- 1Centre for Adolescent Rheumatology Versus Arthritis at University College London (UCL), University College London Hospital (UCLH), Great Ormond Street Hospital (GOSH), London, United Kingdom
- 2Division of Medicine, Centre for Rheumatology Research, University College London (UCL), London, United Kingdom
- 3Department of Paediatric Rheumatology, School of Child and Adolescent Health, Red Cross War Memorial Children’s Hospital, University of Cape Town, Cape Town, South Africa
- 4Crick African Network, The Francis Crick Institute, London, United Kingdom
- 5Department of Paediatric and Adolescent Endocrinology, University College London Hospital (UCLH) and Great Ormond Street Institute of Child Health, University College London, London, United Kingdom
- 6Gender Identity Development Service (GIDS), Tavistock and Portman NHS Foundation Trust, London, United Kingdom
The differences between male and female immune systems are an under-researched field, ripe for discovery. This is evidenced by the stark sex biases seen in autoimmunity and infectious disease. Both the sex hormones (oestrogen and testosterone), as well as the sex chromosomes have been demonstrated to impact immune responses, in multiple ways. Historical shortcomings in reporting basic and clinical scientific findings in a sex-disaggregated manner have led not only to limited discovery of disease aetiology, but to potential inaccuracies in the estimation of the effects of diseases or interventions on females and gender-diverse groups. Here we propose not only that research subjects should include both cis-gender men and cis-gender women, but also transgender and gender-diverse people alongside them. The known interaction between the hormonal milieu and the sex chromosomes is inseparable in cis-gender human research, without the confounders of puberty and age. By inclusion of those pursuing hormonal affirmation of their gender identity- the individual and interactive investigation of hormones and chromosomes is permitted. Not only does this allow for a fine-tuned dissection of these individual effects, but it allows for discovery that is both pertinent and relevant to a far wider portion of the population. There is an unmet need for detailed treatment follow-up of the transgender community- little is known of the potential benefits and risks of hormonal supplementation on the immune system, nor indeed on many other health and disease outcomes. Our research team has pioneered the inclusion of gender-diverse persons in our basic research in adolescent autoimmune rheumatic diseases. We review here the many avenues that remain unexplored, and suggest ways in which other groups and teams can broaden their horizons and invest in a future for medicine that is both fruitful and inclusive.
Introduction
The pertinent sex bias in the human immune system is a phenomenon that may never have come to light, were it not for significant policy changes that enforced the inclusion of female participants alongside males in medical research (1). Historically, clinical trials were conducted predominantly on male subjects only, or failed to discriminate between outcomes experienced by males vs. females (2). Justified by pragmatic reasons, predominantly healthy young males were recruited to avoid potential toxicity risks associated with pregnancy and breastfeeding, while excluding more mature patients of both sexes to decrease the risk of concomitant comorbidity. Little differed in basic scientific research, where male-only mouse models mitigated the outcome variability potentially resulting from the menstrual cycle or pregnancy, and most in vitro human work failed to report the sex of the cell lines used (3). This approach is not only inaccurate in answering research questions relevant to humans, irrespective of sex and gender, but is also potentially harmful in underestimating the effects of interventions on females and other gender-diverse groups. Although medical understanding and subsequent research study design have advanced significantly in recent years, this chronic failure to recognise the importance of sex as a key biological variable has by no means been fully overcome. Anecdotally, in attempting to collect data on global COVID-19 morbidity and mortality between the sexes, it was notable how few countries or local authorities were reliably disaggregating their outcome statistics according to patients’ sex, even at later stages of the pandemic (4). Sophisticated national platforms detailed deaths according to geographical regions, age groups and occupational categories, but frequently neglected to mention sex. Our meta-analysis (5), alongside several other studies (6–8), showed a significant male bias in severe outcomes and deaths from SARS-CoV-2; a pattern mirrored in the vast majority of infectious diseases (9–11) and variously suggested to relate to sex hormone levels (12–14). The enhanced ability of the female immune system to clear invading pathogens is further supported by its ability to mount generally stronger responses to most vaccinations (15–17). For example, in adults given the seasonal Trivalent Inactivated Influenza Vaccine, female responses to a half-dose were comparable to those of males given a full-dose (18). The inverse of this is of course the female predisposition to developing autoimmune disorders associated with a hyper-active immune system, such as systemic lupus erythematosus (SLE), where the male:female ratio is estimated at 4–13:1, according to different studies (19–28).
Both hormonal and chromosomal factors are suggested to contribute to immunological sex differences. Oestradiol is broadly thought of as immunostimulatory, with testosterone having a more regulatory effect (29), though both have demonstrated either capability, as reviewed elsewhere (30–33). Meanwhile the X chromosome encodes the most immune-related genes of any chromosome (34) such as TLR7 [toll like receptor, responsible for sensing viral and endogenous nucleic acids to trigger release of type 1 interferons, and implicated in extrafollicular B cell class switch recombination (35)], CD40-L [co-stimulatory T cell molecule, essential for B cell class switching (36)], FoxP3 [controls regulatory T cells (37)] and CXCR3 [chemokine receptor, recruits effector T cells to sites of inflammation (38)]. This is highlighted by the abundance of X-linked immune disorders such as immunodysregulation polyendocrinopathy enteropathy X-linked (or IPEX) syndrome, X-linked agammaglobulinemia and Wiskott Aldrich Syndrome, which are associated with cellular and humoral immune deficiencies and increased risk of infections from childhood (39). Several immune genes on the X chromosome may escape the X-inactivation of one chromosome in 46,XX individuals, and thus be bi-allelically expressed, potentially resulting in altered immune regulation (40–43). Whilst studies have sought to investigate the contributions of hormonal and/or chromosomal influences on the immune response, it is recognised that it is a complex nexus and mutual interaction of the two that ultimately leads to such notable sex biases in infection and autoimmunity. With this in mind, this review seeks to highlight the importance of including subjects of both sexes, as well as transgender people in immunological research, to enable evaluation of sex-biased clinical outcomes and provide benefit to our understanding of the biology of the immune system with relevance for both science and health.
Gender Identities and Physical Phenotypes
For the majority of the population, the terms sex and gender describe the binary categories of “cisgender male” and “cisgender female”; with experienced gender matching the sex registered at birth, which is itself based upon simple observation of the genitalia of the new-born baby. Frequently assimilated within the category of “other,” however, are a multitude of gender identities and physical phenotypes. By “transgender” we refer broadly to those whose experienced gender identity does not match that in which they were registered at birth. Thus, trans-males, are registered female at birth, typically carry a 46,XX chromosomal background, and may pursue virilisation via testosterone treatment and/or oestradiol blockade. Trans-females, are registered male at birth, typically of 46,XY chromosomal background, and may pursue gender-affirming oestradiol treatment and/or testosterone blockade (44). Specific treatment pathways and medications recommended by the Endocrine Society (45) are summarised in Figure 1. A third main category are those who are non-binary/gender fluid (not identifying exclusively and/or permanently as either gender); some of whom may seek hormonal blockade via treatments such as the gonadotropin releasing hormone analogs (GnRHa), or specific hormonal blockades. There is also the category of differences/disorders of sex development (previously known as ‘intersex’), where people may have physical characteristics of both sexes (gonadal structures, genitalia) and this umbrella term also includes those with karyotype variations of sex development such as Klinefelter syndrome [47,XXY] and Turner syndrome [45,X] (46). Lastly but by no means exhaustively are those classified as “agender”- not identifying with any gender at all. Many other gender-related groupings exist, beyond the scope of this review, but we have included here the main categories pertinent to immunological research.
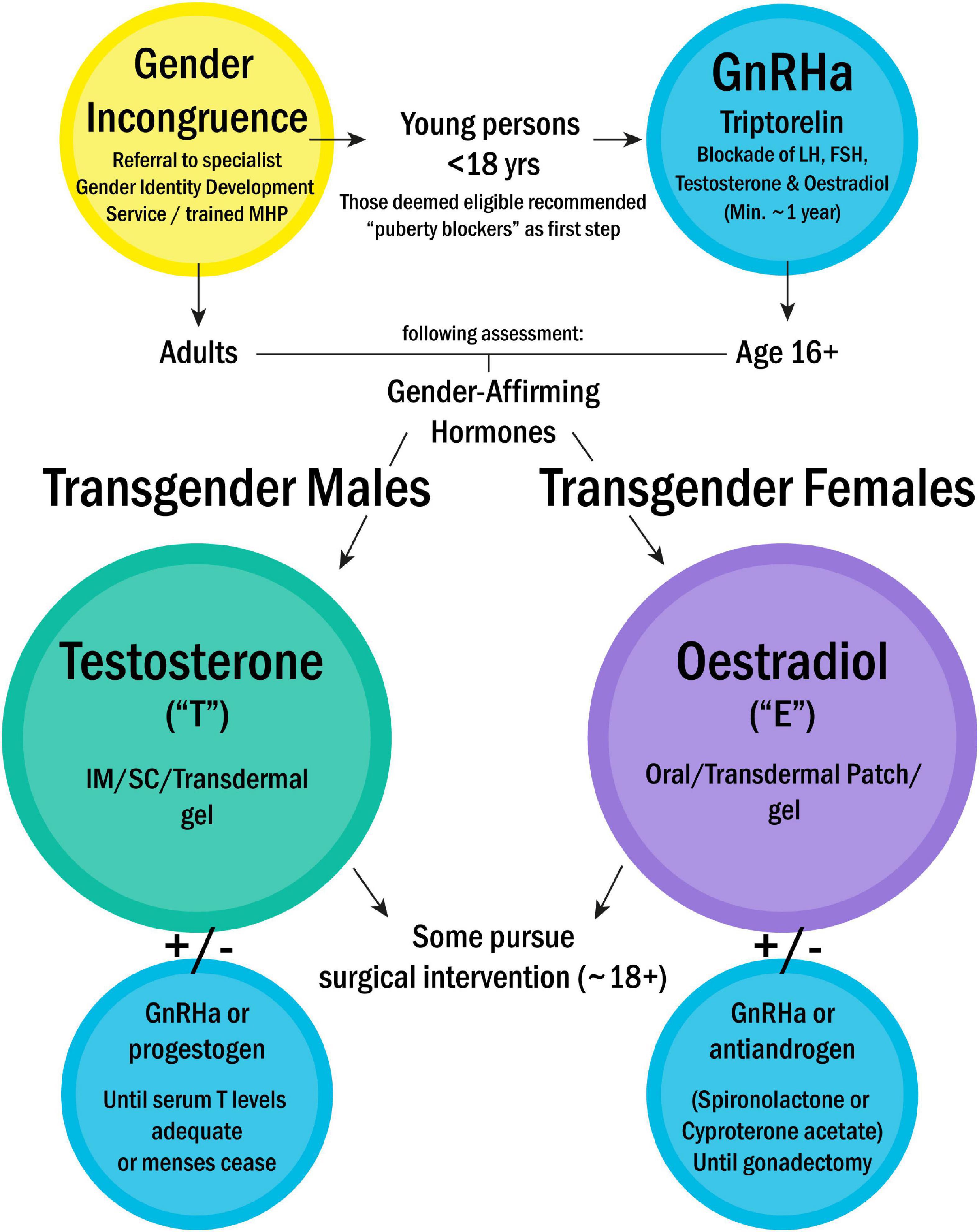
Figure 1. Treatment pathway for gender incongruence, as recommended by the Endocrine Society (42). Treatment is prescribed on a case-by-case basis, based on individual country guidelines. This flowchart outlines the most commonly pursued routes. NB- Parenteral oestradiol not currently used in Europe. MHP, Mental health professional; GnRHa, Gonadotropin releasing hormone analogs; LH, Luteinising hormone; FSH, Follicle stimulating hormone; IM, Intramuscular; SC, Subcutaneous.
