- Department of Obstetrics and Gynecology, Mie University School of Medicine, Tsu, Japan
Background: The aim of this study was to determine the usefulness of placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) in predicting the time for pregnancy termination in pregnant women with known preeclampsia (PE) onset.
Methods: Forty-four pregnant women diagnosed with PE (22 weeks 0 days to 33 weeks 6 days gestation) were included in this study. The levels of sFlt-1 and PlGF, and the sFlt-1/PlGF ratio were compared between the women that delivered in <24 h (T group) and those that delivered in more than 24 h (P group), and between women that delivered in <1 week (T group) and those that delivered in more than 1 week (P group). Cutoff values were calculated for the three markers that were the most significantly correlated with predicting pregnancy termination at <24 h and <1 week.
Results: Among sFlt-1, PlGF, and sFlt-1/PlGF, sFlt-1 was the most significantly associated with the timing of pregnancy termination. sFlt-1 cutoff values of 8682.1 pg/ml (AUC 0.71; 95%Cl, 0.5191–0.9052) and 7,394.5 pg/ml (AUC 0.78; 0.78, 95%Cl, 0.6394–0.9206) for delivery in <24 h and delivery within 1 week, respectively, were important predictive values. The positive predictive value for delivery within 24 h was 43.9%, with a sensitivity of 72.3% and specificity of 69.0%, when sFlt−1 was <8,682 pg/ml. A sFlt-1 level of 7,394 pg/ml or greater would result in delivery within 1 week, with a positive predictive value of 67.2%; the sensitivity was 79.0% and specificity was 72.0%.
Conclusion: This study showed that sFlt-1 may be effective in predicting the timing of pregnancy termination. However, the number of cases was small and, thus, the results were not definitive. This finding should be researched further in order to predict the optimal timing of pregnancy termination in PE to reduce severe maternal complications.
Introduction
Preeclampsia (PE) is a disease with serious consequences for both the mother and fetus. Currently, deaths due to complications from PE are problematic in Japan (1–4). In particular, cerebral hemorrhage is more prevalent than cerebral arteriovenous malformation or Moyamoya disease in patients with PE (1–4). We have previously analyzed cases of PE-associated cerebral hemorrhage in Japan; the prognosis of PE-associated cerebral hemorrhage is determined by the degree of cerebral hemorrhage. Therefore, a backward analysis of those cases concluded that it was difficult to save a patient's life when cerebral hemorrhage was severe (5). Intervention before the onset of cerebral hemorrhage is a means of reducing maternal death from cerebral hemorrhage complicated by PE. Furthermore, Japanese people including to pregnant women are reported to be more prone to cerebral hemorrhage than cerebral infarction (4).
Once PE develops, it progressively worsens; there is currently no treatment other than terminating the pregnancy once PE becomes severe (6–8). Pregnancy cannot be easily terminated at <37 weeks due to fetal prematurity. Once PE develops, various clinical parameters (blood pressure, proteinuria, et al.) that indicate PE deterioration should be carefully monitored. The pregnancy is terminated when PE is determined to have become severe. However, this intervention method does not eliminate the mother's risk of developing cerebral hemorrhage. Furthermore, because the incidence of cerebral hemorrhage itself is very low, it is difficult to design studies to predict cerebral hemorrhage.
It is widely reported that placental growth factor (PlGF), an angiogenic factor involved in placentation, and its inhibitor, soluble fms-like tyrosine kinase-1 (sFlt-1), are involved in the pathogenesis of PE. The sFlt-1/PlGF ratio has attracted attention as an indicator to predict the onset of PE (9, 10). We hypothesized that PlGF and sFlt-1 could be used to predict the timing of PE pregnancy termination in patients who develop PE. Predicting the timing of PE pregnancy termination would allow for earlier intervention in PE cases. Therefore, the purpose of this study was to investigate the use of PlGF and sFlt-1 to predict the timing of pregnancy termination in women with known PE onset.
