- 1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Gastroenterology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Gastrointestinal Surgery, Jinshan Hospital, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4Department of Respiratory and Critical Care Medicine, Chongqing Shapingba District People's Hospital, Chongqing, China
Background: Self-expanding metallic stent (SEMS) is a palliative therapy for patients with malignant central airway obstruction (CAO) or tracheoesophageal fistula (TEF). Despite this, many patients experience death shortly after SEMS placement.
Aims: We aimed to investigate the effect of SEMS on the palliative treatment between malignant CAO and malignant TEF patients and investigate the associated prognostic factors of the 3-month survival.
Methods: We performed a single-center, retrospective study of malignant CAO or TEF patients receiving SEMS placement. Clinical data were collected using the standardized data abstraction forms. Data were analyzed using SPSS 22.0. A two-sided P-value <0.05 was statistically significant.
Results: 106 malignant patients (82 CAO and 24 TEF) receiving SEMS placement were included. The body mass index (BMI), hemoglobin levels, and albumin levels in the malignant TEF group were lower than in the malignant CAO group (all P < 0.05). The procalcitonin levels, C-reactive protein levels, and the proportion of inflammatory lesions were higher in the malignant TEF group than in the malignant CAO group (all P < 0.05). The proportion of symptomatic improvement after the SEMS placement was 97.6% in the malignant CAO group, whereas 50.0% in the malignant TEF group, with a significant difference (P = 0.000). Three months after SEMS placement, the survival rate at was 67.0%, significantly lower in the malignant TEF group than in the malignant CAO group (45.8% vs. 73.2%, P = 0.013). Multivariate analysis revealed that BMI [odds ratio (OR) = 1.841, 95% certificated interval (CI) (1.155-2.935), P = 0.010] and neutrophil percentage [OR = 0.936, 95% CI (0.883–0.993), P = 0.027] were the independent risk factors for patients who survived three months after SEMS placement.
Conclusions: We observed symptom improvement in malignant CAO and TEF patients after SEMS placement. The survival rate in malignant TEF patients after SEMS placement was low, probably due to aspiration pneumonitis and malnutrition. Therefore, we recommend more aggressive treatment modalities in patients with malignant TEF, such as strong antibiotics, nutrition support, and strategic ventilation. More studies are needed to investigate the prognostic factors in patients with malignant airway disorders receiving SEMS placement.
Introduction
Malignant central airway obstruction (CAO) and tracheoesophageal fistula (TEF) were the major malignant airway disorders severely affecting the patients' mobility and quality of life (1, 2). Malignant CAO is the obstruction in the trachea and mainstem bronchi due to extrinsic compression or direct invasion of primary lung cancer, metastatic lesions from distant tumors, or anatomically adjacent airway tumors (3–5). Malignant TEF is the pathological channel between the trachea and mainstem bronchi and esophagus due to esophageal tumor or primary lung cancer (6, 7). Patients with malignant CAO or TEF could present with dyspnea, hemoptysis, fever, cough, or pneumonia, resulting in a poor prognosis (3–7). Self-expanding metallic stent (SEMS) is a palliative therapy for malignant CAO and malignant TEF patients, rapidly relieving the symptoms and improving quality of life but not prolonging survival (8–11). Despite this, many malignant CAO or TEF patients continue to experience disease progression and even death within a short period after SEMS placement (12). Therefore, we aim to investigate the effect of SEMS on the palliative treatment of malignant CAO and malignant TEF and the associated prognostic factors.
Materials and Methods
Patients
We performed a single-center, retrospective study of malignant CAO or TEF patients who received palliative SEMS placement from July 2013 to March 2021 in the First Affiliated Hospital of Chongqing Medical University in Chongqing, China. The inclusion criteria were: (1) patients diagnosed with malignancy pathologically, (2) chest computed tomography (CT) showing airway obstruction or incompleteness and then bronchoscopy with transbronchial lung biopsy confirming malignant CAO or TEF, (3) the risk of airway re-collapse after the debridement or the purpose of fistula closure, (4) written informed consent for airway intervention including SEMS placement; the exclusion criteria were (1) without cytological or histological confirmation of malignancy, (2) follow-up <1 month, (3) lack of clinical or bronchoscopic information, (4) double stent placement in the esophagus and trachea. Standardized abstraction forms were used to gather information from electronic medical records on demographic features, clinical characteristics, laboratory and radiological findings, airway disorders' details (etiology, location, and degree of stenosis et al.), SEMS shapes, and clinical outcomes. The institutional scientific committee approved the publication of this retrospective study.
