- 1Laboratory of Clinical Virology, WHO Regional Reference Laboratory for Poliomyelitis and Measles for the EMR, Institut Pasteur de Tunis, University of Tunis El Manar, Tunis, Tunisia
- 2LR20IPT10 Laboratory of Virus, Host and Vectors, Institut Pasteur de Tunis, University of Tunis El Manar, Tunis, Tunisia
- 3Department of Clinical Biology, Faculty of Pharmacy of Monastir, University of Monastir, Monastir, Tunisia
- 4Centre National de Veille Zoosanitaire, Tunis, Tunisia
- 5Laboratory of Biochemistry, Institut Pasteur de Tunis, University of Tunis El Manar, Tunis, Tunisia
- 6Infectious Diseases Departement, Rabta Hospital, Tunis, Tunisia
- 7Intensive Care Service, Emergence Medical Assistance Center, Tunis, Tunisia
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for COVID-19 disease which is known to have a broad clinical spectrum, from asymptomatic to critical presentation leading to death. Many researchers have investigated the factors impacting the course of the disease. Our previous in silico study suggested a possible protective effect of Hepatitis B, Tetanus and Measles vaccines against COVID-19. In continuity, we conducted a cross-sectional clinical study in order to confirm our in silico assumptions regarding the HBs-Ag antibodies.
Methods: A representative sex- and age-matched sample of patients with confirmed COVID-19 was selected (n = 340). All clinical presentations were equally represented. Using an ELISA test, each patient benefited of a serology for the detection and measurement of the anti-HBs specific IgG antibodies. The obtained results allowed determining the different correlations between these antibody titers and the disease severity. The R® software and the MedCalc® software served to calculate the Spearman's coefficient of rank correlation (rho) for the obtained titers per severity group as well as the different other calculations and figure representations.
Results: A significant positive correlation was found with the anti-HBs titers (rho = 0.107; p = 0.04). High anti-HBs titers were significantly associated with the mild presentation of COVID-19. A significant difference was found between the obtained titers per severity class (chi-2 test, p = 0.03).
Discussion/Conclusion: Our findings demonstrated that anti-HBs titers were significantly higher for patients having mild COVID-19 presentations. We presume that being immunized against the HB may play a protective role in the course of the disease. Our study provided more key elements in understanding the disparity of the clinical spectrum among regions.
Introduction
The emergence and the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for COVID-19, have caused global public health, economic, and social crises (1, 2). While most cases of COVID-19 are mild to moderate, a significant fraction may develop severe to critical clinical presentations leading to death (3). As of 29 March 2022, 6,127,981 COVID-19-related deaths, worldwide, have been reported to the WHO (4). An uneven mortality distribution across the WHO regions is noteworthy. This unequal distribution is even deeper between countries throughout the regions as they are reporting very different case fatality ratios (5). As an example, within the region of the Americas, Venezuela reported only 19 deaths per 100,000 inhabitants, whereas the rates reported by Peru exceeded 600 per 100,000 inhabitants (4). Thomas et al. (6) have gone even further by examining 19 different cities in the United States and concluded to non-uniform COVID-19 severity within the same city level. Presently, almost 2 years after the virus emergence, we are still learning about the risk factors of severe COVID-19 outcomes (7). Demographic risk factors such as advanced age as well as underlying medical conditions have evident impact on the COVID-19 severity (8). In addition, many other assumptions have been made in order to explain these substantial striking disparities such as human leukocyte antigen (HLA) polymorphism, race and ethnicity, environmental conditions, nutrition, and microbiome (9–12). The most plausible hypotheses were those involving the immune response mechanisms (13, 14), and it was suggested that some vaccines could enhance the innate immune response (15, 16). Indeed, even before the emergence of the COVID-19 pandemic, it was described that the large-scale use of the BCG vaccine (Bacillus Calmette-Guérin) has beneficial nonspecific effects on the immune system by protecting against other infectious diseases and reducing the non-tuberculosis child mortality (17). Recently, Curtis et al. (18) suggested that it might be involved in the protection against COVID-19 as it could reduce viraemia and thus enhance rapid recovery and less severity. Anbarasu et al. (19) also suggested that the extensive pediatric vaccination with MMR vaccines (Measles, Mumps, and Rubella vaccines) has induced interferon (IFN) secretion and activated natural killer cells, offering, thereby, natural immunity against SARS-CoV-2 in the young population.
In a previous paper, we investigated the putative protective role against COVID-19 of 12 widely used vaccines, including live attenuated (BCG, Oral Polio Vaccine, MMR vaccines) and inactivated ones [tetanus, Corynebacterium diphtheriae, Bordetella pertussis, Hepatitis B (HB), Hepatitis A, Haemophilus influenzae type B (Hib), and Streptococcus pneumoniae vaccines (PCV10)] (20). A total of 30 antigenic proteins were investigated. Using a package of the in silico analysis tools, we performed amino acid sequence alignments and hot spot analysis. Among the investigated antigenic proteins, 14 proteins presented similar amino acid patterns in eight different vaccines. Structural and antigenicity tests (B-cell and T-cell epitope predictions) identified three segments in Hepatitis B, Measles and Tetanus proteins presented antigenic properties that can induce putative protective effect against SARS-CoV2 (Figure 1).
