
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 07 July 2022
Sec. Pulmonary Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.906364
This article is part of the Research Topic The Effect of Anti-Cancer Drug Therapies in the Treatment of Lung Cancer View all 29 articles
Epidermal growth factor receptor (EGFR)-activating mutations are major oncogenic mechanisms in non-small cell lung cancer (NSCLC). Most patients with NSCLC with EGFR mutations benefit from targeted therapy with EGFR- tyrosine kinase inhibitors (TKIs). One of the main limitations of targeted therapy is that the tumor response is not durable, with the inevitable development of drug resistance. Previous studies demonstrated that the potential resistance mechanisms are diverse, including the presence of EGFR T790M, MET amplification, mesenchymal transformation, and anaplastic lymphoma kinase (ALK) rearrangement. The patient in our report was diagnosed with stage IA lung adenocarcinoma harboring the EGFR L858R mutation and underwent radical surgery. The patient received icotinib for 12 months after recurrence. Subsequent molecular analysis of the left pleural effusion indicated that LCLAT1-ALK fusion might be an underlying mechanism contributing to the acquired resistance to icotinib. Ensartinib was prescribed, but the lesion in the right lung continued to progress. Hence, a re-biopsy and molecular analysis of lesions in the right lung was performed to solve this problem. In contrast to the left pleural effusion, EGFR exon 20 T790M might have mediated the acquired resistance in lesions in the right lung of this patient. The combination of osimertinib and ensartinib has achieved a rapid partial response until now. The complexity and heterogeneity in our case may provide new insights into the resistance mechanisms of targeted therapy.
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related death worldwide (1). Historically, systemic cytotoxic chemotherapy has been the predominant treatment for advanced-stage lung cancer (2). However, lung cancer is a heterogeneous disease requiring personalized treatment. The development of molecular detection technologies has allowed the identification of multiple and potentially targetable oncogene drivers and personalized targeted therapies for lung cancer (3). Some well-established targets include epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and ROS proto-oncogene 1 (ROS1) (4, 5). Over the past two decades, several EGFR inhibitors have been developed. For example, the first-, second-, and third-generation EGFR inhibitors, icotinib, afatinib, and osimertinib, have been developed to treat mutated EGFR (mtEGFR) non-small cell lung cancer (NSCLC) (6, 7). Despite excellent response rates to these drugs, patients invariably experience disease progression due to the emergence of drug-resistant tumors, usually within 9–14 months, which is a major hurdle in EGFR tyrosine kinase inhibitor (TKI) therapy (8). However, the mechanism underlying acquired drug resistance in patients treated with EGFR-TKIs remains unclear. In this study, we report a case of a patient with different drug resistance-associated mutations (EGFR exon 20 T790M and LCLAT1-ALK fusion), which demonstrated the related resistance mechanisms after first-line icotinib treatment to be highly heterogeneous.
A 64-year-old man with no smoking history and no history of cancer presented to our hospital in September 2016 with a primary tumor (1.5 × 1.5 cm) in his upper left lung (Figure 1). A positron emission tomography/computed tomography (PET/CT) scan revealed a mass in the upper left lung with intense uptake of (18F) fluorodeoxyglucose and no distance metastasis. Postoperatively, the patient was diagnosed with adenocarcinoma stage IA (pT1bN0M0). The immunohistochemical staining is shown in Figure 2. Amplification refractory mutation system polymerase chain reaction (EGFR/ALK/ROS1) of the resected tissue identified EGFR exon 21 L858R, and negativity for ALK fusion (Table 1). After 12 months, intrapulmonary metastases were detected by computed tomography (CT). Therefore, treatment with icotinib hydrochloride tablets (125 mg three times daily) was initiated in September 2017. The patient experienced a rapid partial response (PR) according to the Response Evaluation Criteria in Solid Tumors (RECIST).