Methods
Study Population
This study included pregnant women diagnosed with PE (22 weeks 0 days to 33 weeks 6 days gestation) at Mie University Hospital from 1 January 2016, to 30 October 2021, whose pregnancies were terminated for maternal indications (severe PE). Cases in which PlGF and sFlt-1 were measured at the time of diagnosis were enrolled.
Study Design
This was a single-center observational study. The study design was approved by the Ethics Committee of Mie University Hospital (approval No. H2022-013).
The levels of sFlt-1 and PlGF, and the sFlt-1/PlGF ratio were examined to determine which marker most significantly correlated with predicting the severity of PE after PE onset. If preterm delivery is anticipated in clinical practice, steroids should be administered to protect the fetus. Therefore, it is important to predict delivery within 24 h and 1 week in terms of onset and duration of steroid effects. The first group was defined as having delivered at less than 24 h (T group) and the second group as having delivered at more than 24 h (P group). Second, the time from measurement to pregnancy termination near to delivery was divided into two groups: <1 week (T group) and more than 1 week (P group). For the markers that were best correlated, cutoff values were calculated to predict pregnancy termination at <24 h and <1 week.
Diagnostic Criteria for PE
Diagnostic criteria for PE-related disorders were based on the International Society for the Study of Hypertension in Pregnancy (ISSHP) guidelines (11), which are as follows: after 20 weeks of gestation, hypertension (systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or both) and proteinuria (>2+ protein on dipstick urine test, >300 mg protein per 24-h urine collection, >30 mg/dl protein on a spot urine sample, or a protein to creatinine ratio of >30 mg/mM), both of which are defined as new onset (11).
Criteria for Pregnancy Termination
After the diagnosis of PE, the decision to terminate the pregnancy was made if PE became severe. Severe disease was defined as meeting one of the following criteria: persistent hypertension (systolic blood pressure >160 mmHg or diastolic blood pressure >110 mmHg); Hemolysis, Elevated liver enzymes, and Low Platelets (HELLP) syndrome; renal dysfunction (serum creatinine >1.0 mg/dl or oliguria); thrombocytopenia (<100,000/μl); eclampsia; pulmonary edema; or stroke.
Pregnancies were also terminated in case of fetal growth retardation, even if the patient was judged to have a carefully controlled non-reassuring fetal status.
Evaluation of Serum Markers
Serum samples (≥2 ml) collected according to standard operating procedures were retrospectively analyzed in the Obstetrics and Gynecology Laboratory of Mie University. Concentrations of sFlt-1 and PlGF in maternal serum (both measured in pg/ml) were determined using an electrochemiluminescence immunoassay platform (Cobas E Analyzer, Roche Diagnostics) with a fully automated Elecsys assay for sFlt-1 and PlGF. The data obtained from the assay were then used to calculate the sFlt-1/PlGF ratio. The within-run coefficient of variation for control samples was <4% for both assays. Between-run coefficients of variation ranged from 2.3 to 5.6% for the Elecsys sFlt-1 assay and 2.4 to 4.6% for the Elecsys PlGF assay.
Statistical Analysis
Descriptive statistics (mean, standard deviation) were calculated for each group, and inter-group comparisons were made using the two-sided Student's t-test at a significance level of 5%. The cutoff value for predicting that a pregnancy will terminate within 24 h and <1 week was the value with the highest Youden's index.
Results
Maternal Background
Forty-four patients were enrolled in the study. The patient background data is shown in Table 1. The mean age was 34.3 ± 5.6 years. The mean number of weeks for PE onset was 30.5 ± 3.7 weeks, and the mean number of weeks for pregnancy termination was 32.9 ± 4.3 weeks. The method of delivery was cesarean section in 81.1% of cases. The complication rate of fetal growth retardation was 22.7%, and the mean birth weight was 1759.6 ± 757.1 g.
