- Fresenius Medical Care, Dialysis Center in Chicago, Chicago, IL, United States
There have been ongoing debates about resuscitation fluids because each of the current fluids has its own disadvantages. The debates essentially reflect an embarrassing clinical status quo that all fluids are not quite ideal in most clinical settings. Therefore, a novel fluid that overcomes the limitations of most fluids is necessary for most patients, particularly diabetic and older patients. Pyruvate is a natural potent antioxidant/nitrosative and anti-inflammatory agent. Exogenous pyruvate as an alkalizer can increase cellular hypoxia and anoxia tolerance with the preservation of classic glycolytic pathways and the reactivation of pyruvate dehydrogenase activity to promote oxidative metabolism and reverse the Warburg effect, robustly preventing and treating hypoxic lactic acidosis, which is one of the fatal complications in critically ill patients. In animal studies and clinical reports, pyruvate has been shown to play a protective role in multi-organ functions, especially the heart, brain, kidney, and intestine, demonstrating a great potential to improve patient survival. Pyruvate-enriched fluids including crystalloids and colloids and oral rehydration solution (ORS) may be ideal due to the unique beneficial properties of pyruvate relative to anions in contemporary existing fluids, such as acetate, bicarbonate, chloride, citrate, lactate, and even malate. Preclinical studies have demonstrated that pyruvate-enriched saline is superior to 0.9% sodium chloride. Moreover, pyruvate-enriched Ringer’s solution is advantageous over lactated Ringer’s solution. Furthermore, pyruvate as a carrier in colloids, such as hydroxyethyl starch 130/0.4, is more beneficial than its commercial counterparts. Similarly, pyruvate-enriched ORS is more favorable than WHO-ORS in organ protection and shock resuscitation. It is critical that pay attention first to improving abnormal saline with pyruvate for ICU patients. Many clinical trials with a high dose of intravenous or oral pyruvate were conducted over the past half century, and results indicated its effectiveness and safety in humans. The long-term instability of pyruvate aqueous solutions and para-pyruvate cytotoxicity is not a barrier to the pharmaceutical manufacturing of pyruvate-enriched fluids for ICU patients. Clinical trials with sodium pyruvate-enriched solutions are urgently warranted.
Introduction
Fluid therapy is the first and essential treatment in perioperative and critical care patients. However, the selection of crystalloids and/or colloids, which depends on the pathophysiologic mechanism of various diseases; the fluid composition, property, and availability; and even clinicians’ individual preference, remains greatly debatable as each anion, such as acetate, bicarbonate, chloride, citrate, and lactate, in commercial fluid products has its own limitations in the resuscitation of critical care patients, generally contributing to iatrogenic resuscitation injury. In the past 3 decades, it has been well established that pyruvate, a weak acidic anion and the core element of glucose metabolism, holds unique beneficial physiological and pharmacological properties superior to those of the above-mentioned anions in current commercial fluids including intravenous (IV) crystalloids and colloids, as well as oral rehydration solution/salt (ORS). This review focuses on the essence of current fluid debates and the advantages, necessity, and potential clinical uses of sodium pyruvate-enriched fluids.
Non-Optimality of Current Fluids
Normal saline (NS, 0.9% sodium chloride with equal sodium and chloride, 154 mmol/L) has been the most popular fluid in clinical practice for approximately 200 years. High-chloride saline including NS and hypertonic saline has a fatal limitation in that it induces iatrogenic hyperchloremia (1, 2), which leads to metabolic acidosis and kidney dysfunction mainly due to hyperchloremic renal vasocontraction and decline in the glomerular filtration rate (3, 4). In healthy volunteers, infusion of NS at 2 L/h results in hyperchloremia and a decrease in renal blood flow velocity and cortical tissue perfusion but does not cause any damage (5). However, hyperchloremia may exacerbate acid–base disorders and organ dysfunction in intensive care unit (ICU) patients, and hyperchloremia at hospital discharge may still be associated with the risk of 1-year patient mortality (6). Therefore, NS is neither optimal nor suitable for perioperative and ICU patients, but it remains the first choice for the treatment of hypochloremic alkalosis (1). A consensus states that NS is neither a normal nor physiological but an abnormal fluid, which should be replaced by balanced fluids in most patients, if possible.
To overcome the limitations of NS, Ringer’s and lactated Ringer’s solutions were produced around a century ago. Previous evidence shows that balanced fluid (lactated/acetated Ringer’s solution (LR/AR) or acetate-based Plasma-Lytes) is advantageous over NS in critically ill adults (7, 8), specifically in patients with diabetic ketoacidosis (9). However, recent findings with a large sample of patients reveal that balanced crystalloids (LR and AR) do not have advantages over NS regarding hospital-free days in non-critically ill patients (10) and that balanced fluids show no significant superiority over NS in reducing 90-day mortality in critically ill patients and kidney transplant graft function (11, 12). To date, the clinical outcomes of various fluids are still controversial in this respect, and one of the beneficial effects of current fluids may depend on specific subgroups of patient populations.
It has long been known that the serum lactate level is negatively associated with the reversibility of shock and is an independent risk factor of patients’ mortality in ICUs (13, 14). Notably, relative hyperlactatemia within the normal range is also independently associated with mortality in ICU patients (15). Regarding LR, there is a rise of 0.93 mmol/L in the mean serum lactate level in healthy volunteers after a bolus of 30 ml/kg (16). A large LR infusion likely exacerbates lactate accumulation in the resuscitation of patients with severe or decompensated shock, which may interfere with the diagnosis and treatment of lactic acidosis. Thus, LR is not optimally worthy of recommendation in critically ill patients (17). However, it is still controversial whether LR worsens lactic acidosis in ICU patients, including those with septic shock. Although LR generally does not decrease the lactate clearance, the persistence of hyperlactatemia during the first 24 h with a high L/P (lactate/pyruvate) ratio is still associated with a risk of multi-organ failure and death in clinical septic shock (18). The recommended solution is rather the acetate-based Plasma-Lyte solution (17, 19). Nevertheless, in a case report, an AR infusion of sufficient quantity induced lactic acidosis but did not cause any adverse effects (20).
Evidently, there is no consensus on the type of IV fluid, either crystalloids (balanced or non-balanced) or colloids (hydroxyethyl starch: HES 130/0.4, albumin, or plasma with various crystalloids as carriers), that is the best for most patients (21), as each fluid has advantages and disadvantages in the majority of patients.
On the other hand, the volume and speed of fluid delivery are also a critical concern in various clinical settings. Individualized goal-directed therapy (IGDT) is currently optimal in perioperative fluid management and critical care patients, but it still faces challenges. IGDT did not improve early renal function in a recent renal transplant study with a porcine model (22). Although very little or excessive fluid infusion can induce an immediate hemodynamic compromise and cause organ dysfunction (22), a recent study with a large sample of patients found that infusing at a slower (333 ml/h) vs. faster (999 ml/h) rate did not result in a statistical difference in 90-day mortality among ICU patients who were randomized in two groups to receive balanced solutions and NS, respectively (23).
Generally, although current fluids play an important role in healthcare, the ongoing debates about resuscitation fluids essentially reflect a crucial embarrassing clinical status quo that all current fluid products are not quite satisfactory in most clinical settings as an ideal selection, especially in ICU patients with severe and complex comorbidities. Therefore, a novel optimal fluid that overcomes most of the limitations of current fluids is warranted for most patients in a wide variety of severe clinical scenarios, particularly diabetic and elderly patients with or without organ comorbidities.
Advantages of Pyruvate in Future Fluids
Pyruvate is a key metabolite of glycolysis, which is reduced to lactate in anoxia or enters oxidative metabolism in the tricarboxylic acid (TCA) cycle in normoxia and hypoxia. The exogenous pyruvate metabolic profile in the intracellular environment can be simply illustrated, as shown in Figure 1.