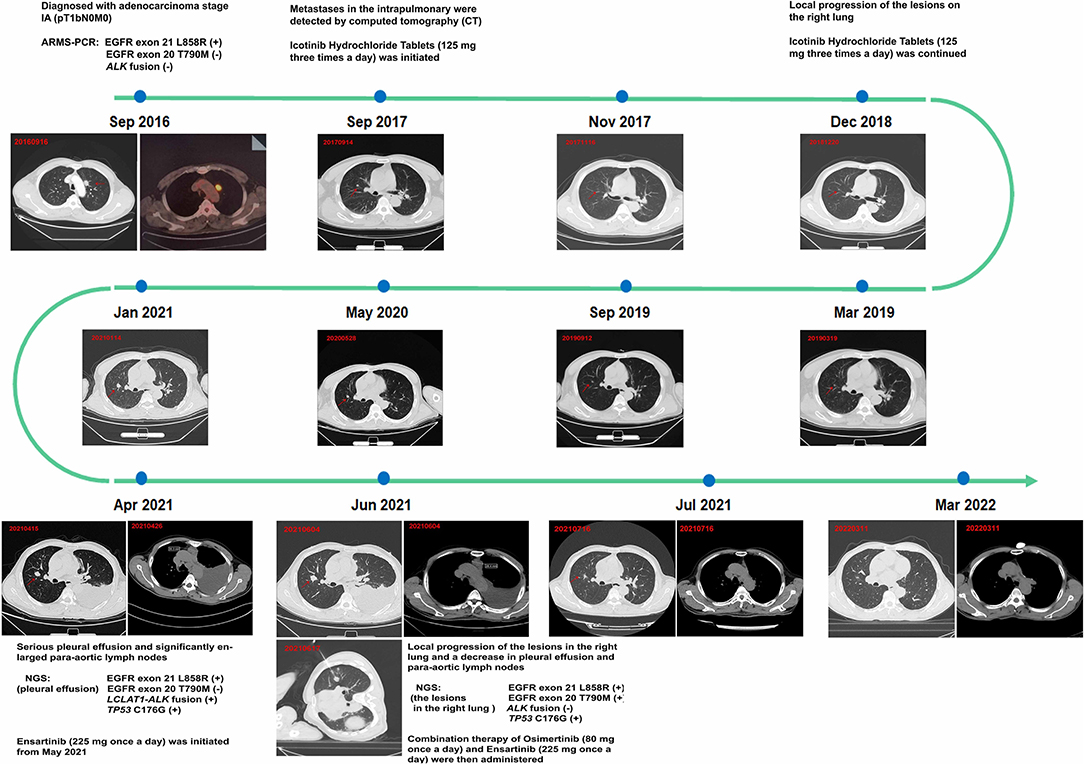
Figure 1. Treatment course with corresponding computed tomography (CT) scans. This figure shows the timeline of progression after 43 months under the treatment with only icotinib; the patient achieved a satisfactory exceptional response by treatment with osimertinib plus ensartinib.
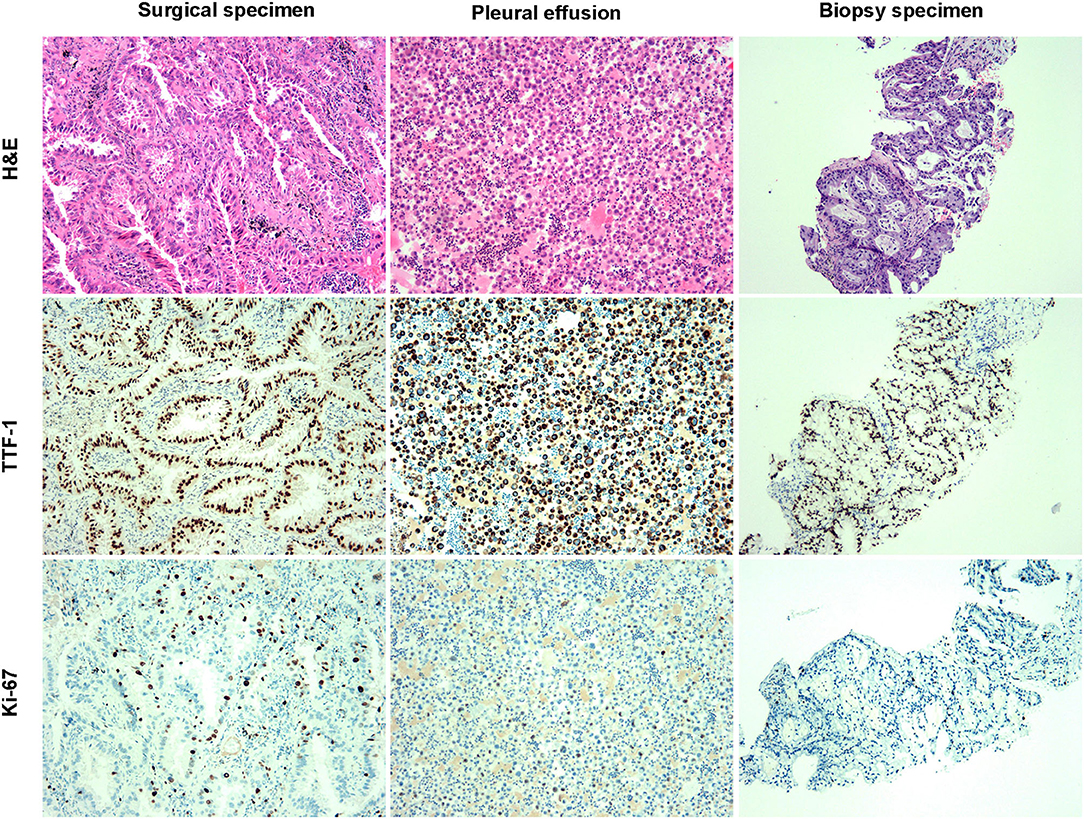
Figure 2. The pathological diagnosis of specimens was lung adenocarcinoma. H and E staining and immunohistochemistry staining of surgical, pleural effusion, and biopsy specimens.
The timeline of the disease progression is shown in Figure 1. After 15 months of icotinib treatment, a CT scan of the patient revealed progressive disease (PD) (Figure 1). Due to the local progression of the lesions in the right lung, icotinib treatment was continued until April 2021. The patient experienced serious pleural effusion on the left side and significantly enlarged para-aortic lymph nodes (6, 30.3 mm) (Figure 1). Pleural drainage was performed to alleviate the clinical symptoms, and next-generation sequencing (48-gene panel) of the pleural effusion was conducted to explore the mechanism of icotinib resistance. The results revealed an LCLAT1-ALK fusion and TP53 C176G in addition to EGFR exon 21 L858R (Table 1). Meanwhile, we verified the result via ARMS-PCR analysis and found that ALK exon 20 fusion-positive. We also detect the ALK protein level by IHC (ALK D5F3). Consequently, the patient started ensartinib (225 mg one time daily) as the next treatment in May 2021, as ensatinib has fewer adverse reactions than ceratinib. After 1 month, chest CT revealed local progression of the lesions in the right lung, as well as a decrease in pleural effusion and para-aortic lymph nodes (6, 28.4 mm). Given the possibility of different drug resistance mechanisms in different lesions, a biopsy specimen was obtained by CT-guided fine-needle aspiration (FNA). Next-generation sequencing (48-gene panel) of the tissue samples obtained from the lesions on the right lung in June 2021 revealed EGFR exon 20 T790M mutation, TP53 C176G, and EGFR exon 21 L858R, with no ALK fusion (Table 1). Hence, combination therapy with osimertinib (80 mg one time daily) and ensartinib (225 mg one time daily) was administered. One month later, a CT scan revealed considerable reductions in the lesion sizes in the right lung, para-aortic lymph nodes, and pleural effusion. Regular follow-up chest CT indicated that the lesion was stable through March 2022 (Figure 1). During the treatment with combination therapy, the patient developed a local rash and transient elevation of his serum creatine kinase level, which required no special treatment.
The patient provided written informed consent for the publication of this case.
