- 1Department of Biopathology, CHRU-ICL, CHRU Nancy, Vandoeuvre-lès-Nancy, France
- 2Faculty of Medicine, Université de Lorraine, Vandoeuvre-lès-Nancy, France
- 3Department of Legal Medicine, CHRU Nancy, Vandoeuvre-lès-Nancy, France
- 4INSERM U1256, NGERE, Vandoeuvre-lès-Nancy, France
- 5Centre de Ressources Biologiques, BB-0033-00035, CHRU, Nancy, France
- 6Department of Maxillofacial and Plastic Surgery, CHRU, Nancy, France
- 7Department of Forensic Medicine, CHU Montpellier, University of Montpellier, Montpellier, France
- 8EDPFM, University of Montpellier, Département de Médecine Légale, Montpellier, France
- 9IRMB, INM, University of Montpellier, INSERM, CHU Montpellier (LBPC-PPC), Montpellier, France
Background: The determination of skin wound vitality based on tissue sections is a challenge for the forensic pathologist. Histology is still the gold standard, despite its low sensitivity. Immunohistochemistry could allow to obtain a higher sensitivity. Upon the candidate markers, CD15 and myeloperoxidase (MPO) may allow to early detect polymorphonuclear neutrophils (PMN). The aim of this study was to evaluate the sensitivity and the specificity of CD15 and MPO, with glycophorin C co-staining, compared to standard histology, in a series of medicolegal autopsies, and in a human model of recent wounds.
Methods: Twenty-four deceased individuals with at least one recent open skin wound were included. For each corpse, a post-mortem wound was performed in an uninjured skin area. At autopsy, a skin sample from the margins of each wound and skin controls were collected (n = 72). Additionally, the cutaneous surgical margins of abdominoplasty specimens were sampled as a model of early intravital stab wound injury (scalpel blade), associated with post-devascularization wounds (n = 39). MPO/glycophorin C and CD15/glycophorin C immunohistochemical double staining was performed. The number of MPO and CD15 positive cells per 10 high power fields (HPF) was evaluated, excluding glycophorin C—positive areas.
Results: With a threshold of at least 4 PMN/10 high power fields, the sensitivity and specificity of the PMN count for the diagnostic of vitality were 16 and 100%, respectively. With MPO/glycophorin C as well as CD15/glycophorin C IHC, the number of positive cells was significantly higher in vital than in non-vital wounds (p < 0.001). With a threshold of at least 4 positive cells/10 HPF, the sensitivity and specificity of CD15 immunohistochemistry were 53 and 100%, respectively; with the same threshold, MPO sensitivity and specificity were 28 and 95%.
Conclusion: We showed that combined MPO or CD15/glycophorin C double staining is an interesting and original method to detect early vital reaction. CD15 allowed to obtain a higher, albeit still limited, sensitivity, with a high specificity. Confirmation studies in independent and larger cohorts are still needed to confirm its accuracy in forensic pathology.
Introduction
The determination of skin wound vitality is a challenge for the forensic pathologist. The detection of an inflammatory infiltration based on histology is to date the gold standard, being highly specific but showing a very low sensitivity in recent wounds. Most notably, in the first minutes or hours after the infliction of a wound, standard histological examination may not determine whether the wound was inflicted in pre- or post-mortem period. Indeed, the delay before the detection of first polymorphonuclear neutrophils (PMN) infiltration may vary from 10 min to 6 h (1, 2). Immunohistochemistry (IHC) is a cost effective and easy-to-use method that could allow to obtain a higher sensitivity (3). Upon the candidate markers, CD15 and myeloperoxidase (MPO) may allow to early detect PMN (4–7). However, one potential pitfall on IHC slide is the difficulty to differentiate passive extravasation of PMN in hemorrhagic infiltration from true active diapedesis. The association with a red blood cells marker, like glycophorin C, could allow to increase specificity, by avoiding the count of inflammatory cells in hemorrhagic areas.
The aim of this study was to evaluate the sensitivity and the specificity of CD15 and MPO, with glycophorin C co-staining, in comparison with standard histology, in a series of recent medicolegal wounds and post-mortem controls, and in a prospective human experimental surgical model.
Materials and Methods
Study Population and Sample Collection
Medicolegal Wounds
Twenty-four individuals (20 men, 4 women, mean age = 51.0 ± 24.3 years) with at least one recent open skin wound were included at the mortuary of the University Hospital of Montpellier. Skin wounds consisted in 20 lacerations from polytrauma cases (traffic accidents, falls from high height) and 4 gunshot wounds. They were mostly located on the lower limbs and the torso. The time interval between trauma and death (survival time) was determined with medical records and police reports, including testimony from witnesses. It varied from a few seconds to 180 min (mean = 47 min). Bodies displaying putrefactive changes were excluded from the study, as well as individuals with severe malnutrition, known immunodeficiencies and immunotherapy.