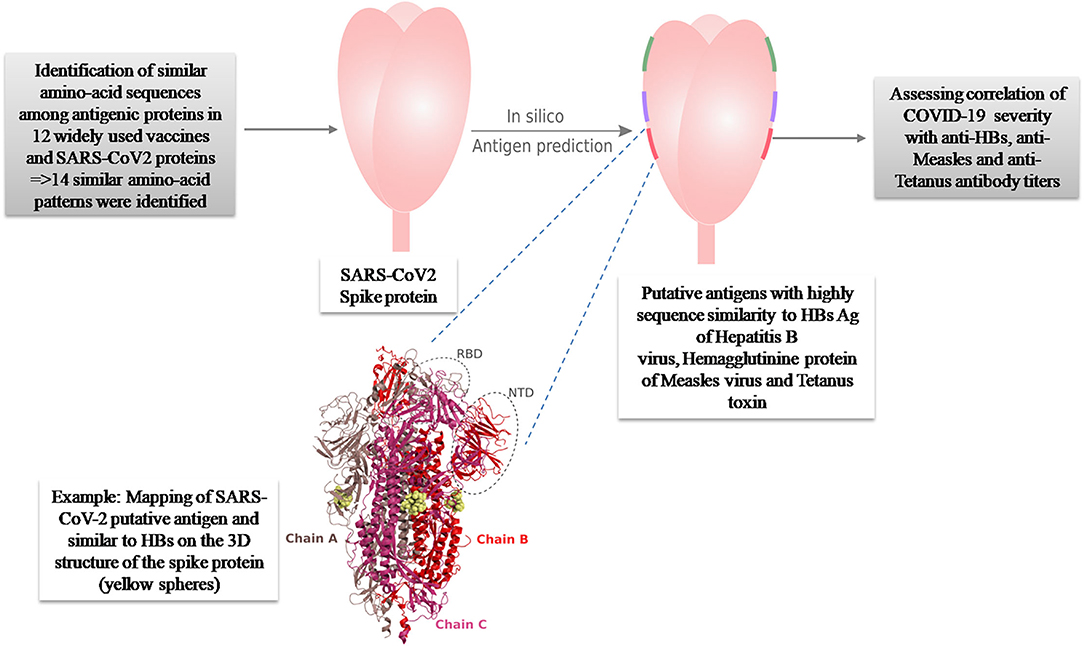
Figure 1. Summary of the original in silico hypothesis suggesting an antigenic potential of three amino-acid patterns present in HBs Ag of Hepatitis B, Hemagglutinine protein of Measles virus and Tetanus toxin, against SARS-CoV-2 (20).
Hepatitis B is a vaccine-preventable disease; however, it is the world's most common liver infection. The WHO estimates that 296 million people were living with chronic HB infection in 2019, with 1.5 million new infections each year (21). National and international efforts are being made in order to improve the vaccine coverage through systematic childhood and health workers vaccination. We were wondering whether this unequal immunization between countries may justify the COVID-19 severity variability. Within this vision, and in continuity to the previous in silico study (20), we investigate in this work the putative protective role of the anti-HBs-Ag specific IgG of the HBV against SARS-CoV-2 using real samples from patients who recovered from COVID-19. Our aim was to identify the statistical correlation between the corresponding antibody titers and the disease severity among a large sample size.
Methods
Ethics Statement
This study was performed under ethical standards according to the 1964 Declaration of Helsinki and its later amendments. The samples were collected in the context of COVID-19 diagnostic activities. They were used in this study after de-identification with respect to patient anonymity and after the approval of the Bio-Medical Ethics Committee of Pasteur Institute of Tunis, Tunisia (2020/14/I/LR16IPT/V1).
Study Population
This cross-sectional study was conducted between May and June, 2021, in the Laboratory of Clinical Virology (LCV) of Pasteur Institute of Tunis. The included sera were randomly selected from the LCV bio-bank; they were collected during the pandemic from patients with different clinical presentations and then carefully stored in a −40°C freezer in accordance with the BAOBAB® LIMS storing application (22). A total of 340 patients with COVID-19 infection were enrolled, matched for age and sex, and classified into “asymptomatic,” “mild,” “moderate,” and “severe” according to the United Stated National Institutes of Health (NIH) definitions update of 19 October 2021 (3). Each group contained 85 patients. All selected patients were not vaccinated against SARS-CoV-2 and the infection with COVID-19 was confirmed either by real-time reverse transcription PCR (RT-qPCR) assessed on nasopharyngeal swab by a WHO-approved in-house protocol (the Hong Kong University, China, RT-qPCR protocol) (23) or by SARS-CoV-2-specific antibody seroconversion detected by the commercial test Elecsys® Roche® total anti-nucleoprotein antibodies.
Clinical Data Collection
The socio-demographic data of patients and information on clinical features, co-morbidities, and exposure or contact history with COVID-19 patients were collected.
