
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 12 July 2022
Sec. Pulmonary Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.900858
 Li-Ting Cheng1
Li-Ting Cheng1 Chi-Hsiang Chung2,3,4
Chi-Hsiang Chung2,3,4 Chung-Kan Peng1
Chung-Kan Peng1 Chin-Chung Shu5
Chin-Chung Shu5 Shu-Yu Wu6
Shu-Yu Wu6 Sheng-Huei Wang1
Sheng-Huei Wang1 Gwo-Jang Wu2,7,8
Gwo-Jang Wu2,7,8 Chang-Huei Tsao2,9
Chang-Huei Tsao2,9 Chien-An Sun10,11,12
Chien-An Sun10,11,12 Wu-Chien Chien2,3,4,12*†
Wu-Chien Chien2,3,4,12*† Shih-En Tang1,6*†
Shih-En Tang1,6*†Some antituberculosis agents may cause hypothyroidism, and thyroid hormones play a vital role in Mycobacterium tuberculosis infection. However, the relationship between tuberculosis (TB) and hypothyroidism has not been clearly established. Therefore, this retrospective, longitudinal cohort study aimed to investigate the association between these two diseases using the 2000–2017 data from the Taiwan's National Health Insurance Research Database. The hypothyroidism and TB cohorts were matched with the control group in a 1:4 ratio. Adjusted hazard ratios (aHRs) were assessed using Cox proportional hazards regression analysis in each cohort. In total, 3,976 individuals with hypothyroidism and 35 120 individuals with TB were included in this study. The risk of developing TB in patients with hypothyroidism was 2.91 times higher than that in those without hypothyroidism (95% confidence interval [CI], 1.50–3.65). The subgroup of thyroxine replacement therapy (TRT) had a 2.40 times higher risk (95% CI, 1.26–3.01), whereas the subgroup of non-TRT had a 3.62 times higher risk of developing TB than those without hypothyroidism (95% CI, 2.19–4.84). On the other hand, the risk of developing hypothyroidism in patients with TB was 2.01 times higher than that in those without TB (95% CI, 1.41–2.38). Our findings provide evidence that TB and hypothyroidism are interrelated. Thus, clinicians and public health authorities should monitor the association between these two diseases to reduce the relevant disease burden.
Tuberculosis (TB) is a severe communicable disease that is among the top 10 causes of death worldwide. According to the World Health Organization, ~10 million new cases and 1.3 million deaths were reported in 2020. Of all TB cases in 2020, the proportion of adult men, adult women, and children is 56, 33, and 11%, respectively (1). Despite achieving a microbiological cure, many survivors face post-TB sequelae, thus increasing the overall disease burden (2).
Taiwan became an aged society in 2018. There were 16,472 and 7,823 new TB cases in 2005 and 2020, respectively, and the incidence rate decreased from 72.5 to 33.2 per 100,000 population during this period. More than half of the new TB cases occur in the elderly population (age ≥ 65 years) since 2005. Besides, there were 460 TB-related deaths in 2020 and the cumulative reduction between 2005 and 2020 was 53.5 %. Since 2006, the coverage rate of directly observed treatment, short course has reached 100%, and ~70% of patients with bacteriologically-positive TB were treated successfully in 2018 (3). Between 2006 and 2013, hypertension (HTN), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), cardiovascular disease, and cancer were identified as common comorbidities in patients with TB in Taiwan. The risk of death in patients with TB and any comorbidity was 2.12 times higher than that in those without comorbidities (4). The Taiwan Centers of Disease Control highlights preventive treatment of high-risk individuals and early diagnosis of TB, and the elimination of TB is expected to be completed gradually by 2035, echoing the World Health Organization End TB Strategy (1).
Hypothyroidism is characterized by decreased thyroid hormone production in the thyroid gland. It can be classified as primary (due to thyroid hormone deficiency) or secondary/tertiary (due to abnormal hypothalamic-pituitary function). The diagnosis of hypothyroidism depends heavily on laboratory testing because of variable non-specific clinical manifestations (5–7). Women are more likely to have hypothyroidism than men (8). More than 95% cases of hypothyroidism are of the primary type (9, 10). Overt hypothyroidism and subclinical hypothyroidism are two degrees of primary hypothyroidism, and the prevalence of these disorders ranges from 0.1 to 3% and 1.6 to 15% (5, 8, 11–15), respectively. Annually, ~2–5% of cases of subclinical hypothyroidism may progress to overt hypothyroidism (6). Although chronic autoimmune thyroiditis is the main cause of primary hypothyroidism (5), other possible causes include iatrogenic diseases, deficiency or excessive consumption of iodine, drugs, and infiltrative diseases (5, 16–18). The common mechanism of drug-induced hypothyroidism includes inhibition of thyroid hormone synthesis, decreased absorption of T4, increased T4 clearance, increased type 3 deiodination, destructive thyroiditis, suppression of TSH, and immune dysregulation. Common drugs can cause hypothyroidism including lithium, amiodarone, omeprazole, lansoprazole, interferon alfa, interleukin-2, tyrosine kinase inhibitors (sunitinib, sorafenib, imatinib, etc.), and checkpoint inhibitors (ipilimumab, pembrolizumab, nivolumab, etc.). Patients taking these drugs should monitor possible hypothyroid symptoms and serum TSH should be measured at least every 6 to 12 months (17).