For each corpse, a post-mortem 2 cm-incision was performed with a scalpel in an uninjured skin area contralateral to the ante-mortem wound, shortly after arrival at the mortuary and before refrigeration. The elapsed time between death and the infliction of the post-mortem wound was comprised between 0 and 180 min (median: 40 min). In patients with multiple ante-mortem wounds, the wound of interest was selected based on its location (no skin sample was collected from the head or hands, for ethical reasons) and on its size (large wounds were preferred to small ones).
At autopsy, a skin sample from the margins of each wound (ante- and post-mortem) and from an uninjured skin area located on the midline incision line (control samples) were collected on each corpse and immediately placed for fixation in 10% buffered formalin solution. A total of 72 skin samples were removed, including 24 samples from each of the conditions: ante-mortem wounds, post-mortem wounds, and healthy skin (control samples). The average post-mortem interval at the time of sampling was 66.3 ± 28.3 h (24–117 h).
Surgical Wounds
As a model of recent vital stab wound injury, the cutaneous surgical margins of abdominoplasty specimens were prospectively collected at the Department of Maxillofacial and Plastic Surgery of the University Hospital of Nancy, France. The precise time interval between incision and devascularization was recorded for each margin, ranging from 0 to 61 min (median: 24 min). As a model of early post-mortem wounding, a wound was inflicted with a sterile scalpel in the center of the specimens, 5 min. after devascularization. In the Pathology Department, tissue sampling was performed perpendicularly to the skin margins, on fresh tissue, before fixation in buffered formalin solution. Thirty-nine samples (26 pre-devascularization and 13 post-devascularization) were obtained from 13 patients.
Standard Histology
After formalin fixation and paraffin embedding, 5-μm sections were stained with hematoxylin, eosin, and saffron (HES), and a blind histological examination of the vital and post-mortem wounds was performed, taking into account the presence or absence of hemorrhagic infiltration and counting the number of PMN in 10 consecutive high-power fields (HPF) (×400 magnification; 0.237 mm2).
Immunohistochemistry
Paraffin 5-μm sections were immersed in a 10 mM sodium citrate buffer (pH6) for 20 min at 97°C for dewaxing and antigen retrieval. The following primary antibodies were used (30 min incubation): CD15 (mouse monoclonal, ready-to-use, DakoCytomation Agilent, Glostrup, Denmark); myeloperoxidase (MPO) (rabbit polyclonal, ready-to-use, Dako); glycophorin C (mouse monoclonal, 1/40, Diagnostic BioSystems). Immunohistochemistry was performed with Dako Autostainer Plus (Dako) using Flex Envision revelation system (Dako) for CD15 or MPO, followed by Envision Magenta (Dako) for glycophorin C. Appropriate positive and negative controls were used throughout the experiment.
A quantitative evaluation of staining for CD15 and MPO was counted in 10 consecutive HPF (0.237 m2/field) on one representative slide per case, in the immediate vicinity of the wound margin, from the superficial dermis to the deep subcutaneous adipose tissue, taking into account all interstitial leucocytes showing stained cytoplasm, excluding intravascular cells and those within hemorrhagic areas, these latter being underlined by the anti-glycophorin C antibody.
Statistical Analyses
The presence of absence of interstitial hemorrhage was considered as a qualitative variable, and the numbers of PMN, positive MPO and positive CD15 cells as quantitative variables. Statistical analysis was performed with IBM SPSS Statistics version 27.0. software. To compare the different groups (ante-mortem vs. post-mortem; pre-devascularization vs. post-devascularization), the Fisher exact test was used for qualitative variables and the Mann-Whitney Wilcoxon test for quantitative variables. The correlation between quantitative variables was evaluated with the Spearman’s coefficient correlation. A p-value lesser than 0.05 was considered as statistically significant.
Ante-mortem and pre-devascularization wounds were considered as vital wounds, whereas post-mortem wounds, control samples, and post-devascularization wounds were defined as non-vital wounds. For the evaluation of sensitivity and specificity, true positivity was defined by a cell count number equal or greater to the defined threshold in vital skin wounds; false positivity by a number equal or greater to the defined threshold in non-vital samples; true negativity by a number lesser to the threshold in non-vital samples; false negativity by a number lesser to the threshold in vital wounds. Receiving operating characteristic (ROC) curves were performed for each marker, in order to screen for the most optimal threshold.
Results
Evaluation of Inflammation With Standard Histology
In the medicolegal wounds, no significant inflammatory reaction was seen on standard histology slides (Figure 1A). In the surgical wounds, a significant inflammatory reaction was found in 2 cases, showing a PMN infiltration (7 and 31 PMN/10 HPF). PMN evaluation showed a median number of 1 PMN/10 HPF (min.-max.: 0–31) in vital wounds and 1 PMN/10 HPF (min.-max.: 0–3) in non-vital wounds (Table 1). No significant difference was found between vital and non-vital wounds, in both autopsy cases and surgical wounds (p = 0.557 and p = 0.294, respectively).