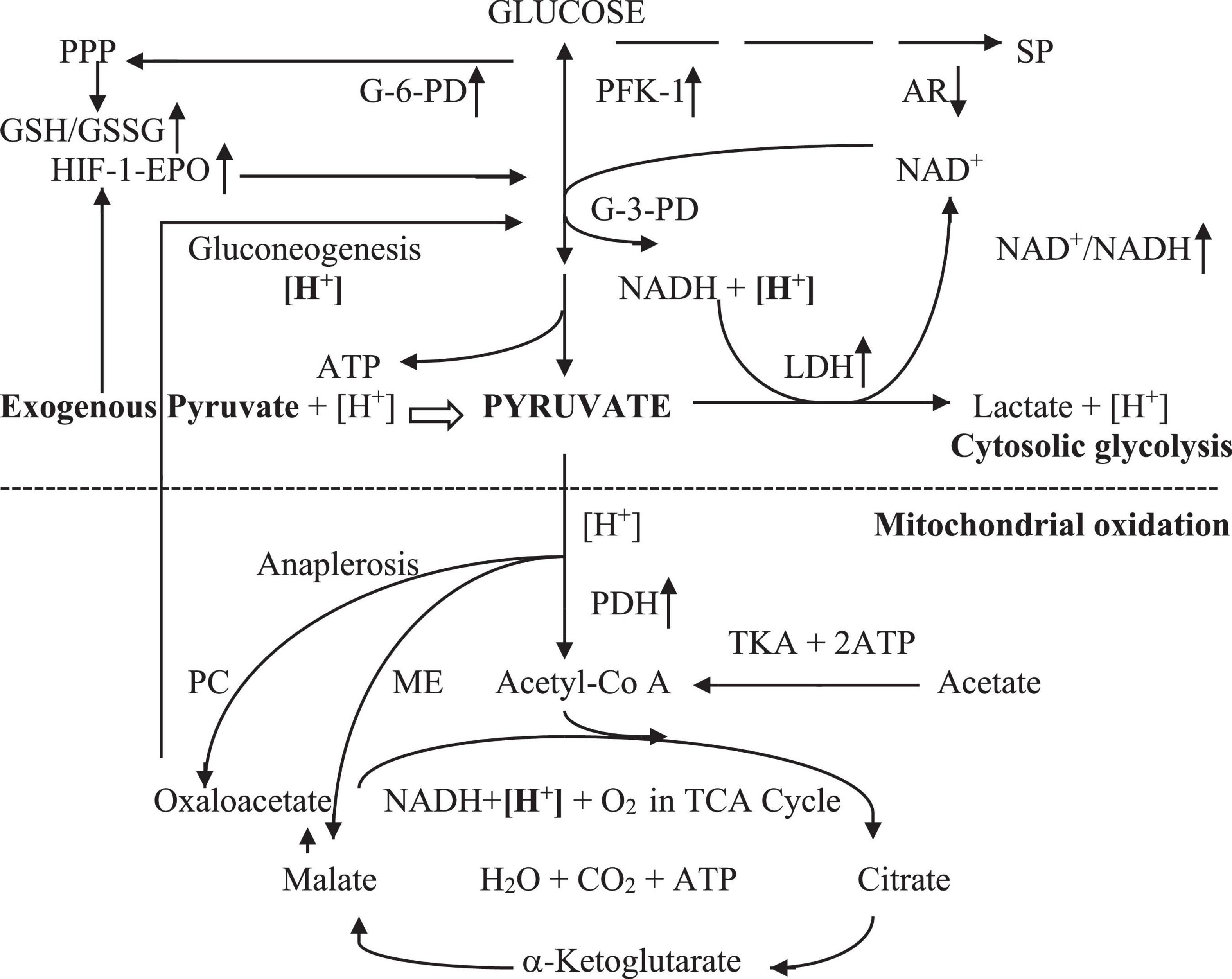
Figure 1. Exogenous pyruvate metabolism in cellular hypoxia. Exogenous pyruvate enters cell plasma with [H+]. The pyruvate or glycolytic pyruvate with [H+] spontaneously reduces to lactate with LDH free of energy in anoxia, leading to [H +] consumption and increment of the NAD+/NADH ratio that promotes the glycolytic pathway at glyceraldehyde-3-phosphate dehydrogenase. Exogenous pyruvate also facilitates glycolysis by stimulating the HIF-1α-EPO signal pathway, increasing G-6PD activity, thereby preserving the PPP pathway and GSH/GSSG ratio. It inhibits AR activity in the sorbitol pathway likely by competing inhibition, enhancing NAD+/NADH also in the second step of sorbitol pathway. Thus, pyruvate sustains canonical glycolytic pathways and glycolytic ATP. It also inhibits LDH-A to decline the pyruvate reduction to lactate. Pyruvate enters mitochondria with [H+] and oxidates in hypoxia and normoxia by renovating inhibited PDH in the TCA cycle, resulting in mitochondrial ATP generation and [H +] consumption. Also, it promotes the TCA cycle via anaplerosis with preservation of PC and ME activities. Hence, pyruvate reverses the Warburg effect. Pyruvate-based gluconeogenesis consumes additional [H +] in relation to lactate-based one in cytosol. Pyruvate has the most powerful energetics with the least oxygen consumption in equal molar ATP generation among lactate, acetate, citrate, and malate oxidation. AR, aldose reductase; ATP, adenosine triphosphate; G-3-PD, glyceraldehyde-3-phosphate dehydrogenase; G-6-PD, glucose-6-phosphate dehydrogenase; GSH/GSSG, glutathione (reduced/oxidized); HIF-1-EPO, hypoxia-inducible factor-1-erythropoienin; [H+], hydrogen in cellular hydrogen pool; [H+], hydrogen consumed in a molar basis; LDH, lactate dehydrogenase; ME, malic enzyme; NADH/NAD+, nicotinamide adenine dinucleotide (reduced/oxidized); PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; PFK-1, phosphofructokinase-1; PPP, pentose phosphate pathway; SP, sorbitol pathway; TCA cycle, tricarboxylic acid cycle with oxidative phosphorylation; TKA, thiokinase.
Exogenous pyruvate in aqueous solutions is a unique anion that has pluripotent beneficial physiological and pharmacological properties to protect multi-organ structures and functions against various noxious insults, such as cardiogenic, hemorrhagic, traumatic, and septic shock. Specifically, pyruvate is a potent alkalizer used in preventing and treating hypoxic lactic acidosis, which is not only lethal but also lacks an ideal treatment agent in clinical practice (17). Recently, its beneficial properties over current anions in medical fluids, as mentioned before, are increasingly being recognized in fluid resuscitation, mainly due to the following beneficial bioactive characteristics.
Increase in Cellular Hypoxia Tolerance
An experiment conducted in 2012 showed that pyruvate protects the function of red blood cells (RBCs), which are the most abundant tissues in humans and exclusively depend on anaerobic glycolysis to produce glycolytic ATP due to the absence of mitochondria (24). Moreover, the delayed decline in ATP levels and ATPase activities of dogs’ RBCs in the extracorporeal circuit primed with sodium pyruvate/chloride saline vs. sodium chloride saline demonstrates that pyruvate alone is beneficial for cell metabolism throughout the body, even in anoxia (24). This investigation validates two previous findings: (1) pyruvate preserved brain ATP levels and prolonged survival in rats subjected to anoxia by exploring a pure nitrogen atmosphere in the 1960s (25), and (2) pyruvate protected against anoxic injury in the anoxic perfusion of hepatocytes, as indicated by decreases in superoxide generation and lactate dehydrogenase (LDH) release, and against reoxygenation injury in rats in the 1990s (26). Recently, infusion of RBCs with pyruvate restoration from storage-induced damage robustly alleviated liver injury in rats, indicating the potential significance of pyruvate protection of RBCs in clinical resuscitation (27). Evidently, it also preserves ATP generation in hypoxia (28, 29). These findings substantiate a fundamental and core fact that exogenous pyruvate preserves canonical glycolysis and glycolytic ATP, which is essential for each cell in both hypoxia and anoxia (see below).
Reactivation of Pyruvate Dehydrogenase and Reversal of Warburg Effect
Pyruvate stimulates pyruvate dehydrogenase (PDH) via the direct inhibition of PDH kinase (PDK), similar to dichloroacetate (DCA, a classic PDH stimulator) (30, 31), and probably via the enhancement of PDH phosphatase 2 (PDP2) expression (32). Recent findings further demonstrated that pyruvate preserved the nicotinamide adenine dinucleotide oxidative form/reduced form (NAD+/NADH) ratio, restored pyruvate kinase (PK), and retarded the sorbitol pathway by the competitive inhibition of aldose reductase, resulting in the promotion of classic glycolytic pathways (Figure 1). It also accelerated glucose oxidative phosphorylation by rejuvenating the suppressed PDH and pyruvate carboxylase (PC) activities in traumatic brain injured and hemorrhagic rats and diabetic db/db mice (31, 33). Alternatively, it can inhibit LDH-A activity, which is usually overexpressed in hypoxia, diabetes, and cancer, facilitating oxidative metabolism in cells (34–36).
Several pieces of preclinical evidence revealed that pyruvate protected from diabetic cataract and retinopathy and increased blood insulin levels (33, 37, 38). Preliminary case reports also confirmed the findings that pyruvate protected against diabetes by reducing daily insulin doses in type 1 diabetes (39, 40). Accordingly, it can reverse glucometabolic disorders in diabetes and trauma and hemorrhage, turning the vicious circle of diabetic glucometabolic disturbance into a virtuous circle (33) as it reversed the Warburg effect by DCA in cancer.
