
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 31 May 2022
Sec. Gene and Cell Therapy
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.900809
 Chenyang Gu1†
Chenyang Gu1† Qiankun Zhang1†
Qiankun Zhang1† Yajing Li2†
Yajing Li2† Rong Li1
Rong Li1 Jia Feng1
Jia Feng1 Wanghao Chen3
Wanghao Chen3 Waqas Ahmed4
Waqas Ahmed4 Ismatullah Soufiany4
Ismatullah Soufiany4 Shiying Huang5*
Shiying Huang5* Jun Long1*
Jun Long1* Lukui Chen1*
Lukui Chen1*Stroke is associated with a high disability and fatality rate, and adversely affects the quality of life of patients and their families. Traditional Chinese Medicine (TCM) has been used effectively in the treatment of stroke for more than 2000 years in China and surrounding countries and regions, and over the years, this field has gleaned extensive clinical treatment experience. The Phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) pathway is important for regulation of cell migration, proliferation, differentiation, and apoptosis, and plays a vital role in vascularization and oxidative stress in stroke. Current Western medicine treatment protocols for stroke include mainly pharmacologic or mechanical thrombectomy to restore blood flow. This review collates recent advances in the past 5 years in the TCM treatment of stroke involving the PI3K/AKT pathway. TCM treatment significantly reduces neuronal damage, inhibits cell apoptosis, and delays progression of stroke via various PI3K/AKT-mediated downstream pathways. In the future, TCM can provide new perspectives and directions for exploring the key factors, and effective activators or inhibitors that affect occurrence and progression of stroke, thereby facilitating treatment.
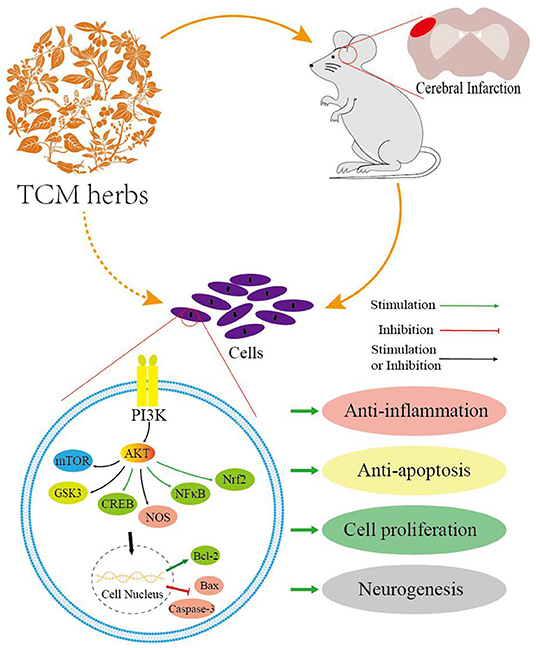
Graphical Abstract. The schematic diagram of neuroprotection of TCM herbs for stroke via the PI3K/AKT-mediated pathways and key downstream molecules.
Stroke, called “Zhongfeng” in Chinese, is still a major disease that seriously endangers human life and health, and is associated with a high rate of disability and fatality (1). Globally, about 80 million patients are afflicted with stroke, and the number of patients in China has reached more than 14 million. Among them, about 1.8 million patients die every year (2, 3). Most of the remaining survivors have varying degrees of disability, which seriously affects the quality of life of patients and their families; stroke takes a toll on the body, spirit, emotions of patients, and also causes economic loss. From the perspective of pathogenesis, 85% of strokes are ischemic strokes, which are caused by intracranial vascular occlusion, leading to apoptosis or necrosis of cells in the brain, and changes in neurovascular units and brain damage. Secondary cell death after ischemic stroke is coordinately mediated by multiple pathophysiological mechanisms, such as inflammation, oxidative stress, vascular dysfunction, energy depletion, excitotoxicity, autophagy, and apoptosis (4, 5).
Current treatment strategies for ischemic stroke mainly include mechanical thrombectomy or intravenous thrombolysis with recombinant tissue-type plasminogen activator to restore blood flow; however, the narrow time window limits the effectiveness and the safety of treatment (6); hence, finding other more effective treatments is imperative.
There is an urgent need for developing new medicines for stroke; new types of chemical compounds or drugs in modern medicine, requiring extensive chemical analysis and animal experiments for confirmation of their therapeutic effect. Based on the various ancient Chinese medicine texts and treatises, there are many prescriptions for treatment of stroke in Traditional Chinese Medicine (TCM), including decoctions, powders, injections, oral solutions, as well as acupuncture and scraping. When applied to animal models, some Chinese medicines reduce cerebral infarction, inhibit autophagy and cell apoptosis, and improve the long-term neurological recovery after stroke.
Phosphotylinosital 3 kinase (PI3K)/Protein kinase B (AKT) is an important pathway that regulates cell migration, proliferation, differentiation, and apoptosis, and plays a vital role in physiological or pathophysiological conditions. PI3K is a family of proteins that can catalyze the G-phosphate transfer of ATP to the phosphatidylinositol of D3 position (7); After activation, the signal sent by AKT can be transmitted to diverse substrates with various functions. Abnormal activity or upregulation or downregulation of the pathway may cause a variety of diseases, such as neurodegenerative diseases, stroke, diabetes, cancer, etc. (8). The activation of PI3K may be mediated by tyrosine kinase growth factor receptors. PI3K transforms phosphatidylinositol 4,5-bisphosphate into phosphatidylinositol 3,4,5-triphosphate, combines AKT and Pyruvate Dehydrogenase Kinase Isozyme 1 (PDK1), and causes PDK1 to phosphorylate AKT, which is the direct target downstream protein. The activation of the PI3K/AKT pathway causes a cascade reaction of downstream proteins that mediate cell function. In various organs, the repair is basically carried out through PI3K/AKT pathway, especially central nervous system.
The PI3K/AKT pathway has several downstream target receptors, for example, c-kit receptor, insulin-like growth factor-1 receptor, vascular endothelial growth factor (VEGF) receptor, etc. Angiogenesis is one of the downstream effects. There are two main routes. One is the PI3K/AKT/nitric oxide synthase (NOS) pathway, which promotes the release of nitric oxide (NO) by vascular parietal cells. NO is released from the endothelium, causing vasodilation by the activity of endothelial NOS (eNOS) (9). NO, fibroblast growth factors (FGFs), VEGFs, along with others, stimulate the generation of new blood vessels. Hypoxia upregulates hypoxia-inducible factor 1α (HIF-1α) levels; epidermal growth factor receptor (EGFR) and PI3K also stimulate HIF-1α production. The other pathway is the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway that induces the release of HIF-1α (10). What is puzzling is that it induces the protein HIF-1α, but the mRNA level is normal. Perhaps the PI3K/AKT/mTOR pathway might enhance HIF-1α translation. This pathway is useful in the current context. Examples include: the synergy of anti-PD-1 and endostar on PI3K/AKT/mTOR-mediated autophagy (11); ginsenoside Rg1 promotes cerebral vascularization via the PI3K/AKT/mTOR pathway after stroke (12).
To deal with oxidative damage, the body is equipped with an efficient defense system that detoxifies and eliminates harmful chemicals and inactivates reactive oxygen species (ROS). Over past the 20 years, people have sought to examine the link between the PI3K/AKT pathway and oxidative stress. Nuclear factor-erythroid 2-related factor 2 (Nrf2), a main regulator of various antioxidant enzymes, is the primary downstream target of the PI3K/AKT pathway. Nrf2 modulates the balance of cell redox and senses the status of oxidative stress by the activity of antioxidant factors, such as ferritin, glutathione reductase, thioredoxin reductase, glutathione peroxidase, superoxide dismutase (SOD), hemeoxygenase-1 (HO-1), and NAD(P)H: quinone oxidoreductase 1 (NQO1) (13, 14). The consensus of some studies on Nrf2 phosphorylation is that the PI3K/AKT/Nrf2 pathway may perhaps be the main pathway that withstands oxidative stress to cells (15, 16). This could be an effective therapeutic target for aging, which has been shown to be linked to oxidative stress.
According to the Yellow Emperor's Classic of Medicine—Suwen (17), the main mechanisms of stroke are related to internal injuries and accumulation, excessive emotion and desire, improper diet, and fat body. Internal injuries and accumulation: as a person's age increase, Yang gradually becomes self-deficient, and Yang turns to Yin. Imbalance of work and rest, prolonged diseases, and Yin causes damage to the qi of the organs. The deficiency of qi causes inability to supply blood and cerebral meridian stasis. Excessive emotions and desires: anger injures the liver, cause qi stagnation in the liver. This qi stagnation transforms liver heat, causing disorder of qi and blood of the liver. The disorder invades the brain, causing blood overflow or blocks the cerebral meridian. Improper diet: the spleen (pancreas), stomach, and liver are usually in harmony. High-fat and high-alcohol diet can hurt this harmony and cause endogenous phlegm fever. The phlegm fever and blood stasis block each other, and heat accumulation leads to cerebral meridian stasis. Fat body: there is deficiency of qi and phlegm-damp develops in fat bodies, which causes the stagnation of qi and blood. Both these also invade the brain, causing cerebral meridian stasis. From the perspective of TCM, therapeutic strategies include clearing the phlegm fever to sputum, draining turbidity of organs, replenishing qi and strengthening the body, removing blood stasis, and promoting blood circulation, nourishing the liver and its harmony. The prescription that is used commonly is the Buyang Huanwu Decoction (BHD), which mainly includes Salvia miltiorrhiza, Scutellaria baicalensis Georgi, Ligusticum wallichii, Astragalus propinquus Schischkin.
Chinese herbal medicines afford therapeutic effects. Many neuroprotective drugs have been widely proven to be effective in animal experiments, but they are not effective when applied to patients. When applied to animal stroke models, several Chinese herbal medicines reduce cerebral infarction, inhibit autophagy and cell apoptosis, improve long-term neurological recovery through similar or identical pathways; the downstream substrates mainly include mTOR, glycogen synthase kinase 3 (GSK3), nuclear factor kappa light chain enhancer of activated B cells (NF-κB), Nrf2, NOS, cyclic AMP-responsive element binding protein (CREB), etc.
As a downstream substrate that transmits signals after AKT activation, mTOR, a serine threonine kinase, mediates cell growth, survival, and metabolism (18).