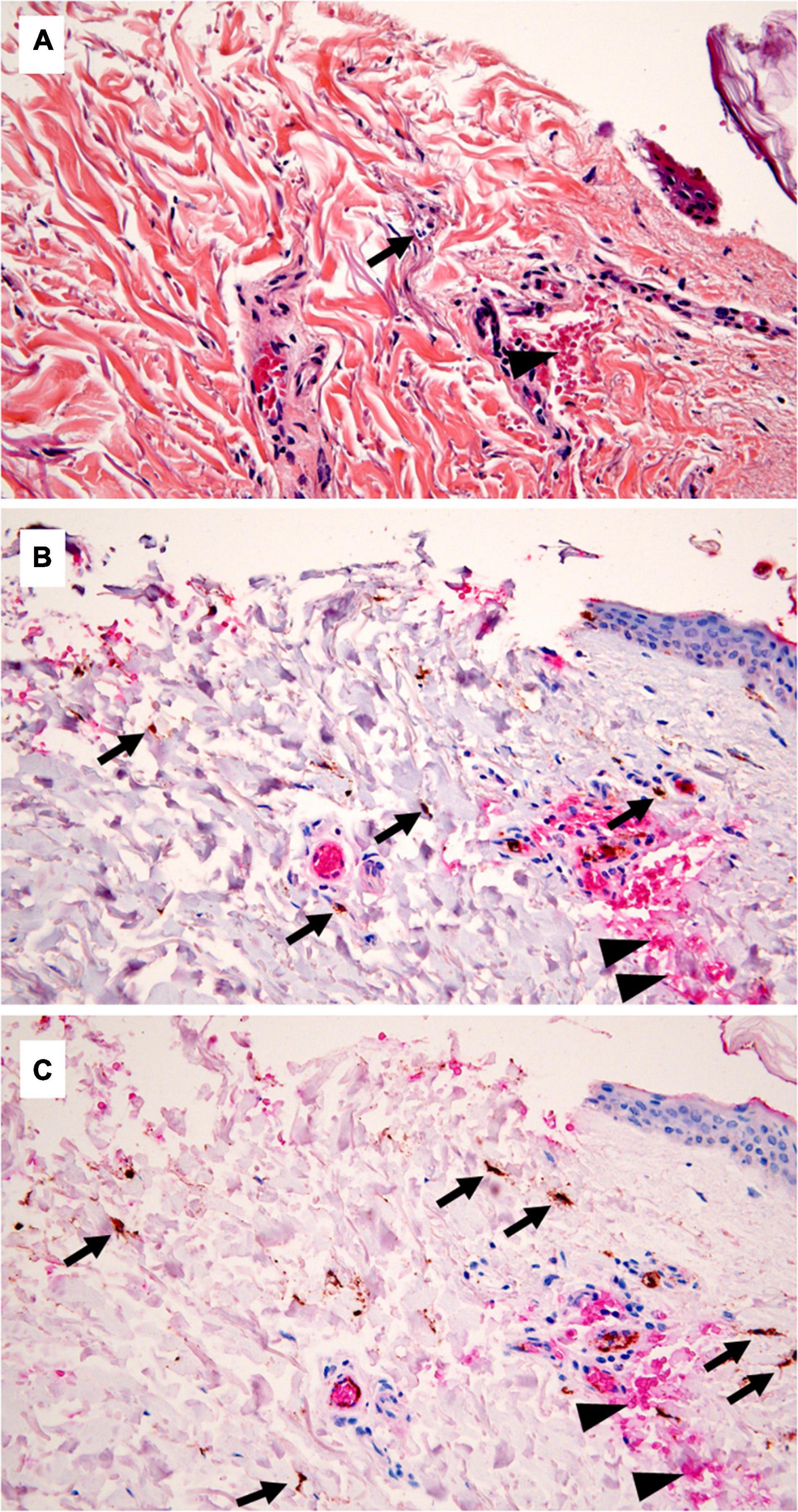
Figure 1. Standard histology and immunohistochemistry (IHC) in a medicolegal wound. (A) Standard histology showing only one polymorphonuclear (PMN) (arrow) close to the wound margin, and hemorrhagic infiltration (arrowhead) (hematoxylin, eosin, and saffron, × 400 magnification). (B) Double staining for myeloperoxidase (MPO) and glycophorin C, underlining the presence of several inflammatory cells (arrows), evaluated outside the hemorrhagic areas (glycophorin C—positive, arrowheads) to avoid the count of passively extravasated leucocytes (IHC, ×400). (C) CD15/glycophorin C double staining, showing a greater number of positive cells, comparing with MPO (IHC, ×400).