Thyroid hormones modulate various immune system functions, including chemotaxis, phagocytosis, production of reactive oxygen species, and the release of cytokine release (19). Hypothyroidism may have a detrimental effect on the immune system and subsequently make patients vulnerable to infection. Recent studies have shown that thyroid hormone signaling plays a vital role in optimal immune response during Mycobacterium tuberculosis (Mtb) infection (20), which could be related to infection or drug-related hypothyroidism. Thyroid tuberculosis, which has a frequency of 0.1–0.4%, may cause hypothyroidism owing to extensive glandular destruction due to caseous necrosis (21). Infectious agents have been reported to trigger autoimmune thyroid diseases by possible mechanisms including molecular mimicry theory and bystander activation theory (22). Rifampin increases T4 clearance, possibly because of enhanced hepatic T4 metabolism and biliary excretion of iodothyronine conjugates. Vaidya (16) and Montanelli (17) reported that rifampin causes primary hypothyroidism and there were few case reports of rifampin-induced hypothyroidism, and most of them had underlying Hashimoto's Thyroiditis, and some of them without underlying thyroid disease. Prior systemic review and meta-analysis showed that ethionamide and para-aminosalicylic acid were the most frequently reported drugs associated with the occurrence of hypothyroidism (23) and these drugs can cause hypothyroidism by inhibiting thyroid hormone synthesis through a mechanism of iodine organification inhibition (24, 25).
These studies may partially explain the causal relationship between TB and hypothyroidism; however, evidence from longitudinal analysis is lacking. Therefore, we conducted a bidirectional, nationwide, population-based cohort study to investigate the association between TB and hypothyroidism.
In 1995, the Taiwanese government adopted the National Health Insurance (NHI) programme, which covers the healthcare data of more than 99% of Taiwanese residents (26). The data analyzed in this study were obtained from the National Health Insurance Research Database (NHIRD). The Longitudinal Health Insurance Database 2005 (LHID2005) (27), which contains the original claims data for 2,000,000 patients randomly sampled from the NHI enrollees registered in 2005 (28).
Previous studies have reported that the data of the LHID are representative of the entire Taiwanese population, and the accuracy of the disease diagnoses have been validated (29–31). Personal identification information from the claims data was encrypted and anonymized to protect the privacy and security of the patients (27). This study was approved by the Institutional Review Board of the Tri-Service General Hospital (approval number B-111-01).
To analyze the bidirectional relationship between hypothyroidism and TB, this study had two main purposes. Diagnoses of both diseases were designated using the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes for the 2000–2015 period and the ICD-10-CM codes for the 2016–2017 period.
For purpose 1, we included patients with hypothyroidism (ICD-9: 243-244, ICD-10: E00, E01.8, E02-E03, E89.0) aged >20 years who had at least three outpatient visits or one hospitalization registered in the LHID2005 between 2000 and 2017. The index date was defined as that on which a new diagnosis of hypothyroidism was made. The exclusion criteria were as follows: history of hypothyroidism before the index date, TB before tracking and without tracking, age <20 years, and unknown sex. For purpose 2, we used validated inclusion criteria for TB patients by ICD-9 code of 010-018 and ICD-10 code of A15-A19 plus prescription of at least two anti-TB drugs (e.g., isoniazid, ethambutol, rifampin, pyrazinamide) for 4 weeks within 180 days of TB diagnosis (32) registered in the LHID2005 between 2000 and 2017. TB outside of the lung, such as the lymphatic system, bones and joints, liver, central nervous system, genitourinary, etc., is called extra-pulmonary TB. Miliary TB is classified as extra-pulmonary TB if the patient has no lesions in the lungs. If the patient has both pulmonary and extra-pulmonary TB, the patient is classified as pulmonary TB (33). The exclusion criteria were similar to purpose 1. Four-fold propensity score matching (34) was employed to match the above mentioned study cohorts with the control cohorts selected from the LHID2005 by sex, age, comorbidities, and index date. A flowchart of the selection of study participants is shown in Figure 1.
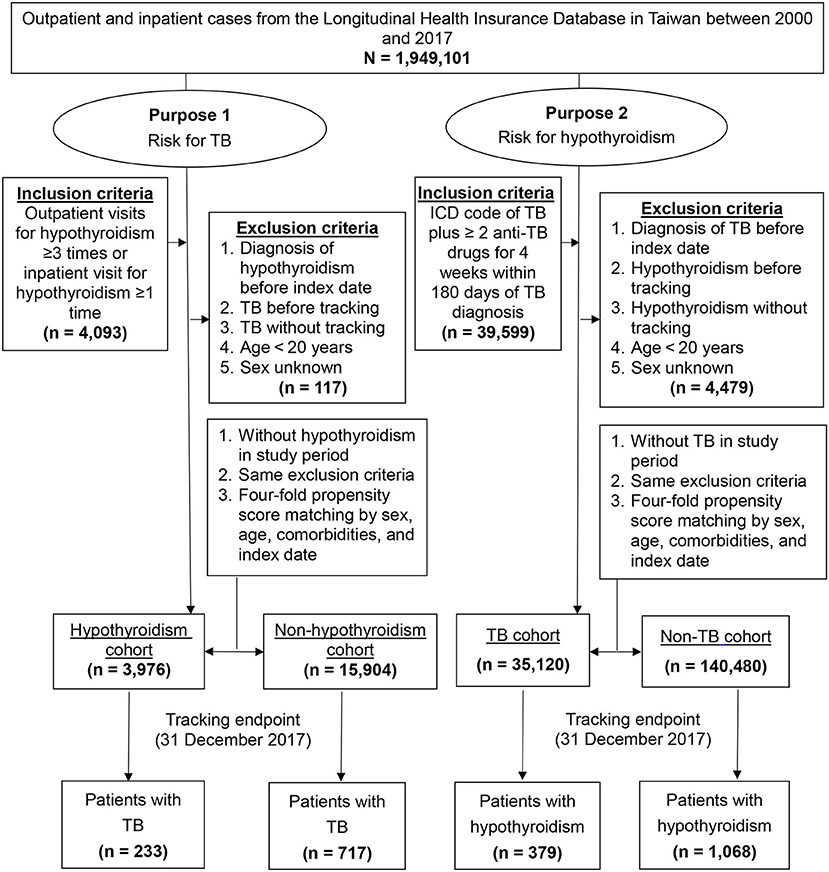
Figure 1. Flowchart of study participant selection. ICD, International Classification of Disease; TB, tuberculosis.
