- 1Department of Medical Education and Research, Kaohsiung Veterans General Hospital, Kaohsiung City, Taiwan
- 2Department of Medical Education and Research, Zuoying Branch of Kaohsiung Armed Forces General Hospital, Kaohsiung City, Taiwan
- 3Institute of Allied Health Sciences, National Cheng Kung University, Tainan City, Taiwan
- 4Department of Rehabilitation Medicine, Cishan Hospital, Ministry of Health and Welfare, Kaohsiung City, Taiwan
- 5Department of Surgery, Kaohsiung Veterans General Hospital, Kaohsiung City, Taiwan
- 6Department of Nursing, Shu-Zen Junior College of Medicine and Management, Kaohsiung City, Taiwan
- 7Department of Physical Therapy, National Cheng Kung University, Tainan City, Taiwan
- 8Department of Physical Medicine and Rehabilitation, Kaohsiung Veteran General Hospital, Kaohsiung City, Taiwan
- 9Department of Internal Medicine, Kaohsiung Armed Forces General Hospital, Kaohsiung City, Taiwan
- 10School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung City, Taiwan
- 11School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei City, Taiwan
- 12Department of Post-Baccalaureate Medicine, National Sun Yat-Sun University, Kaohsiung City, Taiwan
Objectives: Patients with lung cancer pose a high risk of morbidity and mortality after lung resection. Those who receive perioperative cardiopulmonary rehabilitation (PRCR) have better prognosis. Peak oxygen consumption (peak VO2), VO2 at the ventilatory threshold (VO2 at VT), and slope of minute ventilation to carbon dioxide production (VE/VCO2 slope) measured during pre-surgical cardiopulmonary exercise testing (CPET) have prognostic values after lung resection. We aimed to investigate the influence of individualized PRCR on postoperative complications in patients undergoing video-assisted thoracic surgery (VATS) for lung cancer with different pre-surgical risks.
Methods: This was a retrospective study. We recruited 125 patients who underwent VATS for lung cancer between 2017 and 2021. CPET was administered before surgery to evaluate the risk level and PRCR was performed based on the individual risk level defined by peak VO2, VO2 at VT, and VE/VCO2 slope, respectively. The primary outcomes were intensive care unit (ICU) and hospital lengths of stay, endotracheal intubation time (ETT), and chest tube insertion time (CTT). The secondary outcomes were postoperative complications (PPCs), including subcutaneous emphysema, pneumothorax, pleural effusion, atelectasis, infection, and empyema.
Results: Three intergroup comparisons based on the risk level by peak VO2 (3 groups), VO2 at VT (2 groups), and VE/VCO2 slope (3 groups) were done. All of the comparisons showed no significant differences in both the primary and secondary outcomes (p = 0.061–0.910).
Conclusion: Patients with different risk levels showed comparable prognosis and PPCs after undergoing CPET-guided PRCR. PRCR should be encouraged in patients undergoing VATS for lung cancer.
Introduction
Lung cancer is a leading cause of cancer mortality worldwide in both men and women. In 2020, lung cancer was diagnosed in approximately 2.2 million patients and was responsible for an estimated 1.8 million deaths (1). Lung cancer is categorized into small cell lung cancer and non-small cell lung cancer (NSCLC). NSCLC, which includes adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, accounts for the majority of lung cancer cases. Furthermore, smoking is the predominant risk factor for lung cancer (2). Definitive pathologic results are required to make a diagnosis of lung cancer. Therefore, tissue biopsy is necessary for diagnosis and staging (3). For patients with early disease, surgical biopsy is occasionally preferred because it has the potential to achieve diagnosis and curative resection at the same time. However, minimally invasive procedures are typically preferred in patients with a higher disease stage. In addition, lung resection poses a high risk of morbidity and mortality in patients with severe comorbidities or low cardiopulmonary reserve.
According to the 2016 European Society of Cardiology (ESC) guidelines, cardiopulmonary exercise testing (CPET) can be used to assess perioperative and postoperative risks and has a prognostic value in patients undergoing various surgical procedures, including abdominal aortic aneurysm repair (4, 5), radical cystectomy (6), liver transplantation (7), hepatic resection (8), lung resection (9, 10), bariatric surgery, and colorectal surgery (11). In patients undergoing lung resection, three CPET variables have been proven to have prognostic values: peak oxygen consumption (peak VO2) (12), VO2 at the ventilatory threshold (VO2 at VT), and slope of minute ventilation to carbon dioxide production (VE/VCO2 slope) (13, 14). Physiotherapy services for patients with lung cancer have historically been hospital based and have focused on postoperative pulmonary complications (PPCs). Although cardiopulmonary rehabilitation is considered an important component of perioperative care in patients undergoing lung resection surgery, previous studies have not investigated the impact of the application of cardiopulmonary rehabilitation before and after surgery (15). Therefore, in this study, we aimed to investigate the influence of individualized perioperative cardiopulmonary rehabilitation on postoperative complications in patients undergoing video-assisted thoracic surgery (VATS) for lung cancer with different pre-surgical risks.
Methods
Participants
This was a retrospective study. Patients who underwent VATS for lung cancer at a tertiary medical center (Kaohsiung Veterans General Hospital) between May 7, 2017, and May 3, 2020, were enrolled. Patients with cerebrovascular diseases, severe orthopedic disorders, advanced heart failure (functional class IV), severe valvular diseases, or uncontrolled arrhythmia were excluded. Patients with incomplete medical records or postoperative plain chest radiographs were further excluded. Finally, 125 patients were enrolled for analysis. All patients underwent CPET before VATS. After CPET, the patients underwent perioperative cardiopulmonary rehabilitation based on the determined risk level. All patients received education regarding cardiovascular risk factors from a team comprising doctors, nurses, nutritionists, and physical therapists. This study was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital (number: VGHKS17-CT11-11, date of approval: Oct. 17, 2021).
Cardiopulmonary Exercise Test
All patients were tested using Metamax 3B (Cortex Biophysik Co., Leipzig, Germany), which consists of a bicycle ergometer, a gas analyzer, and an electrocardiography (ECG) monitor. The patients pedaled on an upright bicycle ergometer for the assessment of peak VO2, VO2 at VT, and VE/VCO2 slope. The exercise was started at an intensity of 0-W workload for a 1-min warm-up, followed by incremental loading using a ramp protocol (10 W/min) until exhaustion. The patients were tested with the ramp Bruce protocol, following the guidelines of the American College of Sports Medicine (ACSM). All patients safely completed the test.