Delivery in Less Than 24 h
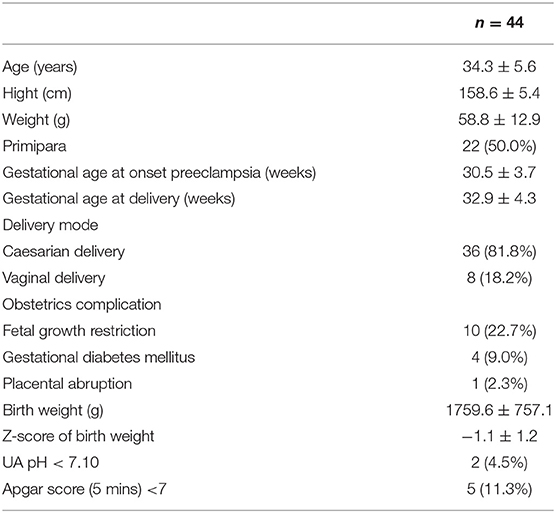
The mean value of sFlt-1 was 12,034.3 ± 1,379.6 pg/ml in the 24 h T group and 7,779.2 ± 796.5 pg/ml in the 24 h P group (P = 0.01). The mean sFlt-1/PlGF ratio was 458.8 ± 112.8 in the 24 h T group and 265.12 ± 66.1 in the 24 h P group (P = 0.12). sFlt-1 was the most significantly associated factor with delivery in <24 h (Figure 1).
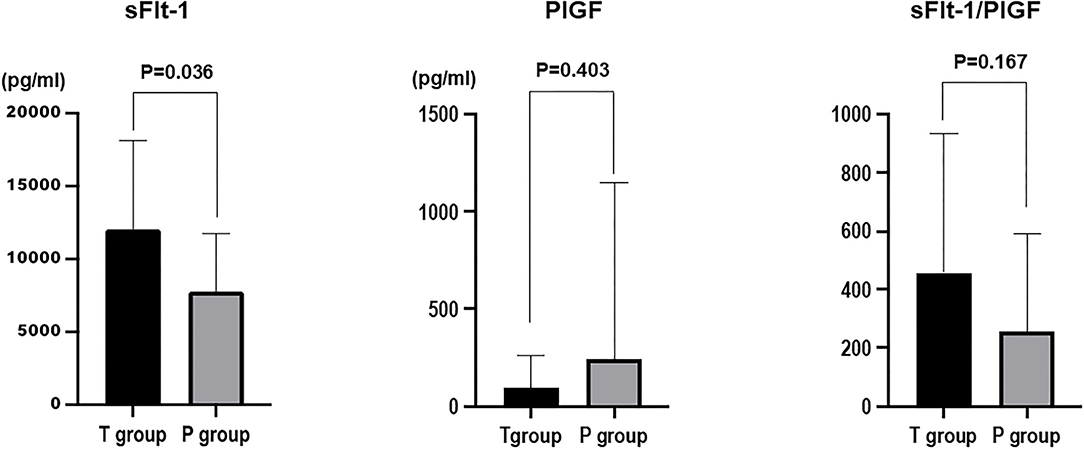
Figure 1. Comparison of sFlt-1, PlGF, and sFlt-1/PlGF ratios between the group that delivered in <24 h (T group) and the group that delivered in more than 24 h (P group).
Delivery in Less Than 1 Week
The mean value of sFlt-1 was 1,1607.5 ± 984.2 pg/ml in the 1-week T group and 6,741.0 ± 858.0 pg/ml in the 1-week P group (P = 0.0006). The mean sFlt-1/PlGF ratio was 515.3 ± 76.7 in the 1-week T group and 149.6 ± 66.9 in the 1-week P group (P=0.0009). sFlt-1 was the most significantly associated factor with delivery in <1 week (Figure 2).
