBD therapies (medications and surgical treatment), the risk of hyperlipidemia was higher in patients with IBD than in the control cohort (aHR, 2.20; 95% CI, 2.05–2.36, P < 0.001). Furthermore, with IBD therapies (medications and surgical treatment), the risk of hyperlipidemia decreased in patients with IBD compared to those without IBD (all aHR ≤ 0.99, not significant). In addition, among patients with IBD, the risk of hyperlipidemia significantly decreased in those that received IBD treatment compared to those that did not (all aHR ≤ 0.45, P < 0.001). Finally, IBD medications in hepatocyte-derived HepaRG cells significantly reduced the expression of lipogenesis-related genes, such as SREBP-1c, SCD, FAS, ACLY, ACC, and LXRα, contributing to improved blood lipid profiles. These findings provide valuable insights for clinical physicians regarding the benefits of IBD therapies for improving blood lipid profiles and CVD prognosis, as well as reducing the risk of CVD through blood lipid control. Our study clarifies the impact of IBD medications on the development of hyperlipidemia, and the decreased risk of hyperlipidemia in patients receiving treatment may be due to drug-induced downregulation of lipogenic genes, especially the master lipogenic transcription factors, SREBP-1c and LXRα.
Our analysis of 29,048 people (14,524 with IBD and 14,524 without) with a mean follow-up period >7 years showed an overall significant increase in risk of hyperlipidemia compared with the general population (aHR, 2.18). Moreover, males, patients >65 years of age, and those with comorbidities were at higher risk than those in the general population matched by age, sex, and comorbidities of T2DM, obesity, CAD, hypertension, and CKD. As such, these groups may require early and aggressive intervention for CVD risk management. Furthermore, a significant association was observed between hyperlipidemia and IBD; however, patients with IBD that received IBD therapy had a decreased risk of hyperlipidemia compared to those that did not receive therapy.
Several large meta-analyses and cohort studies have reported increased rates of CVD in patients with IBD. However, hyperlipidemia was less likely to be assessed among such patients, which could result in the silent progression of atherosclerosis and vascular defects. Moreover, acute myocardial infarction (AMI) was found to be higher in patients with IBD than in those without (OR, 12.05; 95% CI, 11.16–13.01), as was the rate of VTE (12, 18). A prospective study of 24 patients with IBD who had undergone surgical treatment, exhibited increased levels of HDL-C, TC, and LDL-C in patients in remission compared to those with recurrent active IBD (48). In another retrospective study, TC and LDL-C levels were significantly lower in patients with IBD after restorative proctocolectomy than in control subjects (49). These findings may be due to malabsorption from accelerated transit and the exclusion of the terminal ileum caused by the covering ileostomy (49). TC and HDL-C levels were significantly lower, TG levels were significantly higher in patients with IBD compared to controls were also reported (50). Ripollés Piquer et al. suggested that the levels they observed corresponded with those of atherogenesis and may contribute to the development of CVD (24). Nevertheless, more large-scale and epidemiological studies are warranted to evaluate lipid alterations in patients with IBD more in depth.
Lipid profile alterations in patients with IBD have been presented in previous studies. First, Becker and colleagues (51) showed an association of decreased TC levels and increased TG levels with intestinal resection in patients with CD in a study of 22 children with CD and 10 healthy controls. Moreover, a US study (22) concluded that patients with IBD have lower TC and HDL-C levels and higher LDL-C and TG levels compared to controls from the NHANES 2005–2006 database. Another report showed that patients with IBD have lower TC and LDL-C levels compared to control subjects due to systemic inflammation from reduced lipoprotein and hepatic lipase activity and decreased cholesterol absorption (52). The conflicting results of these studies may be partially explained by the differences in study population, methodology, data interpretation, duration of sampling, and baseline characteristics of the study samples (21, 22, 50). While our findings are not consistent with all studies, we performed an in-depth analysis of different treatment modalities over a prolonger follow-up period. Moreover, our study was based on a nationwide population database rather than a single institution.