Interventions
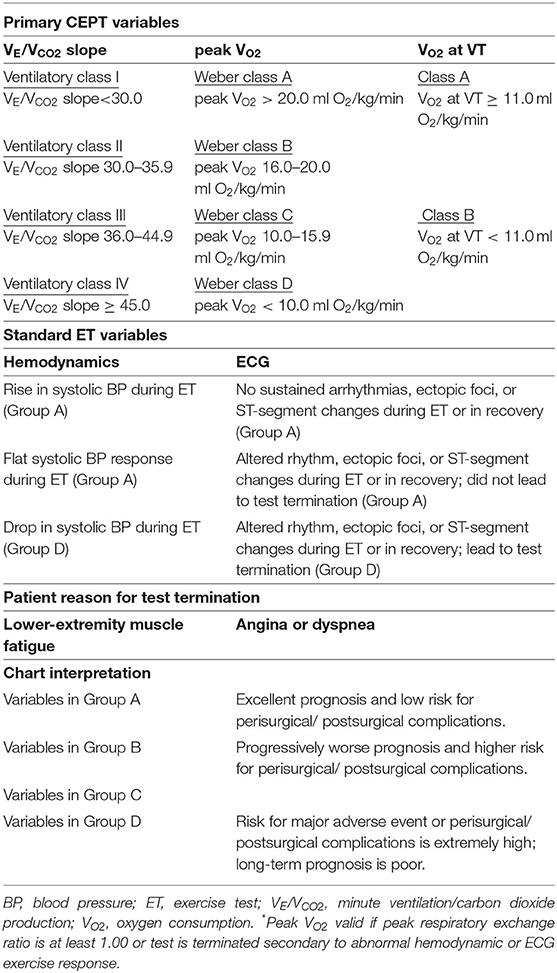
The results of CPET were used to classify the risk levels of the patients in accordance with the criteria from the 2016 ESC guidelines (Table 1). All participants were tested for each of the three CPET variables as follows: (1) peak VO2, divided into four classes [Weber class A (>20 mL/kg/min), Weber class B (16–20 mL/kg/min), Weber class C (10–15.9 mL/kg/min), and Weber class D (<10 mL/kg/min)]; (2) VO2 at VT, divided into two classes [class A (≥11 mL/kg/min) and class B (<11 mL/kg/min)]; and (3) VE/VCO2 slope, divided into four classes [ventilatory class I (<30), ventilatory class II (30–35.9), ventilatory class III (36–44.9), and ventilatory class IV (>45)](11). Thereafter, all participants were classified into four categories (groups A, B, C, and D) according to their risk level for perioperative or postoperative complications. Group A included patients with Weber class A, VO2 at VT class A, ventilatory class I, an increase in systolic blood pressure (SBP) during the exercise test, and ECG results showing no sustained arrhythmias, ectopic foci, or ST-segment changes during the exercise test or recovery phase. Group B included patients with Weber class B and ventilatory class II. Group C included patients with Weber class C, ventilatory class III, flat SBP response during the exercise test, and ECG results showing altered rhythm, ectopic foci, or ST-segment changes during the exercise test or recovery phase without leading to test termination. Group D included patients with Weber class D, VO2 at VT class B, ventilatory class IV, decrease in SBP during the exercise test, and ECG results showing altered rhythm, ectopic foci, or ST-segment changes during the exercise test or recovery phase leading to test termination.
Preoperative Phase
In the preoperative phase, one experienced physical therapist (Jing-Hui Chung) explained the treatment plan and educated the patients regarding common PPCs before starting the training program. All patients were treated with perioperative cardiopulmonary rehabilitation. The perioperative cardiopulmonary rehabilitation used in our study consisted of deep breathing exercises, coughing techniques, early mobilization, and progressive shoulder/thoracic mobility exercises. We used different frequencies and intensities of perioperative cardiopulmonary rehabilitation according to the risk level of each patient before surgery (Figure 1). For example, participants in group B underwent perioperative cardiopulmonary rehabilitation consisting of early extremity and thoracic mobilization, diaphragmatic breathing, Triflow training, and inspiratory muscle training (IMT). The Triflow training was done by a Tri-ball incentive spirometer (Galemed, Taipei, Taiwan) designed to aid in maintaining the lung capacity by encouraging deep and slow breathing. The IMT training was done by using a threshold-type breathing trainer (Dofin DT11/14, Galemed, Taipei, Taiwan). Each IMT session consisted of three sets of 10 breaths with a 2-min break between each set at a target intensity of 30% of the maximal inspiratory pressure (MIP). The MIP were measured using a digital pressure gauge (GB60, Jitto International, Taipei, Taiwan).
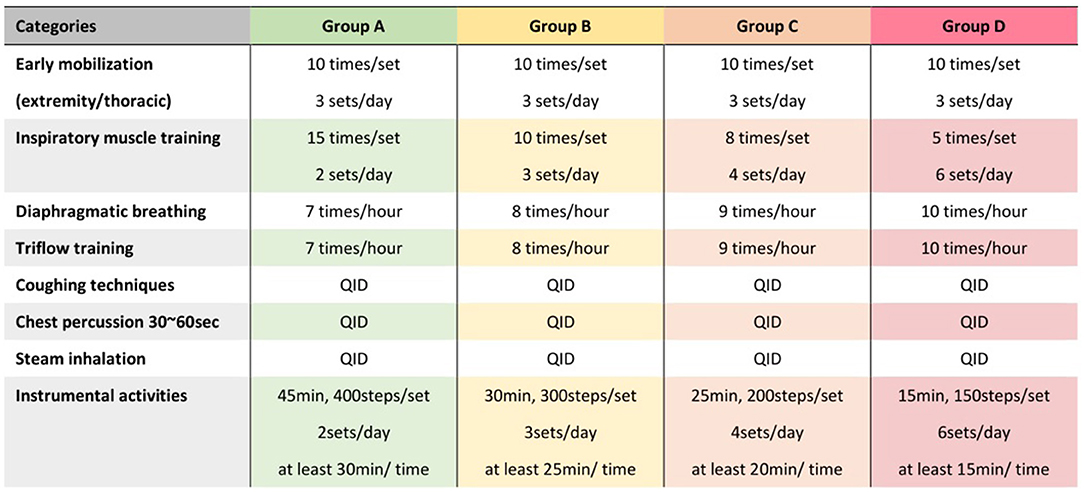
Figure 1. Protocol of perioperative cardiopulmonary rehabilitation for patients with different risk level. Different protocols of perioperative cardiopulmonary rehabilitation were designed for risk levels from group A to group D, respectively, based on the 2016 European Society of Cardiology guidelines (Table 1).