To refer again to international COVID statistics, even fewer countries reported outcomes in those who were not cisgender. In some countries, the catch-all ‘other’ category was reported alongside cisgender males and females; but this was representative of so many diverse groups that granular analyses of differential gender-related outcomes could not be possible. Such is the case for the vast majority of outcome reporting in health and disease, suggesting that better characterisation of populations pertaining to self-reported gender is warranted. In the United Kingdom alone, referrals to the NHS young people’s Gender Identity Development Service (GIDS) have increased by over 2000% in the last 10 years (47); this represents a growing proportion of society who are frequently not even adequately recognised in statistics, let alone included in basic science or relevant clinical research. Here we examine potential ways in which inclusion of a broader spectrum of gender groups can improve our scientific understanding of the pathogenesis of both infectious diseases and autoimmune disorders, as well as providing potentially pertinent clinical information for under-represented groups and the physicians involved in their care.
The multitude of gender-related social factors that may contribute to increased vulnerability to different medical conditions are beyond the scope of this paper and reviewed elsewhere (48). However, the physiological impact of a person’s sex chromosomal makeup combined with their hormonal milieu (be this endogenous or medically supplemented) is what we propose to be an important focus of future research. In cis-gender people, the contributions of sex chromosomes and hormones are inextricably linked. We know both to be of significance, but researchers currently are able to separate these factors to examine how they interact and separately contribute only in animal models and in vitro research. By inclusion of trans or gender-diverse persons pursuing hormonal affirmation of their gender, we are able to investigate the effects of hormonal manipulation on the immune system in healthy individuals of a wide age range (usually older than 16 in the United Kingdom).
Sex Bias in the Epidemiology and Outcomes of Autoimmune Rheumatic Diseases
The majority of autoimmune rheumatic disorders (ARDs) affect cis-females in greater number than cis-males, as is the case with SLE, Sjögren’s syndrome (SS) (49), scleroderma (SSc) (50) and rheumatoid arthritis (RA) (51). SLE predominantly affects females of child-bearing age, with incidence pre-puberty significantly lower (52) and pregnancy associated with increased flares in patients with recently active disease (53, 54). Taken together, these epidemiological observations strongly suggest a role for the sex hormones in disease pathogenesis. However, juvenile rheumatic diseases, defined as having onset before the age of 16–18 years depending on phenotype, such as juvenile idiopathic arthritis (JIA), juvenile lupus (JSLE), juvenile Sjögren’s syndrome (JSS) and juvenile dermatomyositis (JDM) also exhibit sex bias, but this is less prominent than in their corresponding adult-onset phenotypes (55). JIA, for example, has no significant sex bias overall as an umbrella term, but different disease sub-types are characterised by different age at onset and sex-predominance: e.g., Enthesitis Related Arthritis (ERA) affects predominantly boys and has onset around puberty, while subtypes oligo- and poly-arthritis are more common in pre-pubertal and post-pubertal girls, respectively (56). As pre-pubertal cis-boys and cis-girls have similar serum sex hormone levels, a potential role for the sex chromosomes in the disease pathogenesis is thus also supported.
Several studies have investigated the effect of hormonal medications in SLE, where one might expect to see exacerbation of disease upon use of the oral contraceptive (OC), or hormone replacement therapy with oestradiol (HRT) given to alleviate menopausal symptoms. Commonly cited is the Nurse Health Study, which followed thousands of ciswomen, and reported an elevated relative risk for the development of SLE of 1.9 for women who had ever used hormonal OC (57) and of 2.1 in post-menopausal women who had ever used (HRT) (58). Although hormonal treatments have been purported to cause flares in SLE in older studies (59), recent literature has demonstrated little to no impact of OC usage on mild to moderate SLE, with the potential for unplanned pregnancies deemed a more significant risk for patients than OC use (60, 61). Several studies have demonstrated reduced androgen levels in SLE patients (62, 63), and this has been suggested to play a role in disease development or severity. Therein, the use of various forms of androgen as therapeutic agents has been tested in several incidences – with some trials showing mild efficacy (64–68) while others showed no difference from placebo (69). Thus, the current literature on in vivo manipulation of hormones does not provide a conclusive picture. Several case studies (70–77) detail the development of autoimmunity in trans-females upon commencement of gender-affirming oestradiol treatment, or the improvement of symptoms when taking gender-affirming testosterone (78). However, one cannot infer causality from these instances, nor can individual case studies be extrapolated to the wider population. Inclusion of trans people in bigger cohort studies on autoimmunity development is thus strongly supported – whether the increased relative risk seen in post-menopausal cis-females on HRT would be the same or similar in trans-women with an XY chromosomal background is yet unknown.
Although the majority of autoimmune diseases are characterised by female bias, there is evidence that type I diabetes mellitus and Crohn’s disease are characterised by a male predominance, irrespective of age at onset (79, 80). Additionally, some conditions have differential disease phenotypes according to sex, which has implications in disease recognition and epidemiological data collection. This is the case with spondyloarthritis (81), which had been considered a male-predominant disease for many decades before evidence about a different clinical presentation and delays in diagnosing females with spondyloarthritis emerged (82). Further, certain treatments may be more efficacious in one sex compared to the other [recently reviewed extensively by Klein and Morgan (83)], e.g., TNF inhibitors tend to work better for males with RA than for females (84) and female patients may be more likely to stop such drugs following the side effects they experience from them (85). Moreover, there is evidence that spontaneous puberty can completely reverse the sex bias in disorders of immune regulation such as asthma and atopy, characterised by male preponderance prepuberty, followed by a significantly increased female prevalence during reproductive years (86).
Impact of Age, Puberty and Menopause on Autoimmunity
Throughout the various life stages from infancy to old age, the immune system is also subject to great change (87, 88), and these changes are known to differ between cisgender males and females (89). The ageing immune system is a growing area of research, but less is known specifically about the immune changes that may occur during/after puberty and menopause. The coincidence of the average age of onset of several juvenile rheumatic diseases (90) with the average age of puberty onset (91) suggests that it is not merely the maturation process itself that alters one’s immune system, but that the rise in sex hormone levels seen in puberty is also involved. Our systematic review of the bidirectional relationship between puberty and autoimmune rheumatic disorders demonstrated how poorly these relationships are documented in the literature, but highlighted the differences in disease outcome in those with onset pre- vs. post-puberty (92) and symptomatic differences have been noted between different age groups of SLE patients (93), with adolescent onset JSLE noted for its greater severity (94, 95). In the case of menopause, RA (96) and SSc (97) both have their peak incidence in the over 50 age bracket. SLE has classically been considered to have its peak incidence within the childbearing years in females, but a 10-year incidence study of United Kingdom patients found the peak onset to be between 50 and 54 years in females and 70–74 in males (98), and this was supported by two other shorter studies (21, 99). However, these studies were of predominantly white populations, and in studies including black (100), Arab (101) and American Indian (102) patients, younger ages of peak onset between 30.4 and 39.2 have been observed. It is unclear exactly why this might be, but this highlights the complexity of sex-based influences on the immune system, which may interact with both age- and ethnicity-related factors to give rise to autoimmunity. With the inclusion of transgender subjects of different ages and pubertal/menopausal stages among basic and clinical research, these factors could be separated out, and the impact of sex be examined without the confounders of immunosenescence and ethnically inherited risk factors.