Tanshinone IIA, a natural component derived from Salvia miltiorrhiza (Dan-Shen in Chinese), has been proven to have therapeutic effects in cerebrovascular and cardiovascular diseases. Tanshinone IIA treatment promotes the recovery of brain function and increases neuronal viability; furthermore, the glucose concentration in the serum and cultured medium was increased, possibly regulated by an increased glucose uptake ability and activation of the PI3K/AKT/mTOR pathway (19). Sodium tanshinone IIA sulfonate is a water-soluble derivative of tanshinone IIA, which suppresses the expression of HIF-1 and hemoglobin genes via inhibition of the PI3K/AKT/mTOR pathway, thereby protecting against hypoxia in cerebral hemorrhage in zebrafish (20). Catalpol, an iridoid glycoside compound, the main active component of Rehmannia glutinosa Libosch (Di-Huang in Chinese), is commonly used in TCM for treating diseases of aging and stroke (21). Catalpol activates PI3K/AKT/mTOR/neuromodulin and PI3K/AKT/mTOR/brain-derived neurotrophic factor (BDNF) pathways with or without inhibition of miR-124 to further improve neuronal survival, cell viability, and axonal growth after oxygen and glucose deprivation/reoxygenation (OGD/R) (22, 23). Baicalein (5,6,7-trihydroxyflavone), the main active component extracted from the root of Scutellaria baicalensis Georgi (Huang-Qin), has shown promise when administered in the acute phase of ischemic stroke (24). Baicalein significantly inhibited autophagy via activation of the PI3K/AKT/mTOR pathway and subsequently induced changes to B cell lymphoma-2 (Bcl-2), caspase-3, and Bcl-Associated X (Bax) proteins (25). Daucosterol palmitate, the main active component of Alpinia oxyphylla Miq. fruit (Yi-Zhi in Chinese), exhibits therapeutic effects in diarrhea, cognitive impairment, intestinal disorders, and diuresis (26). Daucosterol palmitate protects neurons and inhibits neuronal apoptosis by activating the PI3K/AKT/mTOR pathway-mediated β-actin (27). Cornus officinalis, Shan-Zhu-Yu in Chinese, clinically used in liver and kidney deficiency, has been combined with other herbs to treat stroke. Cornel iridoid glycoside is the main active component extracted from Cornus officinalis; this therapy exerted neuroprotective effects in middle cerebral artery occlusion (MCAO) in rats during acute and chronic phases, thereby improving motor functions and promoting recovery of somatosensory deficits, and alleviated memory deficits. The effects were partially due to promotion of angiogenesis and neurogenesis of gray matter, and anti-neuroinflammatory and anti-apoptotic effects via increased expression of BDNF and neuregulin-1V proteins and consequently activation of the PI3K/pAKT/pmTOR pathway (28). Genistein, a natural phytoestrogen extracted from soybeans, was found to have neuroprotective potential and could help in preventing stroke. Genistein therapy could alleviate neuronal apoptosis induced by I/R injury in ovariectomized rats via activation of the PI3K/AKT/mTOR pathway (29). Ginsenoside Rg1, one of the most active components of ginsenoside that is extracted from ginseng (Ren-Shen in Chinese), is used widely for the treatment of various neurological diseases (30), mediates cell proliferation and differentiation, inhibits inflammation, cell apoptosis, and has other pharmacological effects (31). Ginsenoside Rg1 promotes migration, proliferation, vascularization, and tube formation in endothelial cells through increased expression of VEGF and HIF-1 via activation of the PI3K/AKT/mTOR pathway after ischemic stroke (12). Resveratrol (3,4,5-trihydroxystilbene) is a phenolic ingredient found in Polygonum cuspidatum (Hu-Zhang in Chinese) and also exists abundantly in red grape skin and red wine. Resveratrol improves neurological function and alleviates neurological damage in cerebral infarction, inhibits apoptosis, and protects hippocampal neurons from damage induced by I/R injury via activation of the Janus kinase 2 (JAK2)/PI3K/AKT/mTOR pathway (32). Huperzine A, a lycopodium alkaloid derived from Huperzia serrata (Qian-Ceng-Ta in Chinese), is commonly used for treating Alzheimer's disease in clinical practice because of its inhibitory effect on acetylcholinesterase. Huperzine A reduces oxidative glutamate toxicity in hippocampal cells and inhibits apoptosis via activation of the BDNF/PI3K/AKT/mTOR pathway and the consequent increase in Bcl-2 and decrease in caspase-3 (33). Withaferin A is a steroidal lactone extracted from Withania somnifera (Ashwagandha, Zui-Qie in Chinese) (34), which exhibits immunomodulatory, anti-inflammatory, cardioprotective, and neuroprotective effects (35). Withaferin A exhibits neuroprotective effects, including anti-apoptotic effects and promotes cell proliferation by suppressing phosphatase and tensin homolog deleted on chromosome ten (PTEN) and subsequent activation of the PI3K/AKT/mTOR and PI3K/AKT/GSK3β pathways, and inhibition of migration of vascular smooth muscle cells (36). The molecular mechanism and effect of TCM active components in this section through the PI3K/AKT/mTOR pathway is briefly summarized in Figures 1A, 2A.
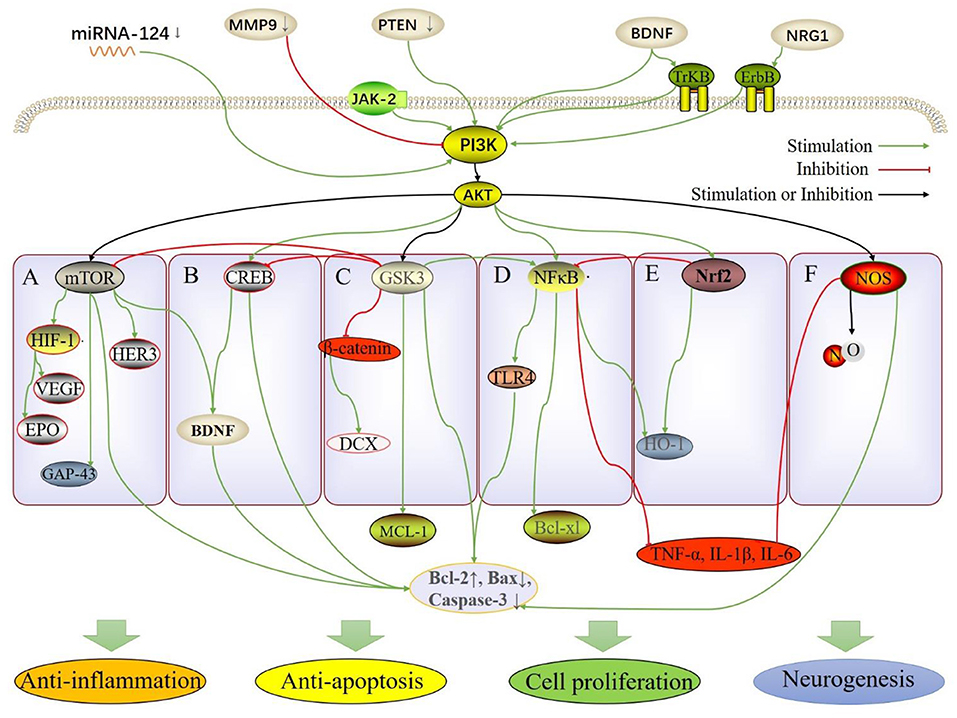
Figure 1. The molecular and pathway mechanisms involved in the neuroprotective effect of TCM herbs, including anti-inflammation, anti-apoptosis, cell proliferation and neurogenesis. (A) PI3K/AKT/mTOR; (B) PI3K/AKT/CREB; (C) PI3K/AKT/GSK3; (D) PI3K/AKT/NF-κB; (E) PI3K/AKT/Nrf2; (F) PI3K/AKT/NOS.
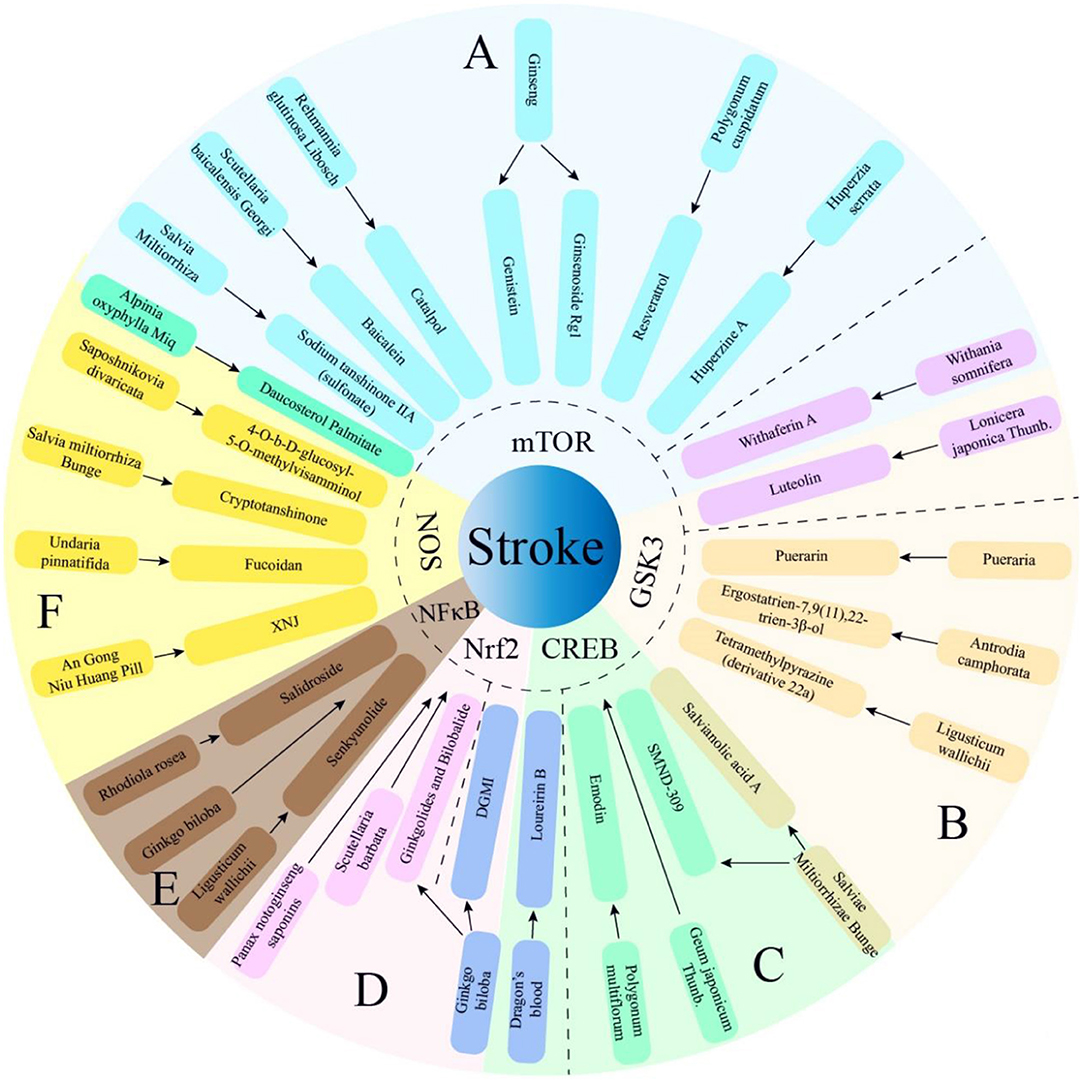
Figure 2. Multiple active components from TCM herbs exert therapeutic effects via PI3K/AKT -mediated 6 main pathways. The TCM herbs of manuscript not mentioned above almost exert therapeutic effects via PI3K/AKT -mediated Bcl-2 pathway. SMND-309, a chemical derivant of propenoic acid of Sal A; DGMI, Diterpene ginkgolides meglumine injection; XNJ, Xingnaojing. (A) PI3K/AKT/mTOR; (B) PI3K/AKT/GSK3; (C) PI3K/AKT/CREB; (D) PI3K/AKT/Nrf2; (E) PI3K/AKT/NF-κB; (F) PI3K/AKT/NOS.