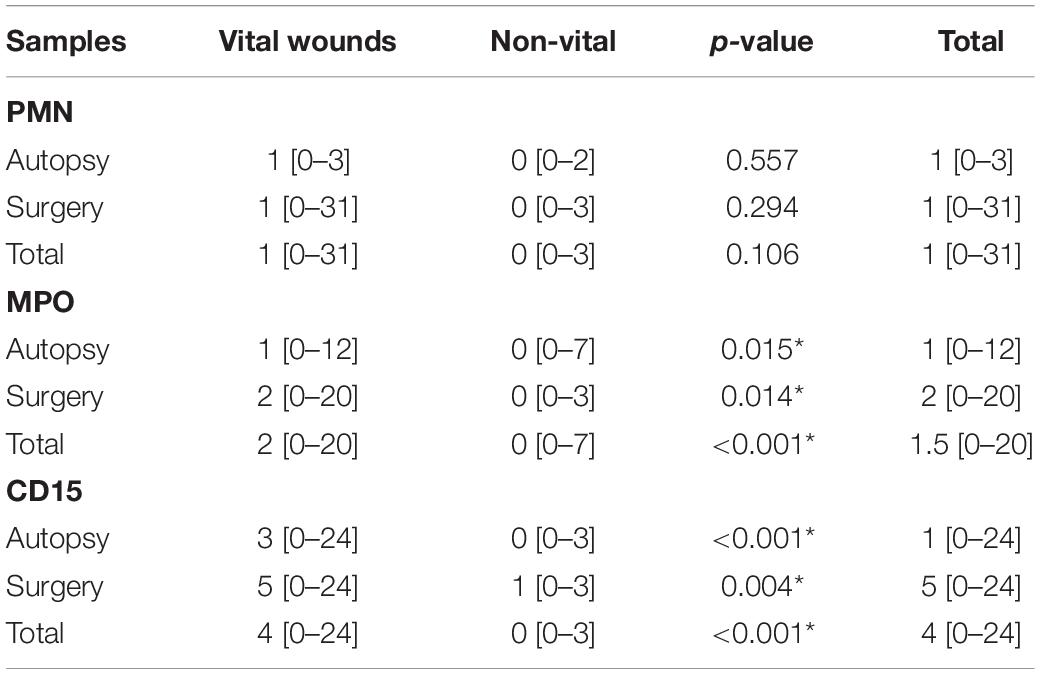
Table 1. Evaluation of the median number of polymorphonuclears neutrophils (PMN) on standard histology and results of anti- myeloperoxydase (MPO) and CD15 immunohistochemistry, in the autopsy cohort and in the surgical model [median (min.-max.); * statistically significant, p < 0.05; Mann-Whitney Wilcoxon test].
A significant correlation between the number of PMN/10 HPF and the survival/pre-devascularization time was found in surgical wounds (rho = 0.424; p = 0.031), but not in autopsy wounds (rho = 0.135; p = 0.511).
With a threshold of at least 4 PMN/10 HPF, the sensitivity and specificity of the PMN count for the diagnostic of vitality were 16 and 100%, respectively.
Interstitial Hemorrhage
With standard histology, a significant interstitial hemorrhage was noticed in 88% of ante-mortem wounds (Figure 1A) vs. 44% of post-mortem wounds and 17% of control skin samples. Using the anti-glycophorin C antibody, an interstitial hemorrhage was noticed in 96% of ante-mortem wounds vs. 56% of post-mortem wounds and 25% of control skin samples. In the surgical model, standard histology and anti-glycophorin C antibody showed an interstitial hemorrhage in, respectively, 88 and 96% of pre-devascularization wounds vs. 54 and 84% of post-devascularization wounds.
For the diagnosis of vitality, the sensitivity and specificity of the identification of interstitial hemorrhage on HES slides were 88 and 66%, respectively. With the anti-glycophorin C antibody, sensitivity raised to 96%, with a specificity of 50%.
Myeloperoxidase and CD15 Immunohistochemistry
With MPO/glycophorin C IHC (Figure 1B), the number of positive cells was significantly higher in vital than in non-vital wounds [p < 0.001; median (min.-max.): 2 (0–20) vs. 0 (0–7)] (Table 1). This difference was still significant in the autopsy and surgery subgroups (p = 0.015 and p = 0.014, respectively). Similarly, with CD15/glycophorin C IHC (Figure 1C), the number of positive cells was significantly higher in vital than in non-vital wounds [p < 0.001; median (min.-max.): 4 (0–24) vs. 0 (0–3)]. This difference was still significant when considering the autopsy (p < 0.001) and surgery (p = 0.004) subgroups.
The ROC curve for the diagnosis of vitality showed that the area under the curve was higher for CD15 (0.78) than MPO (0.69) and standard PMN count (0.58) (Figure 2). With a threshold of at least 4 positive cells/10 HPF, the sensitivity and specificity of CD15 immunohistochemistry were 53 and 100%, respectively; with the same threshold, MPO sensitivity and specificity were 28 and 95%. With a threshold of at least 2 positive cells/10 HPF, the sensitivity of CD15 reached 65%, but with a lower specificity (81%). For MPO, sensitivity and specificity were 51 and 81%.