Postoperative Phase
In the postoperative phase, the physiotherapy exercises performed on the first postoperative day included early mobilization, sitting out of bed, stepping and walking in the ward, breathing exercise, incentive spirometry, chest physical therapy, and supported coughing. On postoperative days 1 to 3, the levels of activities were progressively increased (e.g., walking for 5–10 min in the hallway two to three times per day on the first day and then three to four times per day on the next day). The first walking session was supervised by a physical therapist. Deep breathing exercise, thoracic expansion exercise, shoulder/thoracic stretch, and range-of-motion exercise were conducted to improve functional ability in the short term. For example, after surgery, we provided the patients with an IMT protocol in which each session consisted of two sets of 30 breaths with a 2-min break between each set at a target intensity of 15% of the maximal inspiratory pressure, which was incrementally increased by 2 cm H2O per day depending on the patients' ability. The patients underwent daily progressive strength and endurance training with aerobic exercise, breathing exercise for lung expansion, and chest physiotherapy intervention before discharge.
Post-surgical Pain Management
All the recruited patients received acute post-surgical pain management by multimodal approach, which consists in the concomitant use of different analgesic drugs to guarantee the greatest pain relief together with opioid-sparing effect (16). The patients received systemic analgesia, including acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), and opioids (from weak opioid, such as tramadol and codeine, to morphine). If the systemic analgesia failed, additional local regional analgesia via thoracic paravertebral block (TPB) would be done by anaesthesiologists after evaluation is provided and reviewed by the team and regional analgesia (17). The analgesic management ceased once the chest tube was removed.
Outcomes Measured
To evaluate surgical prognosis and PPCs, we collected data on several variables assessed during hospitalization. The primary outcomes of this study were variables related to prognosis, including intensive care unit (ICU) length of stay, hospital length of stay, endotracheal intubation time (ETT), and chest tube insertion time (CTT). ICU length of stay was defined as the duration between the surgery end time according to the operation record and the precise time of transporting the patient to the general ward according to the treatment order, calculated in minutes. Hospital length of stay was defined as the interval between the admission time and discharge time, calculated in days. ETT was defined as the duration between the surgery starting time according to the operation record and the precise time of endotracheal tube removal according to the treatment order. CTT was defined as the duration between the surgery end time according to the operation record and the precise time of chest tube removal according to the treatment order.
The secondary outcomes were postoperative complications determined by clinical assessment or imaging evaluations, including subcutaneous emphysema, pneumothorax, pleural effusion, atelectasis, infection, and empyema. To assess postoperative complications, two experienced doctors (Wei-Hao Chao and Ko-Long Lin) investigated all hospitalization records, including progression notes, discharge notes, and nursing records, for detailed physical examination data during hospitalization, especially during the postoperative period. For imaging-diagnosed postoperative complications, one doctor (Wei-Hao Chao) investigated the formal reports of preoperative and postoperative plain chest radiography (CXR) evaluations, which were conducted by an expert radiologic technologist and were interpreted by a radiologist. Another doctor (Ko-Long Lin) confirmed the plain imaging findings on a picture archiving and communication system workstation. We compared the preoperative and postoperative images to identify differences in imaging findings. Finally, clinical complications were divided into four aspects: air (subcutaneous emphysema, pneumothorax, and continuous air leakage observed in a water-seal bottle), fluid (pleural effusion), lung (atelectasis), and infection (fever and empyema). CXR-diagnosed complications were also divided into four aspects: air (subcutaneous emphysema, pneumothorax, pneumomediastinum, and hydropneumothorax), fluid (pleural effusion and pulmonary edema), lung (atelectasis), and infection (pneumonia and empyema). Each aspect accounted for 1 point. Clinical or CXR-diagnosed complications were divided into four aspects: air, fluid, lung, and infection. Each aspect accounted for 1 point. The patients were assessed for either clinical complications, CXR-diagnosed complications, or both.
Statistical Analysis
SPSS for Windows (version 19.0, released in 2010; IBM Corp., Armonk, NY, USA) was used for all analyses. Continuous variables were expressed as mean ± standard deviation, and categorical variables were presented as absolute numbers and percentages. Data were tested for normality and homoscedasticity before each analysis. The independent t test was used to compare outcomes between two different groups. For outcome comparisons among three or more groups, we used one-way analysis of variance, except for gender, type of lung resection, and use of post-surgical pain management, which were done by Chi square test. Moreover, given that there were more than 20% cells have expected frequency <5 when comparing the difference of type of lung resection between different groups based on the classification by ESC, we collapsed the number of lobectomy and pneumonectomy into one row. Statistical significance was set at p < 0.05.
Results
Basic Characteristics
Patients who underwent VATS for lung cancer during the inclusion period were identified. Patients with cerebrovascular diseases, severe orthopedic disorders, advanced heart failure (functional class IV), severe valvular diseases, or uncontrolled arrhythmia were excluded. Those with incomplete medical records and plain radiographs were also excluded. Finally, 125 patients were included for analysis. Among the 125 participants, 74 (59.2%) were men and 51 (40.8%) were women. The average age, height, weight, and body mass index (BMI) were 59.88 ± 9.61 years, 161.00 ± 8.32 cm, 62.86 ± 11.02 kg, and 24.16 ± 3.22 kg/m2, respectively. In terms of the type of lung cancer, 7 (5.6%), 86 (68.8%), and 32 (25.6%) patients had squamous cell carcinoma, adenocarcinoma, and other types, respectively. In terms of types of VATS, 65 (52.0%), 56 (44.8%), and 4 (3.2%) patients received lobectomy, wedge resection, and pneumonectomy, respectively.