GSK3 is a ubiquitous serine-threonine kinase with various cellular and neurophysiological functions, including neurogenesis, neurotrophicity, neuroprotection, mood stabilization, and anti-inflammation (37, 38).
As mentioned above, Withaferin A, from Withania somnifera, exhibits neuroprotection by suppressing PTEN and subsequently activating the PI3K/AKT/mTOR and PI3K/AKT/GSK3β pathways, among which the GSK3 pathway is also a key pathway for neuroprotection in TCM (36). Similarly, Luteolin, 3′,4′,5,7-tetrahydroxyflavone, is a flavonoid, a natural compound, present widely in fruits and vegetables (39). Luteolin enhances cell viability and inhibits apoptosis by downregulating the matrix metalloproteinase 9 expression and activating the PI3K/AKT-mediated mTOR and GSK3β pathways, which alleviate cerebral infarction (40). Tetramethylpyrazine (TMP) is an active component of Ligusticum chuanxiong (Chuan-Xiong in Chinese), which is commonly used in TCM clinically for the treatment of cardio- and cerebrovascular diseases (41). TMP functionally increases transcription of redox proteins, scavenges free radicals, and protects antioxidant enzymes (42). Besides, TMP very effectively penetrates the blood–brain barrier (BBB). A compound called α-tetramethylpyrazinyl-N-tert-butyl nitrone (TBN), a TMP nitrone conjugate, shows potent free radical scavenging ability and crosses the BBB easily, protecting neurons from ischemic stroke (43). Caffeic acid, a hydroxyl extractive of cinnamic acid possessing a phenolic moiety (44), and its ester derivatives show excellent neuroprotection (45). The chemical combination of TBN and caffeic acid is compound 22a, which maintains mitochondrial membrane potential and inhibits cell apoptosis by activating the PI3K/AKT/GSK3β pathway, thereby protecting against glutamate-induced cerebellar granule neuronal damage (46, 47). The compound 7,9(11),22-ergostatrien-3β-ol (EK-100) is the main active component extracted from the ethyl acetate crude extract of Antrodia Camphorate (Aphyllophorales, Niu-Zhang-Zhi in Chinese) (EtOAc-AC), a well-known TCM (48). EtOAc-AC and EK100 reduce apoptosis and inflammation by decreasing NF-κB and caspase 3, and promoting Bcl-2 and double-cortin (DCX) by activating the PI3K/AKT pathway, inhibiting GSK3, and activating β-catenin (49). Puerarin is a phytoestrogen extracted from Pueraria (Ge-Gen in Chinese). Puerarin affords protection in various diseases, such as cardiac dysfunction, liver injury, and neurological diseases (50); it is especially neuroprotective in Parkinson's disease (51), Alzheimer's disease (52), and ischemic stroke (53). Puerarin improves cell viability in the hippocampus significantly, inhibits cell apoptosis, and protects the hippocampus by activating the PI3K/AKT/GSK-3β pathway and consequently increase in myeloid cell leukemia 1, and inhibition of caspase-3 (54). Salvianolic acid A (Sal A) is another main active component of Salvia miltiorrhiza that is similar to Tanshinone IIA. Studies suggest that SalA has potent protective effects against ischemia-induced injury both in vitro and in vivo (55). Sal A attenuates inflammation/nitrosative stress and preserves the integrity of the BBB by inhibiting NF-κB. Sal A concomitantly promotes neurogenesis by activating the PI3K/AKT pathway to induce GSK3 to enhance catenin/DCX and Bcl-2 expression (56). Sal A involves CREB when it has anti-inflammatory effects via the GSK3-mediated activated Bcl-2 pathway. The molecular mechanism and effect of TCM active components in this section through the PI3K/AKT/GSK3 pathway is briefly showed in Figures 1C, 2B.
CREB, a nuclear transcription factor, can be stimulated by phosphorylated AKT, which facilitates CREB-binding protein translocation to the promoter and triggers the formation of protective proteins (57).
Similarly, a compound obtained from Salvia miltiorrhiza, SMND-309, a chemical derivative of propenoic acid of Sal A (58), is a novel metabolite produced in the brains and hearts after oral administration of Sal A, which elicits strong neuroprotective effects by promoting angiogenesis and inhibiting cell apoptosis and cerebral edema. SMND-309 prevents apoptosis of differentiated SH-SY5Y cells by increasing cell viability and reducing lactate dehydrogenase (LDH) activity after OGD/R by activating the PI3K/AKT/CREB pathway (59). Geum japonicum Tunb. var. chinense (Lan-Bu-Zheng in Chinese) has been commonly used in the treatment of dizziness and headaches in China. As the main active components, Tellimagrandin II, 5-desgalloylstachyurin, and 3,4,5-Trihydrox-ybenzaldehyde have multiple therapeutic effects, including anti-inflammation (60), anti-apoptosis (61), and so on. The extracts protect astrocytes from OGD/R-induced injury by inhibiting apoptosis and astrocyte reactivity, via activation of the BDNF/PI3K/AKT/CREB pathway (62). Emodin, an anthraquinone derivative derived from Polygonum multiflorum Thunb. (He-Shou-Wu in Chinese), is an effective apoptotic agent promoting oxidative damage at high concentrations (63). Use of emodin appears counterintuitive due to its toxicity; however, it also affords protection to multiple organs (64). Natural emodin exerts neuroprotective effects and is used in the treatment of neurodegenerative disease (65). Emodin inhibits glutamate-induced apoptosis and has significant neuroprotective effects via activation of the PI3K/AKT-mediated Bcl-2 and CREB/BDNF pathways, and has been shown to enhance behavioral function in I/R injury (66). Dragon's blood (DB, Long-Xue in Chinese) is a rare and precious traditional medicine in China. It is abundant in four different plant genera: Dracaena, Croton, Daemonorops, and Pterocarpus (67). DB has the properties of anti-inflammation and anti-oxidative stress (68). Loureirin B, the phenolic extract of DB, is beneficial in patients diagnosed with ischemic stroke clinically (69). Meantime, Longxuetongluo capsule (the main ingredient is Loureirin B) was approved by the Chinese government as a new drug for treating ischemic stroke (70). Loureirin B activates the PI3K/AKT/Nrf2 and CREB pathways, which increase HO-1 and Bcl-2 expression to protect cells from OGD/R injury. Ginkgo biloba, Yin-Xing in Chinese, has various therapeutic effects, including “dispersing toxin” (clearance of inflammation and oxidative state), treatment of stroke and myocardial infarction; this has been recorded in the “Compendium of Materia Medica, Bencao Gangmu in Chinese,” written with Li Shizhen in the Ming Dynasty (71). Ginkgolides, the major component of Diterpene ginkgolides meglumine injection derived from Ginkgo biloba, inhibits neuronal apoptosis for protection against cell injury induced by OGD/R by activation of the PI3K/AKT-mediated CREB and Nrf2 pathways (57). The molecular mechanism and effect of TCM active components in this section through the PI3K/AKT/CREB pathway is briefly concluded in Figures 1B, 2C.
Nrf2, a transcription factor mediating endogenous anti-oxidants, plays a key role in intracellular defense against ROS (72). Furthermore, the activation of Nrf2 exerts protective effects on BBB integrity after brain injury (73).
As mentioned above, extract of Ginkgo biloba, including bilobalide and ginkgolides, belong to ginkgolide terpenoid lactones. Bilobalide and ginkgolides upregulate the levels of antioxidant proteins, including HO-1, SOD, and NQO1 via activation of the important PI3K/AKT/Nrf2 pathway to protect neurons against oxidative stress. Scutellaria barbata D. Don (SBD), which belongs to the Scutellaria genus (Ban-Zhi-Lian in Chinese), is a perennial herb in China with therapeutic effects, including anti-oxidation and anti-inflammation (74). SBD treatment promotes cell proliferation and viability, reduces cell apoptosis after OGD/R injury, and improves mitochondrial dysfunction and oxidative injury via activation of the PI3K/AKT-dependent Nrf2 pathway (75). Panax notoginseng saponins (PNS) are the main active components of Xue-Sai-Tong Injection, commonly used in the treatment of cardiac diseases and ischemic stroke in China (76), which protect neurons against I/R injury (77), and inhibit efficiently oxidant activity combined with above ginsenosides (12, 78). As an extrinsic regulator, Panax notoginseng saponins protect against OGD/R-induced BBB disruption via activation of the PI3K/AKT/Nrf2/HO-1 pathway (79). Salidroside is a key bioactive component of Rhodiola rosea (Hong-Jing-Tian in Chinese), which has neuroprotective effects and improves cognitive functions (80). Salidroside reduces cerebral infarction and neurological deficits in MCAO; it reduces neuroinflammation and neural damage via activation of the PI3K/AKT/Nrf2/NF-κB pathway (81). Salidroside exerts its neuroprotective effects through the PI3K/AKT pathway that involves another key factor, NF-κB. The molecular mechanism and effect of TCM active components in this section through the PI3K/AKT/Nrf2 pathway is generalized in Figures 1E, 2D.
NF-κB is a key transcription factor that, respectively, regulate cellular responses to inflammation (82).
Identically, it has been reported that terpenoids and flavonoids in Ginkgo biloba possess potent anti-inflammation, anti-oxidative, and free radical scavenging activity (83). Ginkgetin aglycone treatment reduces oxidative stress and inflammation to protect against neuronal injury in stroke via activation of the PI3K/AKT/NF-κB/TLR4/Bcl-2 pathway (84). Moreover, Senkyunolide-H (SEH) is another main bioactive component of Ligusticum wallichii (85). SEH inhibits inflammatory factor release and exerts anti-apoptotic effects via activation of the PI3K/AKT/ NF-κB pathway and has therapeutic potential in ischemic stroke (86). Senkyunolide-I, the stereoisomer of SHE, exhibits definite anti-apoptotic activity in I/R injury and has anti-inflammatory effects against endotoxin insult (87). The molecular mechanism and effect of TCM active components in this section through the PI3K/AKT/ NF-κB pathway is briefly concluded in Figures 1D, 2E.
Nitric oxide, synthesized from L-arginine, plays a crucial role in controlling blood pressure, blood flow, and oxygen delivery (88). The NOS isoforms include inducible NOS (iNOS), endothelial NOS (eNOS) and neuronal NOS (nNOS) (89).