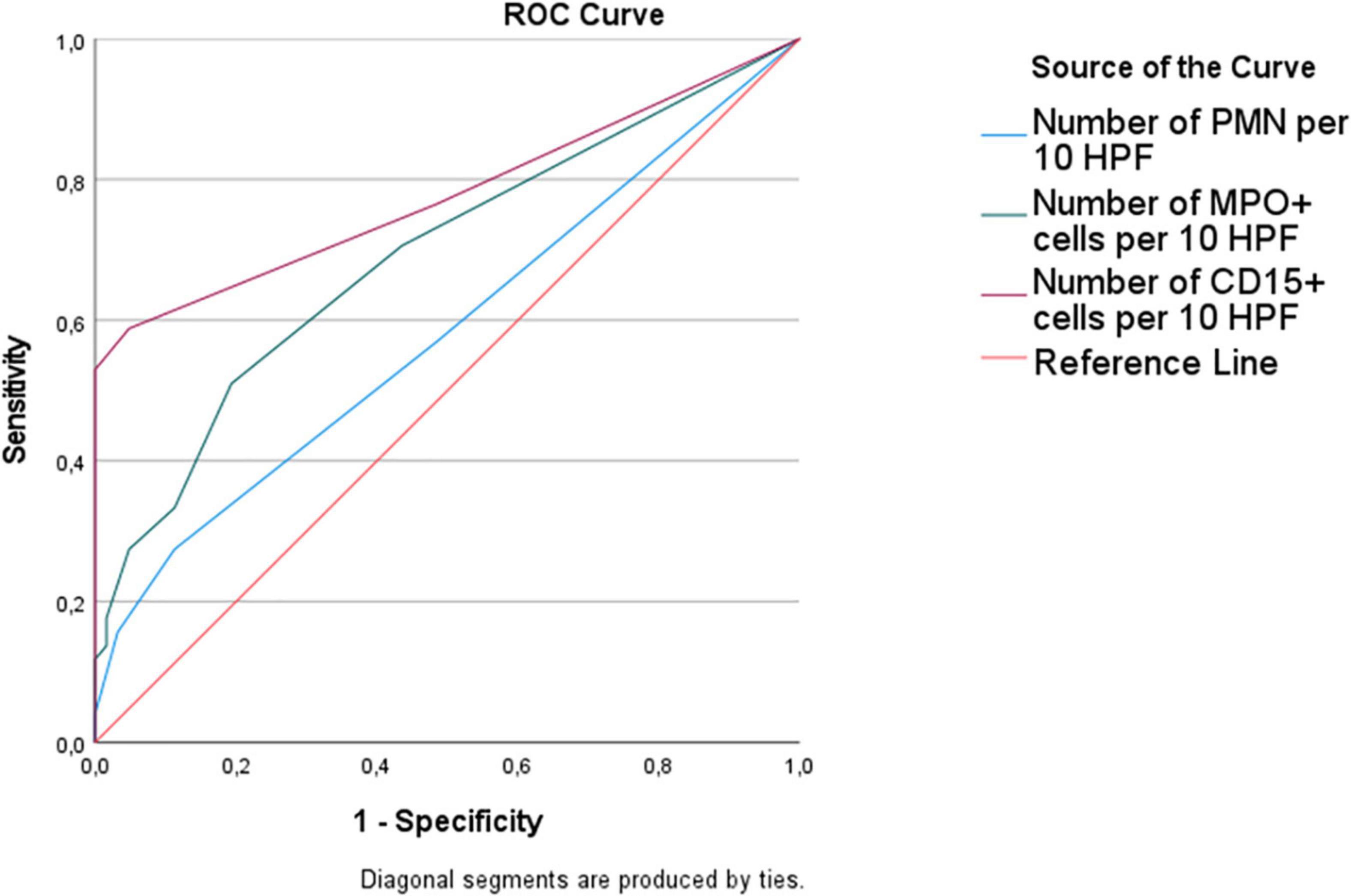
Figure 2. Receiver operating characteristic (ROC) curve for the diagnosis of wound vitality. The area under curve was higher for CD15 (0.78) than MPO (0.69), followed by standard histological count (0.58).
The numbers of MPO and CD15 positive cells were significantly correlated with the standard histological count for PMN (rho = 0.339, p < 0.001; and rho = 0.333, p < 0.001, respectively).
In the surgical model, the numbers of MPO and CD15 positive cells were significantly correlated with survival/pre-devascularization time (rho = 0.400, p = 0.043; and rho = 0.559, p = 0.003, respectively), but not in autopsy wounds (p > 0.50).
Discussion
In the first minutes or hours, the standard histological examination may not be able to determine whether the wound was inflicted in the pre- or post-mortem period. While hemorrhagic infiltration was classically considered as a sign of vital reaction, several studies have shown that the extravasation of blood cells can also occur after death and does not represent a reliable marker in wound vitality diagnosis (8–10). Various methods can be used to detect markers of vitality, such as study of mRNAs or microRNAs (RT-PCR, in situ hybridization) and proteins (ELISA technique, Western blot, immunofluorescence, immunohistochemistry), focusing on the different phases of inflammation and wound healing (3). Most notably, in skin lesions, several studies about cell adhesion molecules (ICAM-1, VCAM-1, P-selectin, E-selectin, fibronectin, …) were published, reporting for some markers a good sensitivity in recent wounds, but limited by a significant risk of post-mortem false positivity (3, 11–16). More recently, the use of microRNAs in forensic science has been proposed in various applications, including wound vitality, showing for few microRNAs interesting but still preliminary results (17–19).