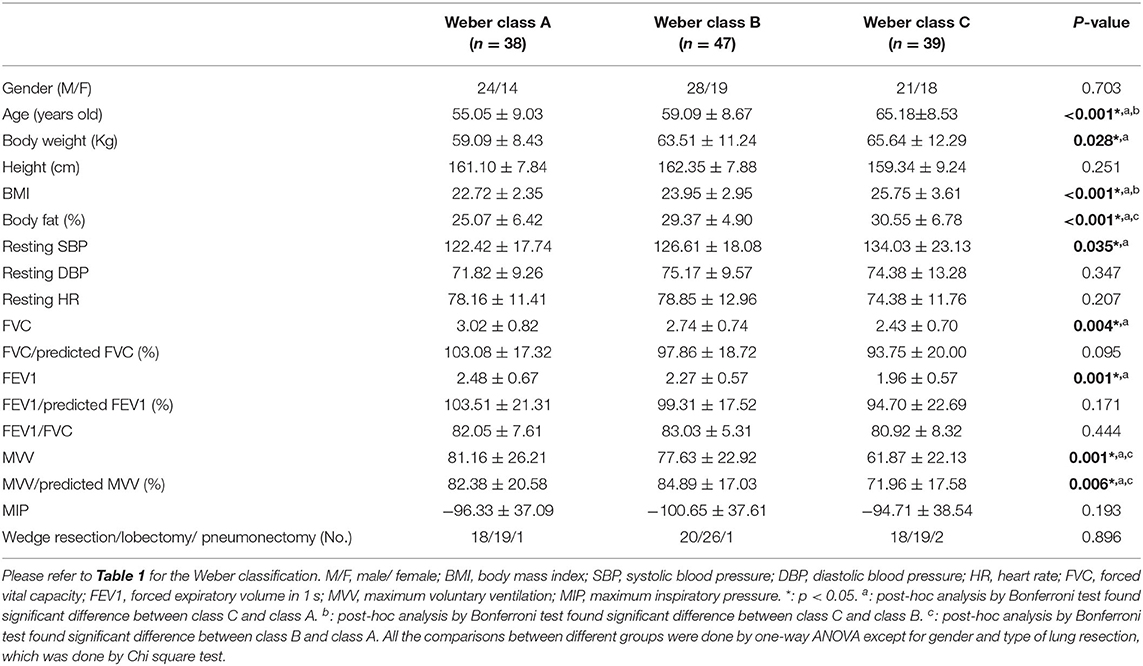
The baseline characteristics of each group defined by peak VO2 are shown in Table 2A. We found significant differences in age, body weight, BMI, body fat, resting SBP, forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and maximum voluntary ventilation (MVV). Patients with lower peak VO2 had older age; higher body weight, BMI, body fat, and resting SBP; and lower FVC, FEV1, and MVV. The baseline characteristics of each group defined by VO2 at VT are shown in Table 2B. Significant differences were found in age, body weight, BMI, FVC/predicted FVC, FEV1/predicted FEV1, and MVV/predicted MVV. Patients with lower VO2 at VT had older age, higher body weight and BMI, and lower FVC/predicted FVC, FEV1/predicted FEV1, and MVV/predicted MVV. The baseline characteristics of each group defined by VE/VCO2 slope are shown in Table 2C. Significant differences were found in age, FEV1, and MVV. Patients with higher VE/VCO2 slope had older age, lower FEV1, and lower MVV. In addition, no significant difference of the type of lung resection was noted between each group defined by peak VO2 (p = 0.896), VO2 at VT (p = 0.821), and VE/VCO2 slope (p = 0.894).
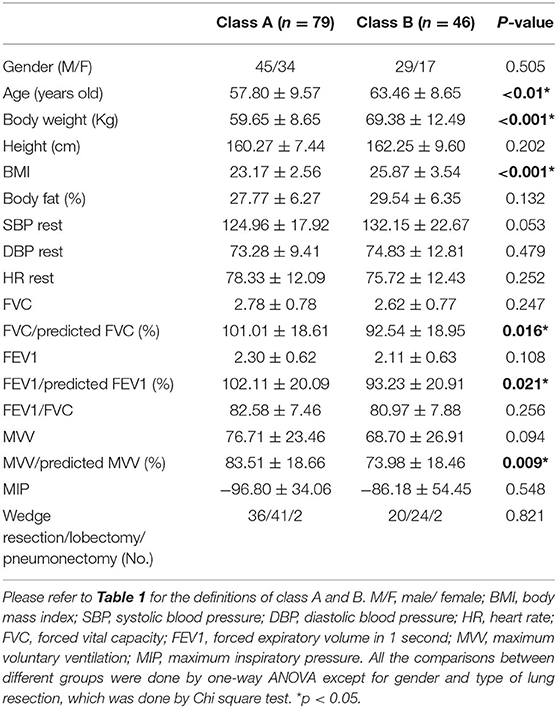
Table 2B. Baseline characteristics of each group defined by oxygen consumption at anaerobic threshold (VO2 at VT).
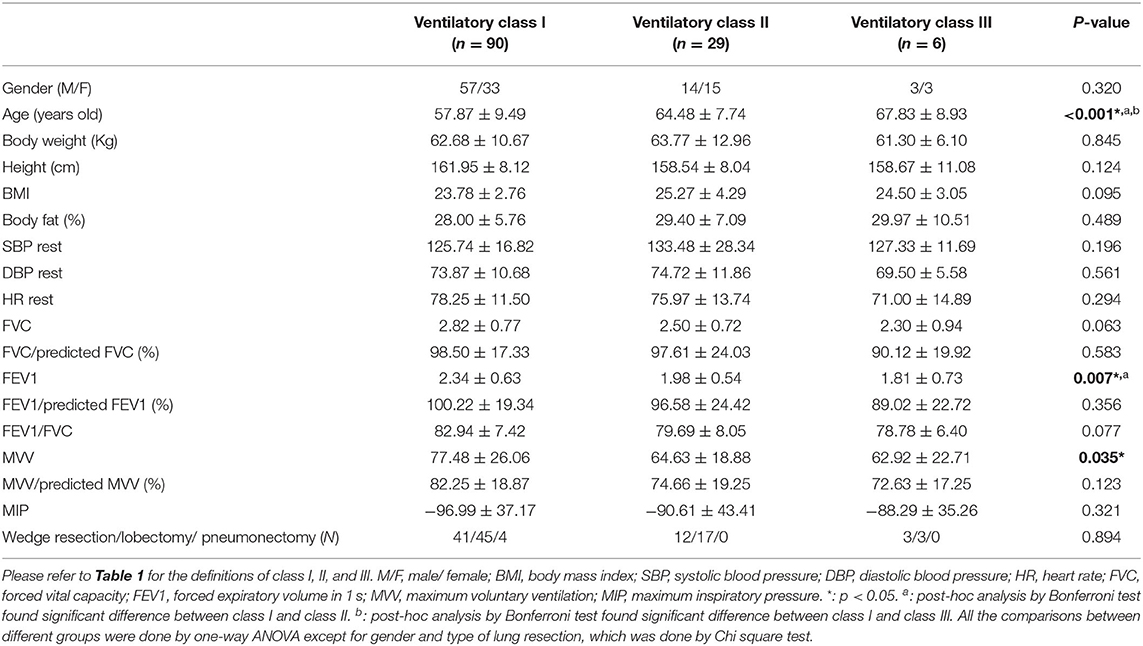
Table 2C. Baseline characteristics of each group defined by slope of minute ventilation and carbon dioxide production (VE/VCO2 slope).