Procedures
Flexible bronchoscopy confirmed the malignant CAO or TEF and visualized the lesion location (upper, middle, or lower third trachea, carina, right main bronchus, and proximal or distal left main bronchus), the stenosis type (intrinsic stenosis, extrinsic stenosis, mixed stenosis), and the stenosis degree (<25%, 25–50, 51–75%, 76–90%, and 90% to complete obstruction in cross-sectional area of the target airway, respectively) (13, 14). The SEMS from Micro-tech Co. Ltd or Boston Scientific Co. Ltd was used in the management of malignant CAO or TEF. The SEMS was accustomed according to the airway 3D reconstruction and the bronchoscopic manifestation. The airway 3D reconstruction at the end of inspiration enabled the diameter detection of the stenosed segment and the adjacent normal airway (15, 16). The SEMS diameter was 2 mm smaller than the average normal diameter of the target airway in patients with malignant CAO; the SEMS diameter was 2 mm greater than the average normal diameter of the target airway in patients with malignant TEF, reducing the possibility of stent migration. The target airway diameter was measured simultaneously at the proximal and distal ends of the adjacent normal airway. The length of SEMS was 10 mm longer both proximally and distally than the edges of the lesion estimated from bronchoscopy. The type of stent was accustomed according to the location of the disorders and their relationship to the surrounding branch: Y-shaped SEMS for malignant airway disorders involving the carina or main bronchi, and straight SEMS for disorders involving the upper and middle third trachea and distal left main bronchus. Covered or uncovered SEMS was preferred in patients with malignant CAO, and covered SEMS was chosen to seal the fistula in patients with malignant TEF. The covered SEMS was not recommended if it might lay across a patent airway side branch, causing the post-obstructive pneumonitis.
Mechanical debulking with rigid bronchoscopy and tumor ablation with laser, electrocautery, brachytherapy, and cryotherapy were applied under general anesthesia before the SEMS placement. The guidewire was passed via a flexible bronchoscope, and the SEMS delivery device was passed through the guidewire. Then, the SEMS was deployed under direct bronchoscopic observation, achieving a 100% immediate technical success rate. The forceps were used to adjust the SEMS into the desired position by grabbing the proximal suture of the SEMS. Balloon dilatation was employed within the stent to speed the enlarge of the SEMS if necessary.
Statistical Analysis
Normally distributed data were expressed as mean ± standard deviation ( ± s), and a t-test or ANOVA was used to compare between groups. Non-normally distributed data were expressed as a median value and interquartile ranges (IQR, 25–75th quartiles), and Mann Whitney test or Kruskal-Wallis test was used to compare between groups. Qualitative data were compared using the Fisher exact test or Chi-squared test. Multivariate logistic regression analysis was performed to determine the risk factors for the 3-month survival in those patients receiving SEMS placement, including the variables that had reached statistical significance in the univariate analysis. We estimated the variance inflation factors to check for multicollinearity before running the multivariate logistic regression analysis. Data were analyzed using SPSS 22.0 statistical software. A two-sided P-value <0.05 was considered to be statistically significant.
Results
Demographic Features and Clinical Characteristics
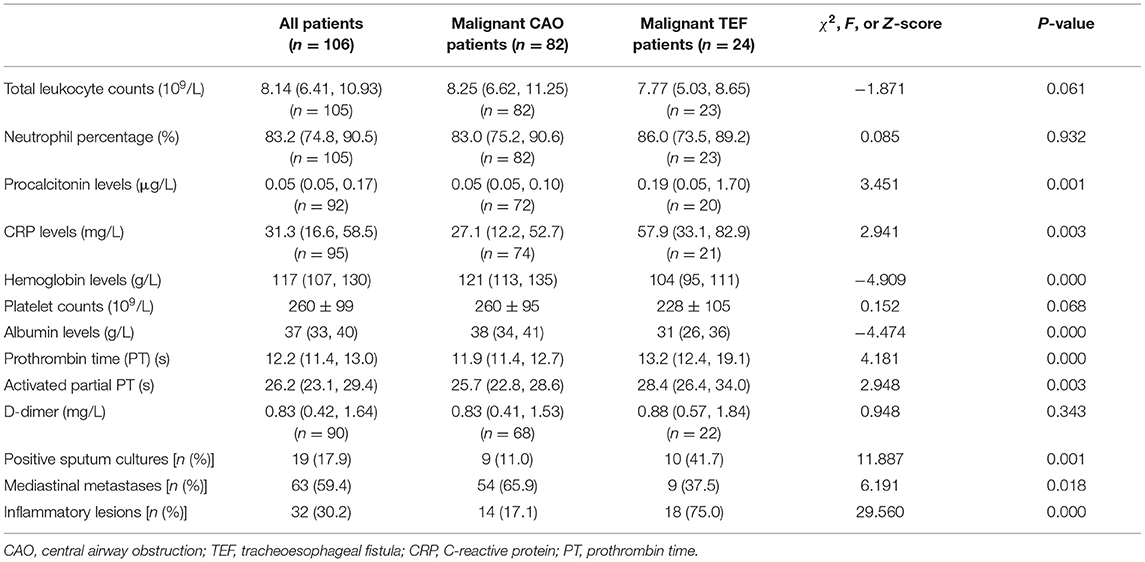
We wound consider the SEMS placement if there has been the risk of airway re-collapse after the debridement or the purpose of fistula closure. Patients who did not require SEMS placement were not included in this study. Among 128 patients receiving SEMS placement in our department, a total of 106 patients (73 male and 43 female), including 82 malignant CAO and 24 malignant TEF patients, were finally enrolled according to the above inclusion and exclusion criteria. Twenty two patients were excluded for the following reasons: benign CAO (n = 10), benign TEF (n = 2), without clinical or bronchoscopic information (n = 4), follow-up <1 month (n = 6). The demographic features and clinical characteristics of these patients are listed in Table 1. The mean age of these patients was 62.6 ± 8.9 years, median height 1.65 m (range 1.50–1.77 m) and median weight 56.00 kg (range 30.0–90.0 kg), with no significant difference between the malignant CAO and malignant TEF groups. The body mass index (BMI) was significantly lower in the malignant TEF group than in the malignant CAO group (19.00 ± 1.74 vs. 21.61 ± 3.56 kg/m2, P = 0.013).