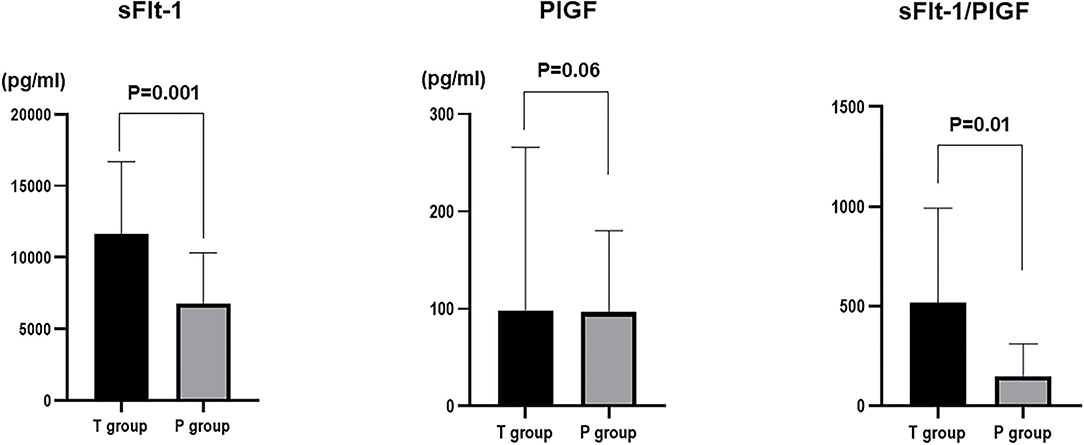
Figure 2. Comparison of sFlt-1, PlGF, and sFlt-1/PlGF ratios in the group that delivered in <1 week (T group) and the group that delivered in more than 1 week (P group).
Cutoff Values for Delivery in <24 h and <1 Week
Among sFlt-1, PlGF, and sFlt-1/PlGF, sFlt-1 was the parameter that was the strongest statistical predictor. Therefore, we used sFlt-1 as the predictor. In this study, cutoff values of 8,682.1 pg/ml (AUC 0.71; 95%Cl, 0.5191–0.9052) and 7,394.5 pg/ml (AUC 0.78; 0.78, 95%Cl, 0.6394–0.9206) for sFlt-1 for delivery in <24 h and within 1 week, respectively, were identified as important predictive values (Figures 3, 4). sFlt-1 below 8,682 pg/ml had a positive predictive value of 43.9% for delivery in <24 h, with a sensitivity of 72.3% and specificity of 69.0%. sFlt-1 >7,394 pg/ml would result in delivery within a week, with a positive predictive value of 67.2%, sensitivity of 79.0%, and specificity of 72.0%.
Figure 3. Cutoff values for delivery in <24 h; Cutoff values of 8,682.1 pg/ml (AUC 0.71; 95%Cl, 0.5191–0.9052).
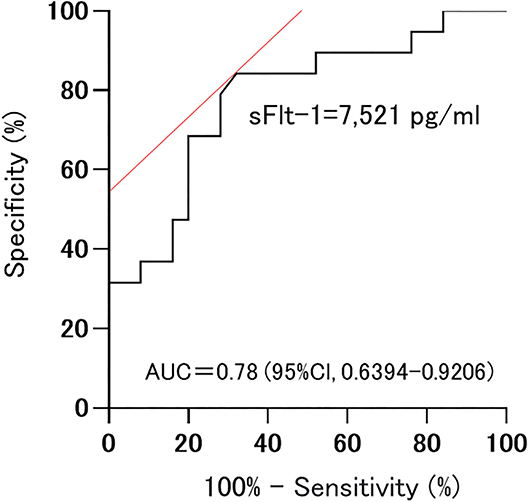
Figure 4. Cutoff values for delivery in <1 week; 7,394.5 pg/ml (AUC 0.78; 0.78, 95%Cl, 0.6394–0.9206).
Discussion
This study highlights two key novel findings. First, sFlt-1 may be more effective than PlGF or the sFlt-1/PlGF ratio in predicting the time of termination in pregnant women with known PE. Second, sFlt-1 cutoff values were calculated to predict the time of termination of pregnancy (<24 h and <1 week) after PE.