In this study, we propose an original method for the detection of early inflammation, based on an immunohistochemical double staining of leucocytes and red blood cells. We tested two markers of PMN: MPO and CD15. CD15 showed a higher sensitivity than MPO, which may be explained by its ability to also detect activated monocytes (20), in addition to PMN. Staining for CD15 was previously reported as a marker of early vital reaction, focusing in most of studies on brain trauma or other organs (7, 21, 22). However, as the timing and the intensity of inflammation may be influenced by the type of the trauma and the nature of the lesioned tissue (1, 23), these studies cannot be applicable to skin injuries. In skin, we previously studied this marker in surgical and medicolegal wounds (5), and it was also reported to be an interesting marker in ligature marks (6, 24) or for the assessment of the vitality in corpse dismemberment (25) and in decomposed bodies (26).
Comparing with other methods, IHC staining of inflammatory cells allows the pathologist to have a morphological control of the signal, i.e., the recognition of leucocyte shape and the precise localization within the sample. In addition to anti-CD15 or MPO antibodies, we performed a double staining with the anti-glycophorin C antibody. Glycophorin C, like glycophorin A and D, is a sialylated glycoprotein in human erythrocytes membranes. Anti-glycophorin immunohistochemistry has been proposed in various studies for the identification of the hemorrhagic infiltration, most notably in decomposed bodies or in specific conditions, such as Amussat’s sign or retinal hemorrhage (27–31). In our study, the association with the anti-glycophorin C antibody limits the risk of counting leukocytes originating from a passive extravasation of PMN in hemorrhagic infiltration, because red blood cells are more difficult to detect on IHC slides. It may also allow to detect more easily the wound margins.
The limit of this method is a relatively low sensitivity in very recent wounds, albeit higher than standard histology. The sensitivity is closely related to the type of wound or experimental model. We included in the present study only recent wounds, with a survival or pre-devascularization time of few seconds or minutes in a significant number of cases, which may explain the low sensitivity, whatever the method. In the same series of medicolegal wound, we previously found a sensitivity of 21% for the evaluation of IL8 staining, which reached 46% when using IL8 in a multiplex immunoassay, normalized on healthy skin levels (32, 33). Hence, we can conclude that IHC is less sensitive that immunoassay, but the latter has the disadvantage of needing fresh frozen tissue and to be a method which is not as largely developed as IHC, requiring training sets and data normalization, without morphological control.
Measures of test accuracy such as sensitivity and specificity depend crucially on the selected threshold, and the optimal value of this threshold is a key question for forensic practice. Given the fact that a 100% sensitivity is probably unreachable in very recent wound, we aimed to obtain a theatrical 100% specificity, to strictly avoid false positivity and obtain a high positive predictive value for vitality assessment. A threshold of 4 CD15-positive per 10 HPF cells allowed to obtain a 100% specificity and would be probably more relevant that a lower threshold, which exposes to false positivity, albeit with better sensitivity. In a previous study in which we compared CD15, FVIIIra and tryptase in medicolegal stab wounds showing inflammation and in surgical specimens (breast reductions), we found similar results for CD15, with the same threshold of 4 cells per 10 HPF (sensitivity: 47%; specificity: 100%) (5). CD15 had also the advantage to show a very good inter-observer reproducibility (0.90) (5).
Conclusion
In conclusion, based on a series of recent medicolegal wounds associated with post-mortem controls and an experimental human model of surgical wounds, we showed that combined CD15/glycophorin C double IHC staining is an interesting and original method to detect early vital reaction. In comparison with standard histology and MPO staining, CD15 allowed to obtain a significantly higher, albeit still limited, sensitivity, with a high specificity. Confirmation studies in independent and larger cohorts are still needed in order to confirm its accuracy in forensic pathology.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The post-mortem study protocol was approved by the French Agency of Biomedicine, Nr. PFS15-003. The surgical model protocol was approved by the review board of the Direction of Research and Innovation, CHRU of Nancy, France (CPRC2013, DRCI, CHRU Nancy) and a written consent was obtained from patients for using surgical specimens (study promoter: CHRU Nancy, CPRC2012; sample collection DC2008-459). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GG and P-AP designed the study, collected the samples, analyzed the data, and wrote the manuscript. AB collected the samples and the data and analyzed the histological preparations. SC, MB, and ES collected the samples. LM, EL, and PC participated to the elaboration of study design. All authors agree to be accountable for the content of the work.
Funding
This work was supported by the CHRU Nancy, INSERM U1256 NGERE, and by CHRU Montpellier.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Quentin Kopp and Michael Cohet for patients recruitment; Amélie Deremarque and Mélina Marechal for their help in immunohistochemistry; all the team of the Biopathology Department (CHRU—ICL, Nancy) for technical support.