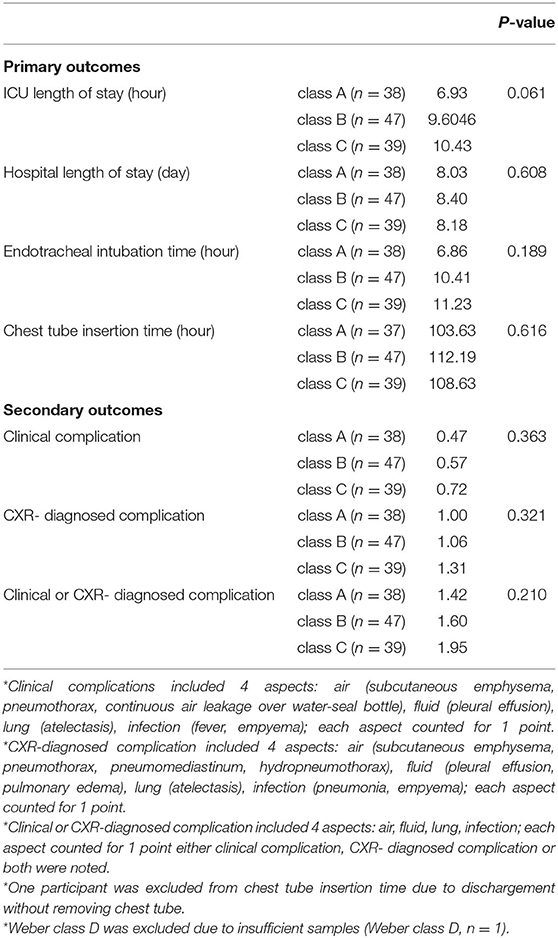
Comparisons of Outcomes According to Peak VO2
On the basis of the 2016 ESC guidelines, 38 patients were in class A (peak VO2 > 20 mL/kg/min), 47 patients were in class B (16 < peak VO2 <20 mL/kg/min), 39 patients were in class C (10 < peak VO2 <15.9 mL/kg/min), and one patient was in class D (peak VO2 <10 mL/kg/min). Patients in class D were excluded from outcome comparisons owing to their small number. As for post-surgical pain management, 9, 10, and 11 patients received acetaminophen in class A, class B, and class C, respectively (p = 0.306). All the patients in each class received NSAIDs (regular use) and opioids (use on requirement). The mean number of using of different types of NSAIDs and opioids was 0.97 ± 0.28 and 1.39 ± 0.75 in class A, 1.02 ± 0.15 and 1.81 ± 0.92 in class B, and 1.03 ± 0.43 and 1.79 ± 0.95 class C, respectively (p = 0.868 and 0.132, respectively). One patient in class B and one patient in class C received additional local regional analgesia via TPB.
The outcome comparisons according to peak VO2 are shown in Table 3A. In terms of the primary outcomes, the three different peak VO2 classes showed no significant differences in ICU length of stay (p = 0.061), hospital length of stay (p = 0.608), ETT (p = 0.189), and CTT (p = 0.616). In terms of the secondary outcomes, no significant differences were observed in clinical complications (p = 0.363), CXR-diagnosed complications (p = 0.321), and clinical or CXR-diagnosed complications (p = 0.210).
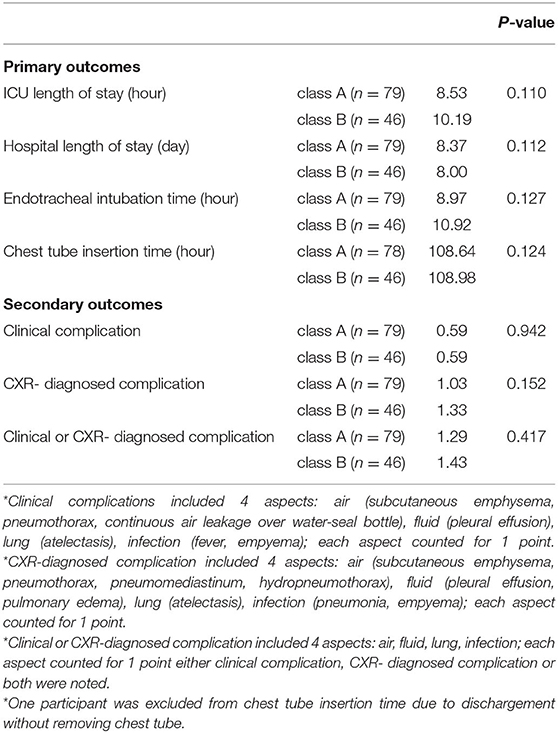
Comparisons of Outcomes According to VO2 at VT
On the basis of the 2016 ESC guidelines, 79 patients were in class A (VO2 ≥ 11 mL/kg/min) and 46 patients were in class B (VO2 <11 mL/kg/min). As for post-surgical pain management, 20 and 11 patients received acetaminophen in class A and class B, respectively (p = 0.861). All the patients in each class received NSAIDs (regular use) and opioids (use on requirement). The mean number of using of different types of NSAIDs and opioids was 1.01 ± 0.25 and 1.58 ± 0.91 in class A, 1.00 ± 0.37 and 1.85 ± 0.84 in class B, respectively (p = 0.820 and 0.110, respectively). One patient in class A and One patient in class B received additional local regional analgesia via TPB.