Extracted from Salvia miltiorrhiza identically, cryptotanshinone, another main active component, possesses various pharmacological activities, including anti-cancer (90), anti-inflammation (91), anti-oxidative effects (92). Cryptotanshinone exhibits anti-apoptotic and blood vessel protective activities and is neuroprotective against cerebral stroke via inhibition of the PI3K/AKT/eNOS pathway and subsequent increased Bcl-2 and NO in both the cerebral cortex and the peripheral blood (93). As a nutritious food in East Asia, Undaria pinnatifida, Qun-Dai-Cai in Chinese, is traditionally used in soup recipes to promote breast milk production during dyslipidemia and the postnatal period (94). Fucoidan, extracted from Undaria pinnatifida, contains sulfate and L-fucose, and possesses bioactivities, including anti-inflammation, anti-coagulation, anti-tumor, and neuroprotection (95). Xingnaojing (XNJ) is extracted from An Gong Niu Huang Pill and is used for treating stroke; it contains She-Xiang (Moschus), Jiang-Huang (Curcuma longa L.), Bing-Pian (Borneol), and Zhi-Zi (Gardenia), and is approved by the Chinese Government (96). XNJ reverses brain injury, promotes functional recovery, and exerts neuroprotective effects after stroke (97). XNJ inhibits human brain microvascular endothelial cell (HBMEC) apoptosis after I/R injury and OGD injury via activation of the PI3K/AKT/eNOS pathway and consequently causes increase in Bcl-2/Bax and decrease in caspase-3 (98). 40-O-b-D-glucosyl-5-O-methylvisamminol (OGOMV), derived from Saposhnikovia divaricate (Fang-Feng in Chinese), is commonly used in the treatment of cancer, inflammation, and cardiac events in China (99). OGOMV attenuates cleaved caspase-3a,−9a related apoptosis, and inflammation to exert neuroprotection via inhibition of the P13K/AKT/iNOS pathway (100). The molecular mechanism and effect of TCM active components in this section through the PI3K/AKT/NOS pathway is briefly introduced in Figures 1F, 2F.
There are many active components of TCMs that directly increase Bcl-2 and decrease Bax via activating PI3K/AKT pathway to exert neuroprotective effects.
Ligustilide, derived from Ligustilide walliichi, attenuates cerebral infarction, and reverses neurological injury, thereby affording neuroprotection, reverses apoptosis of hippocampal cells via activation of the PI3K/AKT pathway and consequently increases Bcl-2, and decreases Cyt C, Bax, and caspase-3 (101). Tetramethylpyrazine protects Bone Mesenchymal Stem Cells (BMSC) against H2O2-induced apoptosis via activation of the PI3K/AKT pathway; this was used to improve cell survival in combination with BMSCs in ischemic stroke (102). Tetramethylpyrazine leads to neural progenitor cell (NPC) migration and survival by inducing stromal cell derived factor 1 secretion, stimulating the PI3K/AKT pathway (103). Chrysophanol (CHR), the main active component of Rhubarb (Da-Huang in Chinese), promotes cell viability and inhibits cell apoptosis by increasing miR-216a expression and activating the PI3K/AKT pathway (104). Kaempferol-3-O-rhamnoside, an active ingredient extracted from Schima wallichii Korth (Hong-Mu-He in Chinese) leaves, reduces cerebral infarction and water content, and inhibits apoptosis by activating the PI3K/AKT pathway (105). Astilbin, a dihydroflavonol derivative from Rhizoma Smilacis glabrae (Tu-Fu-Ling in Chinese), significantly improves cerebral infarction and neurological deficits, and inhibits apoptosis and inflammation after I/R injury by activating the PI3K/AKT pathway (106). Icariin is a quinlizidine flavone extracted from Epimedium L. (Yin-Yang-Huo in Chinese); this in combination with MSCs greatly improves cerebral infarction, neurologic deficits of motor and somatosensory function, and promotes angiogenesis and neurogenesis via activation of PI3K/AKT pathway and consequently increases VEGF and BDNF (107).
Besides these, there are several TCMs and their active ingredients that exert neuroprotection via different PI3K/AKT-mediated pathways.
Salidroside, derived from Rhodiola rosea, activates the PI3K/AKT pathway to induce production of HIF-α subunits and erythropoietin, one or more of which mediate anti-inflammatory effects after IR injury (108). Baicalin, a flavonoid compound derived from Scutellaria baicalensis root, effectively inhibits apoptosis, and reduces cerebral infarction and neuron loss via activation of the PI3K/AKT-mediated glutamate transporter 1 (109). Vinpocetine is an alkaloid derivative isolated from the leaves of Phyllostachys pubescens (Mao-Zhu in Chinese), which possesses anti-inflammation and anti-platelet aggregation properties, and improves cognition, cerebral blood flow, and metabolism (110). Vinpocetine reduces cerebral infarction and edema in I/R injury, and reduces apoptosis, inflammation, and oxidative stress induced by I/R injury via activation of the PI3K/AKT-mediated connexin43 phosphorylation pathway (111). Artesunate is a water-soluble derivative of artemisinin, found in the aboveground dry part of Artemisia annua L (Qing-Hao in Chinese). Artesunate enhances NSC proliferation in the infarcted cortex and alleviates I/R injury via inhibition of the Forkhead box protein O 3a transcription by inducing phosphorylation and downregulating p27 through the PI3K/AKT pathway (112). Formononetin is one of the main components of astragalus isoflavones, derived from Astragalus membranaceus (Huang-Qi), widely used for neuroprotection and neurological functional recovery (113). Formononetin helps in recovery of injured nerve functions, and mediates therapeutic effects through neuronal differentiation and synaptic plasticity after ischemic stroke via activation of the PI3K/AKT/ERK (extracellular regulated protein kinases) pathway (114). DL-3-n-butylphthalide is an oily component extracted from Apium graveolens Linn (Chinese celery), which attenuates neurovascular damage (115), improves cognitive functions and neurological outcomes (116), and reduces brain edema (117), inflammation and oxidation (118), and cerebral infarction. DL-3-n-butylphthalide activates primary and secondary dendrites and dendritic tips via inactivation of the PI3K/AKT pathway, without involving the above mentioned up/downstream pathways (119).
Reports suggest that composite Chinese medicines have multiple pharmacological interactions. There are many prescriptions for stroke treatment in TCM, including decoctions, powders, injections, oral solutions, in combination with acupuncture and scraping.
BHD is the most representative TCM formula for stroke treatment, first documented in Yilin Gaicuo of Wang Qingren in the Qing Dynasty; it includes Astragalus membranaceus, Dang-Gui (Angelica sinensis), Ligusticum chuanxiong, Shao-Yao (Paeonialactiflora), Hong-Hua (Carthamustinctorius), Tao-Ren (seed of Prunus persica), and Di-Long (Lumbricus). BHD attenuates neurological deficits, promotes proliferation (120), neurorehabilitation (121) and the migration of NPCs to ischemic areas (122), and induces synaptic plasticity (121). BHD significantly improves the proliferation and differentiation of NSCs, cerebral infarction, and neuron viability, and decreases cell apoptosis via activation of the PI3K/AKT/Bad and Jak2/Stat3/Cyclin D1 pathways (123). Hou's Black Pulvis (Houshi Heisan, HBP), a classic compound prescription, was first documented in “Synopsis of Golden Chamber (Jingui Yaolue)” by Zhang Zhongjing in the Han Dynasty (216 A.D.); it is widely used for recovery of neuronal function in China, and mainly includes Ju-Hua (chrysanthemum), Bai-Zhu (Atractylodes macrocephala Koidz), Fang-Feng (Saposhnikovia divaricate), Jie-Geng (Platycodon grandifloras). HBP inhibits amyloid precursor protein deposition (124) and reduces the inflammatory reaction (125) by multiple mechanisms, including blunting of abnormal activation of astrocytes and activation of the endogenous nerve growth factor. HBP treatment enhances the reorganization and regeneration of cerebral blood circuits through the BDNF/PI3K/AKT pathway, which also overcomes the growth-inhibitory signals, RhoA/ROCK and Nogo-A/NgR to facilitate plasticity after stroke (126). Tongnao Decoction (TND) is a Chinese decoction approved and used in the treatment of ischemic stroke in Jiangsu Province Hospital of TCM; it is composed of Jiu-Jie-Chang-Pu (Rhizoma Anemones altaicae), Tian-Nan-Xing (Rhizoma arisaematis), Ligusticum chuanxiong, Gou-Teng (Ramulus Uncariae Cum Uncis), Tian-Ma (Rhizoma Gastrodiae), Jiang-Can (Bombyx batryticatus), Shui-Zhi (Hirudo), and Hong-Jing-Tian (Radix et Rhizoma Rhodiolae Crenulatae). TND promotes the migration, proliferation, and tube formation of cells, and restores neurovascular function by promoting angiogenesis in the ischemic cerebral microvasculature, via activation of the PI3K/AKT and Raf/MEK/ERK pathways (127). Taohong Siwu Decoction (TSD) comes from the “Golden Mirror of Medicine” of the Qing Dynasty. It consists of six TCM herbs: seed of Prunus persica, Carthamustinctorius, Angelica sinensis, Ligusticum chuanxiong, Rehmannia glutinosa Libosch and Paeonialactiflora. TSD has significant effects in treating the rats with I/R, the mechanism of which may be involved in promoting the angiogenesis and recovering the nerve function by activating PI3K/AKT signal pathway (128). Nao-shu-ning (NSN) is approved by Zhang Jianfu of Shaanxi University of Chinese Medicine, composed of Leonurus artemisia, Radix Cyathulae, Hirudo, Radix Notoginseng, Rhizoma Imperatae, Raw Rhubarb, Fructus Forsythiae and Rhizoma Acori Tatarinowii. The possible mechanism of effect of NSN on nerve regeneration is the inhibition of AKT and inhibiting phosphorylation of Raf-1 during reperfusion (129). In clinical study, Chaigui Wendan Dingzhi decoction (CWDD) is composed of 16 TCM herbs, mainly including, Bupleurum chinense (Chai-Hu), Paeonialactiflora, Poria cocos, Pinellia ternate (Ban-Xia) and seed of Ziziphus jujuba Mill. CWDD has a significant effect on the recovery of cognitive function and depressive mood in stroke patients after stroke which may be related to the activation of PI3K/AKT-mediated Bcl and BAX pathway (130).