Correction of Severe Metabolic Acidosis
Pyruvate is a potent alkalizer via the rapid metabolic consumption of hydrogen ions (proton, [H+]) through the LDH reduction reaction, which is a systemic alkalizing enzymatic reaction, coupled with an increase in the NAD+/NADH ratio, and the gluconeogenesis pathway in the cytosol in addition to oxidative phosphorylation in the mitochondria (Figure 1). Although it is a weaker acidic anion of sodium salt with a low buffer capacity of pKa 2.49, pyruvate favors a rise in blood plasma pH accordingly (17, 41). Pyruvate has the potential to effectively correct hypoxic lactic acidosis in critically ill patients, as repeatedly demonstrated with IV or oral pyruvate in small or large animal studies, which resulted in approximately doubled survival (42–45). A case report described the effectiveness of a high dose of oral sodium pyruvate in robustly attenuating continuous severe lactic acidosis in a child with Leigh syndrome due to a novel mutation in the PDH E1α gene (46). Another study involving 11 adult patients with the mitochondrial disease also revealed significant decreases in plasma and lateral ventricular lactate and the L/P ratio accompanied by clinical improvements as a result of pyruvate therapy (47). Therefore, pyruvate enriched IV and oral solutions are excellent in preventing and treating severe metabolic lactic acidosis in various severe clinical scenarios, although no data from clinical resuscitation have yet been reported.
Inhibition of Oxidative/Nitrosative Stress and Inflammation
Endogenous pyruvate is a natural and potent antioxidant agent. It is widely known that it can effectively exert a dual effect of antioxidant/nitrosative stress by directly interacting with reactive oxygen/nitrogen species via a non-enzymatic stoichiometric reaction and indirect action with redox potentials, mainly NAD+/NADH and GSH/GSSG (glutathione-reduced form/oxidative form) (48). A recent comprehensive review clearly illustrated the critical significance of reducing equivalents, including NAD+ and GSH, in maintaining cellular redox homeostasis and modulating cellular metabolism (49). There are numerous pieces of evidence that show pyruvate robustly increases both NAD+/NADH and GSH/GSSG ratios in tissues in various injuries (33, 42, 50). In addition, it inhibits inflammatory reactions, including both infiltration of inflammatory cells and secretion of inflammatory mediators, such as cytokines including IL-2 and IL-6, TNF-α, and high-mobility group box-1 (HMGB-1) (43).
Stimulation of HIF-1 and Protection of the Mitochondria
As previously discovered, pyruvate can directly stimulate the hypoxia-inducible factor-1α-erythropoietin (HIF-1α-EPO) signal pathway by inhibiting the HIF-prolyl hydroxylase domain (PHD) to avoid HIF-1α proteasomal degradation in both hypoxia and normoxia and the subsequent elevation of the gene expression and content of EPO (51, 52). The activation of the HIF-1α-EPO pathway further stimulates the downstream enzymes to improve energy metabolism and mitochondrial energetics. On the other hand, it also protects the mitochondrial structure and endoplasmic reticulum function and protects against cellular apoptosis in various insults (53–55). Therefore, the effects of pyruvate can continue for at least several hours after the rapid decline in the peak plasma level to normal in approximately 1 h following the termination of pyruvate infusion (51). In addition, pyruvate can inhibit the formation and deposition of advanced glycation end products (AGEs), which are one of the major pathogenic triggers in organ complications, in hypoxia and diabetes as an AGE antagonist (33, 56).
Stimulation of Insulin Secretion
Sodium pyruvate like methyl pyruvate works as an insulin stimulator. Pyruvate metabolism in the mitochondria is intimately involved in glucose-stimulated insulin secretion (GSIS) and the PC activity, on which pyruvate oxidative metabolism partially depends. PC facilitates the anaplerotic flux, which also plays a critical role in insulin secretion from pancreatic islets (57, 58). It can effectively control diabetes progression and organ complications with restored insulin levels in db/db mice (33). Therefore, exogenous pyruvate enhances insulin secretion in islet β-cells even in type 1 diabetic patients to the extent of hypoglycemia (39, 40). Intriguingly, in children with citrin deficiency, oral pyruvate induces significant enhancements of fasting insulin levels for months (59). Furthermore, in rats subjected to severe scald with multiple organ dysfunction syndrome, direct peritoneal resuscitation with pyruvate-based peritoneal dialysis solution (P-PDS) containing glucose still effectively protects the function of the islet β-cells, as demonstrated by a higher homeostasis model assessment of β-cell (HOMA-β) level without hyperglycemia (60). Thus, pyruvate is beneficial in the resuscitation of critical care patients, specifically those with diabetes and elders (61).
Exertion of Anti-aging
The decrease in NAD+ cellular levels and the increase in senescence cells (a permanent cell cycle arrest but living cell) in tissues are closely associated with natural aging (62, 63). Pyruvate can spontaneously generate NAD+, mainly through the LDH reduction reaction coupled with NADH oxidation free of oxygen and energy, enhancing NAD+ levels in the cytosol on an equimolar basis. Alternatively, the clearance of senescence cells by a senolytic improved multi-organ (kidney and heart) functions and extended the healthy lifespan in mice. A pilot study in patients with idiopathic pulmonary fibrosis first showed promising clinical improvements with oral senolytics (64). Importantly, a cellular study found that pyruvate prevented cellular senescence in normal human fibroblasts by increasing NAD+ generation in vitro and mimicking human skin in vivo (53). Notably, it also showed the protection of DNA repair in rodents and human cells (65). Therefore, pyruvate acts generally as both a novel NAD+ substitute and a new senolytic substance, although further exploration and demonstrable data are warranted (66).
The unique properties of pyruvate described above are superior to those of the anions currently found in fluids used for ICU patients, which lack the properties of pyruvate or have much inferior ones; even malate shows the capacity to effectively eliminate hypoxic lactic acidosis in sufficient amounts cannot be metabolized in anoxia (67), leading to no protection of RBCs. It also possesses the lowest oxygen consumption rate per ATP generation among acetate, citrate, lactate, and malate (17). Therefore, pyruvate is more metabolically protective, especially regarding glucometabolic and acid–base balance and multi-organ function, predominantly in the heart, brain, liver, kidney, and intestine, than current anions in various pathogenic attacks. Alternatively, hypoxia, glucometabolic disturbance, metabolic acidosis, oxidative stress and inflammation, mitochondrial dysfunction, and cellular apoptosis, against which pyruvate protects in multiple organs, are all pathophysiological processes that are shared in most critical illnesses. Accordingly, the favorable pleiotropic bioactivities of pyruvate effectively meet the clinical needs and are beneficial in most patients with or without parenchymatous organ comorbidities.
Pyruvate Saline Superior to Normal Saline
Intriguingly and significantly, pyruvate saline, sodium pyruvate 50 mM plus sodium chloride 104 mM, is advantageous over 0.9% sodium chloride (NS, 154 mM) in preserving ATP generation and ATPase activity in the RBCs of dogs simulated bypass surgical procedures in vitro (24). The ATP product from RBCs is glycolytic ATP, an essential component of ATPase in all cells in the body for several basic cellular functions, such as ion polarization of plasma membranes and maintenance of membrane integrity by Na+-K+-ATPase, and organelle pH regulation by vacuolar ATPase (V-ATPase). The data also demonstrate the inhibition of inflammatory reactions of RBCs by pyruvate, as shown by the reduction of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) in plasma (24). RBCs as an oxygen sensor that triggers the dilation of the microvascular circulation by releasing glycolytic ATP in addition to oxygen delivery are intimately associated with oxygen supply in tissues (68). As aforementioned, RBCs rejuvenated by pyruvate could attenuate liver injury after the blood infusion, indicating the improvement of tissue hypoxia in rats (27). In this respect, its protective effects on stored RBCs were also displayed early in rat models of renal oxygenation and in clinical bypass surgery studies (24). Furthermore, either a high or regular (28 mM) pyruvate concentration preserved the partial pressure of arterial oxygen (paO2) and systemic and cerebral oxygen delivery and consumption in shock IV resuscitation, further demonstrating the attenuation of tissue hypoxia by pyruvate (42, 44). Oral pyruvate also resulted in the preservation of paO2 in severe shock rehydration (69). Additionally, preliminary data showed that it might also improve the RBC oxygen–hemoglobin dissociation curve against peroxide stress (70). The pyruvate effect on paO2 in shock resuscitation should be specifically and intensively investigated.