No study has previously reported the correlation of IBD therapies with hyperlipidemia. We found that IBD treatments can prevent patients from developing hyperlipidemia. Thus, while IBD can neither be prevented nor cured, hyperlipidemia can be partially prevented by appropriate interventions such as diet control, regular exercise, and reduced body weight. In view of hyperlipidemia-related CVD, physicians who participate in the long-term care of patients with IBD should consider aggressive modification of risk factors associated with hyperlipidemia. Finally, we conclude that the association of IBD and hyperlipidemia remains to be explored due to conflicting results of each study.
The association between IBD and the increased risk of CVD has been previously reported in a systemic review. The review observed 6,478 coronary events in 123,907 patients with IBD and found a 19% higher risk of CVD in those with IBD (53). Additionally, patients with IBD exhibited a higher risk of carotid atherosclerotic plaque, warranting evaluation of carotid intima-media thickness through ultrasonography for this high-risk subpopulation. Moreover, Kristensen et al. (13) found that compared with controls, patients with IBD presented an increased risk of stroke [relative risk (RR), 1.15; 95% CI, 1.05–1.27] and hospitalization due to heart failure (RR, 1.37; 95% CI, 1.26–1.49) (13). Since, hyperlipidemia is a major risk factor for stroke, which is a leading cause of death (54, 55), may play a role in this situation. In addition, a case-control study reported an increased risk of stroke in patients with IBD (aOR, 2.93; 95% CI, 1.44–5.98) especially in those younger than 50 years of age (56). Thus, the periodic and continuous monitoring of CVD risk in this population is necessary. However, no specific guidelines exist for patients with IBD regarding the prevention and management of CVD (9, 53). Although the current American Heart Association/American College of Cardiology guidelines on blood cholesterol management showed that chronic inflammatory disorders increase the risk of CVD, patients with IBD have not been specifically assessed (57).
Our findings show that patients with IBD were likely to have more comorbidities. For example, several pathophysiological factors are involved in the development of hyperlipidemia in these patients and may increase the risk of CVD. However, lipid profile monitoring in IBD patients is not routinely performed in Taiwan. Additionally, awareness of hyperlipidemia is lower than that of diabetes and hypertension; therefore, hyperlipidemia is diagnosed only when physical complications, such as CVD accompanied by chest pain (58). Thus, hyperlipidemia is rarely managed in patients with IBD. We suggest that clinicians perform blood lipid profiling for patients with IBD due to their increased risk of hyperlipidemia to provide suitable treatment and complete medical care.
Although a previous report showed that body mass index and blood lipid levels are often low in patients with IBD due to poor absorption of bile during active inflammation and after surgery (59). Our data showed that obesity is pronounced in these patients, which may further increase the risk of hyperlipidemia. Another report revealed that the inflammation-based increase of TGs is caused by increased hepatic lipoprotein production and decreased lipoprotein clearance. TGs are mediated by apolipoprotein C-III, which is a main risk factor for atherosclerogenesis (60). Several studies have indicated that chronic inflammation is associated with altered blood lipid profiles and CVD and generates inflammatory cytokines such as TNFα, reduces NOS expression, decreases NO bioavailability, impairs endothelium vasodilation, and increases cell adhesion molecules and stimulating leukocyte migration, therefore promoting atherosclerosis (61). Inflammatory cytokines, such as TNF, IL-6, and IFN-γ may downregulate lipolytic enzyme activity (8); however, the pathophysiological activities in IBD are more complex because of the chronic inflammation, malnutrition, and lipid malabsorption due to intestinal damage or resection (25).
Previous study found that low TC and LDL-C levels were correlated with systemic inflammation and CRP levels in IBD (52). Meanwhile, one study that observed a significant association of low TC and high TGs levels with disease severity failed to present any association with increased levels of inflammatory biomarkers (CRP, ESR, and albumin) (21). They reported increased risk of CVD in patients with IBD along with lower TC levels and chronic inflammation reflects a complicated and poorly understood pathogenic mechanism, described as a “lipid paradox” or dyslipidemia. A better understanding of dyslipidemia in IBD will help prevent and manage atherosclerosis and CVD.