The outcome comparisons according to VO2 at VT are shown in Table 3B. For the primary outcomes, no significant differences were found in ICU length of stay (p = 0.110), hospital length of stay (p = 0.112), ETT (p = 0.127), and CTT (p = 0.124) between the two classes. For the secondary outcomes, no significant differences between the two classes were observed in clinical complications (p = 0.942), CXR-diagnosed complications (p = 0.152), and clinical or CXR-diagnosed complications (p = 0.417).
Comparisons of Outcomes According to VE/VCO2 Slope
On the basis of the 2016 ESC guidelines, 90, 29, and 6 patients were in class I (VE/VCO2 slope <30), class II (30 < VE/VCO2 slope <35.9), and class III (36 < VE/VCO2 slope <44.9), respectively. None of the patients belonged to class IV (VE/VCO2 slope > 45). As for post-surgical pain management, 22, 9, and 0 patients received acetaminophen in class I, class II, and class III, respectively (p = 0.274). All the patients in each class received NSAIDs (regular use) and opioids (use on requirement). The mean number of using of different types of NSAIDs and opioids was 1.0 ± 0.28 and 1.63 ± 0.88 in class I, 0.93 ± 0.37 and 1.93 ± 0.88 in class II, and 1.00 ± 0.00 and 1.17 ± 0.98 class III, respectively (p = 0.275 and 0.105, respectively). One patient in class I and one patient in class III received additional local regional analgesia via TPB.
The outcome comparisons according to VE/VCO2 slope are shown in Table 3C. No significant differences were found in ICU length of stay (p = 0.414), hospital length of stay (p = 0.661), ETT (p = 0.364), and CTT (p = 0.722) among classes I, II, and III. The comparisons of secondary outcomes showed no significant differences in clinical complications (p = 0.269), CXR-diagnosed complications (p = 0.896), and clinical or CXR-diagnosed complications (p = 0.910).
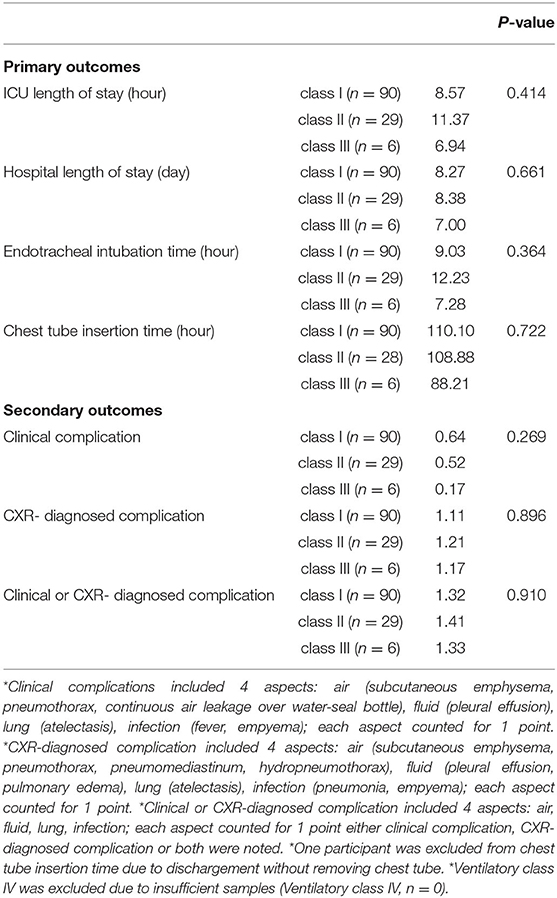
Table 3C. Outcomes comparisons by slope of minute ventilation and carbon dioxide production (VE/VCO2 slope).
Discussion
In this study, we aimed to investigate the influence of exercise testing-guided perioperative cardiopulmonary rehabilitation by applying physiotherapy interventions in the perioperative period. Our results showed that tailored perioperative cardiopulmonary rehabilitation based on CPET risk stratifications allowed patients with lung cancer with different risk levels to achieve comparable clinical and imaging outcomes after surgery. A peak VO2 of <10 mL/kg/min or 35% of the predicted value has been considered the threshold for prohibiting major surgeries (18). Among all variables, high values of VE and VCO2, expressed as the VE/VCO2 slope, imply ventilatory inefficiency and have long been associated with poor outcomes in patients with chronic heart failure (19). A higher VE/VCO2 slope has also been proven to be highly correlated with respiratory complications and mortality after pulmonary resection (13). Furthermore, the ESC guidelines have established adequate criteria for the preoperative assessment of perioperative and postoperative risks and long-term prognosis. Postoperative complications can be well predicted using three CPET variables [peak VO2 (10, 20–27), VO2 at VT (28), and VE/VCO2 slope (13, 29, 30). as reported in several articles published from 2001 to 2021. Therefore, CPET is currently recommended as part of the preoperative evaluation of lung cancer patients with respiratory comorbidities and/or functional limitations (18, 31). Under current guidelines, CPET is recommended only for those patients with lower FEV1, diffusing capacity of the lung for carbon monoxide (DLCO), or their predicted postoperative (ppo) values, that is FEV1 or DLCO <80% predicted by European Respiratory Society/European Society of Thoracic Surgeons (18), or ppoFEV1 or ppoDLCO <30% by American College of Chest Physician (31). We thought that even though the spirometric values correlate strongly with the severity of lung obstruction, they can't provide direct information regarding the degree of gas exchange and cardiovascular reserve (32). On the contrary, CPET reflects interactions between pulmonary function, cardiovascular status and oxygen uptake and utilization by the peripheral tissues (12). It is probable that some lung cancer patients could undergo surgery if CPET permits it even though they once excluded from surgery based on FEV1 and FVC or DLCO results (12). CPET is not only a tool for diagnosing suspected cardiovascular and respiratory diseases or for making decision to proceed to major surgery, but also a guidance of care required postoperatively (33, 34). CPET is feasible, safe, and recommended for patients with cancer prior to a physical exercise program (35). For patients with lung cancer, though only few available studies, the physician can prescribe tailored PRCR and can also asses the effectiveness of the rehabilitation based on CPET parameters (36–38). Our team has performed CPET-guided PRCR since 2017, based on the results of this current study and the low risk of major adverse events associated with performing CPET (33), routine pre-operative CPET should be highlighted for all lung cancer patients pending lung resection if it is performed in a controlled environment with continuous monitoring, appropriate equipment and well-trained personnel, without contraindications proposed by ACSM (39).