References
1. Oehmichen M. Vitality and time course of wounds. Forensic Sci Int. (2004) 144:221–31. doi: 10.1016/j.forsciint.2004.04.057
2. van de Goot FRW. The chronological dating of injury. In: Rutty GN, editor. Essentials of Autopsy Practice. London: Springer-Verlag (2008). p. 167–81.
3. Casse J-M, Martrille L, Vignaud J-M, Gauchotte G. Skin wounds vitality markers in forensic pathology: an updated review. Med Sci Law. (2016) 56:128–37. doi: 10.1177/0025802415590175
4. Fronczek J, Lulf R, Korkmaz HI, Witte BI, van de Goot FRW, Begieneman MPV, et al. Analysis of inflammatory cells and mediators in skin wound biopsies to determine wound age in living subjects in forensic medicine. Forensic Sci Int. (2015) 247:7–13. doi: 10.1016/j.forsciint.2014.11.014
5. Gauchotte G, Wissler M-P, Casse J-M, Pujo J, Minetti C, Gisquet H, et al. FVIIIra, CD15, and tryptase performance in the diagnosis of skin stab wound vitality in forensic pathology. Int J Legal Med. (2013) 127:957–65. doi: 10.1007/s00414-013-0880-1
6. Turillazzi E, Vacchiano G, Luna-Maldonado A, Neri M, Pomara C, Rabozzi R, et al. Tryptase, CD15 and IL-15 as reliable markers for the determination of soft and hard ligature marks vitality. Histol Histopathol. (2010) 25:1539–46. doi: 10.14670/HH-25.1539
7. Hausmann R. Age determination of brain contusions. Forensic Sci Med Pathol. (2006) 2:85–93. doi: 10.1385/FSMP:2:2:85
8. Strejc P, Pilin A, Klír P, Vajtr D. [The origin, distribution and relocability of supravital hemorrhages]. Soud Lek. (2011) 56:18–20.
9. Prinsloo I, Gordon I. Post-mortem dissection artifacts of the neck; their differentiation from ante-mortem bruises. S Afr Med J. (1951) 25:358–61.
10. Pollanen MS, Perera SDC, Clutterbuck DJ. Hemorrhagic lividity of the neck: controlled induction of postmortem hypostatic hemorrhages. Am J Forensic Med Pathol. (2009) 30:322–6. doi: 10.1097/PAF.0b013e3181c17ec2
11. Dressler J, Bachmann L, Koch R, Müller E. Enhanced expression of selectins in human skin wounds. Int J Legal Med. (1998) 112:39–44. doi: 10.1007/s004140050196
12. Dressler J, Bachmann L, Koch R, Müller E. Estimation of wound age and VCAM-1 in human skin. Int J Legal Med. (1999) 112:159–62. doi: 10.1007/s004140050223
13. Dressler J, Bachmann L, Müller E. Enhanced expression of ICAM-1 (CD 54) in human skin wounds: diagnostic value in legal medicine. Inflamm Res. (1997) 46:434–5. doi: 10.1007/s000110050220
14. Dressler J, Bachmann L, Strejc P, Koch R, Müller E. Expression of adhesion molecules in skin wounds: diagnostic value in legal medicine. Forensic Sci Int. (2000) 113:173–6. doi: 10.1016/s0379-0738(00)00258-9
15. Grellner W, Dimmeler S, Madea B. Immunohistochemical detection of fibronectin in postmortem incised wounds of porcine skin. Forensic Sci Int. (1998) 97:109–16. doi: 10.1016/s0379-0738(98)00147-9
16. Ortiz-Rey JA, Suárez-Peñaranda JM, San Miguel P, Muñoz JI, Rodríguez-Calvo MS, Concheiro L. Immunohistochemical analysis of P-selectin as a possible marker of vitality in human cutaneous wounds. J Forensic Leg Med. (2008) 15:368–72. doi: 10.1016/j.jflm.2008.02.011
17. Manetti AC, Maiese A, Baronti A, Mezzetti E, Frati P, Fineschi V, et al. MiRNAs as new tools in lesion vitality evaluation: a systematic review and their forensic applications. Biomedicines. (2021) 9:1731. doi: 10.3390/biomedicines9111731
18. Silva SS, Lopes C, Teixeira AL, Carneiro de Sousa MJ, Medeiros R. Forensic miRNA: potential biomarker for body fluids? Forensic Sci Int Genet. (2015) 14:1–10. doi: 10.1016/j.fsigen.2014.09.002
19. Rocchi A, Chiti E, Maiese A, Turillazzi E, Spinetti I. MicroRNAs: an update of applications in forensic science. Diagnostics (Basel). (2020) 11:E32. doi: 10.3390/diagnostics11010032
20. Lo SK, Golenbock DT, Sass PM, Maskati A, Xu H, Silverstein RL. Engagement of the lewis X antigen (CD15) results in monocyte activation. Blood. (1997) 89:307–14. doi: 10.1182/blood.v89.1.307.307_307_314