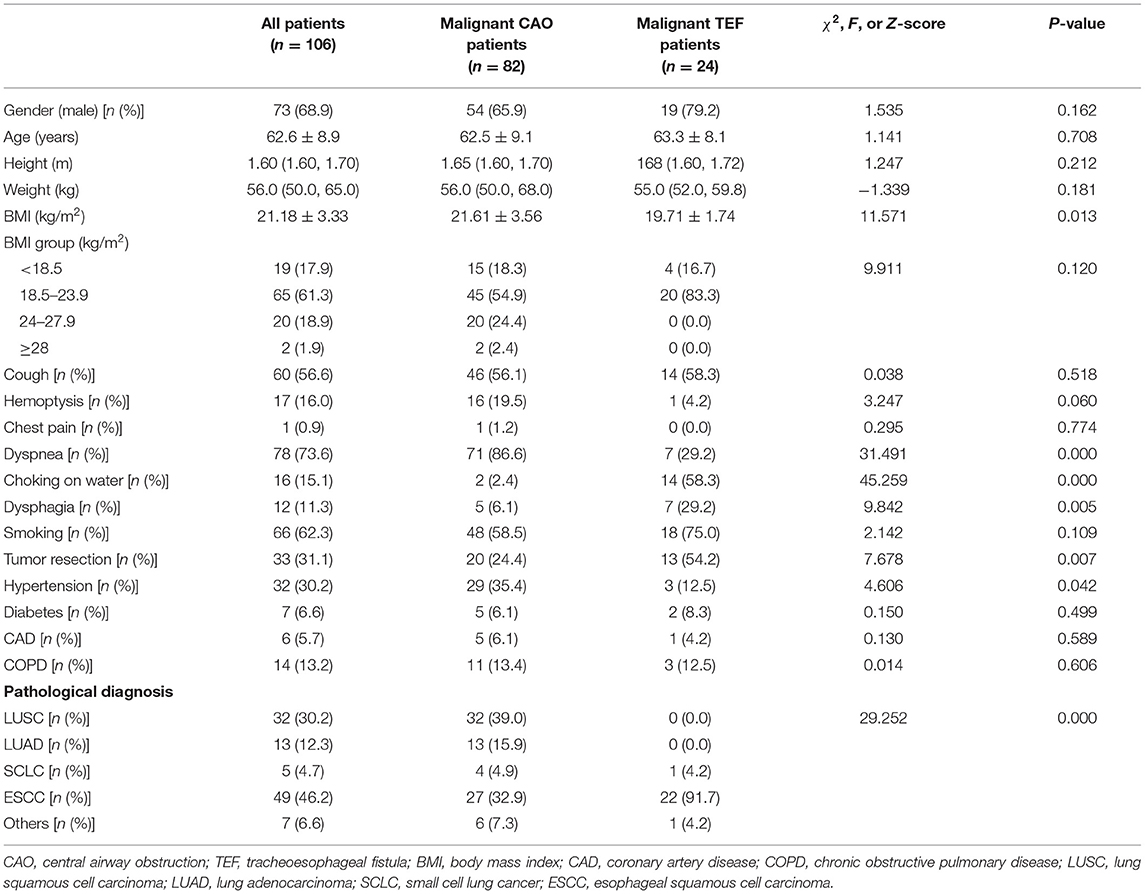
Table 1. Demographic features and clinical characteristics between malignant CAO and malignant TEF patients.
The most common symptoms in the malignant CAO group were dyspnea (71/82, 86.6%), cough (46/82, 56.1%), and hemoptysis (16/82, 19.5%), whereas in the malignant TEF group were choking on water (14/24, 58.3%), cough (14/24, 58.3%), dysphagia (7/24, 29.2%), and dyspnea (7/24, 29.2%). Malignant TEF group reported more frequent symptoms of choking on water (58.3% vs. 2.4%, P = 0.000) and dysphagia (29.2% vs. 6.1%, P = 0.005) than malignant CAO group. The proportion of coexisting hypertension was higher in the malignant CAO group than in the malignant TEF group (35.4% vs. 12.5, P = 0.042). The proportion receiving tumor resection surgery was higher in the malignant TEF group than in the malignant CAO group (54.2% vs. 24.4%, P = 0.007). The most common pathological type in the malignant TEF group was esophagus squamous cell carcinoma (22/24, 91.7%), and in the malignant CAO group was lung squamous cell carcinoma (32/82, 39.0%), followed by esophagus squamous cell carcinoma (27/82, 32.9%) and lung adenocarcinoma (13/82, 15.9%,), with a significant difference between the pathological types in the malignant TEF and malignant CAO groups (P = 0.000).