Detection and Measurement of the Anti-HBs
Detection and measurement of the anti-HBs IgG-specific antibodies were carried out by the commercial in vitro diagnostics (IVD)-validated immunoassay: an anti-HBs enzyme immunoassay kit, ETI-AB-AUK-3, manufactured by the DiaSorin® S.p.A., Italy (REF: P001603). This assay is based on a direct and non-competitive sandwich ELISA. It enables detection of anti-HBs IgG-specific antibodies using wells coated with heat-treated human HBs-Ag. The measurement of the anti-HBs specific antibodies depends on the use of standardized calibrators that were referenced against WHO anti-Hepatitis B Immunoglobulin 1st International Reference Preparation, 1977. This kit is recommended for measuring the anti-HBs titers whether acquired as a result of natural HBV infection or after vaccination. The sensitivity and specificity of this kit are 99.11% CI 95% (98.18–99.64%) and 98.21% CI 95% (97.07–99.00%), respectively, following the manufacturer's instructions. The testing procedures and result interpretation were conducted according to the kit instructions: a titer lower than 10 mIU/mL indicates that the patient is not immunized against the HBV; a titer higher than 10 mIU/mL is correlated to an efficient immunity against the virus; however, a titer more than 100 mIU/mL is recommended for the vulnerable populations such as the health care workers. This classification also aligns with the WHO recommendations (24).
Statistical Analysis
To explore the relationship between anti-HBs specific antibody titers and the severity of COVID-19 cases, the sample size was first calculated with 80% power of the test, an alpha value set to 0.05, and a correlation value of 0.3 using Epitools® (25). The sample size was calculated using the Sample Size Calculators website (26). The Spearman's rank correlation coefficient was used to determine the correlation between anti-HBs specific antibody levels and the SARS-CoV-2 severity of cases. Continuous data were presented in median and ranges and categorical data were presented in numbers and percentages. All statistical analyses were performed using the MedCalc® Software (version 18.2.1) and the R® software (version 3.4). A p-value of <0.05 was considered statistically significant.
Results
Demographic Features of the Tested Population
This study included 340 patients that were stratified according to COVID-19 disease severity into four groups: “asymptomatic,” “mild,” “moderate,” and “severe.” The required sample size was 85 patients in each group. Among the 340 patients, 160 were female (47%) and 180 (53%) were male. The sex ratio male/female (M/F) was estimated at 1.1. The mean age for all groups was 54 years (1–94 range; Figure 2).
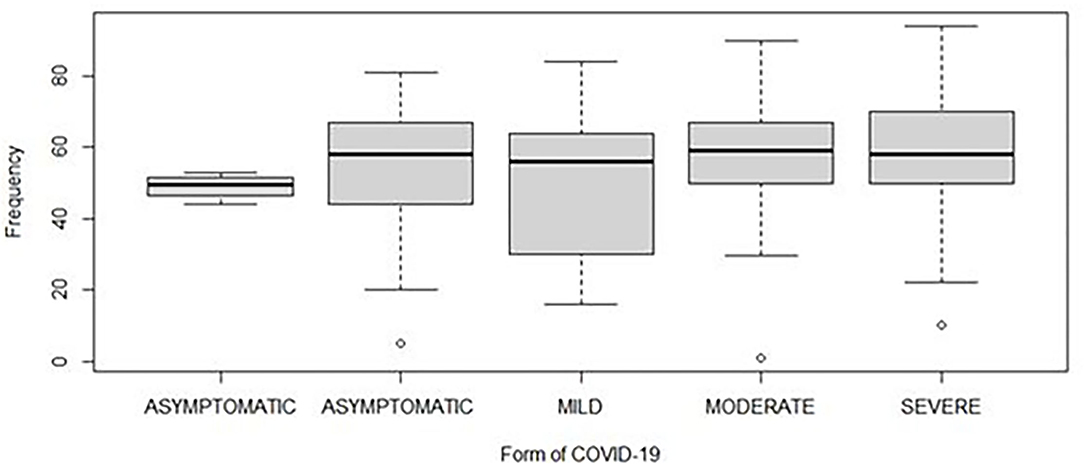
Figure 2. Distribution of the age of patients according to different severities of COVID-19. The horizontal line inside each box represents the median value of the age with interquartile ranges. The dots represent patients.