Differential Effect of Sex Determinants on Immune Activation Pathways
The investigation of the impact of sex-determinants on certain immune activation pathways, such as specific cell populations or pro-inflammatory pathways, where both sex chromosomal and hormonal elements have been separately suggested to be of influence is an area with great scope for new discovery. Work from our lab, published in 2019 (103), pioneered the inclusion of gender-diverse cohorts to address questions relevant to SLE, using a cohort of healthy trans- (n = 13 male, 7 female) and cisgender (n = 48 male, 51 female) young volunteers, alongside individuals with Turner Syndrome (n = 9), who are missing an X chromosome (45,X). Young transgender healthy controls were recruited from the University College London Hospital GIDS and treatment pathways are shown in Figure 1. Production of the antiviral cytokine family known as type 1 interferons (IFN)- predominantly by plasmacytoid dendritic cells (pDC)- is known to contribute significantly to the pathogenesis of both SLE and JSLE. We demonstrated that pDC from healthy cis-females produced more T1 IFN in response to TLR-7 signalling than pDC from cis-males, even before puberty. Using our inclusive volunteer cohort, we were additionally able to show that this related to X chromosome dosage and serum testosterone concentration, in a manner that was dependent upon the number of X chromosomes present. Overall, we showed that both factors were associated not just individually, but also interactively with the T1 IFN response.
More recently, we used a similar cohort (n = 17 cis-male; 22 cis-female; 10 trans-male and 10 trans-female) to examine the effects of sex and hormones on regulatory and responder CD4 + T cells (Tregs and Tresps, respectively) (104). Sex differences in Tregs are well-reported (105–109), and we firstly confirmed the observation that healthy cis-males have higher levels of Tregs compared to Tresps than cis-females both pre- and post-puberty. We then demonstrated that the ability of cis-male Tregs to suppress the division of Tresps was significantly enhanced compared to that of cis-female Tregs, supporting the concept of a pro-inflammatory phenotype in females that could contribute to autoimmunity. Then, using RNA sequencing (RNAseq), we found a significant number of differentially expressed genes (DEGs) in sorted Tregs from cis-males compared to females. Using our transgender healthy controls, we observed significant differences in related immune pathways following hormone treatment, demonstrating the potential for both oestradiol and testosterone to impact Tregs at a transcriptional level, even at the early stages of their treatment.
The COVID-19 pandemic has prompted several interesting studies on sex differences in viral responses, and how these translate into clinical outcomes. Takahashi et al. (8) demonstrated a more robust T cell response in females with the disease, compared to males- with poor T cell responses associated with a worse disease trajectory in males. Meanwhile males had higher levels of innate inflammatory cytokines, but higher levels of these in females were associated with more severe outcomes. Supporting these findings, Liu et al. (110) compared transcriptional differences in healthy males and females, demonstrating that males had higher expression of proinflammatory cytokines and chemokines, which they hypothesise may contribute to the ‘cytokine storm’ that is detrimental in COVID-19 pathogenesis. Females in this study were found to have higher expression of IFN genes, supporting what is already known about the sex bias in IFN production in health and in autoimmunity. These data demonstrate a clear link between sexual dimorphism in the immunological systems that serve to protect us, that may also lead to damage in the context of an autoimmune disease. Inclusion of trans and gender-diverse cohorts in infection response studies, is thus equally warranted alongside those in autoimmunity.
There remain myriad of cell types and mechanisms that have been identified as potentially influenced by sex hormones or chromosomes, thus meriting in vivo interrogation. In addition to the further work necessitated on pDCs, the T1 IFN pathway, and Tregs/Tresps, obvious suggestions for future research directions (based on preliminary evidence of sex hormonal/chromosomal effect in animal or non-diverse cohorts) are B cells and antibody/autoantibody production (111–120), B regulatory (Breg) cells (121), CD4 T cells (116, 122–124), and specific T helper subsets (89, 125–131), CD8 cytotoxic T cells (122, 132–135), dendritic cells (136–140), Natural Killer (NK) cells (116, 141–145), neutrophils (146–149), monocytes (150) and macrophages (149, 151, 152). Table 1 summarises a selection of notable effects of sex determinants on immune processes and cell types known to be relevant to autoimmune rheumatic disease- this is by no means an exhaustive review of the literature, and many extensive reviews are available (89, 182, 183). As a field in its relative infancy, there remain so many avenues ripe for gender-disaggregated interrogation and scintillating project proposals.
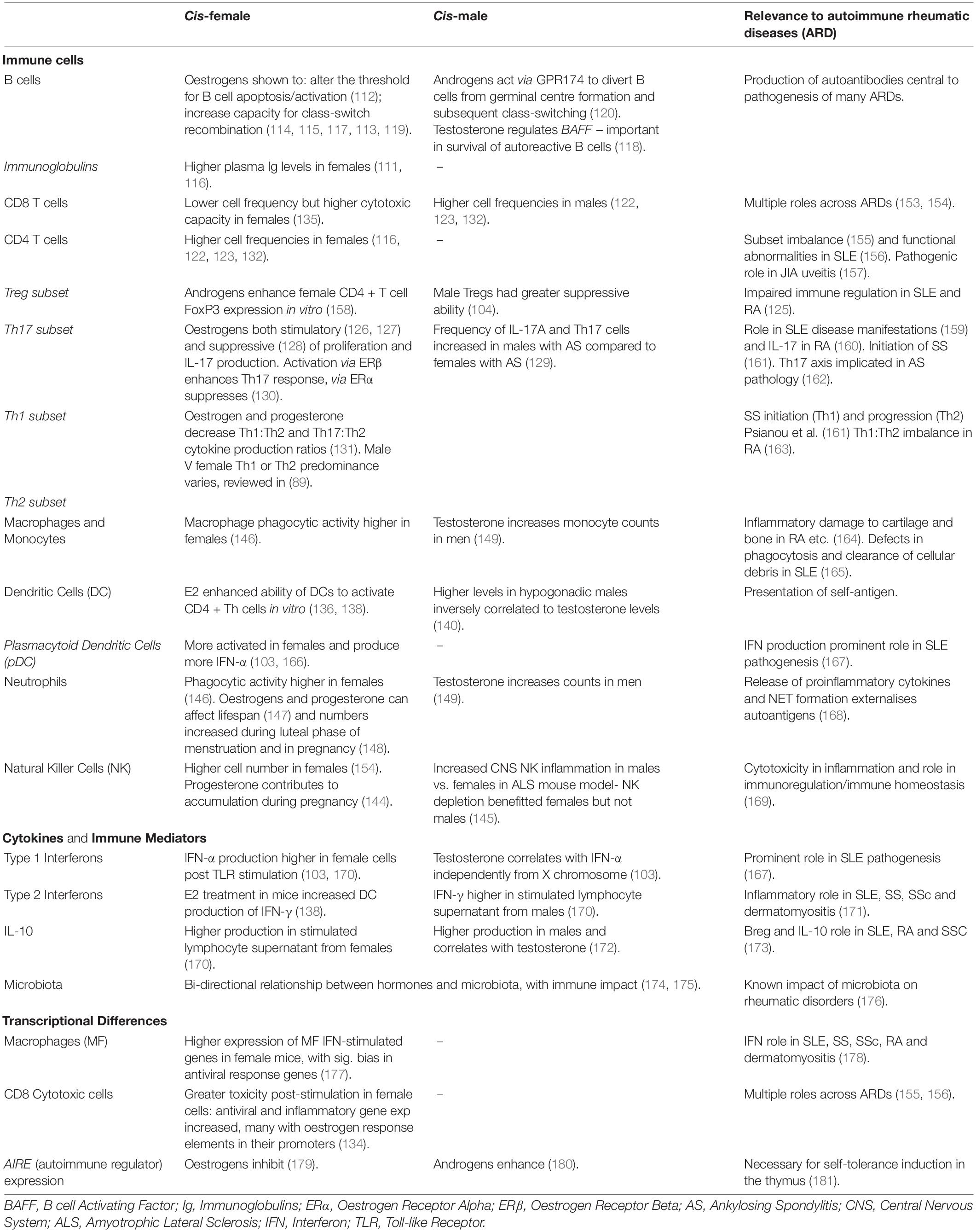
Table 1. Summary of notable immune system elements known to be regulated by sex determinants and their relevance to autoimmune rheumatic disease.
Unanswered Questions and Future Directions
There is an unmet need for better understanding of the long-term outcomes of sex hormone manipulation on the health of trans and gender-diverse people. This includes the effects of gender-affirming treatment on responses to natural and vaccine immunisations, on bone and muscle health, as well as their impact on mental health and quality of life, before moving into investigating infective and autoimmunity risk in these populations. Without accurate gender classifications in population studies, these relevant outcomes cannot be studied. There are many specific questions which need answering in relation to the impact of sex determinants on immune system functions, in particular around exposure to and timings of exposure to sex hormones. We do not know if the length of exposure to/blockade of a particular sex hormone is different from the physiological sex hormone fluctuations, especially those related to menstruation, pregnancy, or early stages of puberty/menopause. There is no research into the impact of age at which a person is first exposed to (or begins blocking) sex hormones on their risk of infections, autoimmunity, or other adverse health outcomes. Our group identified a significant impact of sex hormones in driving a pro-atherogenic lipid profile in healthy cis- and trans-male adolescents post-puberty (184). Therefore, investigating the impact of sex-affirming hormone therapy on the cardio-vascular risk of trans people has a clear clinical rationale. Further research is needed to investigate the effects of lifetime exposure to higher exogenous oestrogen or androgen therapies, especially in the context of potential reversibility and dose-dependent long-term effects. In some countries, young people are able to commence puberty blockade and gender-affirming sex hormones prior to the commencement of their natural puberty. Meanwhile in the United Kingdom, only those aged 16 + and thus likely already post-pubertal can legally be consented to start on gender-affirming hormone treatments. Others still, may not access treatment until much later into adulthood. It is important to establish whether outcomes (immunological or otherwise) would be similar or different in these groups, when their hormonal transitions have commenced at such widespread life stages. Furthermore, it is possible that different routes of hormone administration (oral, patch, gel, IM, SC.) and dosages of these may impact the systems of the human body differently. Innovative clinical trial study design, including volunteers of all gender categories, across various age ranges is required to be able to examine the relative importance of sex hormone exposure at different stages of life, against both sex chromosomal backgrounds, on various interventions or health and disease outcomes. In addition, the inclusion of subjects with altered sex chromosomal complement (such as Klinefelter and Turner syndromes) could provide suitable controls for these studies aiming to tease out the distinct effects of various sex chromosome determinants.