21. Hausmann R, Kaiser A, Lang C, Bohnert M, Betz P. A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int J Legal Med. (1999) 112:227–32. doi: 10.1007/s004140050241
22. Neri M, Frati A, Turillazzi E, Cantatore S, Cipolloni L, Di Paolo M, et al. Immunohistochemical evaluation of aquaporin-4 and its correlation with CD68, IBA-1, HIF-1α, GFAP, and CD15 expressions in fatal traumatic brain injury. Int J Mol Sci. (2018) 19:E3544. doi: 10.3390/ijms19113544
23. Grellner W, Madea B. Demands on scientific studies: vitality of wounds and wound age estimation. Forensic Sci Int. (2007) 165:150–4. doi: 10.1016/j.forsciint.2006.05.029
24. Mansueto G, Feola A, Zangani P, Porzio A, Carfora A, Campobasso CPA. Clue on the skin: a systematic review on immunohistochemical analyses of the ligature mark. Int J Environ Res Public Health. (2022) 19:2035. doi: 10.3390/ijerph19042035
25. Maiese A, Scopetti M, Santurro A, La Russa R, Manetti F, D’Errico S, et al. Corpse dismemberment: a case series. Solving the puzzle through an integrated multidisciplinary approach. J Forensic Leg Med. (2020) 74:102005. doi: 10.1016/j.jflm.2020.102005
26. Bertozzi G, Ferrara M, La Russa R, Pollice G, Gurgoglione G, Frisoni P, et al. Wound vitality in decomposed bodies: new frontiers through immunohistochemistry. Front Med (Lausanne). (2021) 8:802841. doi: 10.3389/fmed.2021.802841
27. Baldari B, Vittorio S, Sessa F, Cipolloni L, Bertozzi G, Neri M, et al. Forensic application of monoclonal anti-human glycophorin a antibody in samples from decomposed bodies to establish vitality of the injuries. A preliminary experimental study. Healthcare (Basel). (2021) 9:514. doi: 10.3390/healthcare9050514
28. Luigi Crudele GD, Galante N, Fociani P, Del Gobbo A, Tambuzzi S, Gentile G, et al. The forensic application of the glycophorin A on the Amussat’s sign with a brief review of the literature. J Forensic Leg Med. (2021) 82:102228. doi: 10.1016/j.jflm.2021.102228
29. Tabata N, Morita M. Immunohistochemical demonstration of bleeding in decomposed bodies by using anti-glycophorin A monoclonal antibody. Forensic Sci Int. (1997) 87:1–8. doi: 10.1016/s0379-0738(97)02118-x
30. Taborelli A, Andreola S, Di Giancamillo A, Gentile G, Domeneghini C, Grandi M, et al. The use of the anti-glycophorin A antibody in the detection of red blood cell residues in human soft tissue lesions decomposed in air and water: a pilot study. Med Sci Law. (2011) 51(Suppl. 1):S16–9. doi: 10.1258/msl.2010.010107
31. Plowey ED, Egbert PR. Histologic artifacts of autolytic müller cell foot process swelling in postmortem examination of infant eyes: potential pitfall in the evaluation of traumatic retinal hemorrhages. JAMA Ophthalmol. (2015) 133:706–9. doi: 10.1001/jamaophthalmol.2015.0493
32. Peyron P-A, Baccino É, Nagot N, Lehmann S, Delaby C. Looking for new biomarkers of skin wound vitality with a cytokine-based multiplex assay: preliminary study. Ann Biol Clin (Paris). (2017) 75:53–60. doi: 10.1684/abc.2016.1214
Keywords: CD15, myeloperoxidase (MPO), glycophorin, vitality, wound datation, histology, immunohistochemistry, forensic
Citation: Gauchotte G, Bochnakian A, Campoli P, Lardenois E, Brix M, Simon E, Colomb S, Martrille L and Peyron P-A (2022) Myeloperoxydase and CD15 With Glycophorin C Double Staining in the Evaluation of Skin Wound Vitality in Forensic Practice. Front. Med. 9:910093. doi: 10.3389/fmed.2022.910093
Received: 31 March 2022; Accepted: 25 April 2022;
Published: 17 May 2022.
Edited by:
Vittorio Fineschi, Sapienza University of Rome, ItalyReviewed by:
Michael Bohnert, Julius Maximilian University of Würzburg, GermanyAniello Maiese, Sapienza University of Rome, Italy
Copyright © 2022 Gauchotte, Bochnakian, Campoli, Lardenois, Brix, Simon, Colomb, Martrille and Peyron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guillaume Gauchotte, Zy5nYXVjaG90dGVAY2hydS1uYW5jeS5mcg==
†These authors share first authorship
 Guillaume Gauchotte
Guillaume Gauchotte Agathe Bochnakian1,2†
Agathe Bochnakian1,2† Sophie Colomb
Sophie Colomb Pierre-Antoine Peyron
Pierre-Antoine Peyron