We found that, each group of patients that received IBD medications showed a significant decrease in risk of hyperlipidemia (all aHR < 0.46) after adjusting for age, sex, and comorbidities with T2DM, obesity, CAD, hypertension, and CKD. Whether IBD medications play an important role in reducing the risk of hyperlipidemia and CVD had not been previously elucidated. Moreover, although it has been reported that corticosteroids may increase the risk of atherosclerosis and acute coronary syndrome resulting from hypertension, hyperlipidemia, and thromboembolism (11, 26), they overall reduce the risk of CVD through inhibition of inflammatory response. In the current study, we also found that corticosteroids may inhibit hepatic lipogenic gene expression. Thus, the contribution of corticosteroids to the reduced risk of CVD remains controversial. Nevertheless, methotrexate has been reported to rarely induce steatohepatitis associated with acute coronary syndrome and thromboembolic events (62, 63). We found that among the immunomodulators, methotrexate and 6-mercaptopurine inhibited the expression of lipogenic genes. Moreover, cyclosporine was associated with increased risk of acute coronary syndrome and heart failure (64). In addition, 5-aminosalicylates, a standard first-line treatment for IBD, may be used to prevent CVD and VTE in patients with IBD (14, 65, 66) due to their anti-inflammatory effects and ability to inactivate platelets. Moreover, we found that most 5-aminosalicylates were shown to inhibit lipogenic gene expression.
Increased carotid intimal thickness, wall stiffness, and endothelial dysfunction observed in patients with IBD may be due to the increase in circulating inflammatory cytokines and CRP (67). IBD medications are beneficial for their dual effects of decreasing the expression of lipogenic genes and treating IBD symptoms; however, the net effects of IBD medications on the lipid profile in terms of CVD risk remain uncertain. Thus, the metabolic profiles of patients with IBD should be considered when making therapeutic decisions. Nevertheless, prospective studies are needed to evaluate the dual effects of IBD medications in the general IBD population.
We performed a longitudinal population-based cohort study with cases and controls matched for sex and age to explore the possible correlation of hyperlipidemia and IBD in Taiwan. We also evaluated the effect of IBD medications on the risk of developing hyperlipidemia. Nevertheless, our study had several limitations. First, the database we used may have misclassified IBD and hyperlipidemia, limiting the reliability and validity. However, in Taiwan, universal health insurance is distributed, and as a result, a peer review system is enacted by specialists to reduce the possibilities of false positives. Second, several potential risk factors of hyperlipidemia, such as smoking habits, alcohol intake, body mass index, lifestyle and dietary habits, and family history of hyperlipidemia were not included in this study as they are not provided in the NHIRD. In addition, the risk of hyperlipidemia according to the severity of IBD could not be estimated, as the database had no information regarding the severity of IBD, such as Harvey-Bradshaw Index and Mayo score. Third, this claim database was initially created for charging purposes, and as some information was anonymized, we could not contact the patients directly to get individual data from them. Fourth, several clinical laboratory data were not included in this database, and as such, we could not assess the degree of hyperlipidemia in patients with IBD. For example, serum TC, TGs, HDL-C, and LDL-C. Cases of hyperlipidemia were defined indirectly according to the physicians' records based on lipid levels under IBD medication therapy. In this study, both IBD and hyperlipidemia were accurately analyzed and coded (ICD-9-CM codes) by specialists according to the standard symptomatic criteria by considering the normal side effects, signs, research facility information, and imaging findings. Additionally, this study reduced the confounding effect of medications by adjusting for comorbidities. However, more information should be obtained from other databases to conduct a comprehensive prospective study or randomized controlled trial to further investigate the relationships of IBD, IBD medications, and hyperlipidemia. Most crucially, evidence derived from a retrospective cohort study is typically lower in statistical quality because of numerous sources of inherent bias, including the classification bias. However, the NHI program has a high coverage rate, and medical reimbursement specialists and peer reviewers scrutinized all insurance claims, ensuring that the diagnoses and coding of diseases were highly reliable in the NHIRD. The classification bias was also supposed to be non-differential, and this should not invalidate our result. Primary human hepatocytes (PHHs) are a well defined in vitro hepatic model to predict drug responses with respect to metabolism and toxicity, based on the proper maintenance of metabolism, transport, and biological signaling pathways. However, the significant variability among individuals and high cost of PHHs has led to the development of alternative cell line models. Thus, HepaRG cells have emerged as a promising alternative to PHHs as an in vitro model, as this cell model, upon reaching phenotypic maturity, can grow to confluence and differentiate over 4 weeks (from progenitor cells) into cocultures of hepatocyte-like and cholangiocyte-like cells (68). However, it must be kept in mind that cell lines do not behave identically to primary cells and do not necessarily completely replace and reflect the effects and outcomes of primary cells.