Furthermore, IV pyruvate saline protection of the kidney was first preliminarily reported in China a decade ago in rats subjected to burn shock with 50% TBSA III (total body surface area, full-thickness scald), compared to an equal volume of NS infusion, although pyruvate renoprotection had been earlier investigated (71, 72); the kidney vascular permeability, tissue water content, hematocrit, and serum creatinine levels 4 h after the scald were significantly increased in the NS group compared to the pyruvate group, while no significant difference was found between the pyruvate group and the sham group (72). Although the histopathological alteration was not investigated, the results apparently revealed that pyruvate might not only prevent kidney injury generally induced by NS due to hyperchloremia in severe shock resuscitation, but also protect the kidney function from burn shock. Its renoprotection was further appreciated afterward, including the protection against diabetic nephropathy (33, 73). In addition, pyruvate also protects systemic endothelial cells in addition to RBCs and neutrophils against oxidative stress (74). These findings provide a convincing basis for using pyruvate saline, rather than NS in ICU patients in future clinical practice, although further intensive studies are required. Therefore, the predictable advantages of pyruvate saline are that it prevents iatrogenic hyperchloremia and protects multi-organ function as a therapeutic agent of organ metabolic and functional aberrances and a volume expander in fluid resuscitation. In these terms, 1.7% sodium pyruvate (154 mM) also showed more promising results than 0.9% sodium chloride by 90 vs. 30% survival at 90 min after fluid infusion during resuscitation for a severe hemorrhagic shock in a rodent model (75).
On the other hand, studies have demonstrated the advantages of PR over LR in shock resuscitation, particularly in effectively correcting hypoxic lactic acidosis, inhibiting apoptosis, and prolonging survival in animal studies (43, 44, 76).
It is worth noting that the regular pyruvate concentration (28 mM) with a low dose was as efficient as high doses for metabolic improvement and multi-organ protection in early reports (42, 43).
Importantly, a very small amount of pyruvate in cardioplegia showed apparent cardio-protection in clinical bypass surgery (77). In parallel, pyruvate-enriched ORS (Pyr-ORS; 0.35%: equimolar pyruvate replacement of bicarbonate or citrate in WHO-ORS I, II, and III) is superior to the latter three ORSs in correcting lactic acidosis, protecting multiorgan, and increasing survival in the shock rehydration of rats and dogs (Table 1) (45, 51, 69, 78).
Furthermore, early studies documented that pyruvate-based PDS (P-PDS) was much more effective than regular commercial lactate-based PDS (L-PDS) in improving human blood neutrophils’ intracellular pH and superoxide generation (79, 80). Regarding direct peritoneal resuscitation, recent animal studies also found that P-PDS was significantly advantageous over L-PDS in reversing visceral hypoperfusion, correcting metabolic acidosis, and protecting the intestinal barrier (81–83). Furthermore, as an example of colloids, pyruvate as a novel carrier in HES 130/0.4 significantly demonstrated kidney protection (as well as the intestinal barrier preservation: unpublished data) in the fluid resuscitation of rats subjected to lethal burn shock, compared to regular carriers in commercial HES 130/0.4 products (21).
All these indicate that pyruvate replacement of current anions, such as acetate, chloride, and lactate, in carrier solutions of colloids would robustly improve the clinical benefits of synthetic or natural colloids, although malate has not been compared till now.
It is rational and expected that on a monolithic view, pyruvate as a novel component in fluid therapy would greatly ameliorate the prognosis of diseases and clinical outcomes, particularly under IGDT, in perioperative fluid management and ICU patients, especially those with diabetes and elders, as the initial clinical indication. For example, clinical studies demonstrate that major abdominal surgery can induce a substantial PDH decrease with glucose metabolic dysregulation in muscles, probably due to an increase in PDK activity and insulin resistance (84, 85). Thus, pyruvate-enriched fluids would be an optimal selection to prevent postoperative hyperglycemia, probably due to its preservation of PDH and islet function (30–33, 60). At present, if pyruvate solutions were compassionately used in severe COVID-19 patients, clinical outcomes might have greatly improved (86, 87). In the future, pyruvate-enriched fluids may encompass other medical solutions specifically used for RBC storage, cell salvage, organ preservation, cardioplegia, peritoneal dialysis, priming fluid for cardiopulmonary bypass circuits, and others (24, 27, 77, 80, 88).
Safety and Feasibility of Pyruvate Fluids
Sodium pyruvate has been intravenously infused in humans since the 1930s. One early study used IV 3.5 g% pyruvate at 10 mg/kg (20 ml/70 kg) for 1–1.5 min in 21 healthy subjects and 27 patients subjected to Vit B1 deficiency (89); another study used 18.8 g of 12% pyruvate in three non-psychotic and four schizophrenic patients (90). Then, 10 g of pyruvate (100 ml of 10% solution) was infused in 18 non-diabetic subjects and 19 diabetic patients to investigate the secretion of pyruvate in urine in 1960 (91). No unexpected effects were observed in all these subjects. Subsequently, many clinical reports with high pyruvate doses in product qualities at the time demonstrated its safety with the absence of adverse effects. In 1996, the first IV pyruvate treatment of chronic liver diseases was reported in 11 patients with pyruvate 54–86.4 g/d for 10 days, followed by a consecutive report; the results showed a promising improvement in liver functions and pathological alterations (92, 93). The first intracoronary pyruvate infusion (totally 1.53 g and 3.05 g in 30 min, according to the calculation) was studied in eight patients with dilated cardiomyopathy in the Lancet in 1999; the hemodynamic measurements, including increases in the cardiac index and stroke volume and a decrease in pulmonary capillary wedge pressure, demonstrated the clinical cardio-protection due to pyruvate with a favorable inotropic effect (94). Additional reports on severe heart failure with intracoronary pyruvate of approximately 6.0 g in 30 min and others with cardioplegia on small doses of pyruvate and several IV loading tests of 10.0 g/4 min or 0.5 g/kg in 10 min all further demonstrated its clinical effectiveness and safety (77, 95–97). The highest oral dose (initially 0.25 g/kg t.i.d. with a maintenance dose of 0.5 g/kg t.i.d.) in 11 patients subjected to mitochondriopathy for 11 months and the same dose in a case with Leigh syndrome for years showed clinical improvements without adverse effects (46, 47). The reported clinical side effects were local pain induced by IV pyruvate at high concentrations and gut irritation or dizziness due to oral pyruvate at high doses (39, 91). The only report presents the case of a child with restrictive cardiomyopathy who received pyruvate infusion and died shortly after the pyruvate loading test (98); however, the causation between pyruvate and death was not confirmed (99).
However, the U.S. FDA has not verified its clinical use to date. The FDA approved a pyruvate-based product (Rejuvesol) in the 1990s, which was the sole commercial pyruvate-compounded solution stored at 4°C for the rejuvenation of stored RBCs in vitro before infusion (100). Early data regarding its acute toxicology showed that oral pyruvate LD50 was over 10.0 g/kg in rats, and IV pyruvate LD50 was over 1.25 g/kg in mice; thus, pyruvate was considered non-toxic in humans (39, 92).
The most crucial issue is the instability of pyruvate aqueous solutions at room temperature (101). Several patents on pyruvate clinical uses including fluid therapy were filed or issued during the last decades (102, 103), strongly indicating that the pivotal role of pyruvate in clinical medicine has been well recognized. However, no pharmaceutical manufacturers can produce pyruvate aqueous solutions worldwide to date. Although pyruvate dimers, such as para-pyruvate, which are cytotoxic in cell experiments in vitro (104), are spontaneously generated in pyruvate aqueous solutions at room temperature, the appropriate acidic environment can inhibit the non-enzymatic aldol-type condensation reaction (101). A patent on the long-term stability of pyruvate aqueous solutions was issued a decade ago following the experimental data of over 99.0% of pyruvate in water with a pH of approximately 4.5 at 25°C for 2 years (US patent: 8,835,508 B2, 2014). To date, there have not been any data regarding para-pyruvate cytotoxicity in humans in vivo. The purity of sodium pyruvate powders was merely over 98.0%, which probably included approximately 1.0% para-pyruvate, several decades ago when high doses of the product were safely infused in several hundreds of patients and healthy adults without acute adverse effects, as mentioned before, indicating that the toxicity of pyruvate aqueous solutions in humans is not realistically true, which is inaccurately referred in many references of ethyl pyruvate (EP) studies (105). Accordingly, pyruvate saline and pyruvate Ringer’s solution with pH 4.5–5.0 would be a long-term stable solution at room temperature without cytotoxicity as 5% glucose-NS but with a similar acidic pH in the clinical scenario. Furthermore, the pyruvate raw product is cheaper, and thus there are many suppliers. It is highly possible and essential to consider pharmaceutically manufacturing pyruvate-enriched fluids with a long-term shelf life for clinical practices today. At least, an IV preparation of sodium pyruvate powder injection should first be feasibly considered for emergency or compassionate use (77, 86).