To further assess the association between TRT and the TB risk in patients with hypothyroidism, subgroup analyses were conducted stratified of data by the duration of TRT (6 weeks−3 months, 3 months−1 year, and ≥1 year). The TRT group was defined as the patients with hypothyroidism who had received levothyroxine sodium prescription for at least 6 weeks during the study period. The non-TRT group included patients with hypothyroidism who had never received levothyroxine sodium prescription or had been on medication for <6 weeks during the study period. The ICD and Anatomical Therapeutic Chemical codes used in this study are presented in Supplementary Table S1.
The endpoints of this study were new diagnoses of TB (purpose 1) and hypothyroidism (purpose 2). The definitions of new TB and hypothyroidism cases were the same as the inclusion criteria mentioned above. In Taiwan, clinicians must report new TB cases to the Taiwan Centers for Disease Control within seven days and submit the final histopathology and TB culture results for document review and medical reimbursement. In addition, the high accuracy of TB diagnoses in the LHID has been validated previously (32).
The follow-up period was defined as the time interval from the index date to the diagnosis of TB (purpose 1) or hypothyroidism (purpose 2), 31 December, 2017, or withdrawal from the NHI programme, whichever occurred first.
Data on demographic variables, including sex, age, insurance premium, and urbanization level, were collected. Comorbidities or possible risk factors for TB or hypothyroidism, including DM (ICD-9: 250, ICD-10: E10-E14), HTN (ICD-9: 401-405, ICD-10: I10-I15), hyperlipidaemia (ICD-9: 272, ICD-10: E74-75, E77-E78, E88), ischaemic heart disease (IHD; ICD-9: 401-414, ICD-10: I20-I25), congestive heart failure (ICD-9: 428, ICD-10: I50), cancer (ICD-9: 140-208, ICD-10: C00-C96), COPD (ICD-9: 490-496, ICD-10: J40-J47), stroke (ICD-9: 430-438, ICD-10: I60-I69), chronic kidney disease (CKD; ICD-9: 585, ICD-10: N18), human immunodeficiency virus (HIV) infection (ICD-9: 042, V08, ICD-10: B20-B24, Z21), and cirrhosis (ICD-9: 517.2, 571.5-571.6, ICD-10: K73-K74, K76), were identified.
The chi-square test was used to compare the differences in categorical variables between the two purpose and control groups. An independent-samples t-test was used for continuous variables. The cumulative risks for TB (purpose 1) and hypothyroidism (purpose 2) were computed using the Kaplan–Meier method, and the log-rank test was used to compare differences between the curves. The incidence rate was calculated per 1000 person-years. The hazard ratios and 95% CI were calculated using the multivariable Cox proportional hazards regression analysis, which was adjusted for sex, age, insured premium, comorbidities, and urbanization level. We further evaluated the interaction effect of hypothyroidism and comorbidities on the risk of TB. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA), and a two-tailed P-value < 0.05 was considered statistically significant.
After 1:4 propensity score matching for sex, age, comorbidities, and index date, we identified 3,976 patients in the hypothyroidism cohort, 15,904 patients in the non-hypothyroidism cohort, 35,120 patients in the TB cohort, and 140,480 patients in the non-TB cohort (Figure 1). Between two purposes and control cohorts, baseline characteristics showed no significant differences in sex, age, and comorbidities (Table 1).
Female was predominated (64.2%) in the purpose 1 and more than half of the participants were age <65 years. At the time of diagnosis of TB, the hypothyroidism cohort had a higher prevalence of DM, HTN, hyperlipidemia, IHD, cancer, COPD, stroke, and CKD than the non-hypothyroidism cohort (Supplementary Table S2). The mean follow-up periods in the hypothyroidism and non-hypothyroidism cohorts were 10.64 and 10.85 years, respectively (Supplementary Table S3). The mean duration before TB development were 7.74 and 8.25 years in the hypothyroidism and non-hypothyroidism cohorts, respectively (Supplementary Table S4).
Multivariate Cox proportional hazard analysis revealed that the hypothyroidism cohort has a significantly higher risk of developing TB than the non-hypothyroidism cohort (Table 2). Furthermore, the hypothyroidism cohort exhibited a significantly higher risk for TB, considering nearly all variables, i.e., male sex, all age groups, comorbidities (except for congestive heart failure and cancer), and urbanization level (except for level 3). Table 3 demonstrated an interaction effect between hypothyroidism and comorbidities including DM (aHR = 3.46, 95% CI 2.25–4.01) and COPD (aHR = 4.33, 95% CI 2.35–6.11) on the risk of developing TB. At the end of the follow-up period, the hypothyroidism cohort had a higher cumulative risk for TB than those in the non-hypothyroidism cohort (Figure 2A).