This study had several strengths. First, we used a nationwide, population-based cohort of patients with anonymized data to minimize selection bias. Additionally, we evaluated the effect of IBD and IBD medications on the risk of hyperlipidemia over a prolonged follow-up period. Moreover, by adjusting for age and sex in a 1:1 ratio, we accounted for confounders that may affect the occurrence of hyperlipidemia. Finally, although detection bias may have occurred if patients had more hospital visits than the control population by increasing the possibility of detecting hyperlipidemia, the risk of hyperlipidemia was still increased 2.2-fold in patients with ≥2 hospital visits per year. Overall, this is the first study to investigate the IBD-associated risk for developing hyperlipidemia and evaluate the effects of IBD medications on lipogenic gene expression.
In conclusion, patients with IBD have a significantly increased risk of developing hyperlipidemia compared to non-IBD controls. However, under all IBD medications and surgical treatment, patients with IBD experienced a reduced risk of hyperlipidemia compared with non-treated IBD patients. We further confirmed that the effects of IBD medications on the decreased expression of lipogenic-related genes contributed to the beneficial effects on the blood lipid profiles. Therefore, regular monitoring of blood lipid levels should be considered for the early detection of hyperlipidemia in patients with IBD, especially in elderly patients, to decrease the risk of CVD. Large, population-based studies or randomized clinical trials are warranted to confirm our observation that IBD and IBD medications play an important role in the development of hyperlipidemia. In addition, early detection, monitoring, aggressive, and comprehensive treatment for metabolic disturbances, and alleviation of acquired risk factors are highly recommended. Further investigations into the possible biological and pathological mechanisms underlying the relationship between hyperlipidemia and the use of IBD medications are essential.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The NHIRD encrypts patient personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Therefore, patient consent is not required to access the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH-104-REC2-115-R5). The IRB also specifically waived the consent requirement.
NT, T-YW, Y-JF, and Y-PL: conceptualization, methodology, investigation, supervision, and funding acquisition. C-LL, C-JW, and C-YH: software and formal analysis. NT and Y-PL: validation. C-LL, C-YH, and Y-PL: resources and data curation. C-YH and Y-PL: project administration. All authors: writing—original draft preparation, writing—review and editing, and visualization. All authors contributed to the article and approved the submitted version.
This study was supported by the Ministry of Science and Technology, Taiwan, R.O.C. (MOST110-2320-B-039-016-MY3), China Medical University, Taichung, Taiwan (CMU110-MF-29), China Medical University Hospital, Taichung, Taiwan (DMR-111-105; DMR-111-228), partially supported by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW110-TDU-B-212-124004), Taichung Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TTCRD111-31), Show Chwan Memorial Hospital, Changhua, Taiwan (SRD-110027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACLY, ATP citrate lyase; ACP, acid phosphatase; AMI, acute myocardial infarction; aOR, adjusted odds ratio; CAD, coronary artery disease; CD, Crohn's disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CVD, cardiovascular disease; DMSO, dimethyl sulfoxide; ESR, erythrocyte sedimentation rate; FAS, fatty acid synthase; HDL-C, high density lipoprotein cholesterol; HR, hazard ratio; IBDs, inflammatory bowel diseases; ICD-9-CM, International Classification of Diseases, Clinical Modification; IFN-γ, interferon γ; IL-6, interleukin 6; LDL-C, low density lipoprotein cholesterol; LHID, Longitudinal Health Insurance Database; LXRs, Liver X receptors; NAFLD, non-alcoholic fatty liver disease; NHIRD, National Health Insurance Research Database; NHRI, National Health Research Institutes; NO, nitric oxide; NOS, nitric oxide synthase; PNPP, para-nitrophenylphosphate; qRT-PCR, quantitative real-time polymerase chain reaction; RR, relative risk; SCD, stearoyl CoA desaturase; SD, standard deviation; SE, standard error; SREBP-1c, sterol regulatory element binding protein 1c; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TGs, triglycerides; TNF, tumor necrosis factor; UC, ulcerative colitis; VTE, venous thromboembolism.