Serological Results
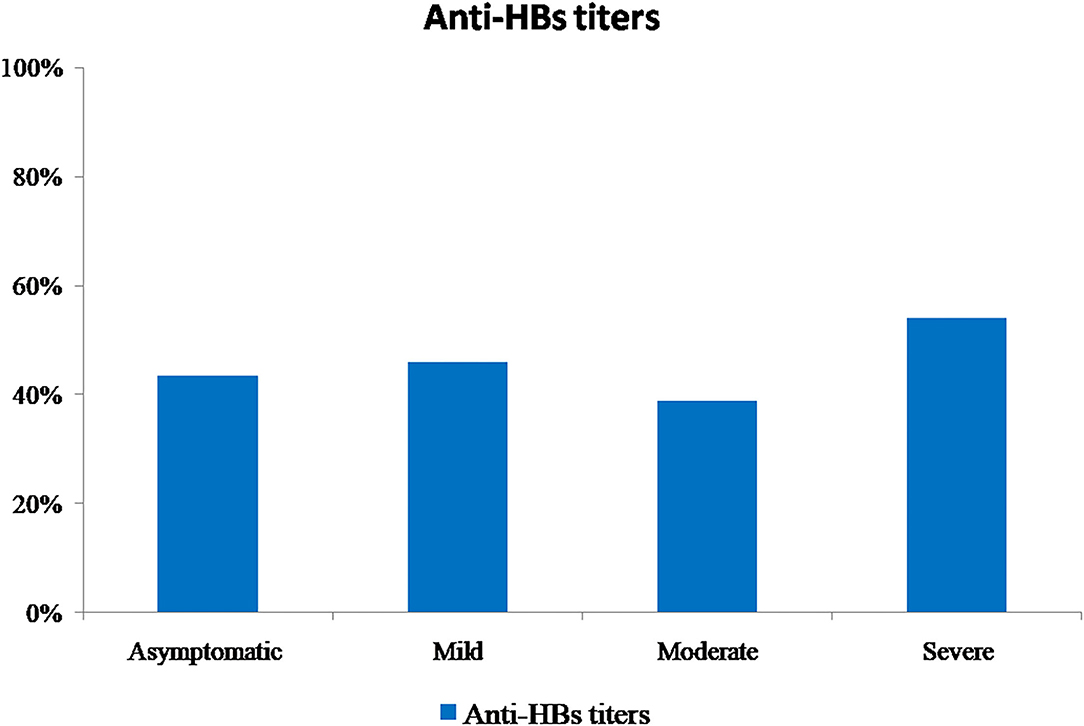
All sera were tested for the presence of anti-HBs antibodies. In total, 54.4% (n = 185) of patients did not have detectable anti-HBs antibodies. For the remaining proportion of patients who had positive anti-HBs antibodies (n = 155, 45.6%), it was distributed according to the COVID-19 disease severity group as shown in Figure 3. Regarding the antibody measurement, the titers ranged from 14 to 2,390 mIU/mL, with a median of 100 mIU/mL. For the “asymptomatic” class, titers ranged from 15 to 2,380 mIU/mL; for the “mild” class, titers ranged from 15 to 2,390 mIU/mL; for the “moderate” class, titers ranged from 15 to 2,220 mIU/mL; and for the “severe” class, titers ranged from 14 to 2,265 mIU/mL. A significant difference was found between the obtained titers per severity class (chi-2 test, p = 0.03). In addition, a significant reverse correlation was found between the patients' ages and the obtained anti-HBs antibody titers (rho = −0.176; p < 0.05); this association demonstrated that higher anti-HBs titers were detected in children and the adult patients than that in older age.
Correlation Analysis
Taking into consideration the different obtained titers, the correlation with the COVID-19 severity classes was performed. A significant positive correlation was found between the anti-HBs titers and the COVID-19 severity classes (rho = 0.107; p = 0.04; Figure 4). High anti-HBs titers were significantly associated with mild presentation of COVID-19. In contrast, patients with severe clinical presentations had lower antibody titers.
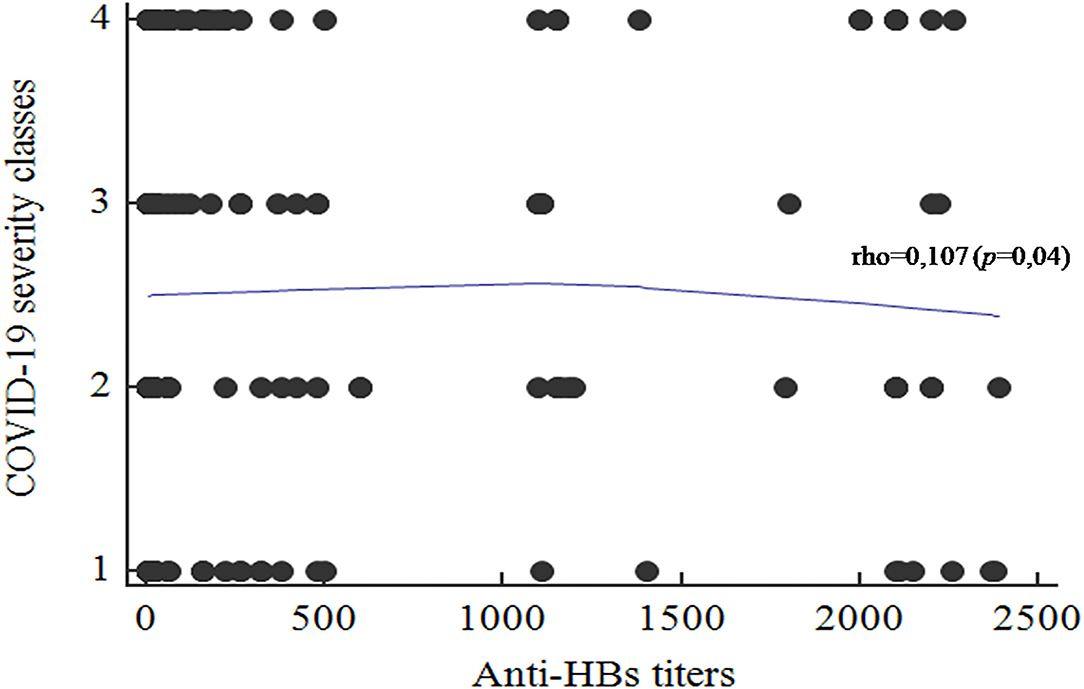
Figure 4. Correlation between the anti-HBs titers and the COVID-19 disease severity classes (1, Asymptomatic; 2, Mild; 3, Moderate; 4, Severe.