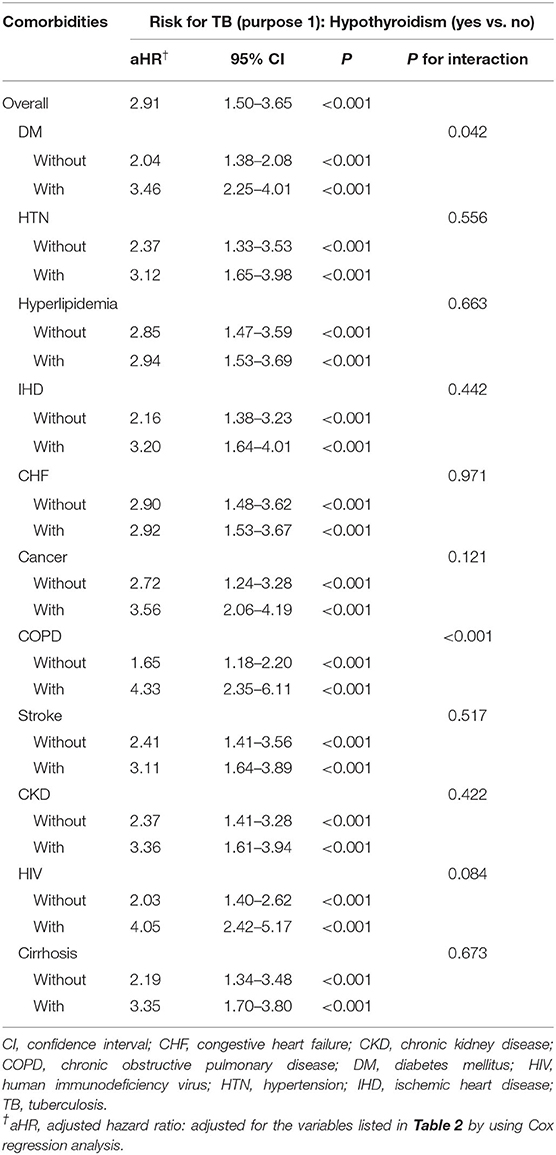
Table 3. Cox proportional hazard regression analysis for the hypothyroidism-linked TB risk with the interaction of comorbidity.
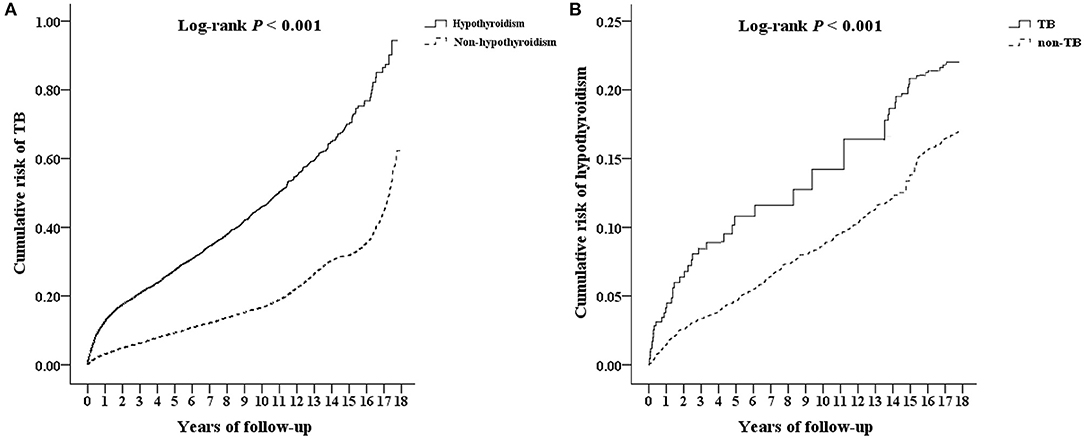
Figure 2. Kaplan–Meier analysis for cumulative risk of developing (A) TB and (B) hypothyroidism among patients aged ≥20 years using log-rank test. TB, tuberculosis.
The hypothyroidism cohort was stratified according to the duration of TRT (Table 4). Patients with hypothyroidism who had never received TRT during the study period had a 3.62 times higher risk of developing TB than those without hypothyroidism. In the TRT group, the highest risk occurred in the treatment period lasting from 6 weeks to 3 months. The hypothyroidism-linked TB risk may be attenuated gradually by long-term TRT. There was no significant difference between the groups in the treatment categories with ≥1-year treatment period. The hypothyroidism cohort had a significantly higher aHR for TB during the follow-up periods of <3 months, 3–6 months, 6–12 months, and 1–5 years than the non-hypothyroidism cohort (Table 6).
Male was predominated (56.9%) in the purpose 2 and more than half of the participants were age ≥ 65 years. At the time of diagnosis of TB, the TB cohort had a higher prevalence of DM, HTN, hyperlipidemia, and IHD than the non-TB cohort (Supplementary Table S2). The mean follow-up periods in the TB and non-TB cohorts were 10.89 and 10.97 years, respectively (Supplementary Table S3). The mean period before hypothyroidism development was 7.30 years in the TB cohort and 8.18 years in the non-TB cohort (Supplementary Table S4).