We took advantage of the prognostic value of the CPET variables to investigate the impact of perioperative cardiopulmonary rehabilitation. According to the ESC guidelines, significant differences in prognosis and postoperative complications can be expected in patients with different risk levels. However, regardless of the risk level categories defined by peak VO2, VO2 at VT, or VE/VCO2 slope, no significant differences in outcomes were observed after perioperative cardiopulmonary rehabilitation in this study. Our results showed comparable prognosis and postoperative complications after exercise testing-guided perioperative cardiopulmonary rehabilitation in patients with different risk levels, suggesting that perioperative cardiopulmonary rehabilitation results in better prognosis and fewer postoperative complications. To clarify this phenomenon, we attempted to compare our results with those of relevant previous studies. Cavalheri et al. (40) published a meta-analysis in 2017, focusing on the effect of preoperative exercise training on postoperative outcomes in patients with NSCLC. The population in this meta-analysis was patients scheduled to undergo lung resection for NSCLC, divided into the preoperative exercise training and no exercise training groups. In terms of CTT and hospital length of stay, our study patients showed significantly superior outcomes to those of the control group (no exercise training group) and comparable outcomes to those of the experimental group (preoperative exercise training group) in the meta-analysis. The last decades have seen increasing interest in investigating the impact of the application of cardiopulmonary rehabilitation in candidates for lung resection or other surgical procedures, and studies have demonstrated that perioperative cardiopulmonary rehabilitation has beneficial effects on postoperative prognosis and complications.
Some systematic review and meta-analysis studies demonstrated that superior preoperative CPET values, especially peak VO2, were significantly associated with improved postoperative outcomes in patients undergoing cancer surgery (41). The reason for this phenomenon remains uncertain but is gradually being elucidated. As the VO2 level is influenced by the combined contribution of the heart, lungs, oxygen transport system, and skeletal muscles to external work, it is a comprehensive indicator of the general physical status. Meanwhile, the VE/VCO2 slope is a more specific expression of ventilatory efficiency (42). Through perioperative cardiopulmonary rehabilitation consisting of deep breathing exercises, coughing techniques, early mobilization, and progressive shoulder/thoracic mobility exercises, we provided appropriate cardiopulmonary training to our participants in the preoperative phase, resulting in improved ventilatory efficiency and physical status and reducing the individual risks of perioperative or postoperative complications. Overall, postoperative cardiopulmonary rehabilitation was performed to improve the patients' functional ability in the short term and to accelerate their physical recovery, especially cardiopulmonary function.
Limitations
This study must be viewed in light of some limitations. First, our study was a retrospective analysis. The results on the relationship between risk levels and PPCs in patients with lung cancer should be interpreted with caution. Conclusions on the direction of the relationships cannot be drawn. Second, the patients were randomly recruited from a single medical center in Southern Taiwan. Therefore, the results may be generalizable only to similar populations, although the distributions of patients according to lung cancer type and age were similar to the data from the national survey in Taiwan. Third, the diagnosis of postoperative complications may be different and subjective depending on the doctor or radiologist. Although we collected the data of each participant as comprehensively as possible, artificial errors in progression notes, discharge notes, or nursing records might exist. Forth, all the patients with lung cancer in this study presented with relatively normal FEV1, regardless of absolute or measured to predicted values. This finding was not surprising given that patients are suitable for lobectomy if FEV1 is >1.5L according to current guideline. However, given that our team has performed CPET-guided PRCR since 2017 with well-equipment, well-experienced physiatrist and allied-health staffs, all the patients in this study received pre-operative CPET which was against current guideline that CPET is recommended for those with either FEV1 or DLCO <80% predicted. Our results in this study should be interpreted carefully in different clinical settings.
Conclusion
In our study, patients with lung cancer undergoing VATS with different risk levels showed comparable prognosis and postoperative complications after undergoing exercise testing-guided perioperative cardiopulmonary rehabilitation. We highly recommend performing preoperative CPET if it could be performed in a controlled environment with continuous monitoring, appropriate equipment, and well-trained personnel, and starting perioperative cardiopulmonary rehabilitation as soon as possible in patients undergoing surgery for lung cancer. Future larger and randomized control studies are warranted to confirm the clinical effectiveness of exercise testing-guided perioperative cardiopulmonary rehabilitation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
This study was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital (Number: VGHKS17-CT11-11). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: S-HT and K-LL. Data curation: W-HC, E-KT, and J-HC. Methodology: W-HC and Y-JT. Resources: K-LL. Supervision: E-KT and K-LL. Writing—original draft: W-HC and S-HT. Writing—review and editing: Y-JT and G-BC. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the grant of Ministry of Science and Technology of Taiwan (R.O.C.), Grant Number: MOST 110-2314-B075B-005-MY2.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to all participants in this study. We sincerely acknowledge department of chest surgery of Kaohsiung Veterans General Hospital for their kindly patient referral and the help of statistical analysis from Professor Huih-Sien Lin of Foo-Ying University, Kaohsiung, Taiwan.
Abbreviations
NSCLC, non-small cell lung cancer; ESC, European Society of Cardiology; CPET, cardiopulmonary exercise testing; Peak VO2, peak oxygen consumption; VO2 at VT, VO2 at the ventilatory threshold; VE/VCO2 slope, slope of minute ventilation to carbon dioxide production; PPC, postoperative pulmonary complication; VATS, video-assisted thoracic surgery; IMT, inspiratory muscle training; ICU, intensive care unit; ETT, endotracheal intubation time; CTT, chest tube insertion time; CXR, plain chest radiography; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; MVV, maximum voluntary ventilation.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. (2007) 75:56–63.