First steps would be establishing national and international registries with associated biological sample repositories capturing patients of various gender categories, sex chromosomal backgrounds and demographic diversity to enable long-term follow-up. A number of social barriers exist, well-documented in the United States, that prevent the trans population from accessing healthcare and thus participating in research (185). Thus, it is important for such registries to be set up with advice and input from transgender charities and organisations such as WPATH (World Professional Association for Transgender Health) on how to overcome these barriers. This should include ensuring that all health professionals and researchers involved are trained in LGBTQ + cultural competency (186), so that all elements of study design- from language used on questionnaires, to subtlety when approaching people for recruitment- are optimised to help participants feel secure and respected. Further, recruitment must extend beyond private healthcare patients, encompassing public healthcare clinics as well as community support groups, in order to capture the true breadth of the trans population. Hospital and clinic record databases must be updated in order to capture gender definitions and associated medications more accurately, and reference ranges for clinical and laboratory tests need to be reviewed and established for gender-diverse people, as it is highly likely that they may differ from those appropriate for cis persons (187). If these changes were made across the world, they would not only facilitate far more impactful retrospective review of outcomes, but would vastly improve the lives and healthcare of transgender persons, who have tolerated systems that weren’t designed to accommodate them for far too long. In Figure 2, we propose several streams of research, both clinical and immunological, as starting points for future projects. Researchers and clinicians should join forces to give people of all gender identities a voice and create opportunities for their involvement in clinical data collection and research. As more countries develop their gender identity services, and adapt to the changes outlined above, we look forward to seeing the results from further large studies such as 2021 Michelson Prize recipient Dr. Camila Consiglio’s multi-parameter analysis of the effect of testosterone treatment on the immune systems of trans-men at the Karolinska Institutet, Sweden (188), and that of Professor Guy T’Sjoen’s ENIGI consortium across Ghent, Oslo, Florence, and Amsterdam (189, 190), where long-term follow-up of participants pursuing hormonal gender affirmation will provide us with a wealth of information, pertinent to everyone – not just those it is convenient to study.
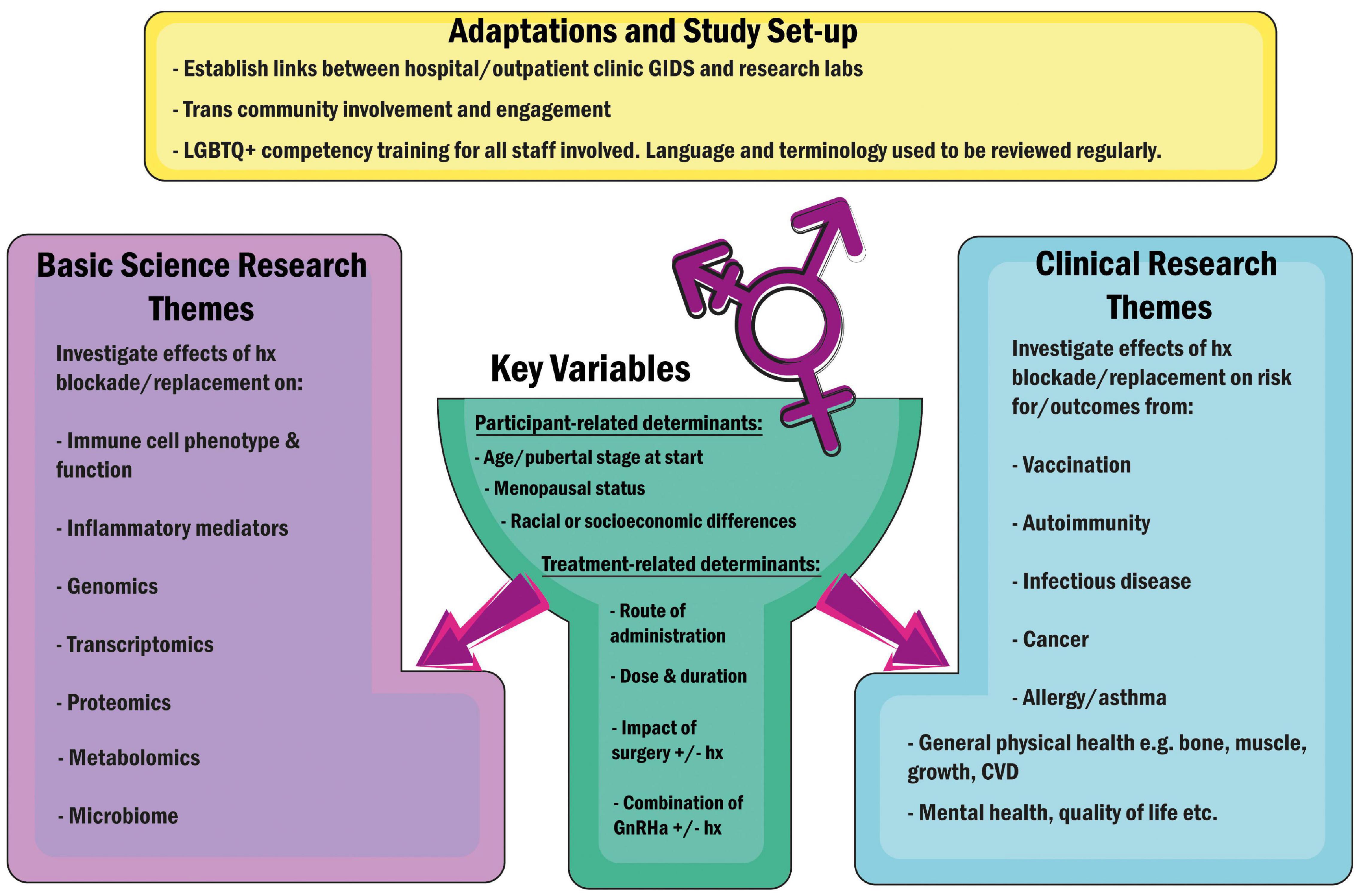
Figure 2. Suggested adaptations to facilitate future research encompassing trans and gender-diverse individuals, and key research pathways proposed. Hx, Hormones; GnRHa, Gonadotropin Releasing Hormone Agonists (“Blockers”); CVD, Cardiovascular Disease.
Concluding Remarks
We advocate that research should celebrate gender diversity and be as inclusive as possible to ensure that it is relevant to human society as a whole. We can only hope that in coming years, more labs and clinical teams will join us in the interrogation of sex determinants as biological variables. As personalised medicine becomes an increasingly viable and beneficial approach to healthcare, it is research like this that will be equipped to inform and steer innovation in the appropriate direction.
Disclaimer
Gender-related terminology is continually evolving, and terms vary in their usage between individuals and between groups across the world. Language and definitions used throughout this article have been adapted from the Gender Identity Research and Education Society (GIRES) website at time of writing (191) – we have made every effort to be inclusive, but acknowledge that these may not capture the preferences and experiences of all.
Author Contributions
CC, GB, and HP contributed to conception of the review. HP wrote the first draft of the manuscript and designed the figures. CC, GB, KW, and ER wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by as grants from the NIHR UCLH Biomedical Research Centre (Grant Nos: BRC772/III/EJ/101350 and BRC773/III/CC/101350), Lupus United Kingdom and was performed within the Centre for Adolescent Rheumatology Versus Arthritis at UCL, UCLH, and GOSH supported by grants from Versus Arthritis (21593 and 20164). HP was supported by a Versus Arthritis Studentship to CC (22203). KW was supported by the Crick African Network which receives its funding from the United Kingdom’s Global Challenges Research Fund (MR/P028071/1), and by the Francis Crick Institute which receives its core funding from Cancer Research United Kingdom (FC1001647), the United Kingdom Medical Research Council (FC1001647), and the Wellcome Trust (FC1001647). ER was supported by a Medical Research Foundation Lupus Fellowship to ER (MRF-057-0001-RG-ROSS-C0797). The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank to Hannah-Louise Hayman (University of Glasgow), for her thoughtful comments and insight on the manuscript.
References
1. Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J. (2016) 30:519–24. doi: 10.1096/fj.15-279554
2. Geller SE, Koch AR, Roesch P, Filut A, Hallgren E, Carnes M. The more things change, the more they stay the same: a study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Acad Med. (2018) 93:630–5. doi: 10.1097/ACM.0000000000002027
3. Johnson J, Sharman Z, Vissandjée B, Stewart DE. Does a change in health research funding policy related to the integration of sex and gender have an impact? PLoS One. (2014) 9:e99900. doi: 10.1371/journal.pone.0099900
4. Kocher K, Delot-Vilain A, Spencer DA, LoTempio J, Délot EC. Paucity and disparity of publicly available sex-disaggregated data for the COVID-19 epidemic hamper evidence-based decision-making. Arch Sex Behav. (2021) 50:407–26. doi: 10.1007/s10508-020-01882-w
5. Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. (2020) 11:6317. doi: 10.1038/s41467-020-19741-6
6. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. (2020) 20:442–7. doi: 10.1038/s41577-020-0348-8
7. Klein SL, Dhakal S, Ursin RL, Deshpande S, Sandberg K, Mauvais-Jarvis F. Biological sex impacts COVID-19 outcomes. PLoS Pathog. (2020) 16:e1008570. doi: 10.1371/journal.ppat.1008570
8. Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. (2020) 588:315–20.