Laboratory and Radiological Findings
The Laboratory and radiological findings of these patients are listed in Table 2. The differences in total leukocyte counts and neutrophil ratios between the malignant TEF and malignant CAO groups were not statistically significant (P > 0.05). The procalcitonin levels [0.19 (0.05, 1.70) vs. 0.05 (0.05, 0.10) μg/L, P = 0.001] and C-reactive protein levels [57.90 (33.10, 82.95) vs. 27.09 (12.20, 52.65) mg/L, P = 0.003] were significantly higher in the malignant TEF group than in the malignant CAO group. In contrast, hemoglobin levels [103.5 (95.25, 110.5) vs. 121.00 (112.75, 135.00) g/L, P = 0.000] and albumin levels [30.50 (26.25, 35.75) vs. 38.00 (34.00, 41.00) g/L, P = 0.000] were significantly lower in the malignant TEF group than in the malignant CAO group. The proportion of positive sputum cultures was significantly higher in the malignant TEF group than in the malignant CAO group (41.7% vs. 11.0%, P = 0.002). The etiological agents most frequently isolated in sputum cultures were gram-negative bacilli (Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii), followed by Candida albicans in these patients (data not shown). The prothrombin time [13.2 (12.4, 19.1) vs. 11.9 (11.4, 12.7) s, P = 0.000] and activated partial prothrombin time [28.4 (26.4, 34.0) vs. 25.7 (22.8, 28.6) s, P = 0.003] were longer in the malignant TEF group than in the malignant CAO group. The proportion of mediastinal metastases found on chest CT was significantly higher in the malignant CAO group than in the malignant TEF group (65.9% vs. 37.5%, P = 0.018). In contrast, the proportion of inflammatory lesions (patchy shadows or solid shadows) found on chest CT was significantly higher in the malignant TEF group than in the malignant CAO group (75.0% vs. 17.1%, P = 0.000).
Airway Disorders' Details, SEMS Choices, and Clinical Outcomes
The airway disorders, SEMS choices, and clinical outcomes of these patients are listed in Table 3. The most frequently involved location in the airway was the lower trachea (56/106, 52.8%), followed by proximal left main bronchus (42/106, 39.6%), right main bronchus (41/106, 38.7%), and carina (36/106, 34.0%). The proportion of multiple-location involvement was higher in the malignant CAO group than in the malignant TEF (72.0% vs. 25.0%, P = 0.000). The most common stenosis type was extrinsic stenosis (54/106, 50.9%), followed by mixed stenosis (40/106, 37.7%) and intrinsic stenosis (12/106, 11.3%), with a significant difference between stenosis types in the malignant TEF and malignant CAO groups (P = 0.000). There was a significant difference in the stenosis degrees between the malignant TEF and malignant CAO groups (P = 0.000).
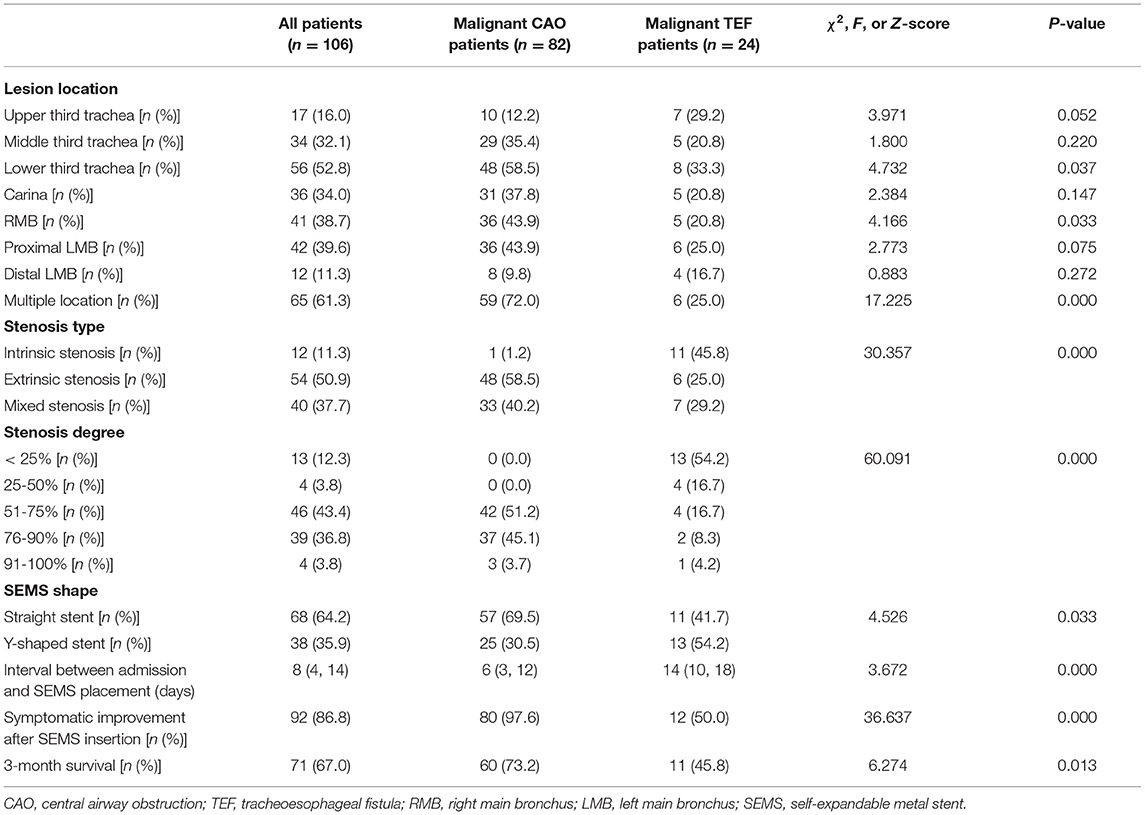
Table 3. Airway disorders' details, SEMS choices, and clinical outcomes between malignant CAO and malignant TEF patients.