Discussion
In this article, we investigated the potential role of a routinely used vaccine against HBV in preventing severe clinical presentations of the COVID-19 disease. Our aim was to confirm our previous in silico findings. Indeed, we identified similar amino acid pattern in HBs-Ag protein of HBV and SARS-CoV-2 with antigenic properties (20) (Figure 1). We have attempted to identify whether there is a correlation between HBV antibody titers and the severity of SARS-CoV-2 infection, using sera of patients who recovered from COVID-19.
Several studies have previously suggested a potential cross protection of post-vaccine antibodies toward severe to critical outcomes of the SARS-CoV-2 infection (17–19, 27, 28) and, as the experimental identification of the incriminated epitopes is challenging, many studies had recourse to in silico approach. Indeed, bioinformatic and computational biology provides a valuable contribution in the current COVID-19 emergency context, thanks to its rapidity and low cost in comparison to wet-laboratory and clinical investigations (29). However, in vitro investigations remain a more solid way to support conclusions. Thus, a total of 340 patients with confirmed SARS-CoV-2 infection were enrolled in this study, matched for age and sex, and classified into different clinical presentations (n = 85/group) according to the National Institute of Health definitions (3). To our knowledge, our study investigated the largest population for the determination of vaccine correlation as compared to previous ones and reported, for the first time, that the presence of high anti-HBs titers in patients' sera may be associated with a significant protective role against the COVID-19 disease. Gold et al. investigated only a total of 80 patients with COVID-19 for the relationship with MMR vaccine (30). Al Balakosy et al. also determined the anti-Measles IgG antibodies on a total of only 78 patients with COVID-19 (31).
The active substance in the HB vaccine is the viral surface protein HBs-Ag, obtained by the yeast-derived recombinant vaccine biotechnology, in most commercially available vaccines (32). The immunogenic fraction is the amino acid hydrophilic region, referred as the common a determinant present in the HBs-Ag (subtype HBs-Ag-adw2 or HBs-Ag-adr) (32, 33). These two antigenic epitopes were investigated in our previous in silico study and we showed that the amino-acid sequence PGTNTSN in the Spike protein of SARS-CoV-2 (position 600–606) matches with the predicted epitope TNTSN in the HBs-Ag-adr (20). The pattern PGTNTSN corresponded to an exposed site in the S protein with a high accessible surface area value and presented probing spheres mimicking the CDR antibodies, that was in line with a potential implication in the B-cell mediated response. Furthermore, it was also described by Tajiri et al. (34) that two regions of HBsAg (residues 104–123 and 108–123), containing the epitope matching the PGTNTSN segment of SARS-CoV-2, were able to bind with two human monoclonal antibodies. This highlighted the immunogenic ability of these segments. Indeed, high-antibody titers were found among the studied population within the mildly infected group, with a significant positive correlation (rho = 0.107; p = 0.04) which is in concordance with our previous in silico findings (20). Therefore, we conclude that epitopes in regions of HBsAg (residues 104–123 and 108–123) matching the PGTNTSN segment of RBD may induce a cross protection against SARS-CoV-2. In vivo, this might lead either to a direct inhibition of the early cell-entry phases or to an indirect non-specific steric clutter. Nevertheless, only experiments such as sero-neutralization assay may support such findings.
Relying on clinical observations, Chen et al. (35) and Wu et al. (36) reported that SARS-CoV-2 infection in patients infected with HBV could facilitate the development of liver injury which is associated with adverse outcomes. Accordingly, it can also be suggested that HBV vaccination may also indirectly protect patients from these adverse outcomes (37). Furthermore, the possible protective role of HBV vaccine against other diseases, such as lymphoma, was reported by two previous studies (38, 39). Presently, the safe and efficient HB vaccination is highly recommended for all children worldwide. However, the WHO reports widely variable coverage rates from country to country, depending on the respective national strategies (24). From another point of view, it is well known, today, that after an initial vaccination, anti-HBs antibody titers may decline over years (40), making elderly less immunized than the other age groups. So, taking into consideration that the severe COVID-19 presentations are more frequent among elderly (8), we attempt to speculate that the loss of anti-HBs antibodies may be in line with our hypothesis. In our study, we performed a serum measurement of the anti-HBs antibodies regardless of the vaccination history because only the presence of anti-HBs at a protective level may indicate an effective protection whatever it was obtained through vaccination or natural infection. Furthermore, the non-response rates to the HBV vaccine range from 5 to 10% in total population and can reach 40% among patients with diabetes and kidney failure (40). For this study, the patients came from Tunisia which is a low-endemic country as result to the introduction of vaccination since 1995. Indeed, on the basis of the most recent national household-based cross sectional and serological survey in 2015, the national point prevalence of HBs-Ag was 1.7% [95% CI (1.6–1.9%)] and the vaccine effectiveness was 88.6% [95% CI (81.5–93.0%)] (41).