1. Jess T, Jensen BW, Andersson M, Villumsen M, Allin KH. Inflammatory bowel diseases increase risk of type 2 diabetes in a nationwide cohort study. Clin Gastroenterol Hepatol. (2020) 18:881–8. doi: 10.1016/j.cgh.2019.07.052
2. Kumar A, Teslova T, Taub E, Miller JD, Lukin DJ. Comorbid diabetes in inflammatory bowel disease predicts adverse disease-related outcomes and infectious complications. Dig Dis Sci. (2021) 66:2005–13. doi: 10.1007/s10620-020-06439-4
3. Wei SC, Chang TA, Chao TH, Chen JS, Chou JW, Chou YH, et al. Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan society of inflammatory bowel disease. Intest Res. (2017) 15:266–84. doi: 10.5217/ir.2017.15.3.266
4. Wei SC, Chang TA, Chao TH, Chen JS, Chou JW, Chou YH, et al. Management of Crohn's disease in Taiwan: consensus guideline of the Taiwan society of inflammatory bowel disease. Intest Res. (2017) 15:285–310. doi: 10.5217/ir.2017.15.3.285
5. Yen HH, Weng MT, Tung CC, Wang YT, Chang YT, Chang CH, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide population-based study. Intest Res. (2019) 17:54–62. doi: 10.5217/ir.2018.00096
6. Loftus EV Jr. Update on the incidence and prevalence of inflammatory bowel disease in the United States. Gastroenterol Hepatol. (2016) 12:704–7.
7. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. (2011) 140:1785–94. doi: 10.1053/j.gastro.2011.01.055
8. Schicho R, Marsche G, Storr M. Cardiovascular complications in inflammatory bowel disease. Curr Drug Targets. (2015) 16:181–8. doi: 10.2174/1389450116666150202161500
9. Tan VP, Chung A, Yan BP, Gibson PR. Venous and arterial disease in inflammatory bowel disease. J Gastroenterol Hepatol. (2013) 28:1095–113. doi: 10.1111/jgh.12260
10. Bollen L, Vande Casteele N, Peeters M, Van Assche G, Ferrante M, Van Moerkercke W, et al. The occurrence of thrombosis in inflammatory bowel disease is reflected in the clot lysis profile. Inflamm Bowel Dis. (2015) 21:2540–8. doi: 10.1097/MIB.0000000000000531
11. Singh S, Singh H, Loftus EV Jr, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2014) 12:382–93. doi: 10.1016/j.cgh.2013.08.023
12. Sridhar ARM, Parasa S, Navaneethan U, Crowell MD, Olden K. Comprehensive study of cardiovascular morbidity in hospitalized inflammatory bowel disease patients. J Crohns Colitis. (2011) 5:287–94. doi: 10.1016/j.crohns.2011.01.011
13. Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp-Pedersen C, et al. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death: a Danish nationwide cohort study. PLoS ONE. (2013) 8:e56944. doi: 10.1371/journal.pone.0056944
14. Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. (2013) 62:689–94. doi: 10.1136/gutjnl-2012-303285
15. Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, Leontiadis GI, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. (2014) 146:835–48. doi: 10.1053/j.gastro.2014.01.042
16. Tsai MS, Lin CL, Chen HP, Lee PH, Sung FC, Kao CH. Long-term risk of acute coronary syndrome in patients with inflammatory bowel disease: a 13-year nationwide cohort study in an Asian population. Inflamm Bowel Dis. (2014) 20:502–7. doi: 10.1097/01.MIB.0000441200.10454.4f
17. Kirchgesner J, Beaugerie L, Carrat F, Andersen NN, Jess T, Schwarzinger M. BERENICE study group. Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut. (2018) 67:1261–8. doi: 10.1136/gutjnl-2017-314015
18. Panhwar MS, Mansoor E, Al-Kindi SG, Sinh P, Katz J, Oliveira GH, et al. Risk of myocardial infarction in inflammatory bowel disease: a population-based national study. Inflamm Bowel Dis. (2019) 25:1080–7. doi: 10.1093/ibd/izy354
19. Card TR, Zittan E, Nguyen GC, Grainge MJ. Disease activity in inflammatory bowel disease is associated with arterial vascular disease. Inflamm Bowel Dis. (2021) 27:629–38. doi: 10.1093/ibd/izaa156
20. Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. (2013) 40:195–211. doi: 10.1016/j.pop.2012.11.003