9. Klein SL, Huber S. Sex Differences in Susceptibility to Viral Infection. Sex Hormones and Immunity to Infection. (2010). p. 93–122. Available online at: https://link.springer.com/chapter/10.1007/978-3-642-02155-8_4 (accessed January 14, 2022).
10. Fischer J, Jung N, Robinson N, Lehmann C. Sex differences in immune responses to infectious diseases. Infection. (2015) 43:399–403. doi: 10.1007/s15010-015-0791-9
11. Ingersoll MA. Sex differences shape the response to infectious diseases. PLoS Pathog. (2017) 13:e1006688. doi: 10.1371/journal.ppat.1006688
12. Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open. (2021) 4:e2111398. doi: 10.1001/jamanetworkopen.2021.11398
13. Al-kuraishy HM, Al-Gareeb AI, Faidah H, Al-Maiahy TJ, Cruz-Martins N, Batiha GES. The looming effects of estrogen in Covid-19: a rocky rollout. Front Nutr. (2021) 8:649128. doi: 10.3389/fnut.2021.649128
14. Raza HA, Sen P, Bhatti OA, Gupta L. Sex hormones, autoimmunity and gender disparity in COVID-19. Rheumatol Int. (2021) 41:1375–86. doi: 10.1007/s00296-021-04873-9
15. Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. (2008) 26:3551–5. doi: 10.1016/j.vaccine.2008.04.054
16. Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. (2015) 109:9–15. doi: 10.1093/trstmh/tru167
17. Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. (2017) 33:577–99. doi: 10.1146/annurev-cellbio-100616-060718
18. Engler RJ, Nelson MR, Klote MM, VanRaden MJ, Huang CY, Cox NJ, et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Arch Intern Med. (2008) 168:2405–14. doi: 10.1001/archinternmed.2008.513
19. Hochberg MC. The incidence of systemic lupus erythematosus in Baltimore, Maryland, 1970-1977. Arthritis Rheum. (1985) 28:80–6. doi: 10.1002/art.1780280113
20. Gudmundsson S, Steinsson K. Systemic lupus erythematosus in Iceland 1975 through 1984. A nationwide epidemiological study in an unselected population. J Rheumatol. (1990) 17:1162–7.
21. Jonsson H, Nived O, Sturfelt G, Silman A. Estimating the incidence of systemic lupus erythematosus in a defined population using multiple sources of retrieval. Rheumatology. (1990) 29:185–8. doi: 10.1093/rheumatology/29.3.185
22. Nossent JC. Systemic lupus erythematosus on the Caribbean island of Curaçao: an epidemiological investigation. Ann Rheum Dis. (1992) 51:1197–201. doi: 10.1136/ard.51.11.1197
23. Hopkinson ND, Doherty M, Powell RJ. Clinical features and race-specific incidence/prevalence rates of systemic lupus erythematosus in a geographically complete cohort of patients. Ann Rheum Dis. (1994) 53:675–80. doi: 10.1136/ard.53.10.675
24. Johnson AE, Gordon C, Palmer RG, Bacon PA. The prevalence and incidence of systemic lupus erythematosus in Birmingham. England. Arthritis Rheum. (1995) 38:551–8. doi: 10.1002/art.1780380415
25. Mccarty DJ, Manzi S, Medsger TA, Ramsey-Goldman R, Laporte RE, Kwoh CK. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. (1995) 38:1260–70. doi: 10.1002/art.1780380914
26. Uramoto KM, Michet CJ, Thumboo J, Sunku J, O’Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus, 1950-1992. Arthritis Rheum. (1999) 42:46–50. doi: 10.1002/1529-0131(199901)42:1<46::AID-ANR6>3.0.CO;2-2
27. Nossent HC. Systemic lupus erythematosus in the Arctic region of Norway. J Rheumatol. (2001) 28:539–46.
28. Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. (2002) 16:847–58. doi: 10.1053/berh.2002.0259
29. Trigunaite A, Dimo J, Jørgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol. (2015) 294:87–94. doi: 10.1016/j.cellimm.2015.02.004
30. Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. Am J Hum Biol. (2005) 17:527–58. doi: 10.1002/ajhb.20419
31. Cutolo M, Capellino S, Straub RH. Oestrogens in rheumatic diseases: friend or foe? Rheumatology. (2008) 47(Suppl. 3):iii2–5. doi: 10.1093/rheumatology/ken150
32. Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. (2016) 6:635. doi: 10.3389/fimmu.2015.00635
33. Foo YZ, Nakagawa S, Rhodes G, Simmons LW. The effects of sex hormones on immune function: a meta-analysis. Biol Rev Camb Philos Soc. (2017) 92:551–71. doi: 10.1111/brv.12243
34. Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. (2012) 38:J187–92. doi: 10.1016/j.jaut.2011.11.012
35. Saitoh SI, Abe F, Kanno A, Tanimura N, Mori Saitoh Y, Fukui R, et al. TLR7 mediated viral recognition results in focal type I interferon secretion by dendritic cells. Nat Commun. (2017) 8:1–12. doi: 10.1038/s41467-017-01687-x
36. Lederman S, Yellin MJ, Cleary AM, Pernis A, Inghirami G, Cohn LE, et al. T-BAM/CD40-L on helper T lymphocytes augments lymphokine-induced B cell Ig isotype switch recombination and rescues B cells from programmed cell death. J Immunol. (1994) 152:2163–71.
37. Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. (2017) 17:703–17. doi: 10.1038/nri.2017.75
38. Kuo PT, Zeng Z, Salim N, Mattarollo S, Wells JW, Leggatt GR. The role of CXCR3 and its chemokine ligands in skin disease and cancer. Front Med. (2018) 5:271. doi: 10.3389/fmed.2018.00271
39. Aytekin G, Vaqar S. X-Linked Immunodeficiency. StatPearls. (2022). Available online at: http://europepmc.org/books/NBK562182 (accessed February 18, 2022).
40. Qu K, Zaba LC, Giresi PG, Li R, Longmire M, Kim YH, et al. Individuality and variation of personal regulomes in primary human T cells. Cell Syst. (2015) 1:51–61. doi: 10.1016/j.cels.2015.06.003
41. Wang J, Syrett CM, Kramer MC, Basu A, Atchison ML, Anguera MC. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc Natl Acad Sci U S A. (2016) 113:E2029–38. doi: 10.1073/pnas.1520113113
42. Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. (2018) 3:eaa8855. doi: 10.1126/sciimmunol.aap8855
43. Oghumu S, Varikuti S, Stock JC, Volpedo G, Saljoughian N, Terrazas CA, et al. Cutting edge: CXCR3 escapes X chromosome inactivation in T cells during infection: potential implications for sex differences in immune responses. J Immunol. (2019) 203:789–94. doi: 10.4049/jimmunol.1800931
44. Butler G, De Graaf N, Wren B, Carmichael P. Assessment and support of children and adolescents with gender dysphoria. Arch Dis Child. (2018) 103:631–6. doi: 10.1136/archdischild-2018-314992
45. Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2017) 102:3869–903. doi: 10.1210/jc.2017-01658
46. Griffiths DA. Shifting syndromes: sex chromosome variations and intersex classifications. Soc Stud Sci. (2018) 48:125. doi: 10.1177/0306312718757081
47. GIDS. Referrals to GIDS 2015-2020. (2021). Available online at: https://gids.nhs.uk/number-referrals (accessed February 14, 2022).
48. Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. (2000) 24:627–38. doi: 10.1016/S0149-7634(00)00027-0
49. Kvarnström M, Ottosson V, Nordmark B, Wahren-Herlenius M. Incident cases of primary Sjögren’s syndrome during a 5-year period in Stockholm County: a descriptive study of the patients and their characteristics. Scand J Rheumatol. (2015) 44:135–42. doi: 10.3109/03009742.2014.931457
50. Hughes M, Pauling JD, Armstrong-James L, Denton CP, Galdas P, Flurey C. Gender-related differences in systemic sclerosis. Autoimmun Rev. (2020) 19:102494. doi: 10.1016/j.autrev.2020.102494
51. Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. (2010) 62:1576–82. doi: 10.1002/art.27425
52. Siegel M, Lee SL. The epidemiology of systemic lupus erythematosus. Semin Arthritis Rheum. (1973) 3:1–54. doi: 10.1016/0049-0172(73)90034-6
53. Eudy AM, Siega-Riz AM, Engel SM, Franceschini N, Howard AG, Clowse MEB, et al. Effect of pregnancy on disease flares in patients with systemic lupus erythematosus. Ann Rheum Dis. (2018) 77:855–60. doi: 10.1136/annrheumdis-2017-212535
54. Petri MA. Pregnancy and systemic lupus erythematosus. Best Pract Res Clin Obstet Gynaecol. (2020) 64:24–30. doi: 10.1016/j.bpobgyn.2019.09.002
55. Cattalini M, Soliani M, Caparello MC, Cimaz R. Sex differences in pediatric rheumatology. Clin Rev Allergy Immunol. (2017) 56:293–307. doi: 10.1007/s12016-017-8642-3
56. Fisher C, Sen D. Juvenile idiopathic arthritis: in adolescence and beyond. Br J Hosp Med. (2012) 73:564–70. doi: 10.12968/hmed.2012.73.10.564
57. Sanchez-Guerrero J, Karlson EW, Liang MH, Hunter DJ, Speizer FE, Colditz GA. Past use of oral contraceptives and the risk of developing systemic lupus erythematosus. Arthritis Rheum. (1997) 40:804–8. doi: 10.1002/art.1780400505
58. Sánchez-Guerrero J, Liang MH, Karlson EW, Hunter DJ, Colditz GA. Postmenopausal estrogen therapy and the risk for developing systemic lupus erythematosus. Ann Intern Med. (1995) 122:430–3. doi: 10.7326/0003-4819-122-6-199503150-00005
59. Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. (2005) 142(Pt 1):953–62. doi: 10.7326/0003-4819-142-12_Part_1-200506210-00004
60. Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. (2005) 353:2550–8. doi: 10.1056/NEJMoa051135
61. Culwell KR, Curtis KM. Contraception for women with systemic lupus erythematosus. J Fam Plan Reprod Heal Care. (2013) 39:9–11. doi: 10.1136/jfprhc-2012-100437
62. Lahita RG, Bradlow HL, Ginzler E, Pang S, New M. Low plasma androgens in women with systemic lupus erythematosus. Arthritis Rheum. (1987) 30:241–8. doi: 10.1002/art.1780300301
63. Mok CC, Lau CS. Profile of sex hormones in male patients with systemic lupus erythematosus. Lupus. (2000) 9:252–7. doi: 10.1191/096120300680198926
64. Van Vollenhoven RF, Engleman EG, Mcguire JL. An open study of dehydroepiandrosterone in systemic lupus erythematosus. Arthritis Rheum. (1994) 37:1305–10. doi: 10.1002/art.1780370906
65. Van Vollenhoven RF, Morabito LM, Engleman EG, McGuire JL. Treatment of systemic lupus erythematosus with dehydroepiandrosterone: 50 patients treated up to 12 months. J Rheumatol. (1998) 25:285–9.