Epitopic similarity between different virus' antigens is a well-described phenomenon that has various implications on pathogenicity comprehension, diagnosis methods, and even treatment opportunities. It has been demonstrated during the emergence of Zika virus, for example, that there is a strong structural similarity with the matrix and the envelope antigens of dengue and West Nile viruses, i.e., within the same family of Flaviviridae (41). It has been reported, recently, that these antigenic cross-reactivities have impeded the IgM-specific antibody serology assays (42). Furthermore, beyond the viruses, it was demonstrated that there is a molecular mimicry between self-human and viral antigens, which might trigger autoimmune diseases in genetically predisposed individuals (43). For instances, viruses such as Coxsackievirus, Mumps virus, Rubella virus, and Hepatitis C virus were incriminated in inducing the type 2 diabetes, again by exhibiting molecular mimicry with the host proteins (44). For the SARS-CoV-2, the previously reported epitopic similarity (20) and the current findings may suggest a possible cross protection. Nevertheless, it should be supported by larger statistic investigation and further lab experiments such as sero-neutralization assays. Our findings may be unique and encourage other studies targeting previously used antiviral to be tested against SARS-CoV-2 infection, especially those used against HBV chronic infection. Indeed, García-Trejo et al. published an argument repurposing the Lamivudine (nucleotide/nucleoside analogs); they provided in silico docking evidence suggesting that Lamivudine may bind and possibly work as an inhibitor of the SARS-CoV-2 RdRp RNA polymerase (45). The telbivudine also was suggested as a fighting drug for COVID-19 (46). These data underline the possible similarity with gene sequences between HBV and SARS-CoV-2.
Conclusion
In the COVID-19 crisis context, clinical research is escalating and providing mounting evidence that immunity background plays a crucial role in deciding the course of the disease. Our findings have placed emphasis on HBV, linking anti-HBs high sero-positivity to the COVID-19 minor severity, as its antigenic properties were consistent with its putative neutralizing capacity. Although findings were significant, larger population investigation may further support the obtained correlation. Also, it will be interesting to investigate the sero-neutralization effects of anti-HBs antibodies using different SARS-CoV-2 variants of interest.
The observed associations between anti-HBs antibody titers and the COVID-19 disease course may explain the geographical disparity worldwide of the COVID-19 severity, along with all the suggested risk factors. We believe that it is still important to dig into the protective and risk factors that have led to the large number of deaths inherent to COVID-19 especially in the context of SARS-CoV-2 variant emergence.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Bio-Medical Ethics Committee of Pasteur Institute of Tunis, Tunisia. Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin.
Author Contributions
MG, SH-B, and SK: conceptualization, methodology, and original draft preparation. RT, RS, and NH: investigation. RT, SK, and NF: data curation. MM, LA, NB, and AJ: sample and data collection. MG, SH-B, SK, and HT: writing—review and editing, supervision, and project administration. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Tunisian Ministry of Higher Education and Scientific Research (LR20IPT10).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully acknowledge Houcemeddine Othman from Sydney Brenner Institute for Molecular Bioscience, University of the Witwatersrand, Johannesburg, South Africa, for his valuable scientific contribution in this work, Ali Bouattour for logistical support, Ezzeddine Nouiri and Nejla Mekki for technical advises, Ali Neili and Wafa Chamsa for the design of the bio-bank by the BAOBAB® LIMS, and Habib Halouani and Donia Sahli for the database entry.
References
1. Pollard CA, Morran MP, Nestor-Kalinoski AL. The COVID-19 pandemic: a global health crisis. Physiol Genomics. (2020) 52:549–57. doi: 10.1152/physiolgenomics.00089.2020
2. Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. (2020) 78:185–93. doi: 10.1016/j.ijsu.2020.04.018
3. Clinical Spectrum. COVID-19 Treatment Guidelines. Available online at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed Mars 3, 2022).
4. WHO Coronavirus (COVID-19) Dashboard. Available online at: https://covid19.who.int (accessed Mars 3, 2022).
5. Mortality Analyses. Johns Hopkins Coronavirus Resource Center. Available online at: https://coronavirus.jhu.edu/data/mortality (accessed Févr 26, 2022).
6. Thomas LJ, Huang P, Yin F, Luo XI, Almquist ZW, Hipp JR, et al. Spatial heterogeneity can lead to substantial local variations in COVID-19 timing and severity. Proc Natl Acad Sci USA. (2020) 117:24180–7. doi: 10.1073/pnas.2011656117
7. CDC. Healthcare Workers. Centers for Disease Control and Prevention. (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed mars 3, 2022).