The proportion of Y-shaped SEMS (54.2% vs. 30.5%) in the malignant TEF group was higher than in the malignant CAO group. The interval between admission and SEMS placement was significantly longer in the malignant TEF group than in the malignant CAO group [14 (10,18) vs. 6 (3, 12) days, P = 0.000)]. The symptomatic improvement was seen in 92 out of 106 patients immediately after the SEMS placement. The symptomatic improvement rate after SEMS implantation was higher in the malignant CAO group than in the malignant TEF group (97.6% vs. 50.0%, p = 0.000). The 3-month survival rate was lower in the malignant TEF group than in the malignant CAO group (45.8% vs. 73.2%, P = 0.013).
Multivariate Analysis of Risk Factors for the 3-Month Survival
We notice some malignant CAO or TEF patients continue to experience disease progression and even death within a short period after SEMS placement. Therefore, identifying the risk factors contributing to death in patients receiving SEMS placement is essential for the management of malignant airway disorders. A total of 106 patients receiving SEMS in our department were included in this study, of which 35 patients were dead three months after SEMS placement. Table 4 has summarized the comparison of clinical features of those patients who died or survived three months after SEMS placement. The results indicated that dead patients had lower weight, lower BMI, higher neutrophil percentage, a lower proportion of hypertension, a lower proportion of multiple-location involvement, a higher proportion of TEF, a higher proportion of TEF, and a longer duration between admission and SEMS placement than the alive patients (P < 0.05).
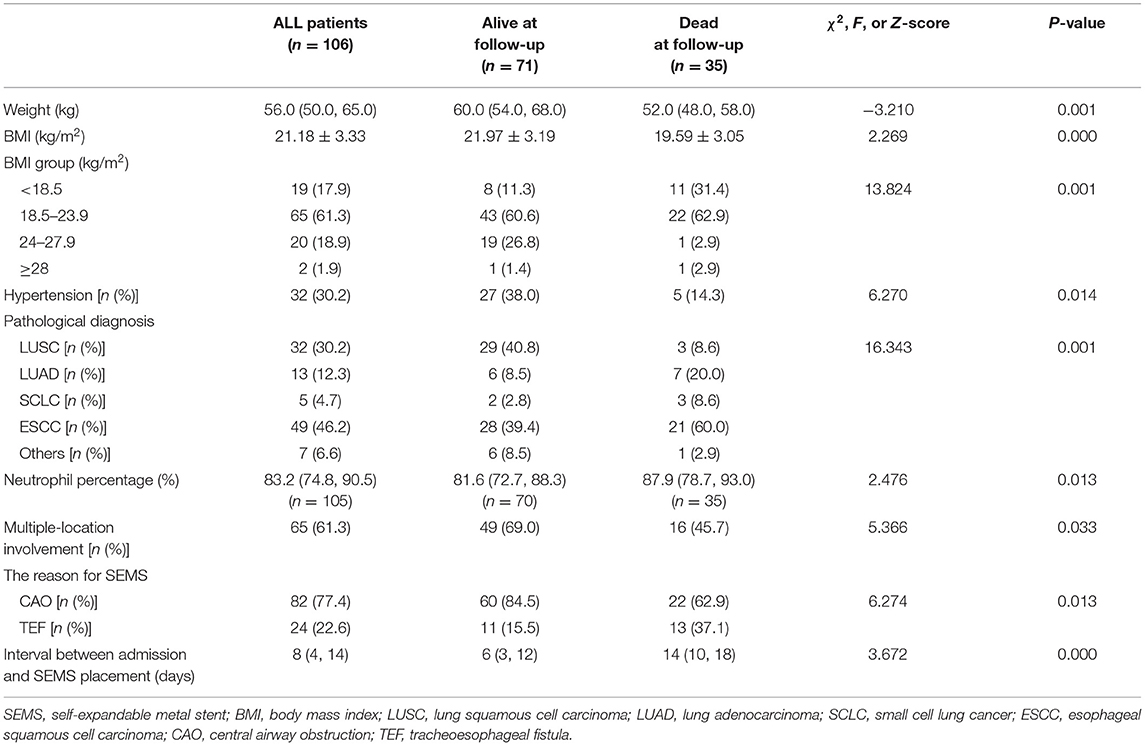
Table 4. The clinical features in patients with malignant airway disorders receiving SEMS placement in which significant differences were found when the patients were divided in two groups (alive or dead three months later).