66. Van Vollenhoven RF, Park JL, Genovese MC, West JP, McGuire JL. A double-blind, placebo-controlled, clinical trial of dehydroepiandrosterone in severe systemic lupus erythematosus. Lupus. (1999) 8:181–7. doi: 10.1191/096120399678847588
67. Chang DM, Lanv JL, Lin HY, Luo SF. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. (2002) 46:2924–7. doi: 10.1002/art.10615
68. Petri MA, Mease PJ, Merrill JT, Lahita RG, Iannini MJ, Yocum DE, et al. Effects of prasterone on disease activity and symptoms in women with active systemic lupus erythematosus. Arthritis Rheum. (2004) 50:2858–68. doi: 10.1002/art.20427
69. Gordon C, Wallace DJ, Shinada S, Kalunian KC, Forbess L, Braunstein GD, et al. Testosterone patches in the management of patients with mild/moderate systemic lupus erythematosus. Rheumatology. (2008) 47:334–8. doi: 10.1093/rheumatology/kem342
70. Troum OM, Quismorio FP. Rheumatoid arthritis in a male transsexual. J Rheumatol. (1985) 12:640–1.
71. Santos-Ocampo AS. New onset systemic lupus erythematosus in a transgender man: possible role of feminizing sex hormones. J Clin Rheumatol. (2007) 13:29–30. doi: 10.1097/01.rhu.0000256169.05087.ad
72. Zandman-Goddard G, Solomon M, Barzilai A, Shoenfeld Y. Lupus erythematosus tumidus induced by sex reassignment surgery. J Rheumatol. (2007) 34:1938–40.
73. Chan KL, Mok CC. Development of systemic lupus erythematosus in a male-to-female transsexual: the role of sex hormones revisited. Lupus. (2013) 22:1399–402. doi: 10.1177/0961203313500550
74. Pakpoor J, Wotton CJ, Schmierer K, Giovannoni G, Goldacre MJ. Gender identity disorders and multiple sclerosis risk: a national record-linkage study. Mult Scler. (2016) 22:1759–62. doi: 10.1177/1352458515627205
75. Campochiaro C, Host LV, Ong VH, Denton C. Development of systemic sclerosis in transgender females: a case series and review of the literature. Clin Exp Rheumatol. (2018) 36:S50–2.
76. Pontes LT, Camilo DT, De Bortoli MR, Santos RSS, Luchi WM. New-onset lupus nephritis after male-to-female sex reassignment surgery. Lupus. (2018) 27:2166–9. doi: 10.1177/0961203318800571
77. Hill BG, Hodge B, Misischia R. Lupus nephritis in a transgender woman on cross-sex hormone therapy: a case for the role of oestrogen in systemic lupus erythematosus. Lupus. (2020) 29:1807–10. doi: 10.1177/0961203320946372
78. Ocon A, Peredo-Wende R, Kremer JM, Bhatt BD. Significant symptomatic improvement of subacute cutaneous lupus after testosterone therapy in a female-to-male transgender subject. Lupus. (2018) 27:347–8. doi: 10.1177/0961203317734921
79. Östman J, Lönnberg G, Arnqvist HJ, Blohmé G, Bolinder J, Schnell AE, et al. Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983-2002. J Intern Med. (2008) 263:386–94. doi: 10.1111/j.1365-2796.2007.01896.x
80. Ishige T, Tomomasa T, Takebayashi T, Asakura K, Watanabe M, Suzuki T, et al. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol. (2010) 45:911–7. doi: 10.1007/s00535-010-0223-7
81. Jovani V, Blasco-Blasco M, Pascual E, Ruiz-Cantero MT. Challenges to conquer from the gender perspective in medicine: the case of spondyloarthritis. PLoS One. (2018) 13:e0205751.
82. Roussou E, Sultana S. Spondyloarthritis in women: differences in disease onset, clinical presentation, and Bath Ankylosing Spondylitis Disease Activity and Functional indices (BASDAI and BASFI) between men and women with spondyloarthritides. Clin Rheumatol. (2011) 30:121–7. doi: 10.1007/s10067-010-1581-5
83. Klein SL, Morgan R. The impact of sex and gender on immunotherapy outcomes. Biol Sex Differ. (2020) 11:1–10. doi: 10.1186/s13293-020-00301-y
84. Jayakumar K, Norton S, Dixey J, James D, Gough A, Williams P, et al. Sustained clinical remission in rheumatoid arthritis: prevalence and prognostic factors in an inception cohort of patients treated with conventional DMARDS. Rheumatology. (2012) 51:169–75. doi: 10.1093/rheumatology/ker250
85. Souto A, Maneiro JR, Gómez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. (2016) 55:523–34. doi: 10.1093/rheumatology/kev374
86. Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. (2003) 88:587–90. doi: 10.1136/adc.88.7.587
87. Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. (2017) 19:10–9. doi: 10.1038/s41590-017-0006-x
88. Sadighi Akha AA. Aging and the immune system: an overview. J Immunol Methods. (2018) 463:21–6. doi: 10.1016/j.jim.2018.08.005
89. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
90. Massias JS, Smith EMD, Al-Abadi E, Armon K, Bailey K, Ciurtin C, et al. Clinical and laboratory characteristics in juvenile-onset systemic lupus erythematosus across age groups. Lupus. (2020) 29:474–81. doi: 10.1177/0961203320909156
91. Whincup PH, Gilg JA, Odoki K, Taylor SJC, Cook DG, Barth JH, et al. Age of menarche in contemporary British teenagers: survey of girls born between 1982 and 1986 Regional Paediatric Endocrinology Clinic, Leeds General Infirmary, Leeds LS1 3EX. BMJ. (2001) 322:1095–101. doi: 10.1136/bmj.322.7294.1095
92. de Gruijter NM, Naja M, Peckham H, Radziszewska A, Kinsella M, Glenister J, et al. A systematic review exploring the bidirectional relationship between puberty and autoimmune rheumatic diseases. Pediatr Rheumatol. (2021) 19:47. doi: 10.1186/s12969-021-00528-y
93. Marder W, Vinet E, Somers EC. Rheumatic autoimmune diseases in women and midlife health. Women’s Midlife Heal. (2015) 1:11. doi: 10.1186/s40695-015-0012-9
94. Tucker LB, Uribe AG, Fernández M, Vilá LM, McGwin G, Apte M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus. (2008) 17:314–22. doi: 10.1177/0961203307087875
95. Ambrose N, Morgan TA, Galloway J, Ionnoau Y, Beresford MW, Isenberg DA. Differences in disease phenotype and severity in SLE across age groups. Lupus. (2016) 25:1542–50. doi: 10.1177/0961203316644333
96. Doran MF, Pond GR, Crowson CS, O’Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. (2002) 46:625–31. doi: 10.1002/art.509
97. Vinet E, Bernatsky S, Hudson M, Pineau CA, Baron M, Pope J, et al. Effect of menopause on the modified Rodnan skin score in systemic sclerosis. Arthritis Res Ther. (2014) 16:1–7. doi: 10.1186/ar4587
98. Somers EC, Thomas SL, Smeeth L, Schoonen WM, Hall AJ. Incidence of systemic lupus erythematosus in the United Kingdom, 1990–1999. Arthritis Care Res (Hoboken). (2007) 57:612–8. doi: 10.1002/art.22683
99. Hopkinson ND, Doherty M, Powell RJ. The prevalence and incidence of systemic lupus erythematosus in nottingham, UK, 1989-1990. Rheumatology. (1993) 32:110–5. doi: 10.1093/rheumatology/32.2.110
100. Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol (Hoboken, NJ). (2014) 66:369–78. doi: 10.1002/art.38238
101. Housey M, De Guire P, Lyon-Callo S, Wang L, Marder W, McCune WJ, et al. Incidence and prevalence of systemic lupus erythematosus among Arab and Chaldean Americans in southeastern Michigan: the Michigan Lupus Epidemiology and Surveillance Program. Am J Public Health. (2015) 105:e74–9. doi: 10.2105/AJPH.2014.302423
102. Ferucci ED, Johnston JM, Gaddy JR, Sumner L, Posever JO, Choromanski TL, et al. Prevalence and incidence of systemic lupus erythematosus in a population-based registry of American Indian and Alaska Native people, 2007-2009. Arthritis Rheumatol (Hoboken, NJ). (2014) 66:2494–502. doi: 10.1002/art.38720
103. Webb K, Peckham H, Radziszewska A, Menon M, Oliveri P, Simpson F, et al. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front Immunol. (2019) 10:3167. doi: 10.3389/fimmu.2018.03167
104. Robinson GA, Waddington KE, Adriani M, Radziszewska A, Peckham H, Isenberg DA, et al. Sex differences in autoimmunity could be associated with altered regulatory T cell phenotype and lipoprotein metabolism. bioRxiv [Preprint]. (2019):760975. doi: 10.1101/760975
105. Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. (2004) 173:2227–30. doi: 10.4049/jimmunol.173.4.2227
106. Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. (2011) 187:1778–87. doi: 10.4049/jimmunol.1003919