On the other hand, oral pyruvate (0.35%) in a modified Pyr-ORS formula, which increases intestinal mucosal blood flow, energy metabolism, and Na+-K+-ATPase activity, protects intestinal barrier structure and function and enhances sodium and water absorption, thus resulting in lactic acidosis correction, multi-organ protection, and survival prolongation, relative to WHO-ORS counterparts (45, 69, 78, 106). Pyr-ORS as a pyruvate-containing beverage (107), which is approved by the FDA as a dietary supplement, may be efficient in prehospital rescue to take advantage of the “golden window” in the rehydration of acutely injured patients, particularly in an ambulance or resource-poor settings, in a large scale such as during earthquakes and terrorist attacks, as pointed out previously (51, 78). Recently, an experienced pyruvate research team strongly recommended the clinical use of pyruvate again (108). Taken together, although prior case reports with pyruvate applications were not sufficiently of high quality and did not have large group sizes, the overall results strongly demonstrate an irrefutable fact that pyruvate is clinically promising relative to all the drawbacks of the major anions found in contemporary resuscitation solutions. Pyruvate-enriched fluids may be a preferred choice via IV, oral, or peritoneal administration in the future for clinical shock resuscitation and the first choice for hemorrhagic shock (109). ORS therapy has been demonstrated in a top hospital to be effective in the patients of the whole hospital including those in the emergency room (110); however, Pyr-ORS has been shown to be more effective than WHO-ORS in various illnesses. Nevertheless, there has been no clinical study yet about the effects of IV or oral pyruvate on shock resuscitation, but novel oral pyruvate combined with nicotinamide has demonstrated its effectiveness in improving retinal ganglion cell function in human glaucoma (111).
Based on the current understanding of fluid therapy in ICU patients, it is recommended to prescribe fluids based on the condition of each individual patient and to understand the benefits of each solution regarding an individual ICU patient, as both are crucial (112). In contrast to existing commercial fluids, novel pyruvate-enriched fluids would be both a volume expander and a therapeutic agent in fluid resuscitation and will be appealing to clinicians as they could simply select them in various clinical settings.
Finally, numerous animal studies demonstrated that EP (a lipophilic ester derivative of pyruvate) in fluid resuscitation, such as the Ringer’s ethyl pyruvate solution (REPS), functions as well as sodium pyruvate (105), which strongly supports the effects of sodium pyruvate in the novel fluids. However, EP cannot correct severe metabolic acidosis, even if hyperlactatemia is improved (113), probably due to its hydrolysis to the pyruvate moiety with [H+] production. It is worth noting that EP works in animals but not in humans (114), and it was failed in a phase II clinical trial in 2009 (115). Moreover, a recent doubt was also raised about its clinical use (116), whereas sodium pyruvate is demonstrably effective and safe in humans, as indicated in many clinical tests over the last half century.
Conclusion
Pyruvate is a multifactorial beneficial anion superior to the current anions, such as acetate, bicarbonate, chloride, citrate, lactate, and others, found in crystalloids and colloid fluids used for resuscitation. Pyruvate can protect metabolic homeostasis and multi-organ function in varying injuries in addition to avoiding iatrogenic adverse effects, such as resuscitation injury, of current fluids used in clinical resuscitation. Regarding the overall concept, the clinical advent of pyruvate-enriched formulations introduces a novel generation of fluid therapy to overcome the limitations of current fluids and be the first preferable fluid employed in most patients. Pyruvate applications would end the fluid debate; profoundly improve prognostic outcomes in ICU patients, especially those with diabetes and older patients; shorten the hospital stay; and enhance the quality of social healthcare. Clinicians and drug manufacturers should recognize that the long-term instability of pyruvate aqueous solutions and para-pyruvate cytotoxicity in vitro are almost not limitations or barriers to the pharmaceutical manufacturing of pyruvate-enriched fluids for clinical use. Randomized clinical trials of pyruvate in fluid resuscitation are urgently warranted.
Author’s Note
The author is a retired independent medical researcher. The opinions or assertions contained herein are not a reflection of the view of Fresenius Medical Care, Dialysis Centers in Chicago, IL, United States.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
FQZ prepared the entire manuscript draft and finally completed and revised the current version of the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the author.
Abbreviations
AGEs, advanced glycation end products; ATP, adenosine triphosphate; AR, acetated Ringer’s solution; DCA, dichloroacetate; EP, ethyl pyruvate; GSH/GSSG, glutathione reduced form/oxidative form; HES, hydroxyethyl starch; HIF-1 α -EPO, hypoxia-inducible factor-1 α -erythropoietin; [H+], hydrogen, proton; ICU, intensive care unit; IGDT, individualized goal-directed therapy; IV, intravenous; LDH, lactate dehydrogenase; L/P, lactate/pyruvate ratio; L-PDS, lactate-based peritoneal dialysis solution; LR, lactated Ringer’s solution; NAD+/NADH, nicotinamide adenine dinucleotide oxidative form/reduced form; NS, normal saline; ORS, oral rehydration solution/salt; paO2, partial pressure of arterial oxygen; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinas; PDP2, pyruvate dehydrogenase phosphatase 2; PC, pyruvate carboxylase; PHD, prolyl hydroxylase domain; PK, pyruvate kinase; P-PDS, pyruvate-based PDS; RBCs, red blood cells; TCA, tricarboxylic acid cycle.
References
1. Hoorn EJ. Intravenous fluids: balancing solutions. J Nephrol. (2017) 30:485–92. doi: 10.1007/s40620-016-0363-9
2. Boer C, Bossers SM, Koning NJ. Choice of fluid type: physiological concepts and perioperative indications. Br J Anaesth. (2018) 120:384–96. doi: 10.1016/j.bja.2017.10.022
3. Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. (1983) 71:726–35. doi: 10.1172/jci110820
4. Prough DS, Bidani A. Hyperchloremic metabolic acidosis is a predictable consequence of intraoperative infusion of 0.9% saline. Anesthesiology. (1999) 90:1247–9. doi: 10.1097/00000542-199905000-00003
5. Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. (2012) 256:18–24.
6. Petnak T, Thongprayoon C, Cheungpasitporn W, Bathini T, Vallabhajosyula S, Chewcharat A, et al. Serum chloride levels at hospital discharge and one-year mortality among hospitalized patients. Med Sci. (2020) 8:22. doi: 10.3390/medsci8020022
7. McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0.9% saline for intra-operative fluid replacement. Anaesthesia. (1994) 49:779–81.
8. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. (2018) 378:829–39. doi: 10.1056/NEJMoa1711584
9. Self WH, Evans CS, Jenkins CA, Brown RM, Casey JD, Collins SP, et al. Clinical effects of balanced crystalloids vs saline in adults with diabetic ketoacidosis: a subgroup analysis of cluster randomized clinical trials. JAMA Netw Open. (2020) 3:e2024596. doi: 10.1001/jamanetworkopen.2020.24596
10. Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. (2018) 378:819–28. doi: 10.1056/NEJMoa1711586
11. Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the basics randomized clinical trial. JAMA. (2021) 326:1–12. doi: 10.1001/jama.2021.11684
12. Jahangir A, Sahra S, Niazi MRK, Siddiqui FS, Anwar MY, Jahangir A, et al. Comparison of normal saline solution with low-chloride solutions in renal transplants: a meta-analysis. Kidney Res Clin Pract. (2021) 40:484–95. doi: 10.23876/j.krcp.21.027
13. Broder G, Weil MH. Excess lactate: an index of reversibility of shock in human patients. Science. (1964) 143:1457–9. doi: 10.1126/science.143.3613.1457
14. Gunnerson KJ, Saul M, He S, Kellum JA. Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Crit Care. (2006) 10:R22. doi: 10.1186/cc3987
15. Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. (2010) 14:R25. doi: 10.1186/cc8888
16. Zitek T, Skaggs ZD, Rahbar A, Patel J, Khan M. Does intravenous lactated Ringer’s solution raise serum lactate? J Emerg Med. (2018) 55:313–8. doi: 10.1016/j.jemermed.2018.05.031
17. Wang Y, Huang Y, Yang J, Zhou FQ, Zhao L, Zhou H. Pyruvate is a prospective alkalizer to correct hypoxic lactic acidosis. Mil Med Res. (2018) 5:13. doi: 10.1186/s40779-018-0160-y
18. Redant S, Hussein H, Mugisha A, Attou R, De Bels D, Honore PM, et al. Differentiating hyperlactatemia type A from type B: how does the lactate/pyruvate ratio help? J Transl Int Med. (2019) 7:43–5. doi: 10.2478/jtim-2019-0010
19. Curran JD, Major P, Tang K, Bagshaw SM, Dionne JC, Menon K, et al. Comparison of balanced crystalloid solutions: a systematic review and meta-analysis of randomized controlled trials. Crit Care Explor. (2021) 3:e0398. doi: 10.1097/CCE.0000000000000398
20. McCague A, Bowman N, Wong DT. Lactic acidosis after resuscitation with sodium acetate. J Surg Res. (2012) 173:362–4. doi: 10.1016/j.jss.2010.10.028
21. Hu S, Dai YL, Gao MJ, Wang XN, Wang HB, Dou YQ, et al. Pyruvate as a novel carrier of hydroxyethyl starch 130/0.4 may protect kidney in rats subjected to severe burns. J Surg Res. (2018) 225:166–74. doi: 10.1016/j.jss.2018.01.003
22. Kampmeier TG, Ertmer C editors. Individualized goal-directed therapy: the challenge with the fluids. Anesth Analg. (2020) 130:596–8. doi: 10.1213/ANE.0000000000004525
23. Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Effect of slower vs faster intravenous fluid bolus rates on mortality in critically ill patients: the basics randomized clinical trial. JAMA. (2021) 326:830–8. doi: 10.1001/jama.2021.11444
24. Gou D, Tan H, Cai H, Zhou F. Pyruvate effects on red blood cells during in vitro cardiopulmonary bypass with dogs’ blood. Artif Organs. (2012) 36:988–91.