Univariate analysis indicated that weight, BMI, hypertension, neutrophil percentage, multiple-location involvement, and the reason for SEMS (CAO or TEF) were potential risk factors for patients who died three months after SEMS placement (P < 0.05). The variance inflation factor was always lower than 10 for these potential risk factors, confirming the weak collinearity. Multivariate analysis revealed that BMI [odds ratio (OR) = 1.841, 95% certificated interval (CI) (1.155–2.935), P = 0.010] and neutrophil percentage [OR = 0.936, 95% CI (0.883–0.993), P = 0.027] were the independent risk factors for patients who survived three months after SEMS placement, suggesting that the prognosis of patients with malignant airway disorders receiving SEMS placement was associated with malnutrition and infection (Table 5).
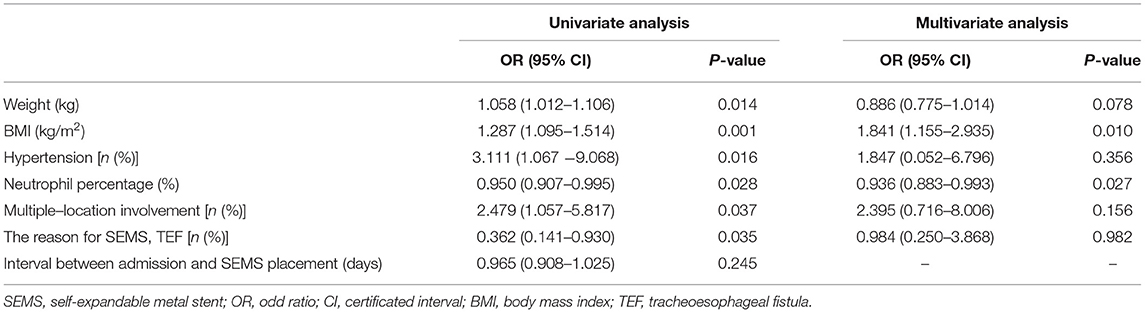
Table 5. Univariate and multivariate analyses of potential risk factors for the survival in malignant airway diseases at 3 months after SEMS placement.
Discussion
Malignant CAO and malignant TEF were the major malignant airway disorders severely affecting the patients' mobility and quality of life. In this study, we retrospectively analyzed the demographic features, clinical characteristics, laboratory and radiological findings, airway disorders' details, SEMS choices, and clinical outcomes. We also identified the BMI and reason for SEMS (CAO or TEF) as risk factors for the 3-month survival in these patients receiving SEMS placement. The malignant CAO should be considered in malignant patients presenting with dyspnea, dry cough, and hemoptysis, and the malignant TEF in malignant patients presenting with choking on water, dysphagia, and cough, especially in those with a BMI <24kg/m2 after the resection surgery of esophagus squamous cell carcinoma. In this study, patients with malignant TEF were often associated with pneumonitis (elevated procalcitonin and C-reactive protein levels, and chest CT showing typical signs of pneumonitis) and malnutrition (decreased hemoglobin and albumin levels), probably leading to a lower survival rate than patients with malignant CAO. Bronchoscopy is the gold standard for the diagnosis of malignant CAO and TEF, determining the pathological types and malignant airway disorders' details while avoiding the risk of worsening the aspiration pneumonitis with repeated gastroscopy in the malignant TEF (2).
SEMS is the palliative treatment to improve mobility and quality of life in patients with malignant CAO or TEF if the radical surgery is contraindicated given the patients' poor clinical status (respiratory distress, pneumonitis, malnutrition) and advanced-stage (stage III–IV) malignancy. SEMS is meth-waved by a shape memory nickel-titanium alloy with substantial elastic formation at a transition temperature of 30°C. The elastic formation of the SEMS permits the easy compression into its delivery device; the shape memory of SEMS allows the resistance to radially compressive forces during coughing; the mesh structure of SEMS enables the conformation to complex and asymmetric lesions in the airway (17). The type, length, diameter, and shape of the SEMS are determined according to the airway 3D reconstruction and the bronchoscopic manifestation. In this study, the deployment of SEMS was observed using bronchoscopy, ensuring a 100% immediate technical success rate and the 97.6% symptom improvement rate in the malignant CAO group and 50% in the malignant TEF group, which was consistent with other studies. In one of the most extensive series (82 patients with CAO, 50 had lung cancer), symptomatic improvement occurred in 87.8% of patients receiving SEMS placement (12). Breitenbücher and colleagues reported a 100% immediate technical success rate and a 100% symptom improvement rate in complex malignant CAO patients (18). In patients with malignant TEF receiving covered SEMS in the tracheobronchial or esophageal under sedatives or general anesthesia, the symptom improvement rate was 80% (19).