107. Afshan G, Afzal N, Qureshi S. CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clin Lab. (2012) 58:567–71.
108. Ahnstedt H, Roy-O’Reilly M, Spychala MS, Mobley AS, Bravo-Alegria J, Chauhan A, et al. Sex differences in adipose tissue CD8+ T cells and regulatory T cells in middle-aged mice. Front Immunol. (2018) 9:659. doi: 10.3389/fimmu.2018.00659
109. Singh RP, Bischoff DS. Sex hormones and gender influence the expression of markers of regulatory T cells in SLE patients. Front Immunol. (2021) 12:619268. doi: 10.3389/fimmu.2021.619268
110. Liu T, Balzano-Nogueira L, Lleo A, Conesa A. Transcriptional differences for COVID-19 disease map genes between males and females indicate a different basal immunophenotype relevant to the disease. Genes (Basel). (2020) 11:1–14. doi: 10.1101/2020.09.30.321059
111. Giltay EJ, Fonk JCM, Von Blomberg BME, Drexhage HA, Schalkwijk C, Gooren LJG. In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J Clin Endocrinol Metab. (2000) 85:1648–57. doi: 10.1210/jcem.85.4.6562
112. Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. (2002) 109:1625–33. doi: 10.1172/JCI0214873
113. Pauklin S, Sernández IV, Bachmann G, Ramiro AR, Petersen-Mahrt SK. Estrogen directly activates AID transcription and function. J Exp Med. (2009) 206:99–111. doi: 10.1084/jem.20080521
114. Mai T, Zan H, Zhang J, Hawkins JS, Xu Z, Casali P. Estrogen receptors bind to and activate the HOXC4/HoxC4 promoter to potentiate HoxC4-mediated activation-induced cytosine deaminase induction, immunoglobulin class switch DNA recombination, and somatic hypermutation. J Biol Chem. (2010) 285:37797–810. doi: 10.1074/jbc.M110.169086
115. White C, Hawkins S, Pone E, Yu E, Al-Qahtani A, Mai T, et al. AID dysregulation in lupus-prone MRL/Faslpr/lpr mice increases class switch DNA recombination and promotes interchromosomal loci translocations: modulation by HoxC4. Autoimmunity. (2011) 44:585–98. doi: 10.3109/08916934.2011.577128
116. Abdullah M, Chai PS, Chong MY, Tohit ERM, Ramasamy R, Pei CP, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. (2012) 272:214–9. doi: 10.1016/j.cellimm.2011.10.009
117. Jones BG, Penkert RR, Xu B, Fan Y, Neale G, Gearhart PJ, et al. Binding of estrogen receptors to switch sites and regulatory elements in the immunoglobulin heavy chain locus of activated B cells suggests a direct influence of estrogen on antibody expression. Mol Immunol. (2016) 77:97–102. doi: 10.1016/j.molimm.2016.07.015
118. Wilhelmson AS, Lantero Rodriguez M, Stubelius A, Fogelstrand P, Johansson I, Buechler MB, et al. Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat Commun. (2018) 9:1–13. doi: 10.1038/s41467-018-04408-0
119. Jones BG, Sealy RE, Penkert RR, Surman SL, Maul RW, Neale G, et al. Complex sex-biased antibody responses: estrogen receptors bind estrogen response elements centered within immunoglobulin heavy chain gene enhancers. Int Immunol. (2019) 31:141–56. doi: 10.1093/intimm/dxy074
120. Zhao R, Chen X, Ma W, Zhang J, Guo J, Zhong X, et al. A GPR174–CCL21 module imparts sexual dimorphism to humoral immunity. Nature. (2020) 577:416–20. doi: 10.1038/s41586-019-1873-0
121. Seifert HA, Benedek G, Liang J, Nguyen H, Kent G, Vandenbark AA, et al. Sex differences in regulatory cells in experimental stroke. Cell Immunol. (2017) 318:49–54. doi: 10.1016/j.cellimm.2017.06.003
122. Lee BW, Yap HK, Chew FT, Quah TC, Prabhakaran K, Chan GS, et al. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry. (1996) 26:8–15. doi: 10.1002/(SICI)1097-0320(19960315)26:1<8::AID-CYTO2>3.0.CO;2-E
123. Uppal SS, Verma S, Dhot PS. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytom Part B Clin Cytom. (2003) 52B:32–6. doi: 10.1002/cyto.b.10011
124. Sankaran-Walters S, Macal M, Grishina I, Nagy L, Goulart L, Coolidge K, et al. Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. (2013) 4:1–12. doi: 10.1186/2042-6410-4-10
125. Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. (2010) 10:849–59. doi: 10.1038/nri2889
126. Smith S, Nígabhann J, McCarthy E, Coffey B, Mahony R, Byrne JC, et al. Estrogen receptor α regulates tripartite motif-containing protein 21 expression, contributing to dysregulated cytokine production in systemic lupus erythematosus. Arthritis Rheumatol (Hoboken, NJ). (2014) 66:163–72. doi: 10.1002/art.38187
127. Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, et al. Estrogen and progesterone decrease Let-7f miRNA expression and increase IL-23/IL-23R signaling and IL-17A production in severe asthma. J Allergy Clin Immunol. (2015) 136:1025. doi: 10.1016/j.jaci.2015.05.046
128. Chen R-Y, Fan Y-M, Zhang Q, Liu S, Li Q, Ke G-L, et al. Estradiol inhibits Th17 cell differentiation through inhibition of RORγT transcription by recruiting the ERα/REA complex to estrogen response elements of the RORγT promoter. J Immunol. (2015) 194:4019–28. doi: 10.4049/jimmunol.1400806
129. Gracey E, Yao Y, Green B, Qaiyum Z, Baglaenko Y, Lin A, et al. Sexual dimorphism in the Th17 signature of ankylosing spondylitis. Arthritis Rheumatol. (2016) 68:679–89. doi: 10.1002/art.39464
130. Qin J, Li L, Jin Q, Guo D, Liu M, Fan C, et al. Estrogen receptor β activation stimulates the development of experimental autoimmune thyroiditis through up-regulation of Th17-type responses. Clin Immunol. (2018) 190:41–52. doi: 10.1016/j.clim.2018.02.006
131. AbdulHussain G, Azizieh F, Makhseed M, Raghupathy R. Effects of progesterone, dydrogesterone and estrogen on the production of Th1/Th2/Th17 cytokines by lymphocytes from women with recurrent spontaneous miscarriage. J Reprod Immunol. (2020) 140:103132. doi: 10.1016/j.jri.2020.103132
132. Lisse IM, Aaby P, Whittle H, Jensen H, Engelmann M, Christensen LB. T-lymphocyte subsets in West African children: impact of age, sex, and season. J Pediatr. (1997) 130:77–85. doi: 10.1016/S0022-3476(97)70313-5
133. Lü FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, et al. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. (2002) 128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x
134. Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. (2009) 10:509–16. doi: 10.1038/gene.2009.12
135. Purnamawati K, Ong JAH, Deshpande S, Tan WKY, Masurkar N, Low JK, et al. The importance of sex stratification in autoimmune disease biomarker research: a systematic review. Front Immunol. (2018) 9:1208. doi: 10.3389/fimmu.2018.01208
136. Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11bintermediate dendritic cells from bone marrow precursors. J Immunol. (2004) 172:1426–36. doi: 10.4049/jimmunol.172.3.1426
137. Butts CL, Shukair SA, Duncan KM, Bowers E, Horn C, Belyavskaya E, et al. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol. (2007) 19:287–96. doi: 10.1093/intimm/dxl145
138. Siracusa MC, Overstreet MG, Housseau F, Scott AL, Klein SL. 17β-Estradiol alters the activity of conventional and IFN-producing killer dendritic cells. J Immunol. (2008) 180:1423–31. doi: 10.4049/jimmunol.180.3.1423
139. Jones LA, Kreem S, Shweash M, Paul A, Alexander J, Roberts CW. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. J Immunol. (2010) 185:4525–34. doi: 10.4049/jimmunol.0901155
140. Corrales JJ, Almeida M, Cordero M, Martín-Martín L, Méndez C, Miralles JM, et al. Enhanced immunological response by dendritic cells in male hypogonadism. Eur J Clin Invest. (2012) 42:1205–12. doi: 10.1111/j.1365-2362.2012.02712.x
141. Hou J, Wu FZ. Effect of sex hormones on NK and ADDC activity of mice. Int J Immunopharmacol. (1988) 10:15–22. doi: 10.1016/0192-0561(88)90145-2
142. Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. (2001) 81:254–62. doi: 10.1006/gyno.2001.6153
143. Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C, et al. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol. (2008) 180:5746–53. doi: 10.4049/jimmunol.180.8.5746
144. Carlino C, Stabile H, Morrone S, Bulla R, Soriani A, Agostinis C, et al. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood. (2008) 111:3108–15. doi: 10.1182/blood-2007-08-105965
145. Murdock BJ, Famie JP, Piecuch CE, Raue KD, Mendelson FE, Pieroni CH, et al. NK cells associate with ALS in a sex- and age-dependent manner. JCI Insight. (2021) 6:e147129.