According to the multivariate Cox proportional hazard analysis, the aHR for the risk of developing hypothyroidism was 2.01 times higher in the TB cohort than in the non-TB cohort (Table 2). After adjusting for variables, patients with comorbidities (DM, HTN, hyperlipidaemia, IHD, COPD, stroke, CKD, HIV, and cirrhosis) in the TB cohort had a significantly higher aHR for hypothyroidism than those in the non-TB cohort. The TB cohort had a significantly higher cumulative risk of developing hypothyroidism than the non-TB cohort at the end of the follow-up period (Figure 2B).
The subgroup analysis showed that patients with pulmonary TB and extra-pulmonary TB have a significantly higher aHR for developing hypothyroidism than those without TB (Table 5). Furthermore, the TB cohort had a significantly higher aHR for hypothyroidism in the follow-up periods of <3, 3–6, 6–12 months, and 1–5 years than the non-TB cohort (Table 6).
To the best of our knowledge, this is the first longitudinal, nationwide, population-based cohort study to investigate the bidirectional relationship between hypothyroidism and TB. We found that patients with hypothyroidism have a 2.91 times higher risk of developing TB than those without hypothyroidism, and that patients with TB have a 2.01 times higher risk of developing hypothyroidism than that those without. Although the pathophysiological association between hypothyroidism and tuberculosis remains unclear, several hypotheses have been proposed.
In our data, hypothyroidism was predominated in the female (64.2%). But male with hypothyroidism had a higher risk of TB development, especially in the age ≥45 years. Besides, the higher urbanization level 1 and 2 also had an impact on TB development. We proposed some explanations for these interesting findings. First, previous studies revealed that male and old age were the risk factors of TB development (35, 36). Testosterone can impair macrophage activation and decrease the production of proinflammatory cytokines, which may increase the susceptibility to Mtb infection. In contrast, estrogen can enhance macrophage activation and induce proinflammatory cytokines, which provide protection against Mtb infection (37). Second, a modeling study in Taiwan showed that immune senescence played an important role in the age disparity between children and elders, which means patients with latent TB infection have an increased risk of developing TB as they age due to a weakening immune system with older age (38). Third, TB is a disease whose transmission is favored by the density of urban populations (39). Taiwan has seen rapid growth in living standards and nationwide coverage of high-quality, publicly funded healthcare services (28). Due to the patient preference, better service quality, increased geographic accessibility, better community awareness, and access to better diagnostics and treatment, the TB case notification rates are higher in urban areas than in rural areas (39). Our findings are compatible with the above explanations.
In Taiwan, more than half of the new TB cases occur in the elderly population (age ≥ 65 years) since 2005 (3) and it can be seen in our TB cohort. In general concept, hypothyroidism development is much more common in female than male, and the incidence rate increase with age (40). However, these findings were not seen in our study. We think there may be other possible causes, with drug-induced hypothyroidism being one of the most likely, and additional study is needed in the future. Besides, this study also showed that patients with hypothyroidism have an interaction effect with comorbidities including DM and COPD toward the risk of developing TB. Individual links between TB and these two comorbidities among the general population are well established among papers (41). This demonstrates a relationship between hypothyroidism and DM and COPD and how all these interrelations remain unexplored.
Previous studies have demonstrated that serum triiodothyronine (T3) and thyroxine (T4) modulate specific immune responses, including innate and adaptive immune responses, which change significantly during aging and in cases of hypothyroidism. The dysregulation of innate and adaptive immune responses may subsequently increase susceptibility to infection (42–44). A recent population-based cohort study showed that patients with hypothyroidism were associated with a risk of pneumonia. In addition, the use of TRT (>30 days) can attenuate the hypothyroidism-linked pneumonia risk (45). However, there was no evidence or mechanism to explain the TB-linked hypothyroidism. In a rabbit Mtb infection model, defective production of thyroid hormones increased susceptibility to Mtb infection (46). Furthermore, T3 and T4 restricted Mtb growth in human monocyte-derived macrophages through interleukin-1α production; therefore, young, healthy household contacts of patients with TB with a decreased production of T4 at baseline have an increased risk of developing active TB (20). Our findings strengthen the evidence that patients with hypothyroidism have an increased risk of developing TB. The non-TRT group showed a significantly higher risk of developing TB, especially pulmonary TB, than the non-hypothyroidism cohort. Additionally, we found that long-term TRT (>1 year) may decrease the risk of developing TB, which supports the findings of a previous study (20). Further prospective cohort studies to evaluate the association between TRT and the risk of TB are warranted.
In our study, patients with TB, pulmonary TB, or extra-pulmonary TB had a significantly higher risk of developing hypothyroidism than those without TB. However, the results of previous, similar studies (20, 47) have been controversial. Kleynhans investigated the changes in the immune system during treatment for TB in patients who were cured and in those in whom it failed. They found that T3 concentrations increase during TB infection, whereas T4 concentrations remain unchanged in the failed group. T4 concentrations were lower in the cured group after 6 months of TB treatment than in the failed group. T4 plays a critical role in Mtb infection and may be a potential biomarker to differentiate treatment outcomes (47). Recently, T3 and T4 concentrations were reported to be elevated in TB progressors (healthy household contacts developing TB during the follow-up period) after 6 months of TB treatment, although T4 concentrations decreased in treated TB progressors 3 years after TB treatment (20). Although previous studies have demonstrated an association between TB and thyroid hormone levels, our findings reinforce that TB is a risk factor for hypothyroidism.