Patients with malignant TEF require the covered SEMS to seal the fistula, resulting in a longer interval between admission and SEMS placement in the malignant TEF group than in the malignant CAO group, as shown in this study. During this interval, more aggressive treatment modalities are needed to improve clinical outcomes and survival in patients with malignant TEF, such as strong antibiotics, nutrition support, and strategic ventilation. Gram-negative bacilli (Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii) and Candida albicans were commonly isolated in the sputum culture or bronchoalveolar lavage fluid in these malignant patients (20, 21). We must note that multi-resistant Acinetobacter baumannii infection is associated with a higher risk of death (22). Broad-spectrum cephalosporins (ceftazidime or cefepime), β-lactam/β-lactamase inhibitor combinations (tazobactam or sulbactam), or carbapenems (meropenem or imipenem) can be used empirically in the initial phase, and if necessary, in combination with antifungal drugs. Targeted antibiotics are guided by microbiological culture and drug sensitivity test results. Rigid bronchoscopy with jet ventilation is required in malignant TEF patients with severe respiratory failure during SEMS placement. Strategic ventilation with lower pause pressure and peak inspiratory pressure permits sufficient oxygenation and carbon dioxide evacuation and reduces the risk of subsequent regurgitation of gastric secretions through the TEF into the lungs, leading to the worsening of aspiration pneumonitis (23, 24). Percutaneous endoscopic feeding tube or gastrojejunal feeding tube for enteric feeding and artificial nutrition support is recommended in patients with malignant patients as we noticed that lower BMI was associated a poor survival in the multivariate analysis (25).
There were some limitations that should be addressed in this study. The patients included in the study were from the First Affiliated Hospital of Chongqing Medical University with certain spatial restrictions. The sample size was small relative to the number of independent variables that might be entered in our multivariate logistic regression, leading to the multivariate logistic regression analysis bias (26). We did not have enough data regarding cancer staging and therapies analysis before SEMS placement in patients with malignant airway disorders. Most patients were not followed up, and this study did not provide bronchoscopic observation of SEMS-associated complications. We did not have enough data to calculate what symptoms improved and what symptoms remained among malignant TEF patients after SEMS placement, either the causes of death. Large-scale prospective studies are needed to investigate the prognostic factors in patients with malignant airway disorders receiving SEMS placement.
Conclusion
We retrospectively analyzed the malignant CAO or TEF patients receiving SEMS placement at the First Affiliated Hospital of Chongqing Medical University, none of which were candidates for surgical treatment. SEMS placement improved symptoms in most malignant CAO patients, whereas in some malignant TEF patients. The survival rate in malignant TEF patients after SEMS placement was low, probably due to malnutrition and infection. Therefore, we recommend more aggressive treatment modalities to improve clinical outcomes and survival in patients with malignant TEF, such as strong antibiotics, nutrition support, and strategic ventilation, especially in those with a BMI <24kg/m2 after the resection surgery of the esophagus squamous cell carcinoma. More studies are needed to investigate the prognostic factors in patients with malignant airway disorders receiving SEMS placement.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SG and YL designed the study. YB, KZ, and JC performed the research and analyzed the data. YB, JC, KZ, and JJ were involved in data discussion, drafting, and editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The present study was funded by the Chongqing Science and Health Joint Medical Research Project (2020FYYX222) and Chongqing Medical University Future Medical Young Innovation Team Development Support Program (W0119).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Mrs. Tairong Tan, Mrs. Meiling Xiao, Mrs. Xia Zhang, Mr. Xingxing Jin, and Mr. Yang Xiao for their sincere and professional support of our work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.902488/full#supplementary-material
References
1. Murgu SD, Egressy K, Laxmanan B, Doblare G, Ortiz-Comino R, Hogarth DK. Central airway obstruction: benign strictures, tracheobronchomalacia, and malignancy-related obstruction. Chest. (2016) 150:426–41. doi: 10.1016/j.chest.2016.02.001
2. Kim HS, Khemasuwan D, Diaz-Mendoza J, Mehta AC. Management of tracheo-oesophageal fistula in adults. Eur Respir Rev. (2020) 29:200094. doi: 10.1183/16000617.0094-2020
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. (2004) 169:1278–97. doi: 10.1164/rccm.200210-1181SO