The mechanism of acquired drug resistance in patients treated with EGFR-TKIs is currently under investigation. The evolutionary pressure acting on cancer cells via spatial and temporal clonal selection, combined with the random acquisition of genetic mutations, contributes to drug resistance (9). The most commonly observed acquired resistance mechanism is the acquisition or outgrowth of the T790M mutation (exon 20 of EGFR), which accounts for approximately 60% of patients receiving gefitinib, erlotinib, or icotinib (10). Osimertinib is an irreversible third-generation EGFR TKI administered for the first-line treatment of common sensitive EGFR mutations, or for second-line treatment of acquired resistance, to first-generation EGFR-TKIs by selectively targeting the T790M mutation (11). The results of the AURA3 (NCT02151981) phase III trial revealed that osimertinib significantly prolonged progression-free survival (PFS) compared to platinum and pemetrexed chemotherapy (10.1 months vs. 4.4 months, P < .001) in patients with the T790M resistance mutation after the failure of prior TKIs (12). In addition to EGFR exon 20 T790M, several gene fusions involving driver oncogenes are rarely acquired resistance mechanisms in patients treated with first-generation EGFR-TKIs. A previous study reported that only 13% of patients with NSCLC-harboring-EGFR mutations acquired EGFR TKI resistance mediated by emerging ALK rearrangements (13). Hu et al. (14) reported a patient with lung adenocarcinoma who displayed alternate drug resistance changes between EGFR and ALK after gefitinib resistance. Similarly, Hou et al. (15) summarized previously reported cases, in which ALK rearrangement or fusion was a rare but critical resistance mechanism to osimertinib. The most common alteration in the ALK gene is chromosomal rearrangements, such as EML4 (chromosome 2)-ALK (chromosome 2) (16). Xia et al. (17) revealed complex ALK gene fusion by targeted-capture DNA-based NGS, RNA-based NGS, RT-PCR, IHC, and FISH. In 343 samples of existing ALK fusions, they identified that intron 1 of LCLAT1 joined between the intron 13 of EML4 and the intron 19 of ALK. In our case, we identified a novel ALK gene fusion, LCLAT1 exon6-ALK exon 20 by DNA-based NGS. LCLAT1 exon6 has not been previously reported as a partner gene of ALK. Unfortunately, due to the limitation of specimens, we could not perform RNA-based NGS to explore the RNA transcript of LCLAT1 exon6-ALK exon 20, which is a limitation of our case. According to the result of DNA-based NGS, IHC, and the effect of ensatinib, we preliminarily speculated that the RNA transcript might be LCLAT1-ALK (exon6: exon20).
The increasing understanding of tumor heterogeneity has revealed that specific resistance mechanisms may occur in different clones. A deeper understanding of the complexity of EGFR-TKI resistance will help to guide clinical decisions. In our case, two different acquired resistance mechanisms occurred in two lesions following the development of icotinib resistance. This phenomenon reflects the heterogeneity of the original tumor. Therefore, when targeted treatment fails, re-biopsy and molecular analysis are considered standard procedures to solve this issue. In our report, the patient achieved a satisfactory response after treatment with osimertinib plus ensartinib. Patients with EGFR/ALK co-alterations may benefit from combined treatment with both TKIs, including long-term survival (18).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WC, WZ, and HD drafted the manuscript. XF, ZL, and BW treated the patient. XS and DJ retrieve the related literature. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Project of Zhejiang Basic Public Benefit Research of Zhejiang Province (No. LGD21H010001 to ZL, No. LGF21H160003 to BW). The foundation supported gene mutational analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We apologize to all researchers whose relevant contributions were not cited due to space limitations.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Group NM-AC. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. (2008) 26:4617–25. doi: 10.1200/JCO.2008.17.7162