21. Koutroumpakis E, Ramos-Rivers C, Regueiro M, Hashash JG, Barrie A, Swoger J, et al. Association between long-term lipid profiles and disease severity in a large cohort of patients with inflammatory bowel disease. Dig Dis Sci. (2016) 61:865–71. doi: 10.1007/s10620-015-3932-1
22. Sappati Biyyani RS, Putka BS, Mullen KD. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J Clin Lipidol. (2010) 4:478–82. doi: 10.1016/j.jacl.2010.08.021
23. Levy E, Rizwan Y, Thibault L, Lepage G, Brunet S, Bouthillier L, et al. Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am J Clin Nutr. (2000) 71:807–15. doi: 10.1093/ajcn/71.3.807
24. Ripollés Piquer B, Nazih H, Bourreille A, Segain JP, Huvelin JM, Galmiche JP, et al. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism. (2006) 55:980–8. doi: 10.1016/j.metabol.2006.03.006
25. Singh S, Kullo IJ, Pardi DS, Loftus Jr EV. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol. (2015) 12:26–35. doi: 10.1038/nrgastro.2014.202
26. Bunu DM, Timofte CE, Ciocoiu M, Floria M, Tarniceriu CC, Barboi OB, et al. Cardiovascular manifestations of inflammatory bowel disease: Pathogenesis, diagnosis, and preventive strategies. Gastroenterol Res Pract Actions. (2019) 2019:3012509. doi: 10.1155/2019/3012509
27. Podolsky DK. Inflammatory bowel disease. N Engl J Med. (2002) 347:417–29. doi: 10.1056/NEJMra020831
28. Yeshi K, Ruscher R, Hunter L, Daly NL, Loukas A, Wangchuk P. Revisiting inflammatory bowel disease: Pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J Clin Med. (2020) 9:1273. doi: 10.3390/jcm9051273
29. Changchien CS, Wang JH, Tsai TL, Hung CH. Correlation between fatty liver and lipidemia in Taiwanese. J Med Ultrasound. (2003) 11:60–5. doi: 10.1016/S0929-6441(09)60043-6
30. Wang J, Su Z, Feng Y, Xi R, Liu J, Wang P. Comparison of several blood lipid-related indexes in the screening of non-alcoholic fatty liver disease in women: a cross-sectional study in the Pearl River Delta region of southern China. BMC Gastroenterol. (2021) 21:482. doi: 10.1186/s12876-021-02072-1
31. Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ, et al. a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. (1995) 9:1033–45. doi: 10.1101/gad.9.9.1033
32. Schultz JR, Tu H, Luk A, Repa JJ, Medina JC Li L, et al. Role of LXRs in control of lipogenesis. Genes Dev. (2000) 14:2831–8. doi: 10.1101/gad.850400
33. Higuchi N, Kato M, Shundo Y, Tajiri H, Tanaka M, Yamashita N, et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. (2008) 38:1122–9. doi: 10.1111/j.1872-034X.2008.00382.x
34. Liu SP, Shibu MA, Tsai FJ, Hsu YM, Tsai CH, Chung JG, et al. Tetramethylpyrazine reverses high-glucose induced hypoxic effects by negatively regulating HIF-1α induced BNIP3 expression to ameliorate H9c2 cardiomyoblast apoptosis. Nutr Metab. (2020) 17:12. doi: 10.1186/s12986-020-0432-x
35. Edsbäcker S, Andersson T. Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn's disease. Clin Pharmacokinet. (2004) 43:803–21. doi: 10.2165/00003088-200443120-00003
36. Elliott PR, Powell-Tuck J, Gillespie PE, Laidlow JM, Lennard-Jones JE, English J, et al. Prednisolone absorption in acute colitis. Gut. (1980) 21:49–51. doi: 10.1136/gut.21.1.49
37. Al-Habet SM, Rogers HJ. Methylprednisolone pharmacokinetics after intravenous and oral administration. Br J Clin Pharmacol. (1989) 27:285–90. doi: 10.1111/j.1365-2125.1989.tb05366.x
38. Jung C, Greco S, Nguyen HH, Ho JT, Lewis JG, Torpy DJ, et al. Plasma, salivary and urinary cortisol levels following physiological and stress doses of hydrocortisone in normal volunteers. BMC Endocr Disord. (2014) 14:91. doi: 10.1186/1472-6823-14-91
39. Van Os EC, Zins BJ, Sandborn WJ, Mays DC, Tremaine WJ, Mahoney DW, et al. Azathioprine pharmacokinetics after intravenous, oral, delayed release oral and rectal foam administration. Gut. (1996) 39:63–8. doi: 10.1136/gut.39.1.63
40. Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, van de Laar M. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. (2004) 31:645–8.