4. Noppen M, Meysman M, D'Haese J, Schlesser M, Vincken W. Interventional bronchoscopy: 5-year experience at the Academic Hospital of the Vrije Universiteit Brussel (AZ-VUB). Acta Clin Belg. (1997) 52:371–80. doi: 10.1080/17843286.1997.11718603
5. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. (2008) 83:584–94. doi: 10.1016/S0025-6196(11)60735-0
6. Zhou C, Hu Y, Xiao Y, Yin W. Current treatment of tracheoesophageal fistula. Ther Adv Respir Dis. (2017) 11:173–80. doi: 10.1177/1753465816687518
7. Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg. (2008) 34:1103–7. doi: 10.1016/j.ejcts.2008.06.025
8. Ong P, Grosu HB, Debiane L, Casal RF, Eapen GA, Jimenez CA, et al. Long-term quality-adjusted survival following therapeutic bronchoscopy for malignant central airway obstruction. Thorax. (2019) 74:141–56. doi: 10.1136/thoraxjnl-2018-211521
9. Kim J, Shin JH, Kim JH, Song HY, Song SY, Park CK. Metallic stent placement for the management of tracheal carina strictures and fistulas: technical and clinical outcomes. AJR Am J Roentgenol. (2014) 202:880–5. doi: 10.2214/AJR.12.10425
10. Qian HW, Zhang P, Wang X, Zhang Y, Li J, Zhong EJ Ji SD, et al. Survival and prognostic factors for patients with malignant central airway obstruction following airway metallic stent placement. J Thorac Dis. (2021) 13:39–49. doi: 10.21037/jtd-20-1520
11. Wang H, Tao M, Zhang N, Li D, Zou H, Zhang J, et al. Airway covered metallic stent based on different fistula location and size in malignant tracheoesophageal fistula. Am J Med Sci. (2015) 350:364–8. doi: 10.1097/MAJ.0000000000000565
12. Saad CP, Murthy S, Krizmanich G, Mehta AC. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. (2003) 124:1993–9. doi: 10.1378/chest.124.5.1993
13. Mudambi L, Miller R, Eapen GA. Malignant central airway obstruction. J Thorac Dis. (2017) 9:S1087–s1110. doi: 10.21037/jtd.2017.07.27
14. Freitag L, Ernst A, Unger M, Kovitz K, Marquette CH. A proposed classification system of central airway stenosis. Eur Respir J. (2007) 30:7–12. doi: 10.1183/09031936.00132804
15. Lee KS, Lunn W, Feller-Kopman D, Ernst A, Hatabu H, Boiselle PM. Multislice CT evaluation of airway stents. J Thorac Imaging. (2005) 20:81–8. doi: 10.1097/01.rti.0000149789.28967.03
16. Nair A, Godoy MC, Holden EL, Madden BP, Chua F, Ost DE, et al. Multidetector CT and postprocessing in planning and assisting in minimally invasive bronchoscopic airway interventions. Radiographics. (2012) 32:E201–232. doi: 10.1148/rg.325115133
17. Stoeckel D, Pelton A, Duerig T. Self-expanding nitinol stents: material and design considerations. Eur Radiol. (2004) 14:292–301. doi: 10.1007/s00330-003-2022-5
18. Breitenbücher A, Chhajed PN, Brutsche MH, Mordasini C, Schilter D, Tamm M. Long-term follow-up and survival after Ultraflex stent insertion in the management of complex malignant airway stenoses. Respiration. (2008) 75:443–9. doi: 10.1159/000119053
19. Shin JH, Song HY, Ko GY, Lim JO, Yoon HK, Sung KB. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology. (2004) 232:252–9. doi: 10.1148/radiol.2321030733
20. Abdul-Aziz MH, Portunato F, Roberts JA. Prolonged infusion of beta-lactam antibiotics for Gram-negative infections: rationale and evidence base. Curr Opin Infect Dis. (2020) 33:501–10. doi: 10.1097/QCO.0000000000000681
21. Souza ESVC, Oliveira VC, Sousa Á FL, Bim FL, Macedo AP, Andrade D, et al. Prevalence and susceptibility profile of Candida spp. isolated from patients in cancer therapy. Arch Oral Biol. (2020) 119:104906. doi: 10.1016/j.archoralbio.2020.104906
22. Lemos EV. de la Hoz FP, Einarson TR, McGhan WF, Quevedo E, Castañeda C, Kawai K: Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. (2014) 20:416–23. doi: 10.1111/1469-0691.12363
23. Putz L, Mayné A, Dincq AS. Jet Ventilation during rigid bronchoscopy in adults: a focused review. Biomed Res Int. (2016) 2016:4234861. doi: 10.1155/2016/4234861
24. Grotberg JC, Hyzy RC, De Cardenas J, Co IN. Bronchopleural fistula in the mechanically ventilated patient: a concise review. Crit Care Med. (2021) 49:292–301. doi: 10.1097/CCM.0000000000004771
25. Bosaeus I. Nutritional support in multimodal therapy for cancer cachexia. Supportive care in cancer. (2008) 16:447–51. doi: 10.1007/s00520-007-0388-7
Keywords: central airway obstruction, tracheoesophageal fistula, malignancy, self-expandable metal stent, management
Citation: Bai Y, Zhan K, Chi J, Jiang J, Li S, Yin Y, Li Y and Guo S (2022) Self-Expandable Metal Stent in the Management of Malignant Airway Disorders. Front. Med. 9:902488. doi: 10.3389/fmed.2022.902488
Received: 23 March 2022; Accepted: 09 June 2022;
Published: 07 July 2022.
Edited by:
Shu-Min Lin, Linkou Chang Gung Memorial Hospital, TaiwanReviewed by:
Kosaku Maeda, Kobe University, JapanYi-Han Hsiao, Taipei Veterans General Hospital, Taiwan
Copyright © 2022 Bai, Zhan, Chi, Jiang, Li, Yin, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yishi Li, Mjg3OTc2MjI1QHFxLmNvbQ==; Shuliang Guo, R1VPU0w5OTlAc2luYS5jb20=
†These authors have contributed equally to this work
 Yang Bai
Yang Bai Ke Zhan2†
Ke Zhan2† Jing Chi
Jing Chi Yishi Li
Yishi Li Shuliang Guo
Shuliang Guo