25. Dahl NA, Balfour WM. Prolonged anoxic survival due to anoxia preexposure: brain ATP, lactate, and pyruvate. Am J Physiol. (1964) 207:452–6. doi: 10.1152/ajplegacy.1964.207.2.452
26. Borle AB, Stanko RT. Pyruvate reduces anoxic injury and free radical formation in perfused rat hepatocytes. Am J Physiol. (1996) 270:G535–40. doi: 10.1152/ajpgi.1996.270.3.G535
27. Xia S, Chen G, Wang B, Yin Y, Sun Z, Zhao J, et al. Addition of sodium pyruvate to stored red blood cells attenuates liver injury in a murine transfusion model. Mediators Inflamm. (2016) 2016:3549207. doi: 10.1155/2016/3549207
28. So PW, Fuller BJ. Enhanced energy metabolism during cold hypoxic organ preservation: studies on rat liver after pyruvate supplementation. Cryobiology. (2003) 46:295–300. doi: 10.1016/s0011-2240(03)00047-6
29. Kumar VHS, Gugino S, Nielsen L, Chandrasekharan P, Koenigsknecht C, Helman J, et al. Protection from systemic pyruvate at resuscitation in newborn lambs with asphyxial cardiac arrest. Physiol Rep. (2020) 8:e14472. doi: 10.14814/phy2.14472
30. Jaimes R 3rd, Kuzmiak-Glancy S, Brooks DM, Swift LM, Posnack NG, Kay MW. Functional response of the isolated, perfused normoxic heart to pyruvate dehydrogenase activation by dichloroacetate and pyruvate. Pflugers Arch. (2016) 468:131–42. doi: 10.1007/s00424-015-1717-1
31. Sharma P, Walsh KT, Kerr-Knott KA, Karaian JE, Mongan PD. Pyruvate modulates hepatic mitochondrial functions and reduces apoptosis indicators during hemorrhagic shock in rats. Anesthesiology. (2005) 103:65–73. doi: 10.1097/00000542-200507000-00013
32. Olenchock BA, Vander Heiden MG. Pyruvate as a pivot point for oncogene- induced senescence. Cell. (2013) 153:1429–30. doi: 10.1016/j.cell.2013.06.001
33. Zhang XM, Deng H, Tong JD, Wang YZ, Ning XC, Yang XH, et al. Pyruvate-enriched oral rehydration solution improves glucometabolic disorders in the kidneys of diabetic db/db nice. J Diabetes Res. (2020) 2020:2817972. doi: 10.1155/2020/2817972
34. Guan LD, Wang ZL, Zhao L, Wang B, Wang GY, Wei GZ, et al. Protective effect of sodium pyruvate on ischemia/reperfusion injury of rats subjected to hemorrhagic shock. Chin J Appl Physiol. (2007) 23:264–8.
35. Sanchez PKM, Khazaei M, Gatineau E, Geravandi S, Lupse B, Liu H, et al. LDHA is enriched in human islet alpha cells and upregulated in type 2 diabetes. Biochem Biophys Res Commun. (2021) 568:158–66. doi: 10.1016/j.bbrc.2021.06.065
36. Rao Y, Gammon ST, Sutton MN, Zacharias NM, Bhattacharya P, Piwnica- Worms D. Excess exogenous pyruvate inhibits lactate dehydrogenase activity in live cells in an MCT1-dependent manner. J Biol Chem. (2021) 297:100775. doi: 10.1016/j.jbc.2021.100775
37. Varma SD, Hegde KR, Kovtun S. Attenuation and delay of diabetic cataracts by antioxidants: effectiveness of pyruvate after onset of cataract. Ophthalmologica. (2005) 219:309–15. doi: 10.1159/000086117
38. Qi YX, Fu JD, Wang YQ, Wang DL. Effects of pyruvate on retinal oxidative damage and retinal ultrastructure in diabetic rats. Int Eye Sci. (2014) 14:2143–6.
39. Petkova I, Hristov V, Petrov K, Thorn W. Oral application of sodium pyruvate in healthy persons and patients with diabetes mellitus type 1. Proc Bulgar Acad Sci. (2007) 60:579–84.
40. Inoue T, Murakami N, Ayabe T, Oto Y, Nishino I, Goto Y, et al. Pyruvate improved insulin secretion status in a mitochondrial diabetes mellitus patient. J Clin Endocrinol Metab. (2016) 101:1924–6. doi: 10.1210/jc.2015-4293
41. Zhou FQ. Pyruvate in the correction of intracellular acidosis: a metabolic basis as a novel superior buffer. Am J Nephrol. (2005) 25:55–63. doi: 10.1159/000084141
42. Mongan PD, Fontana JL, Chen R, Bunger R. Intravenous pyruvate prolongs survival during hemorrhagic shock in swine. Am J Physiol. (1999) 277:H2253–63. doi: 10.1152/ajpheart.1999.277.6.H2253
43. Flaherty DC, Hoxha B, Sun J, Gurji H, Simecka JW, Mallet RT, et al. Pyruvate-fortified fluid resuscitation improves hemodynamic stability while suppressing systemic inflammation and myocardial oxidative stress after hemorrhagic shock. Mil Med. (2010) 175:166–72. doi: 10.7205/milmed-d-09-00161
44. Hu S, Bai XD, Liu XQ, Wang HB, Zhong YX, Fang T, et al. Pyruvate Ringer’s solution corrects lactic acidosis and prolongs survival during hemorrhagic shock in rats. J Emerg Med. (2013) 45:885–93. doi: 10.1016/j.jemermed.2013.04.062
45. Liu R, Hu XH, Wang SM, Guo SJ, Li ZY, Bai XD, et al. Pyruvate in oral rehydration salt improves hemodynamics, vasopermeability and survival after burns in dogs. Burns. (2016) 42:797–806. doi: 10.1016/j.burns.2016.01.012
46. Koga Y, Povalko N, Katayama K, Kakimoto N, Matsuishi T, Naito E, et al. Beneficial effect of pyruvate therapy on Leigh syndrome due to a novel mutation in PDH E1a gene. Brain Dev. (2012) 34:87–91. doi: 10.1016/j.braindev.2011.03.003
47. Koga Y, Povalko N, Inoue E, Nashiki K, Tanaka M. Biomarkers and clinical rating scales for sodium pyruvate therapy in patients with mitochondrial disease. Mitochondrion. (2019) 48:11–5. doi: 10.1016/j.mito.2019.02.001
48. Mallet RT, Sun J, Knott EM, Sharma AB, Olivencia-Yurvati AH. Metabolic cardioprotection by pyruvate: recent progress. Exp Biol Med. (2005) 230:435–43. doi: 10.1177/153537020523000701
49. Xiao W, Loscalzo J. Metabolic responses to reductive stress. Antioxid Redox Signal. (2020) 32:1330–47. doi: 10.1089/ars.2019.7803
50. Liu R, Wang SM, Liu XQ, Guo SJ, Wang HB, Hu S, et al. Pyruvate alleviates lipid peroxidation and multiple-organ dysfunction in rats with hemorrhagic shock. Am J Emerg Med. (2016) 34:525–30. doi: 10.1016/j.ajem.2015.12.040
51. Hu S, Lin ZL, Zhao ZK, Liu R, Ma L, Luo HM, et al. Pyruvate is superior to citrate in oral rehydration solution in the protection of intestine via hypoxia-inducible factor-1 activation in rats with burn injury. J Parenter Enteral Nutr. (2016) 40:924–33. doi: 10.1177/0148607115577817
52. Ryou MG, Liu R, Ren M, Sun J, Mallet RT, Yang SH. Pyruvate protects the brain against ischemia-reperfusion injury by activating the erythropoietin signaling pathway. Stroke. (2012) 43:1101–7. doi: 10.1161/STROKEAHA.111.620088
53. Kim JY, Lee SH, Bae IH, Shin DW, Min D, Ham M, et al. Pyruvate protects against cellular senescence through the control of mitochondrial and lysosomal function in dermal fibroblasts. J Invest Dermatol. (2018) 138:2522–30. doi: 10.1016/j.jid.2018.05.033
54. Zhang XM, Wang YZ, Tong JD, Ning XC, Zhou FQ, et al. Pyruvate alleviates high glucose-induced endoplasmic reticulum stress and apoptosis in HK-2 cells. FEBS Open Bio. (2020) 10:827–34. doi: 10.1002/2211-5463.12834