41. NEORAL (2009). Available online at: https://www.fda.gov/; https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050715s027,050716s028lbl.pdf
42. Lemaitre F, Blanchet B, Latournerie M, Antignac M, Houssel-Debry P, Verdier MC, et al. Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: inside the white blood cells. Clin Biochem. (2015) 48:406–11. doi: 10.1016/j.clinbiochem.2014.12.018
43. Saiz-Rodríguez M, Ochoa D, Belmonte C, Román M, Martínez-Ingelmo C, Ortega-Ruíz L, et al. Influence of thiopurine S-methyltransferase polymorphisms in mercaptopurine pharmacokinetics in healthy volunteers. Basic Clin Pharmacol Toxicol. (2019) 124:449–55. doi: 10.1111/bcpt.13153
44. Astbury C, Taggart AJ, Juby L, Zebouni L, Bird HA. Comparison of the single dose pharmacokinetics of sulphasalazine in rheumatoid arthritis and inflammatory bowel disease. Ann Rheum Dis. (1990) 49:587–90. doi: 10.1136/ard.49.8.587
45. GIAZO (2012). Available online at: https://www.fda.gov/
46. Hussain FN, Ajjan RA, Riley SA. Dose loading with delayed-release mesalazine: a study of tissue drug concentrations and standard pharmacokinetic parameters. Br J Clin Pharmacol. (2000) 49:323–30. doi: 10.1046/j.1365-2125.2000.00164.x
47. Lai SW, Kuo YH, Liao KF. Association between inflammatory bowel disease and diabetes mellitus. Clin Gastro Hepatol. (2020) 18:1002–3. doi: 10.1016/j.cgh.2019.09.016
48. Romanato G, Scarpa M, Ruffolo C, Marin R, Zambon S, Zanoni S, et al. Lipid and phospholipid profile after bowel resection for Crohn's disease. Int J Colorectal Dis. (2008) 23:931–8. doi: 10.1007/s00384-008-0503-3
49. Scarpa M, Romanato G, Manzato E, Ruffolo C, Marin R, Basato S, et al. Restorative proctocolectomy for ulcerative colitis: impact on lipid metabolism and adipose tissue and serum fatty acids. J Gastrointest Surg. (2008) 12:279–87. doi: 10.1007/s11605-007-0380-z
50. Agouridis AP, Elisaf M, Milionis HJ. An overview of lipid abnormalities in patients with inflammatory bowel disease. Ann Gastroenterol. (2011) 24:181–7.
51. Becker SA, McClave SA. Lipid profiles in Crohn's disease patients with and without ileal resection. Am J Gastroenterol. (1996) 91:2452.
52. Romanato G, Scarpa M, Angriman I, Faggian D, Ruffolo C, Marin R, et al. Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment Pharmacol Ther. (2009) 29:298–307. doi: 10.1111/j.1365-2036.2008.03886.x
53. Biondi RB, Salmazo PS, Bazan SGZ, Hueb JC, de Paiva SAR, Sassaki LY. Cardiovascular risk in individuals with inflammatory bowel disease. Clin Exp Gastroenterol. (2020) 13:107–13. doi: 10.2147/CEG.S243478
54. Alloubani A, Nimer R, Samara R. Relationship between hyperlipidemia, cardiovascular disease and stroke: a systematic review. Curr Cardiol Rev. (2021) 17:e051121189015. doi: 10.2174/1573403X16999201210200342
55. Ferrara P, Di Laura D, Cortesi PA, Mantovani LG. The economic impact of hypercholesterolemia and mixed dyslipidemia: a systematic review of cost of illness studies. PLoS One. (2021) 16:e0254631. doi: 10.1371/journal.pone.0254631
56. Andersohn F, Waring M, Garbe E. Risk of ischemic stroke in patients with Crohn's disease: a population-based nested case-control study. Inflamm Bowel Dis. (2010) 16:1387–92. doi: 10.1002/ibd.21187
57. Bigeh A, Sanchez A, Maestas C, Gulati M. Inflammatory bowel disease and the risk for cardiovascular disease: does all inflammation lead to heart disease? Trends Cardiovasc Med. (2020) 30:463–9. doi: 10.1016/j.tcm.2019.10.001
58. Groenendyk JW, Rivera AS, Sinha A, Lloyd-Jones DM, Feinstein MJ. Changes in proportionate cardiovascular mortality in patients with chronic infectious and inflammatory conditions in the United States, 1999-2018. Sci Rep. (2021) 11:23985. doi: 10.1038/s41598-021-03407-4
59. Tigas S, Tsatsoulis A. Endocrine and metabolic manifestations in inflammatory bowel disease. Ann Gastroenterol. (2012) 25:37–44.