146. Spitzer JA. Gender differences in some host defense mechanisms. Lupus. (1999) 8:380–3. doi: 10.1177/096120339900800510
147. Molloy EJ, O’Neill AJ, Grantham JJ, Sheridan-Pereira M, Fitzpatrick JM, Webb DW, et al. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood. (2003) 102:2653–9. doi: 10.1182/blood-2003-02-0649
148. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Updat. (2005) 11:411–23. doi: 10.1093/humupd/dmi008
149. Gagliano-Jucá T, Pencina KM, Guo W, Li Z, Huang G, Basaria S, et al. Differential effects of testosterone on circulating neutrophils, monocytes, and platelets in men: findings from two trials. Andrology. (2020) 8:1324–31. doi: 10.1111/andr.12834
150. Jiang W, Zhang L, Lang R, Li Z, Gilkeson G. Sex differences in monocyte activation in Systemic Lupus Erythematosus (SLE). PLoS One. (2014) 9:e114589. doi: 10.1371/journal.pone.0114589
151. Agostino P, Milano S, Barbera C, Di Bella G, La Rosa M, Ferlazzo V, et al. Sex hormones modulate inflammatory mediators produced by macrophagesa. Ann N Y Acad Sci. (1999) 876:426–9. doi: 10.1111/j.1749-6632.1999.tb07667.x
152. Menzies FM, Henriquez FL, Alexander J, Roberts CW. Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology. (2011) 134:281–91. doi: 10.1111/j.1365-2567.2011.03488.x
153. Walter U, Santamaria P. CD8+ T cells in autoimmunity. Curr Opin Immunol. (2005) 17:624–31. doi: 10.1016/j.coi.2005.09.014
154. Collier JL, Weiss SA, Pauken KE, Sen DR, Sharpe AH. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat Immunol. (2021) 22:809–19. doi: 10.1038/s41590-021-00949-7
155. He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat Med. (2016) 22:991–3. doi: 10.1038/nm.4148
156. Gao X, Liu L, Min X, Jia S, Zhao M. Non-Coding RNAs in CD4+ T cells: new insights into the pathogenesis of systemic lupus erythematosus. Front Immunol. (2020) 11:568. doi: 10.3389/fimmu.2020.00568
157. Clarke SLN, Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol. (2016) 14:1–11. doi: 10.1186/s12969-016-0088-2
158. Walecki M, Eisel F, Klug J, Baal N, Paradowska-Dogan A, Wahle E, et al. Androgen receptor modulates Foxp3 expression in CD4 + CD25 + Foxp3 + regulatory T-cells. Mol Biol Cell. (2015) 26:2845–57. doi: 10.1091/mbc.E14-08-1323
159. Kim BJ, Kim YH, Lee S, Han JH, Lee SY, Seong J, et al. Otological aspects of NLRP3-related autoinflammatory disorder focusing on the responsiveness to anakinra. Rheumatology (Oxford). (2021) 60:1523–32. doi: 10.1093/rheumatology/keaa511
160. Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. (2015) 11:415–29. doi: 10.1038/nrrheum.2015.53
161. Psianou K, Panagoulias I, Papanastasiou AD, de Lastic AL, Rodi M, Spantidea PI, et al. Clinical and immunological parameters of Sjögren’s syndrome. Autoimmun Rev. (2018) 17:1053–64. doi: 10.1016/j.autrev.2018.05.005
162. Simone D, Al Mossawi MH, Bowness P. Progress in our understanding of the pathogenesis of ankylosing spondylitis. Rheumatology. (2018) 57(Suppl. 6):vi4–9. doi: 10.1093/rheumatology/key001
163. Islander U, Jochems C, Lagerquist MK, Forsblad-d’Elia H, Carlsten H. Estrogens in rheumatoid arthritis; the immune system and bone. Mol Cell Endocrinol. (2011) 335:14–29. doi: 10.1016/j.mce.2010.05.018
164. Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. (2016) 12:472–85. doi: 10.1038/nrrheum.2016.91
165. Li Y, Lee PY, Reeves WH. Monocyte and macrophage abnormalities in systemic lupus erythematosus. Arch Immunol Ther Exp (Warsz). (2010) 58:355–64. doi: 10.1007/s00005-010-0093-y
166. Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. (2006) 177:2088–96. doi: 10.4049/jimmunol.177.4.2088
167. Rönnblom L, Alm GV. Systemic lupus erythematosus and the type I interferon system. Arthritis Res Ther. (2003) 5:1–8. doi: 10.1186/ar625
168. Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol. (2016) 12:402–13. doi: 10.1038/nrneph.2016.71
169. Zitti B, Bryceson YT. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev. (2018) 42:37–46. doi: 10.1016/j.cytogfr.2018.08.001
170. Girón-González JA, Moral FJ, Elvira J, García-Gil D, Guerrero F, Gavilán I, et al. Consistent production of a higher T(H)1:T(H)2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol. (2000) 143:31–6. doi: 10.1530/eje.0.1430031
171. Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon-γ and systemic autoimmunity. Discov Med. (2013) 16:123.
172. Torcia MG, Nencioni L, Clemente AM, Civitelli L, Celestino I, Limongi D, et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS One. (2012) 7:e39853. doi: 10.1371/journal.pone.0039853
173. Sakkas LI, Daoussis D, Mavropoulos A, Liossis SN, Bogdanos DP. Regulatory B cells: new players in inflammatory and autoimmune rheumatic diseases. Semin Arthritis Rheum. (2019) 48:1133–41. doi: 10.1016/j.semarthrit.2018.10.007
174. Neuman H, Debelius JW, Knight R, Koren O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. (2015) 39:509–21. doi: 10.1093/femsre/fuu010
175. Rizzetto L, Fava F, Tuohy KM, Selmi C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J Autoimmun. (2018) 92:12–34. doi: 10.1016/j.jaut.2018.05.008
176. Konig MF. The microbiome in autoimmune rheumatic disease. Best Pract Res Clin Rheumatol. (2020) 34:101473. doi: 10.1016/j.berh.2019.101473
177. Gal-Oz ST, Maier B, Yoshida H, Seddu K, Elbaz N, Czysz C, et al. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun. (2019) 10:1–14. doi: 10.1038/s41467-019-12348-6
178. Muskardin TLW, Niewold TB. Type I interferon in rheumatic diseases. Nat Rev Rheumatol. (2018) 14:214. doi: 10.1038/nrrheum.2018.31
179. Dragin N, Bismuth J, Cizeron-Clairac G, Biferi MG, Berthault C, Serraf A, et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest. (2016) 126:1525–37. doi: 10.1172/JCI81894
180. Zhu ML, Bakhru P, Conley B, Nelson JS, Free M, Martin A, et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun. (2016) 7:11350. doi: 10.1038/ncomms11350
182. Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. (2019) 56:308–21.
183. Cutolo M, Straub RH. Sex steroids and autoimmune rheumatic diseases: state of the art. Nat Rev Rheumatol. (2020) 16:628–44. doi: 10.1038/s41584-020-0503-4
184. Robinson GA, Peng J, Peckham H, Radziszewska A, Butler G, Pineda-Torra I, et al. Sex hormones drive changes in lipoprotein metabolism. iScience. (2021) 24:103257. doi: 10.1016/j.isci.2021.103257
185. Redcay A, Bergquist K, Luquet W. On the basis of gender: a medical-legal review of barriers to healthcare for transgender and gender-expansive patients. Soc Work Public Health. (2021) 36:615–27. doi: 10.1080/19371918.2021.1942378
186. Radix A, Maingi S. LGBT cultural competence and interventions to help oncology nurses and other health care providers. Semin Oncol Nurs. (2018) 34:80–9. doi: 10.1016/j.soncn.2017.12.005
187. Greene DN, McPherson GW, Rongitsch J, Imborek KL, Schmidt RL, Humble RM, et al. Hematology reference intervals for transgender adults on stable hormone therapy. Clin Chim Acta. (2019) 492:84–90. doi: 10.1016/j.cca.2019.02.011
188. Chapman J. 2021 Michelson Prize Recipient Dr. Camila Consiglio Explores Differences Between the Sexes to Develop More Targeted Vaccines. Michelson Medical Research Foundation. (2021). Available online at: https://www.michelsonmedicalresearch.org/news/2021-michelson-prize-recipient-dr-camila-consiglio (accessed May 9, 2022).
189. Reardon S. The largest study involving transgender people is providing long-sought insights about their health. Nature. (2019) 568:446–9. doi: 10.1038/d41586-019-01237-z
190. Dekker MJHJ, Wierckx K, Van Caenegem E, Klaver M, Kreukels BP, Elaut E, et al. A European network for the investigation of gender incongruence: endocrine part. J Sex Med. (2016) 13:994–9. doi: 10.1016/j.jsxm.2016.03.371
191. GIRES. Terminology. (2022). Available online at: https://www.gires.org.uk/resources/terminology/ (accessed February 14, 2022).
Keywords: sex, gender, autoimmunity, sex hormones, sex chromosome, transgender
Citation: Peckham H, Webb K, Rosser EC, Butler G and Ciurtin C (2022) Gender-Diverse Inclusion in Immunological Research: Benefits to Science and Health. Front. Med. 9:909789. doi: 10.3389/fmed.2022.909789
Received: 31 March 2022; Accepted: 24 June 2022;
Published: 14 July 2022.
Edited by:
Chris Wincup, King’s College Hospital NHS Foundation Trust, United KingdomReviewed by:
Maurizio Cutolo, Università degli Studi di Genova, ItalyLore M. Dickey, Retired, Flagstaff, AZ, United States
Copyright © 2022 Peckham, Webb, Rosser, Butler and Ciurtin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hannah Peckham, aGFubmFoLnBlY2toYW0uMTRAdWNsLmFjLnVr
 Hannah Peckham
Hannah Peckham Kate Webb3,4
Kate Webb3,4 Elizabeth C. Rosser
Elizabeth C. Rosser Gary Butler
Gary Butler Coziana Ciurtin
Coziana Ciurtin