Moreover, we observed that the TB and hypothyroidism cohorts have a higher risk for hypothyroidism and TB, respectively during a follow-up period of <5 years; the highest risks were noted in the 3–6 month follow-up. To our knowledge, this is the first study to reveal this correlation. We proposed possible explanations for the short-term and long-term risks of this observation. First, rifampin enhances T4 clearance and may cause hypothyroidism (16, 17). Without treatment, ~4–15% of patients with latent TB infection develop TB within 1–5 years after getting infected (48–50). Devalraju et al. found that 17 of 688 healthy household contacts developed active TB, and that 12 of 17 progressors received a diagnosis of TB within the first year (20). These may be the reasons for the short-term risk of both diseases. Second, Mtb infection may trigger autoimmune reactions by molecular mimicry of Mtb antigens with human proteins (51) and infection may subsequently result in autoimmune thyroid diseases (22). Third, asymptomatic populations of hypothyroidism and TB may result in delayed diagnosis. And finally, genetic polymorphism plays a major role in the progression to active TB (52) and two single nucleotide polymorphisms in immune-and inflammation-related genes (interleukin-6 rs2066992 and rs1524107) increased the risk of active TB (53). In addition, interleukin-6 can inhibit thyroid function in vivo (54, 55). The relationship between the long-term risk of TB and hypothyroidism may involve complex interactions between genes, cytokines, and the immune system, and additional studies are warranted to elucidate these mechanisms.
Our study had some limitations. First, we were unable to analyze the type or disease severity of hypothyroidism and TB in both cohorts because of the lack of symptoms and the absence of associated laboratory data, such as concentrations of thyroid-stimulating hormone, free T4, T3, or anti-thyroid peroxidase antibodies, in the NHIRD. Second, similar to issues with all electronic health databases, there can be problems related to coding errors and deliberate upcoding. Although previous studies have demonstrated high accuracy and validity for ICD-9-CM-based LHID disease identification (27, 29–31), we sought to reduce misclassification bias with a stricter definition of hypothyroidism and TB (29). On the other hand, we may underestimate the populations of asymptomatic subclinical hypothyroidism. Third, clinicians did not routinely examine chest radiography scans and thyroid functions in either cohort, which might have resulted in underdiagnosis. Fourth, although we adjusted for the latent variables in the table, there might have been unknown or unmeasured confounding biases. Fifth, although rifampin, ethionamide and para-aminosalicylic acid were the possible cause of hypothyroidism (16, 17, 23), we did not perform an analysis of these drugs. Additional research should be performed in the future.
In conclusion, the current study revealed a bidirectional relationship between TB and hypothyroidism. Patients with hypothyroidism showed a 2.91-fold higher risk of developing TB than the general population, especially those combined with DM or COPD. The hypothyroidism-linked TB risk may be attenuated by long-term TRT. Physicians should be aware of the risk of developing hypothyroidism when treating patients with TB. We recommend that public health authorities should conduct surveillance for both diseases to reduce the associated disease burden.
The data analyzed in this study is subject to the following licenses/restrictions: The data on the study population that were obtained from the NHIRD are maintained in the NHIRD. The National Health Research Institutes (NHRI) is a non-profit foundation established by the government. Only citizens of Taiwan who fulfill the requirements of conducting research projects are eligible to apply for access to the NHIRD. The use of the NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law and the related regulations of the National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and their supervisor upon application submission. All applications are reviewed for approval of data release. Requests to access these datasets should be directed to https://nhird.nhri.org.tw/.
The studies involving human participants were reviewed and approved by Institutional Review Board of the Tri-Service General Hospital (approval number: B-111-01). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
L-TC, C-CS, S-HW, and S-ET contributed to conception and design of the study. C-HC and W-CC organized the database and performed the statistical analysis. L-TC wrote the first draft of the manuscript. C-KP, S-HW, and S-ET wrote sections of the manuscript. L-TC, S-YW, G-JW, C-HT, C-AS, and S-ET were responsible for the critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
This study was supported by the Tri-Service General Hospital Research Foundation (TSGH-E-111230), the sponsor had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, the Ministry of Health and Welfare of Taiwan, and the National Health Research Institutes of Taiwan. The interpretations and conclusions contained herein do not represent those of the aforementioned organizations.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.900858/full#supplementary-material
aHR, Adjusted hazard ratio; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HIV, human immunodeficiency virus; HTN, hypertension; ICD-9-CM, International Classification of Disease, 9th Revision, Clinical Modification; IHD, ischaemic heart disease; LHID2005, Longitudinal Health Insurance Database 2005; Mtb, Mycobacterium tuberculosis; NHI, National Health Insurance; NHIRD, National Health Insurance Research Database; TB, tuberculosis; T3, triiodothyronine; T4, thyroxine.
1. World Health Organization (2021). Global Tuberculosis Report 2021. Available online at: https://www.who.int/publications/i/item/9789240037021 (accessed April 19, 2022).
2. Menzies NA, Quaife M, Allwood BW, Byrne AL, Coussens AK, Harries AD, et al. Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Glob Health. (2021) 9:e1679–87. doi: 10.1016/S2214-109X(21)00367-3
3. Taiwan Centers for Disease Control (2021). CDC Annual Report 2021. Available online at: https://www.cdc.gov.tw/En/InfectionReport/Info/BAkN3lDoa6hdrimSerBQyQ?infoId=s0pQQSgwPQuP4xq5x99Msw (accessed April 19, 2022).
4. Taiwan Centers for Disease Control (2018). Aging Population and Impact to Infectious Diseases and Comorbidities and Pilot Management Model Evaluation. Available online at: https://www.cdc.gov.tw/En/Professional/ProgramResultInfo/ppxd4Xu5zcYwcLHniXKk6w?programResultId=AlYTPfko4hmdzvjF-umW1g (accessed 20 October 2021).
5. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. (2017) 390:1550–62. doi: 10.1016/S0140-6736(17)30703-1
6. Khandelwal D, Tandon N. Overt and subclinical hypothyroidism: who to treat and how. Drugs. (2012) 72:17–33. doi: 10.2165/11598070-000000000-00000
7. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. (2014) 24:1670–751. doi: 10.1089/thy.2014.0028
8. Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid. (2007) 17:1211–23. doi: 10.1089/thy.2006.0235
9. Carlé A, Laurberg P, Pedersen IB, Knudsen N, Perrild H, Ovesen L, et al. Epidemiology of subtypes of hypothyroidism in Denmark. Eur J Endocrinol. (2006) 154:21–8. doi: 10.1530/eje.1.02068
10. Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. (2002) 8:457–69. doi: 10.4158/1934-2403-8.6.457
11. Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf). (1977) 7:481–93. doi: 10.1111/j.1365-2265.1977.tb01340.x
12. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). (1995) 43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x
13. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. (2000) 160:526–34. doi: 10.1001/archinte.160.4.526
14. Tseng FY, Lin WY, Lin CC, Lee LT Li TC, Sung PK, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. (2012) 60:730–7. doi: 10.1016/j.jacc.2012.03.047
15. Wang CY, Chang TC, Chen MF. Associations between subclinical thyroid disease and metabolic syndrome. Endocr J. (2012) 59:911–7. doi: 10.1507/endocrj.EJ12-0076
16. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. (2008) 337:a801. doi: 10.1136/bmj.a801
17. Montanelli L, Benvenga S, Hegedüs L, Vitti P, Latrofa F, Duntas LH. Drugs and other substances interfering with thyroid function. In: Vitti P, Hegedüs L, editors. Thyroid Diseases: Pathogenesis, Diagnosis, and Treatment. Cham: Springer (2018), p. 733–61. doi: 10.1007/978-3-319-45013-1_27
18. Tsai SH, Chien SC, Nguyen PA, Chien PH, Ma HP, Asdary RN, et al. Incidences of hypothyroidism associated with surgical procedures for thyroid disorders: a nationwide population-based study. Front Pharmacol. (2019) 10:1378. doi: 10.3389/fphar.2019.01378
19. Jara EL, Muñoz-Durango N, Llanos C, Fardella C, González PA, Bueno SM, et al. Modulating the function of the immune system by thyroid hormones and thyrotropin. Immunol Lett. (2017) 184:76–83. doi: 10.1016/j.imlet.2017.02.010
20. Devalraju KP, Tripathi D, Neela VSK, Paidipally P, Radhakrishnan RK, Singh KP, et al. Reduced thyroxine production in young household contacts of tuberculosis patients increases active tuberculosis disease risk. JCI Insight. (2021) 6:e148271. doi: 10.1172/jci.insight.148271
21. Majid U, Islam N. Thyroid tuberculosis: a case series and a review of the literature. J Thyroid Res. (2011) 2011:359864. doi: 10.4061/2011/359864
22. Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun. (2009) 32:231–9. doi: 10.1016/j.jaut.2009.02.007
23. Tola HH, Holakouie-Naieni K, Lejisa T, Mansournia MA, Yaseri M, Tesfaye E, et al. Is hypothyroidism rare in multidrug resistance tuberculosis patients on treatment? A systematic review and meta-analysis. PLoS ONE. (2019) 14:e0218487. doi: 10.1371/journal.pone.0218487
24. Drucker D, Eggo MC, Salit IE, Burrow GN. Ethionamide-induced goitrous hypothyroidism. Ann Intern Med. (1984) 100:837–9. doi: 10.7326/0003-4819-100-6-837
25. Macgregor AE, Somner AR. The anti-thyroid action of para-aminosalicylic acid. Lancet. (1954) 267:931–6. doi: 10.1016/S0140-6736(54)92552-0
26. Wu TY, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. Lond J Prim Care (Abingdon). (2010) 3:115–9. doi: 10.1080/17571472.2010.11493315
27. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan's National Health Insurance Research Database: past and future. Clin Epidemiol. (2019) 11:349–58. doi: 10.2147/CLEP.S196293
28. National Health Research Institutes (2021). National Health Insurance Research Database. Available online at: http://nhird.nhri.org.tw/en/index.htm (accessed October 30, 2021).
29. Cheng CL, Kao YHY, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. (2011) 20:236–42. doi: 10.1002/pds.2087
30. Hsieh CY, Chen CH Li CY, Lai ML. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. (2015) 114:254–9. doi: 10.1016/j.jfma.2013.09.009
31. Cheng CL, Chien HC, Lee CH, Lin SJ, Yang YH. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol. (2015) 201:96–101. doi: 10.1016/j.ijcard.2015.07.075
32. Su VY, Su WJ, Yen YF, Pan SW, Chuang PH, Feng JY, et al. Statin use is associated with a lower risk of TB. Chest. (2017) 152:598–606. doi: 10.1016/j.chest.2017.04.170
33. Taiwan Centers for Disease Control (2022). Taiwan Guidelines for TB Diagnosis & Treatment, 7th edition. Available online at: https://www.cdc.gov.tw/En/InfectionReport/Info/BAkN3lDoa6hdrimSerBQyQ?infoId=s0pQQSgwPQuP4xq5x99Msw (accessed 23 May 2022).