3. Ergoren MC, Cobanogullari H, Temel SG, Mocan G. Functional coding/non-coding variants in EGFR, ROS1 and ALK genes and their role in liquid biopsy as a personalized therapy. Crit Rev Oncol Hematol. (2020) 156:103113. doi: 10.1016/j.critrevonc.2020.103113
4. Pirker R, Filipits M. Personalized treatment of advanced non-small-cell lung cancer in routine clinical practice. Cancer Metastasis Rev. (2016) 35:141–50. doi: 10.1007/s10555-016-9612-6
5. Rocco G, Morabito A, Leone A, Muto P, Fiore F, Budillon A. Management of non-small cell lung cancer in the era of personalized medicine. Int J Biochem Cell Biol. (2016) 78:173–9. doi: 10.1016/j.biocel.2016.07.011
6. Han C, Ding X, Li M, Luo N, Qi Y, Wang C. Afatinib, an effective treatment for patient with lung squamous cell carcinoma harboring uncommon EGFR G719A and R776C co-mutations. J Cancer Res Clin Oncol. (2022) 148:1265–8. doi: 10.1007/s00432-021-03864-4
7. Ortega-Franco A, Rafee S. ADAURA: the splash of osimertinib in adjuvant EGFR-mutant non-small cell lung cancer. Oncol Ther. (2022) 10:13–22 doi: 10.1007/s40487-022-00190-8
8. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. (2011) 3:75ra26. doi: 10.1126/scitranslmed.3002003
9. Passaro A, Janne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. (2021) 2:377–91. doi: 10.1038/s43018-021-00195-8
10. Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. (2013) 19:1389–400. doi: 10.1038/nm.3388
11. Jiang T, Zhou C. Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients with EGFR inhibitor-resistant non-small cell lung cancer. Transl Lung Cancer Res. (2014) 3:370–2. doi: 10.3978/j.issn.2218-6751.2014.08.02
12. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. (2017) 376:629–40. doi: 10.1056/NEJMoa1612674
13. Xu H, Shen J, Xiang J, Li H, Li B, Zhang T, et al. Characterization of acquired receptor tyrosine-kinase fusions as mechanisms of resistance to EGFR tyrosine-kinase inhibitors. Cancer Manag Res. (2019) 11:6343–51. doi: 10.2147/CMAR.S197337
14. Hu Y, Xiao L, Yang N, Zhang Y. Tyrosine kinase inhibitor acquired resistance mechanism alternates between EGFR and ALK in a lung adenocarcinoma patient. Thorac Cancer. (2019) 10:1252–5. doi: 10.1111/1759-7714.13015
15. Hou H, Sun D, Zhang C, Liu D, Zhang X. ALK rearrangements as mechanisms of acquired resistance to osimertinib in EGFR mutant non-small cell lung cancer. Thorac Cancer. (2021) 12:962–9. doi: 10.1111/1759-7714.13817
16. Wu W, Haderk F, Bivona TG. Non-canonical thinking for targeting ALK-fusion onco-proteins in lung cancer. Cancers. (2017) 9:164. doi: 10.3390/cancers9120164
17. Xia P, Zhang L, Li P, Liu E, Li W, Zhang J, et al. Molecular characteristics and clinical outcomes of complex ALK rearrangements identified by next-generation sequencing in non-small cell lung cancers. J Transl Med. (2021) 19:308. doi: 10.1186/s12967-021-02982-4
Keywords: lung adenocarcinoma, resistance mechanisms, ALK rearrangement, EGFR exon 20 T790M, combination therapy, case report
Citation: Liu Z, Dong H, Chen W, Wang B, Ji D, Zhang W, Shi X and Feng X (2022) Case Report: Heterogeneity of Resistance Mechanisms in Different Lesions Co-Mediate Acquired Resistance to First-Line Icotinib in EGFR Mutant Non-Small Cell Lung Cancer. Front. Med. 9:906364. doi: 10.3389/fmed.2022.906364
Received: 28 March 2022; Accepted: 06 June 2022;
Published: 07 July 2022.
Edited by:
Pasquale Pisapia, University of Naples Federico II, ItalyReviewed by:
Mau Ern Poh, University Malaya Medical Center, MalaysiaCopyright © 2022 Liu, Dong, Chen, Wang, Ji, Zhang, Shi and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueren Feng, ZnhyOTMwMTAwNkAxNjMuY29t; Xuefei Shi, c2hpeHVlZmVpMTIyM0BhbGl5dW4uY29t; Wei Zhang, NDI2MjkxNDBAcXEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.