8. Rossen LM. Excess deaths associated with COVID-19, by age and race and ethnicity — United States, January 26–October 3, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1522–7. doi: 10.15585/mmwr.mm6942e2
9. Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S, et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths : a systematic review. Ann Intern Med. (2021) 174:362–73. doi: 10.7326/M20-6306
10. Sakuraba A, Haider H, Sato T. Population difference in allele frequency of HLA-C*05 and its correlation with COVID-19 mortality. Viruses. (2020) 12:E1333. doi: 10.3390/v12111333
11. Ganslmeier M, Furceri D, Ostry JD. The impact of weather on COVID-19 pandemic. Sci Rep. (2021) 11:22027. doi: 10.1038/s41598-021-01189-3
12. Kumar P, Chander B. COVID 19 mortality: probable role of microbiome to explain disparity. Med Hypotheses. (2020) 144:110209. doi: 10.1016/j.mehy.2020.110209
13. Melenotte C, Silvin A, Goubet AG, Lahmar I, Dubuisson A, Zumla A, et al. Immune responses during COVID-19 infection. Oncoimmunology. (2020) 9:1807836. doi: 10.1080/2162402X.2020.1807836
14. Esenboga S, Ocak M, Akarsu A, Bildik HN, Cagdas D, Iskit AT, et al. COVID-19 in patients with primary immunodeficiency. J Clin Immunol. (2021) 41:1515–22. doi: 10.1007/s10875-021-01065-9
15. Mina MJ. Measles, immune suppression and vaccination: direct and indirect nonspecific vaccine benefits. J Infect. (2017) 74(Suppl 1):S10–7. doi: 10.1016/S0163-4453(17)30185-8
16. Fidel PL, Noverr MC. Could an unrelated live attenuated vaccine serve as a preventive measure to dampen septic inflammation associated with COVID-19 infection? MBio. (2020) 11:e00907–20. doi: 10.1128/mBio.00907-20
17. Higgins JPT, Soares-Weiser K, López-López JA, Kakourou A, Chaplin K, Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. (2016) 355:i5170. doi: 10.1136/bmj.i5170
18. Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. (2020) 395:1545–6. doi: 10.1016/S0140-6736(20)31025-4
19. Anbarasu A, Ramaiah S, Livingstone P. Vaccine repurposing approach for preventing COVID 19: can MMR vaccines reduce morbidity and mortality? Hum Vaccin Immunother. 16:2217–8. doi: 10.1080/21645515.2020.1773141
20. Haddad-Boubaker S, Othman H, Touati R, Ayouni K, Lakhal M, Ben Mustapha I, et al. In silico comparative study of SARS-CoV-2 proteins and antigenic proteins in BCG, OPV, MMR and other vaccines: evidence of a possible putative protective effect. BMC Bioinformatics. (2021) 22:163. doi: 10.1186/s12859-021-04045-3
21. Hepatitis B. Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed Mai 13, 2022).
22. Bendou H, Sizani L, Reid T, Swanepoel C, Ademuyiwa T, Merino-Martinez R, et al. Baobab laboratory information management system: development of an open-source laboratory information management system for biobanking. Biopreserv Biobank. (2017) 15:116–20. doi: 10.1089/bio.2017.0014
23. WHO IN HOUSE ASSAYS. Available online at: https://www.who.int/docs/default-source/coronaviruse/whoinhouseassays.pdf (accessed Févr 23, 2022).
24. Guidelines_development_recommendations.pdf. Available online at: https://www.who.int/immunization/sage/Guidelines_development_recommendations.pdf?ua=1 (accessed Mars 3, 2022).
25. Epitools - Home. Available online at: https://epitools.ausvet.com.au/ (accessed Mars 7, 2022).
26. Kohn JS Michael. Correlation sample size | Sample Size Calculators. Available online at: https://sample-size.net/correlation-sample-size/ (accessed Mars 7, 2022).
27. Young A, Neumann B, Mendez RF, Reyahi A, Joannides A, Modis Y, et al. Homologous protein domains in SARS-CoV-2 and measles, mumps and rubella viruses: preliminary evidence that MMR vaccine might provide protection against COVID-19. medRxiv. [Preprint]. Infect Dis. (2020). doi: 10.1101/2020.04.10.20053207
28. Marakasova E, Baranova A MMR. Vaccine and COVID-19: Measles protein homology may contribute to cross-reactivity or to complement activation protection. MBio. (2021) 12:e03447–20. doi: 10.1128/mBio.03447-20
29. Basu S, Ramaiah S, Anbarasu A. In-silico strategies to combat COVID-19: a comprehensive review. Biotechnol Genet Eng Rev. (2021) 37:64–81. doi: 10.1080/02648725.2021.1966920
30. Gold JE, Baumgartl WH, Okyay RA, Licht WE, FidelJr PL, Noverr MC, et al. Analysis of Measles-Mumps-Rubella (MMR) titers of recovered COVID-19 patients. mBio. (2020) 11:e02628–20. doi: 10.1128/mBio.02628-20
31. Al Balakosy A, Alfishawy M, Elnabawy O, Hassan A, Shamkh M, Mahmoud M, et al. Measles IgG antibodies: is there a protective role in COVID 19 pandemic?? ? Afro-Egypt J Infect Endem Dis. (2021) 11:306–13. doi: 10.21608/aeji.2021.72615.1144
32. Heijtink RA, Bergen P, Melber K, Janowicz ZA, Osterhaus AD. Hepatitis B surface antigen (HBsAg) derived from yeast cells (Hansenula polymorpha) used to establish an influence of antigenic subtype (adw2, adr, ayw3) in measuring the immune response after vaccination. Vaccine. (2002) 20:2191–6. doi: 10.1016/S0264-410X(02)00145-7
33. Romanò L, Paladini S, Galli C, Raimondo G, Pollicino T, Zanetti AR. Hepatitis B vaccination. Hum Vaccin Immunother. (2015) 11:53–7. doi: 10.4161/hv.34306
34. Tajiri K, Ozawa T, Jin A, Tokimitsu Y, Minemura M, Kishi H, et al. Analysis of the epitope and neutralizing capacity of human monoclonal antibodies induced by hepatitis B vaccine. Antiviral Res. (2010) 87:40–9. doi: 10.1016/j.antiviral.2010.04.006
35. Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, et al. Clinical characteristics of hospitalized patients with SARS-CoV-2 and hepatitis B virus co-infection. Virol Sin. (2020) 35:842–5. doi: 10.1007/s12250-020-00276-5
36. Wu J, Yu J, Shi X, Li W, Song S, Zhao L, et al. epidemiological and clinical characteristics of 70 cases of coronavirus disease and concomitant hepatitis B virus infection: a multicentre descriptive study. J Viral Hepatitis. (2021) 28:80–8. doi: 10.1111/jvh.13404
37. Xiang TD, Zheng X. Interaction between hepatitis B virus and SARS-CoV-2 infections. World J Gastroenterol. (2021) 27:782–93. doi: 10.3748/wjg.v27.i9.782
38. Lia L, Grima D, Amici F, Manzi L, Monaci A, Torre GL. The possible protective effect of hepatitis B vaccine against lymphomas: a systematic review. Curr Pharm Biotechnol. (2022). doi: 10.2174/1389201023666220113111946. [Epub ahead of print].