Stroke has one of the highest mortality rates globally. Currently used medicines and modern medical surgical treatment are not conducive to recovery. This review collates knowledge and experience on stroke management from the realm of TCM with modern molecular scientific research and describes the progress TCM involving PI3K/AKT pathway regarding neuroprotection in the past 5 years. The PI3K/AKT pathway is the key pathway that underpins pathophysiological mechanisms of diseases, also stroke, effects including neurological damage, cell apoptosis, vascularization, oxidative stress, etc. The PI3K/AKT pathway acts as a central mediator of growth factor stimulation during cell growth (131). Previous studies have shown that the PI3K/AKT inhibited Raf/MAPK/ERK pathway-mediated negative effects during cerebral I/R (132). They suggest that the PI3K/AKT pathway contributes to cyto-protection in different cell, tissue, and animal models, including focal ischemia and post-ischemic conditioning. TCM treatment involving the PI3K/AKT pathway has considerable effects in reducing neuronal damage, inhibiting cell apoptosis, and delaying the progression of stroke.
The neuroprotection of herbal single components through the PI3K/AKT pathway is detailed in the main text, some herbs contain multiple active components that synergistically protect the nervous system through PI3K/AKT-mediated downstream molecules. There are four active components of Salvia miltiorrhiza, including Sodium tanshinone IIA sulfonate, Sal A, SMND-309, and Cryptotanshinone, exert neuroprotection synergistically through the four PI3K/AKT-mediated pathways, including increasing neuronal viability, preventing apoptosis and promoting neurogenesis (20, 56, 59). Ginkgo biloba has various therapeutic effects, and contains multiple active components, like Ginkgolides, bilobalide, Ginkgetin aglycone. They inhibit neuronal apoptosis, reduce oxidative stress and inflammation via activation of the PI3K/AKT pathways (57, 84). Compound 22a and Senkyunolide-H, are derived from Ligusticum wallichii. Both maintains mitochondrial membrane potential, inhibits cell apoptosis and inflammatory factor release by activating the PI3K/AKT pathways (46, 47, 86). Extracted from Scutellaria baicalensis Georgi, Baicalein and Baicalin inhibits autophagy, reduces cerebral infarction and neuronal loss via activation of the PI3K/AKT mediated pathway (25, 109).
In the past, the prevention and treatment of stroke was often guided by vascular recanalization, including thrombolysis, mechanical recanalization, etc. This review shows more than 20 common TCM herbs for stroke, each of which has at least one active ingredient that can improve the progression of stroke through PI3K pathway mediated downstream pathways. Some components may also interact with each other in positive or negative feedback during the treatment process to achieve dynamic balance by mutual regulation. Like Salvia miltiorrhiza, Ginkgo biloba, Scutellaria baicalensis Georgi, etc., multiple active components of a single herb can synergistically activate the PI3K/AKT pathway to exert a variety of neuroprotective effects, including maintaining neuronal vitality, neurogenesis, preventing apoptosis, reducing inflammation, and oxidative stress, etc. The compound formula is composed of a variety of traditional Chinese medicines, and different herbs can also play a synergistic effect to form a multi-target and multi-organ protective effect on the body. Perhaps the PI3K/AKT pathway is a potential key pathway of TCM treatment for stroke, which provides targets and thought for exogenous prevention and treatment. Active ingredients of TCM provides a direction for finding key mechanisms and pathways in other diseases (Graphical Abstract). However, the advantages of TCM for stroke are not absolute. The composition of TCM herbs is complex, and it is impossible for TCM herbs to exert effect only through the PI3K/AKT pathway. It is necessary to further explore the specific multi-target and multi-pathway mechanisms of TCM herbs, which cannot be used blindly. The incompatibility of TCM should be avoided, this may cause discomfort in the digestive system, skin mucous membranes, nervous system and cardiovascular system at light level, and may lead to poisoning with dose-effect in severe level.
The global library of TCM and TCM ingredients is huge, and the mechanism research on TCM ingredients is still lacking. We hope that more researchers will devote themselves to TCM research in the future. Everything starts with nature and ends with nature.
CG and QZ collected the data, wrote the manuscript, and conceived the structure of Figures. YL collected the data of manuscript and finished the contents of Figures. RL, JF, and WC summarized and analyzed the data. WA and IS revised the manuscript. LC, JL, and SH launched the viewpoint of manuscript. All authors declared that they materially participated in the article preparation. All authors contributed to the article and approved the submitted version.
This study was supported by National Natural Science Foundation of China (81671819) and Department of Education of Guangdong Province (2021ZDZX2011).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
AKT, protein kinase B; Bax, Bcl-Associated X; BBB, blood-brain barrier; Bcl-2, B cell lymphoma-2; BDNF, brain-derived neurotrophic factor; BHD, Buyang Huanwu Decoction; BMSC, Bone Mesenchymal Stem Cells; CHR, Chrysophanol; CREB, cyclic AMP-responsive element binding protein; DCX, double-cortin; EA, electroacupuncture; EGFR, epidermal growth factor receptor; eNOS, endothelial NOS; EtOAc-AC, ethyl acetate crude extract of Antrodia Camphorate; FGFs, fibroblast growth factors; GSK3, glycogen synthase kinase 3; HBMEC, human brain microvascular endothelial cell; Houshiheisan, HBP, Hou's Black Pulvis; HIF-1α, hypoxia-inducible factor 1α; HO-1, hemeoxygenase-1; iNOS, inducible NOS; I/R, ischemia-reperfusion; JAK2, Janus kinase 2; LC3, micro-tubule-associated protein 1 light chain 3 II/I; LDH, lactate dehydrogenase; MCAO, middle cerebral artery occlusion; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; nNOS, neuronal NOS; NOS, nitric oxide synthase; NQO1, NAD(P)H: quinone oxidoreductase 1; NPC, neural progenitor cell; Nrf2, Nuclear factor-erythroid 2-related factor 2; OGD/R, oxygen and glucose deprivation/reoxygenation; OGOMV, 40-O-b-D-glucosyl-5-O-methylvisamminol; PDK1, Pyruvate Dehydrogenase Kinase Isozyme 1; PI3K, Phosphatidylinositol 3 kinase; PNS, Panax notoginseng saponins; PTEN, phosphatase and tensin homolog deleted on chromosome ten; ROS, reactive oxygen species; SBD, Scutellaria barbata D. Don; SHE, Senkyunolide-H; SOD, superoxide dismutase; TBN, α-tetramethylpyrazinyl-N-tert-butyl nitrone; TMP, Tetramethylpyrazine; TND, Tongnao Decoction; TREM2, microglial triggering receptor expressed on myeloid cells 2; ULK1, Unc-51-like kinase 1; VEGF, vascular endothelial growth factor; XNJ, Xingnaojing.
1. Thomalla G, Boutitie F, Ma H, Koga M, Ringleb P, Schwamm LH, et al. Intravenous alteplase for stroke with unknown time of onset guided by advanced imaging: systematic review and meta-analysis of individual patient data. Lancet. (2020) 396:1574–84. doi: 10.1016/S0140-6736(20)32163-2
3. Rodgers ML, Fox E, Abdelhak T, Franker LM, Johnson BJ, Kirchner-Sullivan C, et al. Care of the patient with acute ischemic stroke (Endovascular/Intensive Care Unit-Postinterventional Therapy): update to 2009 comprehensive nursing care scientific statement: a scientific statement from the American Heart Association. Stroke. (2021) 52:e198–210. doi: 10.1161/STR.0000000000000358
4. Feng J, Zhang Y, Zhu Z, Gu C, Waqas A, Chen L. Emerging exosomes and exosomal MiRNAs in spinal cord injury. Front Cell Dev Biol. (2021) 9:703989. doi: 10.3389/fcell.2021.703989
5. Gu C, Feng J, Waqas A, Deng Y, Zhang Y, Chen W, et al. Technological advances of 3D Scaffold-based stem cell/exosome therapy in tissues and organs. Front Cell Dev Biol. (2021) 9:709204. doi: 10.3389/fcell.2021.709204
6. Adams HP Jr, Del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. (2007) 38:1655–711. doi: 10.1161/STROKEAHA.107.181486
7. Mehra S, Deshpande N, Nagathihalli N. Targeting PI3K pathway in pancreatic ductal adenocarcinoma: rationale and progress. Cancers. (2021) 13:4434. doi: 10.3390/cancers13174434
8. Roudsari NM, Lashgari NA, Momtaz S, Abaft S, Jamali F, Safaiepour P, et al. Inhibitors of the PI3K/Akt/mTOR pathway in Prostate Cancer Chemoprevention and Intervention. Pharmaceutics. (2021) 13:1195. doi: 10.3390/pharmaceutics13081195
9. Duan MX, Zhou H, Wu QQ, Liu C, Xiao Y, Deng W, et al. Andrographolide protects against HG-induced inflammation, apoptosis, migration, and impairment of Angiogenesis via PI3K/AKT-eNOS signalling in HUVECs. Mediators Inflamm. (2019) 2019:6168340. doi: 10.1155/2019/6168340
10. Karar J, Maity A. PI3K/AKT/mTOR pathway in Angiogenesis. Front Mol Neurosci. (2011) 4:51. doi: 10.3389/fnmol.2011.00051
11. Wu J, Zhao X, Sun Q, Jiang Y, Zhang W, Luo J, et al. Synergic effect of PD-1 blockade and endostar on the PI3K/AKT/mTOR-mediated autophagy and angiogenesis in Lewis lung carcinoma mouse model. Biomed Pharmacother. (2020) 125:109746. doi: 10.1016/j.biopha.2019.109746
12. Chen J, Zhang X, Liu X, Zhang C, Shang W, Xue J, et al. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur J Pharmacol. (2019) 856:172418. doi: 10.1016/j.ejphar.2019.172418
13. Sajadimajd S, Khazaei M. Oxidative stress and Cancer: the role of Nrf2. Curr Cancer Drug Targets. (2018) 18:538–57. doi: 10.2174/1568009617666171002144228
14. Yuan H, Xu Y, Luo Y, Wang NX, Xiao JH. Role of Nrf2 in cell senescence regulation. Mol Cell Biochem. (2021) 476:247–59. doi: 10.1007/s11010-020-03901-9
15. Lai TT, Yang CM, Yang CH. Astaxanthin protects retinal photoreceptor cells against high glucose-induced oxidative stress by induction of antioxidant enzymes via the PI3K/Akt/Nrf2 pathway. Antioxidants. (2020) 9:729. doi: 10.3390/antiox9080729
16. Yu C, Xiao JH. The Keap1-Nrf2 System: a mediator between oxidative stress and aging. Oxid Med Cell Longev. (2021) 2021:6635460. doi: 10.1155/2021/6635460
17. Anonymity. The Yellow Emperor's Classic of Internal Medicine (photocopy version). Beijing: People's Medical Publishing House (1956).
18. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. (2006) 124:471–84. doi: 10.1016/j.cell.2006.01.016
19. Wang J, Tong H, Wang X, Wang X, Wang Y. Tanshinone IIA alleviates the damage of neurocytes by targeting GLUT1 in ischaemia reperfusion model (in vivo and in vitro experiments). Folia Neuropathol. (2020) 58:176–93. doi: 10.5114/fn.2020.96983
20. Zhou ZY, Zhao WR, Xiao Y, Zhang J, Tang JY, Lee SM. Mechanism study of the protective effects of Sodium Tanshinone IIA Sulfonate against Atorvastatin-Induced Cerebral Hemorrhage in Zebrafish: transcriptome analysis. Front Pharmacol. (2020) 11:551745. doi: 10.3389/fphar.2020.551745
21. Wan D, Xue L, Zhu H, Luo Y. Catalpol induces neuroprotection and prevents memory dysfunction through the Cholinergic System and BDNF. Evid Based Complement Alternat Med. (2013) 2013:134852. doi: 10.1155/2013/134852
22. Wang J, Wan D, Wan G, Wang J, Zhang J, Zhu H. Catalpol induces cell activity to promote axonal regeneration via the PI3K/AKT/mTOR pathway in vivo and in vitro stroke model. Ann Transl Med. (2019) 7:756. doi: 10.21037/atm.2019.11.101
23. Zhu H, Wang J, Shao Y, Wan D. Catalpol may improve axonal growth via regulating miR-124 regulated PI3K/AKT/mTOR pathway in neurons after ischemia. Ann Transl Med. (2019) 7:306. doi: 10.21037/atm.2019.06.25
24. Liang W, Huang X, Chen W. The effects of Baicalin and Baicalein on Cerebral Ischemia: a review. Aging Dis. (2017) 8:850–67. doi: 10.14336/AD.2017.0829
25. Yang S, Wang H, Yang Y, Wang R, Wang Y, Wu C, et al. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed Pharmacother. (2019) 117:109102. doi: 10.1016/j.biopha.2019.109102
26. Zhang Q, Zheng Y, Hu X, Hu X, Lv W, Lv D, et al. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla Miquel: a review. J Ethnopharmacol. (2018) 224:149–68. doi: 10.1016/j.jep.2018.05.002
27. Zhang H, Song Y, Feng C. Improvement of cerebral ischemia/reperfusion injury by daucosterol palmitate-induced neuronal apoptosis inhibition via PI3K/Akt/mTOR signaling pathway. Metab Brain Dis. (2020) 35:1035–44. doi: 10.1007/s11011-020-00575-6
28. Wang M, Hua X, Niu H, Sun Z, Zhang L, Li Y, et al. Cornel Iridoid Glycoside protects against white matter lesions induced by Cerebral Ischemia in rats via activation of the brain-derived neurotrophic factor/neuregulin-1 pathway. Neuropsychiatr Dis Treat. (2019) 15:3327–40. doi: 10.2147/NDT.S228417
29. Lu LY, Liu Y, Gong YF, Zheng XY. A preliminary report: genistein attenuates cerebral ischemia injury in ovariectomized rats via regulation of the PI3K-Akt-mTOR pathway. Gen Physiol Biophys. (2019) 38:389–97. doi: 10.4149/gpb_2019024
30. Ghaeminia M, Rajkumar R, Koh HL, Dawe GS, Tan CH. Ginsenoside Rg1 modulates medial prefrontal cortical firing and suppresses the hippocampo-medial prefrontal cortical long-term potentiation. J Ginseng Res. (2018) 42:298–303. doi: 10.1016/j.jgr.2017.03.010
31. Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G, et al. Protective effects and target network analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion injury: a comprehensive overview of experimental studies. Cells. (2018) 7:270. doi: 10.3390/cells7120270
32. Hou Y, Wang K, Wan W, Cheng Y, Pu X, Ye X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. (2018) 5:245–55. doi: 10.1016/j.gendis.2018.06.001
33. Mao XY, Zhou HH, Li X, Liu ZQ. Huperzine A Alleviates oxidative glutamate toxicity in Hippocampal HT22 cells via activating BDNF/TrkB-Dependent PI3K/Akt/mTOR signaling pathway. Cell Mol Neurobiol. (2016) 36:915–25. doi: 10.1007/s10571-015-0276-5
34. Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. (2000) 5:334–46.
35. Agarwal R, Diwanay S, Patki P, Patwardhan B. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J Ethnopharmacol. (1999) 67:27–35. doi: 10.1016/S0378-8741(99)00065-3
36. Zhang QZ, Guo YD, Li HM, Wang RZ, Guo SG, Du YF. Protection against cerebral infarction by Withaferin A involves inhibition of neuronal apoptosis, activation of PI3K/Akt signaling pathway, and reduced intimal hyperplasia via inhibition of VSMC migration and matrix metalloproteinases. Adv Med Sci. (2017) 62:186–92. doi: 10.1016/j.advms.2016.09.003
37. Chuang DM, Wang Z, Chiu CT. GSK-3 as a target for lithium-induced neuroprotection against excitotoxicity in neuronal cultures and animal models of Ischemic stroke. Front Mol Neurosci. (2011) 4:15. doi: 10.3389/fnmol.2011.00015
38. Chen Y, Wu Z, Zhu X, Zhang M, Zang X, Li X, et al. OCT4B-190 protects against ischemic stroke by modulating GSK-3β/HDAC6. Exp Neurol. (2019) 316:52–62. doi: 10.1016/j.expneurol.2019.04.005
39. Pandurangan AK, Esa NM. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: a review. Asian Pac J Cancer Prev. (2014) 15:5501–8. doi: 10.7314/APJCP.2014.15.14.5501
40. Luo S, Li H, Mo Z, Lei J, Zhu L, Huang Y, et al. Connectivity map identifies luteolin as a treatment option of ischemic stroke by inhibiting MMP9 and activation of the PI3K/Akt signaling pathway. Exp Mol Med. (2019) 51:1–11. doi: 10.1038/s12276-019-0229-z
41. Xue Y, Tie CR, Li J, Tian T, Li QX. Ligustrazine inhibits lipopolysaccharide-induced proliferation by affecting P27, Bcl-2 expression in rat mesangial cells. Eur J Pharmacol. (2011) 665:8–12. doi: 10.1016/j.ejphar.2011.05.004
42. Zhao H, Xu ML, Zhang Q, Guo ZH, Peng Y, Qu ZY, et al. Tetramethylpyrazine alleviated cytokine synthesis and dopamine deficit and improved motor dysfunction in the mice model of Parkinson's disease. Neurol Sci. (2014) 35:1963–7. doi: 10.1007/s10072-014-1871-9
43. Guo B, Xu D, Duan H, Du J, Zhang Z, Lee SM, et al. Therapeutic effects of multifunctional tetramethylpyrazine nitrone on models of Parkinson's disease in vitro and in vivo. Biol Pharm Bull. (2014) 37:274–85. doi: 10.1248/bpb.b13-00743
44. Zakrzewski J, Huras B. Reactions of nitroxides 15. Cinnamates bearing a nitroxyl moiety synthesized using a Mizoroki-Heck cross-coupling reaction. Beilstein J Org Chem. (2015) 11:1155–62. doi: 10.3762/bjoc.11.130
45. Kim JH, Wang Q, Choi JM, Lee S, Cho EJ. Protective role of caffeic acid in an Aβ25-35-induced Alzheimer's disease model. Nutr Res Pract. (2015) 9:480–8. doi: 10.4162/nrp.2015.9.5.480
46. Chen H, Tan G, Cao J, Zhang G, Yi P, Yu P, et al. Design, synthesis, and biological evaluation of novel tetramethylpyrazine derivatives as potential neuroprotective agents. Chem Pharm Bull. (2017) 65:56–65. doi: 10.1248/cpb.c16-00699
47. Chen H, Cao J, Zhu Z, Zhang G, Shan L, Yu P, et al. A novel tetramethylpyrazine derivative protects against glutamate-induced cytotoxicity through PGC1α/Nrf2 and PI3K/Akt signaling pathways. Front Neurosci. (2018) 12:567. doi: 10.3389/fnins.2018.00567
48. Shao YY, Chen CC, Wang HY, Chiu HL, Hseu TH, Kuo YH. Chemical constituents of Antrodia camphorata submerged whole broth. Nat Prod Res. (2008) 22:1151–7. doi: 10.1080/14786410601132410
49. Wang YH, Chern CM, Liou KT, Kuo YH, Shen YC. Ergostatrien-7,9:22-trien-3β-ol from Antrodia camphorata ameliorates ischemic stroke brain injury via downregulation of p65NF-κ-B and caspase 3, and activation of Akt/GSK3/catenin-associated neurogenesis. Food Funct. (2019) 10:4725–38. doi: 10.1039/C9FO00908F
50. Wei SY, Chen Y, Xu XY. Progress on the pharmacological research of puerarin: a review. Chin J Nat Med. (2014) 12:407–14. doi: 10.1016/S1875-5364(14)60064-9
51. Li X, Sun S, Tong E. Experimental study on the protective effect of puerarin to Parkinson disease. J Huazhong Univ Sci Technolog Med Sci. (2003) 23:148–50. doi: 10.1007/BF02859940
52. Wu HQ, Guo HN, Wang HQ, Chang MZ, Zhang GL, Zhao YX. Protective effects and mechanism of puerarin on learning-memory disorder after global cerebral ischemia-reperfusion injury in rats. Chin J Integr Med. (2009) 15:54–9. doi: 10.1007/s11655-009-0054-4
53. Xu XH, Zheng XX, Zhou Q, Li H. Inhibition of excitatory amino acid efflux contributes to protective effects of puerarin against cerebral ischemia in rats. Biomed Environ Sci. (2007) 20:336–42.