Although the etiology of PE remains controversial, the discovery of cardiovascular angiogenic factors has led to significant advances in both the diagnosis and prediction of prognosis. Of particular interest are the anti-angiogenic factor sFlt-1 and angiogenic factor PlGF. The sFlt-1 level increases in maternal blood in PE, whereas the PlGF level decreases as impaired spiral artery remodeling worsens placental return (12, 13). Therefore, various clinical studies have been conducted using the sFlt-1/PlGF ratio. The sFlt-1/PlGF ratio can be used to predict the development of PE in pregnant women who may be at risk of PE, and for the management planning and decision making in pregnant women with clinical features that are suspicious of PE or those diagnosed with PE. In particular, the sFlt-1/PlGF ratio is extremely useful in predicting the development of PE in pregnant women with suspected PE (9, 14, 15). In Japan, even standard low-risk pregnant women visit the clinic every 1–2 weeks for a checkup. Therefore, pregnant women with PE can be diagnosed at a relatively early stage. In Japan, after PE is diagnosed, the patient is generally hospitalized for observation. For these reasons, we are more interested in the application of the sFlt-1/PlGF ratio to the management and decision-making of pregnant women with known PE than in predicting the onset of PE. Specifically, we are interested in the possibility of terminating pregnancies after the onset of PE and before serious complications develop.
The association between angiogenic factors and adverse events in pregnant women with suspected PE was studied by Rana et al. (16). These studies could reduce unnecessary hospitalizations and inappropriate discharge of pregnant women with suspected PE and reduce the considerable morbidity associated with unplanned preterm delivery. However, it is not currently possible to determine when a pregnancy should be terminated in pregnant women with known PE.
In the Hypertension and Preeclampsia Intervention Trial At near Term-I (HYPITAT I) study in the Netherlands, it was reported that women with gestational hypertension and mild hypertension should be induced to deliver after 37 weeks gestation, although certain angiogenic factors had not been investigated (17). In a study by Verlohren et al. it was possible to identify pregnant women at risk for impending delivery with different hypertension, chronic hypertension, and gestational hypertension (18). However, it was not possible to determine whether induction of labor should be recommended for these pregnant women.
The sFlt-1/PlGF ratio can be used to predict maternal adverse events in pregnant women with known PE. The higher the ratio, the higher the risk of maternal complications requiring hospital intervention, such as acute pulmonary edema, HELLP syndrome, placental abruption, renal failure, refractory hypertension, and eclampsia (10, 16, 18–22). However, these studies have not indicated when the pregnancy should be terminated.
Recent data has shown that the use of an algorithm based on PlGF levels in women with late preterm eclampsia results in a lower rate of progression to severe PE, fewer maternal complications, and no worsening of the neonatal outcome (23). Similarly, the use of the sFlt-1/PlGF ratio in standard practice has been reported to improve clinical accuracy and correctly identify both at-risk women and at-risk babies (24). In the future, it will be necessary to combine this with neonatal conversion to find the appropriate time to terminate the pregnancy.
Predicting the duration of pregnancy after the onset of gestational hypertension nephropathy would allow the obstetrician not to miss the timing of administering steroids before delivery, the obstetrician to observe the patient more intensively as the pregnancy is predicted to be nearing its end, and the neonatologist to be better prepared for the delivery. On the other hand, there is concern that setting a cutoff value may lead to an increase in excessive preterm births beyond the cutoff value. At this time, the indicator of pregnancy termination should be based on clinical findings.
This study had some potential limitations. Although the focus of this study was on the timing of pregnancy termination, it is necessary to examine this issue from the perspective of neonatal prognosis. However, this study was not conducted from the perspective of neonatal prognosis. Another limitation is the retrospective study design and the small number of cases, even though the cases were managed in a uniform manner. An important limitation of this study is the small number of cases. Though this study themselves show that there are different predictive values, the small group of cases considered may be the reason for this result. In the future, more cases should be added to validate the results of this study. sFlt-1 increases modestly with weeks. However, this study did not adjust for this increase, so caution should be exercised in interpreting the cutoff values in this study.