55. Li M, Zhou S, Chen C, Ma L, Luo D, Tian X, et al. Therapeutic potential of pyruvate therapy for patients with mitochondrial diseases: a systematic review. Ther Adv Endocrinol Metab. (2020) 11:2042018820938240. doi: 10.1177/2042018820938240
56. Scott GF, Nguyen AQ, Cherry BH, Hollrah RA, Salinas I, Williams AG Jr., et al. Featured Article: pyruvate preserves antiglycation defenses in porcine brain after cardiac arrest. Exp Biol Med. (2017) 242:1095–103. doi: 10.1177/1535370217703353
57. Patterson JN, Cousteils K, Lou JW, Manning Fox JE, MacDonald PE, Joseph JW. Mitochondrial metabolism of pyruvate is essential for regulating glucose-stimulated insulin secretion. J Biol Chem. (2014) 289:13335–46. doi: 10.1074/jbc.M113.521666
58. Xu J, Han J, Long YS, Epstein PN, Liu YQ. The role of pyruvate carboxylase in insulin secretion and proliferation in rat pancreatic beta cells. Diabetologia. (2008) 51:2022–30. doi: 10.1007/s00125-008-1130-9
59. Nagasaka H, Komatsu H, Inui A, Nakacho M, Morioka I, Tsukahara H, et al. Circulating tricarboxylic acid cycle metabolite levels in citrin-deficient children with metabolic adaptation, with and without sodium pyruvate treatment. Mol Genet Metab. (2017) 120:207–12. doi: 10.1016/j.ymgme.2016.12.011
60. Ning LP, Wang ZK, Zhou FQ, Guo J, Wang QI, Yuan MQ, et al. Protective effect of sodium pyruvate on islet P-cell dysfunction in the mice with multiple organ dysfunction syndrome induced by severe scald. Med Pharm J Chin PLA. (2020) 32:11–5. doi: 10.3969/j.issn.2095-140X.2020.07.003
61. Zhou FQ. Pyruvate may be a novel intervention of diabetes. Intervent Obes Diab. (2021) 5:415–8. doi: 10.31031/IOD.2021.05.000602
62. Katsyuba E, Romani M, Hofer D, Auwerx J. NAD+ homeostasis in health and disease. Nat Metab. (2020) 2:9–31. doi: 10.1038/s42255-019-0161-5
63. Sabbatinelli J, Prattichizzo F, Olivieri F, Procopio AD, Rippo MR, Giuliani A. Where metabolism meets senescence: focus on endothelial cells. Front Physiol. (2019) 10:1523. doi: 10.3389/fphys.2019.01523
64. Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, openlabel, pilot study. EBioMedicine. (2019) 40:554–63. doi: 10.1016/j.ebiom.2018.12.052
65. Han C, Yang H, Li B, Wang Z. Exogenous pyruvate facilitates ultraviolet B- induced DNA damage repair by promoting H3K9 acetylation in keratinocytes and melanocytes. Biomed Pharmacother. (2020) 126:110082. doi: 10.1016/j.biopha.2020.110082
66. Zhou FQ. NAD+, senolytics, or pyruvate for healthy aging? Nutr Metab Insights. (2021) 14:1–8. doi: 10.1177/11786388211053407
67. Dai ZL, Wu J, Meng C, Zeng F, Yang Y, Yao SL. Ringer’s malate solution protects against the multiple organ injury and dysfunction caused by hemorrhagic shock in rats. Shock. (2012) 38:268–74. doi: 10.1097/SHK.0b013e318264e664
68. Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG Jr. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. (2000) 278:H1294–8. doi: 10.1152/ajpheart.2000.278.4.H1294
69. Liu R, Wang SM, Li ZY, Yu W, Zhang HP, Zhou FQ. Pyruvate in reduced osmolarity oral rehydration salt corrected lactic acidosis in sever scald rats. J Surg Res. (2018) 226:173–80. doi: 10.1016/j.jss.2018.01.018
70. Huang Y, Du J, Zhao L, Zhao J, You G, Yang J, et al. Pyruvate ameliorates in vitro oxidative damage resulting from lethal methemoglobinemia. Int J Clin Exp Med. (2018) 11:13055–64.
71. Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo. J Clin Invest. (1991) 88:1886–93. doi: 10.1172/JCI115511
72. Han XC, Hu S, Liu XQ, Fang T, Bai XD. Study on the effect of pyruvate on the kidney vascular permeability in rats with scalded injury. China Med Engine. (2011) 19:4–6.
73. Johnson AC, Zager RA. Renal cortical pyruvate as a potentially critical mediator of acute kidney injury. Nephron Clin Pract. (2014) 127:129–32. doi: 10.1159/000363547
74. Lee YJ, Kang IJ, Bunger R, Kang YH. Mechanisms of pyruvate inhibition of oxidant-induced apoptosis in human endothelial cells. Microvasc Res. (2003) 66:91–101. doi: 10.1016/s0026-2862(03)00052-9
75. Slovin PN, Huang CJ, Cade JR, Wood CE, Nasiroglu O, Privette M, et al. Sodium pyruvate is better than sodium chloride as a resuscitation solution in a rodent model of profound hemorrhagic shock. Resuscitation. (2001) 50:109–15. doi: 10.1016/s0300-9572(01)00325-2
76. Koustova E, Rhee P, Hancock T, Chen H, Inocencio R, Jaskille A, et al. Ketone and pyruvate Ringer’s solutions decrease pulmonary apoptosis in a rat model of severe hemorrhagic shock and resuscitation. Surgery. (2003) 134:267–74. doi: 10.1067/msy.2003.245
77. Olivencia-Yurvati AH, Blair JL, Baig M, Mallet RT. Pyruvate-enhanced cardioprotection during surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. (2003) 17:715–20. doi: 10.1053/j.jvca.2003.09.007
78. Yu W, Hu S, Xie ZY, He ZJ, Luo HM, Lin HY, et al. Pyruvate oral rehydration solution improved visceral function and survival in shock rats. J Surg Res. (2015) 193:344–54. doi: 10.1016/j.jss.2014.06.037
79. Ing TS, Zhou XJ, Yu AW, Zhou FQ, Vaziri ND. Effects of pyruvate- based or lactate-based peritoneal dialysis solutions on neutrophil intracellular pH. Int J Artif Organs. (1997) 20:255–60.
80. Wu YT, Wu ZL, Jiang XF, Li S, Zhou FQ. Pyruvate preserves neutrophilic superoxide production in acidic, high glucose-enriched peritoneal dialysis solutions. Artif Organs. (2003) 27:276–81. doi: 10.1046/j.1525-1594.2003.69962.x
81. Hu S, Ma L, Luo HM, Lin ZL, Wang XQ, Jia YH, et al. Pyruvate is superior to reverse visceral hypoperfusion in peritoneal resuscitation from hemorrhagic shock in rats. Shock. (2014) 41:355–61. doi: 10.1097/SHK.0000000000000113
82. Lu XG, Kang X, Zhou FQ, Wang XZ, Guo S, Fan ZW, et al. Effects of pyruvate-enriched peritoneal dialysis solution on intestinal barrier in peritoneal resuscitation from hemorrhagic shock in rats. J Surg Res. (2015) 193:368–76. doi: 10.1016/j.jss.2014.06.053
83. Zhang JJ, Shen HQ, Deng JT, Jiang LL, Zhang QY, Xiong Y, et al. Effect of peritoneal dialysis solution with different pyruvate concentrations on intestinal injury. Exp Biol Med. (2020) 245:644–53. doi: 10.1177/1535370220909332
84. Varadhan KK, Constantin-Teodosiu D, Constantin D, Greenhaff PL, Lobo DN. Inflammation-mediated muscle metabolic dysregulation local and remote to the site of major abdominal surgery. Clin Nutr. (2018) 37:2178–85. doi: 10.1016/j.clnu.2017.10.020
85. Atkins R, Constantin-Teodosiu D, Varadhan KK, Constantin D, Lobo DN, Greenhaff PL. Major elective abdominal surgery acutely impairs lower limb muscle pyruvate dehydrogenase complex activity and mitochondrial function. Clin Nutr. (2021) 40:1046–51. doi: 10.1016/j.clnu.2020.07.006
86. Zhou FQ. Pyruvate potential effects on Covid-19 virus infection: novel fluid intervention and prevention. London J Med Health Res. (2020) 20:69–77.