54. Tao J, Cui Y, Duan Y, Zhang N, Wang C, Zhang F. Puerarin attenuates locomotor and cognitive deficits as well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3β signaling pathway in an in vivo model of cerebral ischemia. Oncotarget. (2017) 8:106283–95. doi: 10.18632/oncotarget.22290
55. Wang SB, Pang XB, Zhao Y, Wang YH, Zhang L, Yang XY, et al. Protection of salvianolic acid A on rat brain from ischemic damage via soluble epoxide hydrolase inhibition. J Asian Nat Prod Res. (2012) 14:1084–92. doi: 10.1080/10286020.2012.723200
56. Chien MY, Chuang CH, Chern CM, Liou KT, Liu DZ, Hou YC, et al. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free Radic Biol Med. (2016) 99:508–19. doi: 10.1016/j.freeradbiomed.2016.09.006
57. Zhang W, Song JK, Yan R, Li L, Xiao ZY, Zhou WX, et al. Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacol Sin. (2018) 39:1259–72. doi: 10.1038/aps.2017.149
58. Zhu H, Zou L, Tian J, Du G, Gao Y. SMND-309, a novel derivative of salvianolic acid B. protects rat brains ischemia and reperfusion injury by targeting the JAK2/STAT3 pathway. Eur J Pharmacol. (2013) 714:23–31. doi: 10.1016/j.ejphar.2013.05.043
59. Wang Y, Zhang J, Han M, Liu B, Gao Y, Ma P, et al. SMND-309 promotes neuron survival through the activation of the PI3K/Akt/CREB-signalling pathway. Pharm Biol. (2016) 54:1982–90. doi: 10.3109/13880209.2015.1137951
60. Liu M, Liu Y, Xu S, Guo X, Jiang B, Lv J, et al. Influence of Geun japonicum Thunb on the expression of NF-κB and IL-6 protein in hippocampus of vascular dementia mice. Pharmacol Clin Chinese Materia Med. (2017) 2017:33. doi: 10.13412/j.cnki.zyyl.2017.03.031
61. Zulin HU, Zhang Y, Liu Y, Liu M. Effects of Gei herba extract on learning and memory ability of senile dementia model mice and expression of TNF-α and AKT in hippocampus. Chin J Ethnomed Ethnophar. (2019) 2018:78–83. doi: 10.13412/j.cnki.zyyl.2018.06.020
62. Du L, Mei Z, Huang Y, Tao W, Wang K, Huang W, et al. Protection of the Geum japonicum Thunb. var. chinense extracts against oxygen-glucose deprivation and re-oxygenation induced astrocytes injury via BDNF/PI3K/Akt/CREB pathway. Biomed Pharmacother. (2020) 127:110123. doi: 10.1016/j.biopha.2020.110123
63. Tamokou Jde D, Chouna JR, Fischer-Fodor E, Chereches G, Barbos O, Damian G, et al. Anticancer and antimicrobial activities of some antioxidant-rich cameroonian medicinal plants. PLoS ONE. (2013) 8:e55880. doi: 10.1371/journal.pone.0055880
64. Lin L, Ni B, Lin H, Zhang M, Li X, Yin X, et al. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: a review. J Ethnopharmacol. (2015) 159:158–83. doi: 10.1016/j.jep.2014.11.009
65. Zhou X, Wang L, Wang M, Xu L, Yu L, Fang T, et al. Emodin-induced microglial apoptosis is associated with TRB3 induction. Immunopharmacol Immunotoxicol. (2011) 33:594–602. doi: 10.3109/08923973.2010.549135
66. Ahn SM, Kim HN, Kim YR, Choi YW, Kim CM, Shin HK, et al. Emodin from Polygonum multiflorum ameliorates oxidative toxicity in HT22 cells and deficits in photothrombotic ischemia. J Ethnopharmacol. (2016) 188:13–20. doi: 10.1016/j.jep.2016.04.058
67. Mahmood S, Fatima T, Zulfaqar H, Saher R, Rafiq M, Rehman A, et al. Meta-analysis of dragon's blood resin extract as radio-protective agent. J Coastal Life Med. (2017) 5:409–16. doi: 10.12980/jclm.5.2017J7-75
68. Ran Y, Wang R, Hasan M, Jia Q, Tang B, Shan S, et al. Radioprotective effects of dragon's blood and its extracts on radiation-induced myelosuppressive mice. J Ethnopharmacol. (2014) 154:624–34. doi: 10.1016/j.jep.2014.04.036
69. Sun J, Huo H, Song Y, Zheng J, Zhao Y, Huang W, et al. Method development and application for multi-component quantification in rats after oral administration of Longxuetongluo Capsule by UHPLC-MS/MS. J Pharm Biomed Anal. (2018) 156:252–62. doi: 10.1016/j.jpba.2018.04.030
70. Zhou JM, Wang HM, Lv YZ, Wang ZZ, Xiao W. Anti-atherosclerotic effect of Longxuetongluo Capsule in high cholesterol diet induced atherosclerosis model rats. Biomed Pharmacother. (2018) 97:793–801. doi: 10.1016/j.biopha.2017.08.141
72. Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X. Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol. (2017) 54:6006–17. doi: 10.1007/s12035-016-0111-0
73. Li H, Wang P, Huang F, Jin J, Wu H, Zhang B, et al. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol Appl Pharmacol. (2018) 340:58–66. doi: 10.1016/j.taap.2017.12.019
74. Chen J, Zhou J, Chen X, Yang B, Wang D, Yang P, et al. miRNA-449a is downregulated in osteosarcoma and promotes cell apoptosis by targeting BCL2. Tumour Biol. (2015) 36:8221–9. doi: 10.1007/s13277-015-3568-y
75. Wang Y, Li B, Zhang X. Scutellaria barbata D. Don (SBD) protects oxygen glucose deprivation/reperfusion-induced injuries of PC12 cells by up-regulating Nrf2. Artif Cells Nanomed Biotechnol. (2019) 47:1797–807. doi: 10.1080/21691401.2019.1610413
76. Zhang X, Wu J, Zhang B. Xuesaitong injection as one adjuvant treatment of acute cerebral infarction: a systematic review and meta-analysis. BMC Complement Altern Med. (2015) 15:36. doi: 10.1186/s12906-015-0560-4
77. Shi X, Yu W, Yang T, Liu W, Zhao Y, Sun Y, et al. Panax notoginseng saponins provide neuroprotection by regulating NgR1/RhoA/ROCK2 pathway expression, in vitro and in vivo. J Ethnopharmacol. (2016) 190:301–12. doi: 10.1016/j.jep.2016.06.017
78. Huang GD, Zhong XF, Deng ZY, Zeng R. Proteomic analysis of ginsenoside Re attenuates hydrogen peroxide-induced oxidative stress in human umbilical vein endothelial cells. Food Funct. (2016) 7:2451–61. doi: 10.1039/C6FO00123H
79. Hu S, Wu Y, Zhao B, Hu H, Zhu B, Sun Z, et al. Panax notoginseng saponins protect cerebral microvascular endothelial cells against oxygen-glucose deprivation/reperfusion-induced barrier dysfunction via activation of PI3K/Akt/Nrf2 antioxidant signaling pathway. Molecules. (2018) 23:2781. doi: 10.3390/molecules23112781
80. Dimpfel W, Schombert L, Panossian AG. Assessing the quality and potential efficacy of commercial extracts of Rhodiola rosea L. by analyzing the salidroside and rosavin content and the electrophysiological activity in hippocampal long-term potentiation, a synaptic model of memory. Front Pharmacol. (2018) 9:425. doi: 10.3389/fphar.2018.00425
81. Zhang X, Lai W, Ying X, Xu L, Chu K, Brown J, et al. Salidroside reduces inflammation and brain injury after permanent middle cerebral artery occlusion in rats by regulating PI3K/PKB/Nrf2/NFκB signaling rather than complement C3 activity. Inflammation. (2019) 42:1830–42. doi: 10.1007/s10753-019-01045-7
82. Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. (2001) 107:7–11. doi: 10.1172/JCI11830
83. Naik SR, Pilgaonkar VW, Panda VS. Evaluation of antioxidant activity of Ginkgo biloba phytosomes in rat brain. Phytother Res. (2006) 20:1013–6. doi: 10.1002/ptr.1976
84. Xu B, He X, Sui Y, Wang X, Wang X, Ren L, et al. Ginkgetin aglycone attenuates neuroinflammation and neuronal injury in the rats with ischemic stroke by modulating STAT3/JAK2/SIRT1. Folia Neuropathol. (2019) 57:16–23. doi: 10.5114/fn.2019.83827
85. Qi H, Siu SO, Chen Y, Han Y, Chu IK, Tong Y, et al. Senkyunolides reduce hydrogen peroxide-induced oxidative damage in human liver HepG2 cells via induction of heme oxygenase-1. Chem Biol Interact. (2010) 183:380–9. doi: 10.1016/j.cbi.2009.11.029
86. Zhang J, Jiang Y, Liu N, Shen T, Jung HW, Liu J, et al. A network-based method for mechanistic investigation and neuroprotective effect on post-treatment of Senkyunolid-H against cerebral ischemic stroke in mouse. Front Neurol. (2019) 10:1299. doi: 10.3389/fneur.2019.01299
87. Hu YY, Wang Y, Liang S, Yu XL, Zhang L, Feng LY, et al. Senkyunolide I attenuates oxygen-glucose deprivation/reoxygenation-induced inflammation in microglial cells. Brain Res. (2016) 1649:123–31. doi: 10.1016/j.brainres.2016.08.012
88. Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. (2012) 491:473–7. doi: 10.1038/nature11626
89. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. (2012) 33:829–37. doi: 10.1093/eurheartj/ehr304
90. Chen W, Lu Y, Chen G, Huang S. Molecular evidence of cryptotanshinone for treatment and prevention of human cancer. Anticancer Agents Med Chem. (2013) 13:979–87. doi: 10.2174/18715206113139990115
91. Kang BY, Chung SW, Kim SH, Ryu SY, Kim TS. Inhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacology. (2000) 49:355–61. doi: 10.1016/S0162-3109(00)00256-3
92. Wang N, Luo HW, Niwa M, Ji J. A new platelet aggregation inhibitor from Salvia miltiorrhiza. Planta Med. (1989) 55:390–1. doi: 10.1055/s-2006-962037
93. Zhu W, Qiu W, Lu A. Cryptotanshinone exhibits therapeutical effects on cerebral stroke through the PI3K/AKT-eNOS signaling pathway. Mol Med Rep. (2017) 16:9361–6. doi: 10.3892/mmr.2017.7824
94. Khan MN, Cho JY, Lee MC, Kang JY, Park NG, Fujii H, et al. Isolation of two anti-inflammatory and one pro-inflammatory polyunsaturated fatty acids from the brown seaweed Undaria pinnatifida. J Agric Food Chem. (2007) 55:6984–8. doi: 10.1021/jf071791s
95. Gao Y, Dong C, Yin J, Shen J, Tian J, Li C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell Mol Neurobiol. (2012) 32:523–9. doi: 10.1007/s10571-011-9792-0
96. Xu P, Du SY, Lu Y, Bai J, Guo YW, Du Q, et al. The effect of stroke and other components in Xing-Nao-Jing on the pharmacokinetics of geniposide. J Ethnopharmacol. (2014) 152:302–7. doi: 10.1016/j.jep.2013.12.046
97. Ma X, Yang YX, Chen N, Xie Q, Wang T, He X, et al. Meta-analysis for clinical evaluation of Xingnaojing injection for the treatment of cerebral infarction. Front Pharmacol. (2017) 8:485. doi: 10.3389/fphar.2017.00485