Conclusion
In summary, this study showed that sFlt-1 may be effective in predicting the timing of pregnancy termination. However, the number of cases was small and the results were therefore not definitive. In combination with previous reports, we would like to develop this study further to predict the optimal timing of pregnancy termination to prevent cerebral hemorrhage after PE.
Author's Note
The authors of this article are board-certified Obstetrics and Gynecology physicians at Mie University Hospital, which is the largest Obstetrics and Gynecology resident training institution in Japan. The corresponding author is a member of the Japan Maternal Death Exploratory Committee.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Mie University Hospital Medical Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
HT conceived and designed the study, performed data acquisition, and conducted data analysis and interpretation. KT provided statistical expertise. HT wrote the manuscript draft. ST, NE, and TI made major revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to our colleagues who provided their expertise, which greatly assisted the research, although they may not agree with all the conclusions in this paper.
References
1. Hasegawa J, Ikeda T, Sekizawa A, Tanaka H, Nakata M, Murakoshi T, et al. Maternal Death Exploratory Committee, Japan Association of Obstetricians and Gynecologists. Maternal death due to stroke associated with pregnancy-induced hypertension. Circ J. (2015) 79:1835–40. doi: 10.1253/circj.CJ-15-0297
2. Katsuragi S, Tanaka H, Hasegawa J, Nakamura M, Kanayama N, Nakata M, et al. On behalf of the Maternal Death Exploratory Committee in Japan and Japan Association of Obstetricians and Gynecologists. Analysis of preventability of stroke-related maternal death from the nationwide registration system of maternal deaths in Japan. In: J Matern Fetal Neonatal Med. (2018) 31:2097–104. doi: 10.1080/14767058.2017.1336222
3. Hasegawa J, Katsuragi S, Tanaka H, Kurasaki A, Nakamura M, Murakoshi T, et al. Decline in maternal death due to obstetric haemorrhage between 2010 and 2017 in Japan. Sci Rep. (2019) 9:11026. doi: 10.1038/s41598-019-47378-z
4. Katsuragi S, Tanaka H, Hasegawa J, Nakamura M, Kanayama N, Nakata M, et al. Maternal Death Exploratory Committee in Japan and Japan Association of Obstetricians and Gynecologists. Analysis of preventability of hypertensive disorder in pregnancy-related maternal death using the nationwide registration system of maternal deaths in Japan. J Matern Fetal Neonatal Med. (2019) 32:3420–6. doi: 10.1080/14767058.2018.1465549
5. Enomoto N, Tanaka H, Katsuragi S, Hayata E, Hasegawa J, Nakata M, et al. Pregnancy-associated hemorrhagic stroke: a nationwide survey in Japan. J Obstet Gynaecol Res. (2021) 47:2066–75. doi: 10.1111/jog.14786
6. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. (2010) 376:631–44. doi: 10.1016/S0140-6736(10)60279-6
7. Ramos JGL, Sass N, Costa SHM. Preeclampsia. Rev Bras Ginecol Obstet. (2017) 39:496–512. doi: 10.1055/s-0037-1604471
8. Gestational hypertension and preeclampsia: ACOG practice bulletin summary number 222. Obstet Gynecol. (2020) 135:1492–5. doi: 10.1097/AOG.0000000000003892
9. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. (2016) 374:13–22. doi: 10.1056/NEJMoa1414838
10. Dröge LA, Perschel FH, Stütz N, Gafron A, Frank L, Busjahn A, et al. Prediction of preeclampsia-related adverse outcomes with the sFlt-1 (soluble fms-like tyrosine kinase 1)/PlGF (placental growth factor)-ratio in the clinical routine: a real-world study. Hypertension. (2021) 77:461–71. doi: 10.1161/HYPERTENSIONAHA.120.15146
11. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Preg Hypertens. (2018) 13:291–310. doi: 10.1161/HYPERTENSIONAHA.117.10803
12. Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. (2006) 355:992–1005. doi: 10.1056/NEJMoa055352
13. Chau K, Hennessy A, Makris A. Placental growth factor and pre-eclampsia. J Hum Hypertens. (2017) 31:782–6. doi: 10.1038/jhh.2017.61
14. Bian X, Biswas A, Huang X, Lee KJ Li TK, Masuyama H, et al. Short-term prediction of adverse outcomes using the sFlt-1 (soluble fms-like tyrosine kinase 1)/PlGF (placental growth factor) ratio in Asian women with suspected preeclampsia. Hypertension. (2019) 74:164–72. doi: 10.1161/HYPERTENSIONAHA.119.12760
15. Zeisler H, Llurba E, Chantraine FJ, Vatish M, Staff AC, Sennström M, et al. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: Ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet Gynecol. (2019) 53:367–75. doi: 10.1002/uog.19178
16. Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. (2012) 125:911–9. doi: 10.1161/CIRCULATIONAHA.111.054361
17. de Sonnaville CMW, Hukkelhoven CW, Vlemmix F, Groen H, Schutte JM, Mol BW, et al. Impact of hypertension and preeclampsia intervention trial at near term-I (HYPITAT-I) on obstetric management and outcome in the Netherlands. Ultrasound Obstet Gynecol. (2020) 55:58–67. doi: 10.1002/uog.20417
18. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. (2012) 206:58.e1–8. doi: 10.1016/j.ajog.2011.07.037
19. March MI, Geahchan C, Wenger J, Raghuraman N, Berg A, Haddow H, et al. Circulating angiogenic factors and the risk of adverse outcomes among Haitian Women with preeclampsia. PLoS ONE. (2015) 10:e0126815. doi: 10.1371/journal.pone.0126815
20. Lopes Perdigao J, Chinthala S, Mueller A, Minhas R, Ramadan H, Nasim R, et al. Angiogenic factor estimation as a warning sign of preeclampsia-related peripartum morbidity among hospitalized patients. Hypertension. (2019) 73:868–77. doi: 10.1161/HYPERTENSIONAHA.118.12205
21. Gaccioli F, Sovio U, Cook E, Hund M, Charnock-Jones DS, Smith GCS. Screening for fetal growth restriction using ultrasound and the sFLT1/PlGF ratio in nulliparous women: a prospective cohort study. Lancet Child Adolesc Health. (2018) 2:569–81. doi: 10.1016/S2352-4642(18)30129-9
22. Mirkovic L, Tulic I, Stankovic S, Soldatovic I. Prediction of adverse maternal outcomes of early severe preeclampsia. Preg Hypertens. (2020) 22:144–50. doi: 10.1016/j.preghy.2020.09.009
23. Peguero A, Herraiz I, Perales A, Melchor JC, Melchor I, Marcos B, et al. Placental growth factor testing in the management of late preterm preeclampsia without severe features: a multicenter, randomized, controlled trial. Am J Obstet Gynecol. (2021) 225:308.e1–e14. doi: 10.1016/j.ajog.2021.03.044
Keywords: preeclampsia, sFlt-1, PlGF, prediction, Angiogenetic factors
Citation: Tanaka H, Tanaka K, Takakura S, Enomoto N and Ikeda T (2022) Predicting Preeclampsia Pregnancy Termination Time Using sFlt-1. Front. Med. 9:900639. doi: 10.3389/fmed.2022.900639
Received: 21 March 2022; Accepted: 26 May 2022;
Published: 20 June 2022.
Edited by:
Edoardo Sciatti, Local Social Health Agency Garda, ItalyReviewed by:
Joško Osredkar, University Medical Center Ljubljana, SloveniaKazuo Itabashi, Showa University, Japan
Copyright © 2022 Tanaka, Tanaka, Takakura, Enomoto and Ikeda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroaki Tanaka, aF90YW5ha2FAbWVkLm1peWF6YWtpLXUuYWMuanA=
 Hiroaki Tanaka
Hiroaki Tanaka Kayo Tanaka
Kayo Tanaka Sho Takakura
Sho Takakura Naosuke Enomoto
Naosuke Enomoto Tomoaki Ikeda
Tomoaki Ikeda