39. Marcucci F, Spada E, Mele A, Caserta CA, Pulsoni A. The association of hepatitis B virus infection with B-cell non-Hodgkin lymphoma - a review. Am J Blood Res. (2012) 2:18–28.
40. Phattraprayoon N, Kakheaw J, Soonklang K, Cheirsilpa K, Ungtrakul T, Auewarakul C, et al. Duration of hepatitis B vaccine-induced protection among medical students and healthcare workers following primary vaccination in infancy and rate of immunity decline. Vaccines. (2022) 10:267. doi: 10.3390/vaccines10020267
41. Ben Hadj M, Bouguerra H, Saffar F, Chelly S, Hechaichi A, Talmoudi K, et al. Observational study of vaccine effectiveness 20 years after the introduction of universal hepatitis B vaccination in Tunisia. Vaccine. (2018) 36:5858–64. doi: 10.1016/j.vaccine.2018.08.038
42. Stiasny K, Malafa S, Aberle SW, Medits I, Tsouchnikas G, Aberle JH, et al. Different cross-reactivities of IgM responses in dengue, zika and tick-borne encephalitis virus infections. Viruses. (2021) 13:596. doi: 10.3390/v13040596
43. Sousa M de AC, Parana R, Andrade LJ de O. SEQUENCE SIMILARITY BETWEEN THYROID SELF-PROTEIN AND HEPATITIS C VIRUS POLYPROTEIN: possible triggering mechanism of autoimmune thyroiditis. Arq Gastroenterol. (2016) 53:185–91. doi: 10.1590/S0004-28032016000300012
44. Antonelli A, Ferrari SM, Di Domenicantonio A, Ferrannini E, Fallahi P. Viral Infections and Type 1 Diabetes. In: Infection and Autoimmunity [Internet]. Elsevier (2015). p. 877–89. Available online at: https://linkinghub.elsevier.com/retrieve/pii/B9780444632692000477
45. Garcia-Trejo JJ, Ortega R, Zarco-Zavala M. Putative repurposing of lamivudine, a nucleoside/nucleotide analogue and antiretroviral to improve the outcome of cancer and COVID-19 patients. Front Oncol. (2021) 11:664794. doi: 10.3389/fonc.2021.664794
Keywords: COVID-19, correlation, antibody titer, Hepatitis B, SARS-CoV-2
Citation: Gdoura M, Touati R, Kalthoum S, Ben Slama R, Fatnassi N, Mrad M, Ammari L, Brahmi N, Ben Jazia A, Hogga N, Triki H and Haddad-Boubaker S (2022) Presumed Protective Role for Anti-Hepatitis B Virus Antibodies Against COVID-19 Severe Cases: A Clinical Study Confirming in silico Hypothesis. Front. Med. 9:909660. doi: 10.3389/fmed.2022.909660
Received: 31 March 2022; Accepted: 13 June 2022;
Published: 08 July 2022.
Edited by:
Tarek A. Ahmad, Bibliotheca Alexandrina, EgyptReviewed by:
Ahmed Yaqinuddin, Alfaisal University, Saudi ArabiaMohammad Uzzal Hossain, National Institute of Biotechnology (NIB), Bangladesh
Waleed Mahallawi, Taibah University, Saudi Arabia
Copyright © 2022 Gdoura, Touati, Kalthoum, Ben Slama, Fatnassi, Mrad, Ammari, Brahmi, Ben Jazia, Hogga, Triki and Haddad-Boubaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariem Gdoura, bWFyaWVtZ2RvdXJhQGdtYWlsLmNvbQ==; Sondes Haddad-Boubaker, c29uZGVzaGFkZGFkYm91YmFrZXJAZ21haWwuY29t
 Mariem Gdoura
Mariem Gdoura Raoua Touati1,2
Raoua Touati1,2 Sana Kalthoum
Sana Kalthoum Nozha Brahmi
Nozha Brahmi Amira Ben Jazia
Amira Ben Jazia Sondes Haddad-Boubaker
Sondes Haddad-Boubaker