87. Tanwar O, Soni A, Prajapat P, Shivhare T, Pandey P, Samaiya PK, et al. Ethyl pyruvate as a potential defense intervention against cytokine storm in COVID-19? ACS Omega. (2021) 6:7754–60. doi: 10.1021/acsomega.1c00157
88. Peltz M, Milchgrub S, Jessen ME, Meyer DM. Effect of pyruvate and HEPES on rat lung allograft acidosis and cell death after long-term hypothermic storage. Transplant Proc. (2010) 42:2771–6. doi: 10.1016/j.transproceed.2010.06.004
89. Youmans JB, Corlette MB, Patton W. A clinical study of pyruvic acid metabolism with special reference to vitamin B1 deficiency. Trans Am Clin Climatol Assoc. (1938) 54:141–51.
90. Bueding E, Goldfarb W. Blood changes following injections in man glucose, lactate, and pyruvate. J Biol Chem. (1943) 147:33–40.
91. Takanami A, Ohya S, Goto Y. Urinary excretion of pyruvic acid, citric acid and alpha-ketoglutaric acid after the intravenous injection of pyruvate and fructose in diabetic patients. Tohoku J Exp Med. (1960) 72:163–8. doi: 10.1620/tjem.72.163
92. Mateva L, Petkova I, Petrov K, Beniozef D, Bojilova M, Vankova L, et al. Ten-Day course of sodium pyruvate infusions in patients with chronic liver diseases (CLD). Jpn Pharmacol Ther. (1996) 24:2629–39.
93. Petkova I, Mateva L, Beniozef D, Petrov K, Thorn W. Sodium pyruvate infusions in patients with alcoholic liver disease. Preliminary report. Acta Physiol Pharmacol Bulg. (2000) 25:103–8.
94. Hermann HP, Pieske B, Schwarzmuller E, Keul J, Just H, Hasenfuss G. Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study. Lancet. (1999) 353:1321–3. doi: 10.1016/s0140-6736(98)06423-x
95. Schillinger W, Hunlich M, Sossalla S, Hermann HP, Hasenfuss G. Intracoronary pyruvate in cardiogenic shock as an adjunctive therapy to catecholamines and intra-aortic balloon pump shows beneficial effects on hemodynamics. Clin Res Cardiol. (2011) 100:433–8. doi: 10.1007/s00392-010-0261-4
96. Moorhouse JA. Pyruvate-tolerance tests in healthy and diabetic subjects. Lancet. (1964) 1:689–93. doi: 10.1016/s0140-6736(64)91519-3
97. Van Erven PM, Gabreels FJ, Wevers RA, Doesburg WH, Ruitenbeek W, Renier WO, et al. Intravenous pyruvate loading test in Leigh syndrome. J Neurol Sci. (1987) 77:217–27. doi: 10.1016/0022-510x(87)90124-9
98. Matthys D, Van Coster R, Verhaaren H. Fatal outcome of pyruvate loading test in child with restrictive cardiomyopathy. Lancet. (1991) 338:1020–1.
99. Denfield SW, Rosenthal G, Gajarski RJ, Bricker JT, Schowengerdt KO, Price JK, et al. Restrictive cardiomyopathies in childhood. Etiologies and natural history. Tex Heart Inst J. (1997) 24:38–44.
100. Smethurst PA, Jolley J, Braund R, Proffitt S, Lynes T, Hazell M, et al. Rejuvenation of RBCs: validation of a manufacturing method suitable for clinical use. Transfusion. (2019) 59:2952–63. doi: 10.1111/trf.15426
101. Margolis SA, Coxon B. Identification and quantitation of the impurities in sodium pyruvate. Anal Chem. (1986) 58:2504–10. doi: 10.1021/ac00125a033
102. Veech RL. Fluid Therapy with L-Lactate and/or Pyruvate Anions. PCTWO. 87/03808. Munich: European Patent Office (1987).
103. Nath KA. Use of Pyruvate to Treat Acute Renal Failure. US Patent No. 5,210,098. Alexandria, VIR: USPTO (1993).
104. Chang SC, Lee I, Ting H, Chang YJ, Yang NC. Parapyruvate, an impurity in pyruvate supplements, induces senescence in human fibroblastic Hs68 cells via inhibition of the a-ketoglutarate dehydrogenase complex. J Agric Food Chem. (2018) 66:7504–13. doi: 10.1021/acs.jafc.8b01138
105. Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer’s ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. (2001) 29:1513–8. doi: 10.1097/00003246-200108000-00003
106. Hu S, Liu WW, Zhao Y, Lin ZL, Luo HM, Bai XD, et al. Pyruvate- enriched oral rehydration solution improved intestinal absorption of water and sodium during enteral resuscitation in burns. Burns. (2014) 40:693–701. doi: 10.1016/j.burns.2013.09.030
107. Zhou FQ. Pyruvate-enriched fluids as a novel medical solution and beverage. Food Sci Nutri Tech. (2021) 6:1–7.
108. Mallet RT, Olivencia-Yurvati AH, Bunger R. Pyruvate-enriched resuscitation for shock. Exp Biol Med. (2018) 243:663–4. doi: 10.1177/1535370218773717
109. Wu XQ, Li ZB, Chen WX, Wen DL, Zhang ZH, Xiong XM. The influence of different resuscitation solution on lactic acid accumulation after hemorrhagic shock: a network meta-analysis. Eur Rev Med Pharmacol Sci. (2019) 23:6707–17. doi: 10.26355/eurrev_201908_18562
110. Patino AM, Marsh RH, Nilles EJ, Baugh CW, Rouhani SA, Kayden S. Facing the shortage of IV fluids - A hospital-based oral rehydration strategy. New Engl J Med. (2018) 378:1475–7. doi: 10.1056/NEJMp1801772
111. De Moraes CG, John SWM, Williams PA, Blumberg DM, Cioffi GA, Liebmann JM. Nicotinamide and pyruvate for neuroenhancement in open-angle glaucoma: a phase 2 randomized clinical trial. JAMA Ophthalmol. (2022) 140:11–8. doi: 10.1001/jamaophthalmol.2021.4576
112. Ostermann M, Randolph AG. Resuscitation fluid composition and acute kidney injury in critical illness. N Engl J Med. (2022) 386:888–9. doi: 10.1056/NEJMe2200294
113. Venkataraman R, Kellum JA, Song M, Fink MP. Resuscitation with Ringer’s ethyl pyruvate solution prolongs survival and modulates plasma cytokine and nitrite/nitrate concentrations in a rat model of lipopolysaccharide-induced shock. Shock. (2002) 18:507–12. doi: 10.1097/00024382-200212000-00004
114. Bahar FG, Ohura K, Ogihara T, Imai T. Species difference of esterase expression and hydrolase activity in plasma. J Pharm Sci. (2012) 101:3979–88. doi: 10.1002/jps.23258
115. Bennett-Guerrero E, Swaminathan M, Grigore AM, Roach GW, Aberle LG, Johnston JM, et al. A phase II multicenter double-blind placebo-controlled study of ethyl pyruvate in high-risk patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. (2009) 23:324–9. doi: 10.1053/j.jvca.2008.08.005
Keywords: fluid therapy, resuscitation, hypoxia, metabolic acidosis, oral rehydration solution, pyruvate
Citation: Zhou FQ (2022) Pyruvate as a Potential Beneficial Anion in Resuscitation Fluids. Front. Med. 9:905978. doi: 10.3389/fmed.2022.905978
Received: 29 March 2022; Accepted: 15 June 2022;
Published: 12 July 2022.
Edited by:
Raghavan Pillai Raju, Augusta University, United StatesReviewed by:
Pushpa Sharma, Uniformed Services University of the Health Sciences, United StatesBelinda H. McCully, Western University of Health Sciences, United States
Copyright © 2022 Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang-Qiang Zhou, ZnF6aDYwMTMwQHlhaG9vLmNvbQ==
 Fang-Qiang Zhou
Fang-Qiang Zhou