60. Kohan AB. Apolipoprotein C-III: a potent modulator of hypertriglyceridemia and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. (2015) 22:119–25. doi: 10.1097/MED.0000000000000136
61. Steyers CM. 3rd, Miller FJ Jr. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. (2014) 15:11324–49. doi: 10.3390/ijms150711324
62. Rabinowich L, Shibolet O. Drug induced steatohepatitis: An uncommon culprit of a common disease. Biomed Res Int. (2015) 2015:168905. doi: 10.1155/2015/168905
63. He M, Pawar A, Desai RJ, Glynn RJ, Lee H, Weinblatt ME, et al. Risk of venous thromboembolism associated with methotrexate versus hydroxychloroquine for rheumatoid arthritis: A propensity score-matched cohort study. Semin Arthritis Rheum. (2021) 51:1242–50. doi: 10.1016/j.semarthrit.2021.10.001
64. Lazzerini PE, Capecchi PL, Galeazzi M, Laghi-Pasini F. Biologic drugs and arrhythmic risk in chronic inflammatory arthritis: the good and the bad. Immunol Res. (2017) 65:262–75. doi: 10.1007/s12026-016-8833-7
65. Barnes EL, Beery RM, Schulman AR, McCarthy EP, Korzenik JR, Winter RW. Hospitalizations for acute myocardial infarction are decreased among patients with inflammatory bowel disease using a nationwide inpatient database. Inflamm Bowel Dis. (2016) 22:2229–37. doi: 10.1097/MIB.0000000000000899
66. Zuin M, Rigatelli G, Del Favero G, Andreotti AN, Picariello C, Zuliani G, et al. Cardiovascular disease in patients with inflammatory bowel disease: an issue in no guidelines land. Int J Cardiol. (2016) 222:984–5. doi: 10.1016/j.ijcard.2016.08.101
67. Lu Q, Shi R, Mao T, Wang Z, Sun Z, Tan X, et al. Arterial stiffness in inflammatory bowel disease: an updated systematic review and meta-analysis. Turk J Gastroenterol. (2021) 32:422–30. doi: 10.5152/tjg.2021.20293
Keywords: inflammatory bowel disease (IBD), IBD medications, hyperlipidemia, Longitudinal Health Insurance Database (LHID), lipogenesis
Citation: Tien N, Wu T-Y, Lin C-L, Wu C-J, Hsu C-Y, Fang Y-J and Lim Y-P (2022) Impact of Inflammatory Bowel Disease (IBD) and IBD Medications on Risk of Hyperlipidemia and in vitro Hepatic Lipogenic-Related Gene Expression: A Population-Based Cohort Study. Front. Med. 9:910623. doi: 10.3389/fmed.2022.910623
Received: 01 April 2022; Accepted: 16 May 2022;
Published: 13 June 2022.
Edited by:
Antonietta G. Gravina, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Kevin Sheng-Kai Ma, University of Pennsylvania, United StatesCopyright © 2022 Tien, Wu, Lin, Wu, Hsu, Fang and Lim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Ping Lim, bGlteXBAbWFpbDIwMDAuY29tLnR3; bGlteXBAbWFpbC5jbXUuZWR1LnR3; Yi-Jen Fang, ZmFuZzUzMTEwOUBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.