35. Yen YF, Hu HY, Lee YL, Ku PW, Ko MC, Chuang PH, et al. Sexual inequality in incident tuberculosis: a cohort study in Taiwan. BMJ Open. (2018) 8.2:e020142. doi: 10.1136/bmjopen-2017-020142
36. Lee MR, Ho CM, Lee CH, Lee MC, Chang LY, Yu KL, et al. Tuberculosis contact investigation in an intermediate burden setting: implications from a large tuberculosis contact cohort in Taiwan. Eur Respir J. (2017) 50:2. doi: 10.1183/13993003.00851-2017
37. Hertz D, Schneider B. Sex differences in tuberculosis. Semin Immunopathol. (2019) 41:225–37. doi: 10.1007/s00281-018-0725-6
38. Fu H, Lin HH, Hallett TB, Arinaminpathy N. Explaining age disparities in tuberculosis burden in Taiwan: a modelling study. BMC Infect Dis. (2020) 20:1–12. doi: 10.1186/s12879-020-4914-2
39. Datiko D, Hadgu A, Jerene D, Suarez PG. High urban tuberculosis case notification rates can be misleading: evidence from an urban setting in Ethiopia. BMC Public Health. (2020) 20:1–6. doi: 10.1186/s12889-020-8290-z
40. Meng Z, Liu M, Zhang Q, Liu L, Song K, Tan J, et al. Gender and age impacts on the association between thyroid function and metabolic syndrome in Chinese. Medicine. (2015) 94:e2193. doi: 10.1097/MD.0000000000002193
41. Stevenson CR, Critchley JA, Forouhi NG, Roglic G, Williams BG, Dye C, et al. Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn. (2007) 3:228–45. doi: 10.1177/1742395307081502
42. De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. (2011) 21:879–90. doi: 10.1089/thy.2010.0429
43. Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. (2011) 4:252–60. doi: 10.1038/mi.2011.13
44. Perrotta C, Buldorini M, Assi E, Cazzato D, De Palma C, Clementi E, et al. The thyroid hormone triiodothyronine controls macrophage maturation and functions: protective role during inflammation. Am J Pathol. (2014) 184:230–47. doi: 10.1016/j.ajpath.2013.10.006
45. Huang H-K, Wang J-H, Kao S-L. Risk of developing pneumonia associated with clinically diagnosed hypothyroidism: a nationwide population-based cohort study. Fam Pract. (2021) 38:630–6. doi: 10.1093/fampra/cmab027
46. Lurie MB, Zappasodi P, Levy RS, Blaker RG. On the role of the thyroid in native resistance to tuberculosis. I. The effect of hyperthyroidism. Am Rev Tuberc. (1959) 79:152–79.
47. Kleynhans L, Ruzive S, Ehlers L, Thiart L, Chegou NN, Conradie M, et al. Changes in host immune-endocrine relationships during tuberculosis treatment in patients with cured and failed treatment outcomes. Front Immunol. (2017) 8:690. doi: 10.3389/fimmu.2017.00690
48. Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. (1974) 99:131–8. doi: 10.1093/oxfordjournals.aje.a121593
49. Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. (1997) 119:183–201. doi: 10.1017/S0950268897007917
50. Kiazyk S, Ball TB. Latent tuberculosis infection: an overview. Can Commun Dis Rep. (2017) 43:62–6. doi: 10.14745/ccdr.v43i34a01
51. Starshinova A, Malkova A, Kudryavtsev I, Kudlay D, Zinchenko Y, Yablonskiy P. Tuberculosis and autoimmunity: Common features. Tuberculosis. (2022) 102202. doi: 10.1016/j.tube.2022.102202
52. Aravindan PP. Host genetics and tuberculosis: theory of genetic polymorphism and tuberculosis. Lung India. (2019) 36:244–52. doi: 10.4103/lungindia.lungindia_146_15
53. Chen Y, Zeng Y, Wang J, Meng C. Immune and inflammation-related gene polymorphisms and susceptibility to tuberculosis in Southern Xinjiang population: a case-control analysis. Int J Immunogenet. (2021). doi: 10.1111/iji.12564
54. Yamazaki K, Yamada E, Kanaji Y, Shizume K, Wang DS, Maruo N, et al. Interleukin-6 (IL-6) inhibits thyroid function in the presence of soluble IL-6 receptor in cultured human thyroid follicles. Endocrinology. (1996) 137:4857–63. doi: 10.1210/endo.137.11.8895357
Keywords: hypothyroidism, international classification of diseases, levothyroxine, longitudinal study, mycobacterium tuberculosis, risk factors, tuberculosis
Citation: Cheng L-T, Chung C-H, Peng C-K, Shu C-C, Wu S-Y, Wang S-H, Wu G-J, Tsao C-H, Sun C-A, Chien W-C and Tang S-E (2022) Bidirectional Relationship Between Tuberculosis and Hypothyroidism: An 18-Year Nationwide Population-Based Longitudinal Cohort Study. Front. Med. 9:900858. doi: 10.3389/fmed.2022.900858
Received: 21 March 2022; Accepted: 22 June 2022;
Published: 12 July 2022.
Edited by:
Shu-Min Lin, Linkou Chang Gung Memorial Hospital, TaiwanReviewed by:
Chia-Hsiang Li, China Medical University, TaiwanCopyright © 2022 Cheng, Chung, Peng, Shu, Wu, Wang, Wu, Tsao, Sun, Chien and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wu-Chien Chien, Y2hpZW53dUBuZG1jdHNnaC5lZHUudHc=; Shih-En Tang, bXNldGFuZ0BnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.