98. Zhang YM, Qu XY, Zhai JH, Tao LN, Gao H, Song YQ, et al. Xingnaojing injection protects against cerebral ischemia reperfusion injury via PI3K/Akt-mediated eNOS phosphorylation. Evid Based Complement Alternat Med. (2018) 2018:2361046. doi: 10.1155/2018/2361046
99. Yan Z, Yang X, Wu J, Su H, Chen C, Chen Y. Qualitative and quantitative analysis of chemical constituents in traditional Chinese medicinal formula Tong-Xie-Yao-Fang by high-performance liquid chromatography/diode array detection/electrospray ionization tandem mass spectrometry. Anal Chim Acta. (2011) 691:110–8. doi: 10.1016/j.aca.2011.02.046
100. Chang CZ, Wu SC. 4'-O-β-D-Glucosyl-5-O-Methylvisamminol, A Natural Histone H3 Phosphorylation Epigenetic Suppressor, exerts a neuroprotective effect through PI3K/Akt signaling pathway on focal cerebral ischemia in rats. World Neurosurg. (2016) 89:474–88. doi: 10.1016/j.wneu.2016.01.061
101. Wu Q, Mao Z, Liu J, Huang J, Wang N. Ligustilide attenuates ischemia reperfusion-induced hippocampal neuronal apoptosis via activating the PI3K/Akt Pathway. Front Pharmacol. (2020) 11:979. doi: 10.3389/fphar.2020.00979
102. Fang Y, Chu L, Li L, Wang J, Yang Y, Gu J, et al. Tetramethylpyrazine protects bone marrow-derived mesenchymal stem cells against hydrogen peroxide-induced apoptosis through PI3K/Akt and ERK1/2 pathways. Biol Pharm Bull. (2017) 40:2146–52. doi: 10.1248/bpb.b17-00524
103. Kong X, Zhong M, Su X, Qin Q, Su H, Wan H, et al. Tetramethylpyrazine promotes migration of neural precursor cells via activating the phosphatidylinositol 3-Kinase pathway. Mol Neurobiol. (2016) 53:6526–39. doi: 10.1007/s12035-015-9551-1
104. Liu Y, Liu C, Zhang X, Liu Z, Yan X. Chrysophanol protects PC12 cells against oxygen glucose deprivation-evoked injury by up-regulating miR-216a. Cell Cycle. (2020) 19:1433–42. doi: 10.1080/15384101.2020.1731655
105. Sun J, Wang J, Hu L, Yan J. K-3-Rh protects against cerebral ischemia/reperfusion injury by anti-apoptotic effect through PI3K-Akt signaling pathway in rat. Neuropsychiatr Dis Treat. (2020) 16:1217–27. doi: 10.2147/NDT.S233622
106. Li Y, Wang R, Xue L, Yang Y, Zhi F. Astilbin protects against cerebral ischaemia/reperfusion injury by inhibiting cellular apoptosis and ROS-NLRP3 inflammasome axis activation. Int Immunopharmacol. (2020) 84:106571. doi: 10.1016/j.intimp.2020.106571
107. Liu D, Ye Y, Xu L, Yuan W, Zhang Q. Icariin and mesenchymal stem cells synergistically promote angiogenesis and neurogenesis after cerebral ischemia via PI3K and ERK1/2 pathways. Biomed Pharmacother. (2018) 108:663–9. doi: 10.1016/j.biopha.2018.09.071
108. Wei Y, Hong H, Zhang X, Lai W, Wang Y, Chu K, et al. Salidroside inhibits inflammation through PI3K/Akt/HIF signaling after focal cerebral ischemia in rats. Inflammation. (2017) 40:1297–309. doi: 10.1007/s10753-017-0573-x
109. Zhou ZQ, Li YL, Ao ZB, Wen ZL, Chen QW, Huang ZG, et al. Baicalin protects neonatal rat brains against hypoxic-ischemic injury by upregulating glutamate transporter 1 via the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neural Regen Res. (2017) 12:1625–31. doi: 10.4103/1673-5374.217335
110. Zhang F, Yan C, Wei C, Yao Y, Ma X, Gong Z, et al. Vinpocetine inhibits NF-κB-dependent inflammation in acute ischemic stroke patients. Transl Stroke Res. (2018) 9:174–84. doi: 10.1007/s12975-017-0549-z
111. Zhao M, Hou S, Feng L, Shen P, Nan D, Zhang Y, et al. Vinpocetine protects against cerebral ischemia-reperfusion injury by targeting Astrocytic Connexin43 via the PI3K/AKT signaling pathway. Front Neurosci. (2020) 14:223. doi: 10.3389/fnins.2020.00223
112. Zhang K, Yang Y, Ge H, Wang J, Chen X, Lei X, et al. Artesunate promotes the proliferation of neural stem/progenitor cells and alleviates Ischemia-reperfusion Injury through PI3K/Akt/FOXO-3a/p27(kip1) signaling pathway. Aging. (2020) 12:8029–48. doi: 10.18632/aging.103121
113. Lu MC, Yao CH, Wang SH, Lai YL, Tsai CC, Chen YS. Effect of Astragalus membranaceus in rats on peripheral nerve regeneration: in vitro and in vivo studies. J Trauma. (2010) 68:434–40. doi: 10.1097/TA.0b013e31819adb38
114. Wu QL, Cheng YQ, Liu AJ, Zhang WD. Formononetin recovered injured nerve functions by enhancing synaptic plasticity in ischemic stroke rats. Biochem Biophys Res Commun. (2020) 525:67–72. doi: 10.1016/j.bbrc.2020.02.015
115. Li J, Li Y, Ogle M, Zhou X, Song M, Yu SP, et al. DL-3-n-butylphthalide prevents neuronal cell death after focal cerebral ischemia in mice via the JNK pathway. Brain Res. (2010) 1359:216–26. doi: 10.1016/j.brainres.2010.08.061
116. Jiang MJ, Chen YH, Li L, Xu L, Liu H, Qu XL, et al. Protective effects of DL-3-n-butylphthalide in the lipopolysaccharide-induced mouse model of Parkinson's disease. Mol Med Rep. (2017) 16:6184–9. doi: 10.3892/mmr.2017.7352
117. Feng L, Sharma A, Niu F, Huang Y, Lafuente JV, Muresanu DF, et al. TiO(2)-Nanowired delivery of DL-3-n-butylphthalide (DL-NBP) attenuates blood-brain barrier disruption, brain edema formation, and neuronal damages following concussive head injury. Mol Neurobiol. (2018) 55:350–8. doi: 10.1007/s12035-017-0746-5
118. He Z, Zhou Y, Lin L, Wang Q, Khor S, Mao Y, et al. Dl-3-n-butylphthalide attenuates acute inflammatory activation in rats with spinal cord injury by inhibiting microglial TLR4/NF-κB signalling. J Cell Mol Med. (2017) 21:3010–22. doi: 10.1111/jcmm.13212
119. Zhang P, Xu R, Guo Y, Qin J, Dai Y, Liu N, et al. DL-3-n-butylphthalide promotes dendrite development in cortical neurons subjected to oxygen-glucose deprivation/reperfusion. Cell Biol Int. (2018) 42:1041–9. doi: 10.1002/cbin.10980
120. Hao CZ, Wu F, Shen J, Lu L, Fu DL, Liao WJ, et al. Clinical efficacy and safety of buyang huanwu decoction for acute ischemic stroke: a systematic review and meta-analysis of 19 randomized controlled trials. Evid Based Complement Alternat Med. (2012) 2012:630124. doi: 10.1155/2012/630124
121. Pan R, Cai J, Zhan L, Guo Y, Huang RY, Li X, et al. Buyang Huanwu decoction facilitates neurorehabilitation through an improvement of synaptic plasticity in cerebral ischemic rats. BMC Complement Altern Med. (2017) 17:173. doi: 10.1186/s12906-017-1680-9
122. Cai G, Liu B, Liu W, Tan X, Rong J, Chen X, et al. Buyang Huanwu Decoction can improve recovery of neurological function, reduce infarction volume, stimulate neural proliferation and modulate VEGF and Flk1 expressions in transient focal cerebral ischaemic rat brains. J Ethnopharmacol. (2007) 113:292–9. doi: 10.1016/j.jep.2007.06.007
123. Chen X, Chen H, He Y, Fu S, Liu H, Wang Q, et al. Proteomics-guided study on Buyang Huanwu Decoction for its neuroprotective and neurogenic mechanisms for transient ischemic stroke: involvements of EGFR/PI3K/Akt/Bad/14-3-3 and Jak2/Stat3/Cyclin D1 signaling cascades. Mol Neurobiol. (2020) 57:4305–21. doi: 10.1007/s12035-020-02016-y
124. Wang H, Wang L, Zhang N, Zhang Q, Zhao H, Zhang Q. Houshiheisan compound prescription protects neurovascular units after cerebral ischemia. Neural Regen Res. (2014) 9:741–8. doi: 10.4103/1673-5374.131580
125. Zhang Q, Zhao H, Wang L, Zhang Q, Wang H. Effects of wind-dispelling drugs and deficiency-nourishing drugs of Houshiheisan compound prescription on astrocyte activation and inflammatory factor expression in the corpus striatum of cerebral ischemia rats. Neural Regen Res. (2012) 7:1851–7. doi: 10.3969/j.issn.1673-5374.2012.24.002
126. Chang J, Yao X, Zou H, Wang L, Lu Y, Zhang Q, et al. BDNF/PI3K/Akt and Nogo-A/RhoA/ROCK signaling pathways contribute to neurorestorative effect of Houshiheisan against cerebral ischemia injury in rats. J Ethnopharmacol. (2016) 194:1032–42. doi: 10.1016/j.jep.2016.11.005
127. Wang J, Zhang XH, Xu X, Zhu Q, Yao B, Liang S, et al. Pro-angiogenic activity of Tongnao decoction on HUVECs in vitro and zebrafish in vivo. J Ethnopharmacol. (2020) 254:112737. doi: 10.1016/j.jep.2020.112737
128. Zhang Y. Effect of Taohongsiwu decoction on the angiogenesis and PI3K/AKT signal pathway in brain tissue of rats with cerebral ischemia reperfusion injury. J Bengbu Med Coll. (2017) 42:6. doi: 10.13898/j.cnki.issn.1000-2200.2017.01.010
129. Jing L. Studies on the Effect of Naoshuning on Nerve Regeneration in Ischemic Stroke and Its Mechanism (2020). doi: 10.27667/d.cnki.gzymu.2020.000087
130. Liu X, Liu W, Li C, Li N. Effect of Chaigui Wendan Dingzhi Decoction on depression and cognitive function of stroke patients. World Chin Med. (2018) 13:4. doi: 10.3969/j.issn.1673-7202.2018.05.013
131. Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. (2006) 34:249–70. doi: 10.1385/MN:34:3:249
Keywords: Traditional Chinese Medicine, stroke, herb, PI3K/Akt pathway, neuroprotection
Citation: Gu C, Zhang Q, Li Y, Li R, Feng J, Chen W, Ahmed W, Soufiany I, Huang S, Long J and Chen L (2022) The PI3K/AKT Pathway—The Potential Key Mechanisms of Traditional Chinese Medicine for Stroke. Front. Med. 9:900809. doi: 10.3389/fmed.2022.900809
Received: 21 March 2022; Accepted: 26 April 2022;
Published: 31 May 2022.
Edited by:
Xiang Cao, Nanjing Drum Tower Hospital, ChinaReviewed by:
Hailong Yu, Yangzhou University Guangling College, ChinaCopyright © 2022 Gu, Zhang, Li, Li, Feng, Chen, Ahmed, Soufiany, Huang, Long and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukui Chen, bmV1cm9fY2xrQGhvdG1haWwuY29t; Jun Long, c3BpbmVsb25nQDE2My5jb20=; Shiying Huang, ZGFyb29uQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.