3. Toumazis I, Bastani M, Han SS, Plevritis SK. Risk-based lung cancer screening: a systematic review. Lung Cancer. (2020) 147:154–86. doi: 10.1016/j.lungcan.2020.07.007
4. Goodyear SJ, Yow H, Saedon M, Shakespeare J, Hill CE, Watson D, et al. Risk stratification by pre-operative cardiopulmonary exercise testing improves outcomes following elective abdominal aortic aneurysm surgery: a cohort study. Perioper Med. (2013) 2:10. doi: 10.1186/2047-0525-2-10
5. Barakat HM, Shahin Y, McCollum PT, Chetter IC. Prediction of organ-specific complications following abdominal aortic aneurysm repair using cardiopulmonary exercise testing. Anaesthesia. (2015) 70:679–85. doi: 10.1111/anae.12986
6. Tolchard S, Angell J, Pyke M, Lewis S, Dodds N, Darweish A, et al. Cardiopulmonary reserve as determined by cardiopulmonary exercise testing correlates with length of stay and predicts complications after radical cystectomy. BJU Int. (2015) 115:554–61. doi: 10.1111/bju.12895
7. Mancuzo EV, Pereira RM, Sanches MD, Mancuzo AV. Pre-transplant aerobic capacity and prolonged hospitalization after liver transplantation. GE Port J Gastroenterol. (2015) 22:87–92. doi: 10.1016/j.jpge.2015.02.001
8. Kasivisvanathan R, Abbassi-Ghadi N, McLeod AD, Oliver A, Rao Baikady R, Jhanji S, et al. Cardiopulmonary exercise testing for predicting postoperative morbidity in patients undergoing hepatic resection surgery. HPB. (2015) 17:637–43. doi: 10.1111/hpb.12420
9. Choi H, Mazzone P. Preoperative evaluation of the patient with lung cancer being considered for lung resection. Curr Opin Anaesthesiol. (2015) 28:18–25. doi: 10.1097/ACO.0000000000000149
10. Bobbio A, Chetta A, Internullo E, Ampollini L, Carbognani P, Bettati S, et al. Exercise capacity assessment in patients undergoing lung resection. Eur J Cardiothorac Surg. (2009) 35:419–22. doi: 10.1016/j.ejcts.2008.11.004
11. Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. (2018) 39:1144–61. doi: 10.1093/eurheartj/ehw180
12. Kallianos A, Rapti A, Tsimpoukis S, Charpidou A, Dannos I, Kainis E, et al. Cardiopulmonary exercise testing (CPET) as preoperative test before lung resection. In Vivo. (2014) 28:1013–20.
13. Brunelli A, Belardinelli R, Pompili C, Xiume F, Refai M, Salati M, et al. Minute ventilation-to-carbon dioxide output (VE/VCO2) slope is the strongest predictor of respiratory complications and death after pulmonary resection. Ann Thorac Surg. (2012) 93:1802–6. doi: 10.1016/j.athoracsur.2012.03.022
14. Torchio R, Guglielmo M, Giardino R, Ardissone F, Ciacco C, Gulotta C, et al. Exercise ventilatory inefficiency and mortality in patients with chronic obstructive pulmonary disease undergoing surgery for non-small-cell lung cancer. Eur J Cardiothorac Surg. (2010) 38:14–9. doi: 10.1016/j.ejcts.2010.01.032
15. Stubblefield MD. Cancer rehabilitation. Semin Oncol. (2011) 38:386–93. doi: 10.1053/j.seminoncol.2011.03.008
16. Kumar K, Kirksey MA, Duong S, Wu CL. A review of opioid-sparing modalities in perioperative pain management: methods to decrease opioid use postoperatively. Anesth Analg. (2017) 125:1749–60. doi: 10.1213/ANE.0000000000002497
17. Piccioni F, Ragazzi R. Anesthesia and analgesia: how does the role of anesthetists changes in the ERAS program for VATS lobectomy. J Vis Surg. (2018) 4:9–9. doi: 10.21037/jovs.2017.12.11
18. Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J. (2009) 34:17–41. doi: 10.1183/09031936.00184308
19. Corra U, Mezzani A, Bosimini E, Giannuzzi P. Cardiopulmonary exercise testing and prognosis in chronic heart failure: a prognosticating algorithm for the individual patient. Chest. (2004) 126:942–50. doi: 10.1378/chest.126.3.942
20. Kasikcioglu E, Toker A, Tanju S, Arzuman P, Kayserilioglu A, Dilege S, et al. Oxygen uptake kinetics during cardiopulmonary exercise testing and postoperative complications in patients with lung cancer. Lung Cancer. (2009) 66:85–8. doi: 10.1016/j.lungcan.2008.12.024
21. Brunelli A, Belardinelli R, Refai M, Salati M, Socci L, Pompili C, et al. Peak oxygen consumption during cardiopulmonary exercise test improves risk stratification in candidates to major lung resection. Chest. (2009) 135:1260–7. doi: 10.1378/chest.08-2059
22. Fang Y, Ma G, Lou N, Liao W, Wang D. Preoperative maximal oxygen uptake and exercise-induced changes in pulse oximetry predict early postoperative respiratory complications in lung cancer patients. Scand J Surg. (2014) 103:201–8. doi: 10.1177/1457496913509235
23. Licker M, Schnyder JM, Frey JG, Diaper J, Cartier V, Inan C, et al. Impact of aerobic exercise capacity and procedure-related factors in lung cancer surgery. Eur Respir J. (2011) 37:1189–98. doi: 10.1183/09031936.00069910
24. Loewen GM, Watson D, Kohman L, Herndon JE, Shennib H, Kernstine K, et al. Preoperative exercise Vo2 measurement for lung resection candidates: results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol. (2007) 2:619–25. doi: 10.1097/JTO.0b013e318074bba7
25. Mao YS, He J, Yan SP, Dong JS, Cheng GY, Sun KL, et al. Cardiopulmonary exercise testing in the evaluation of high risk patients with lung cancer. Chin Med J. (2010) 123:3089–94.
26. Villani F, Busia A. Preoperative evaluation of patients submitted to pneumonectomy for lung carcinoma: role of exercise testing. Tumori. (2004) 90:405–9. doi: 10.1177/030089160409000408
27. Win T, Jackson A, Sharples L, Groves AM, Wells FC, Ritchie AJ, et al. Cardiopulmonary exercise tests and lung cancer surgical outcome. Chest. (2005) 127:1159–65. doi: 10.1016/S0012-3692(15)34462-7
28. Nagamatsu Y, Terazaki Y, Muta F, Yamana H, Shirouzu K, Ishitake T. Expired gas analysis during exercise testing pre-pneumonectomy. Surg Today. (2005) 35:1021–5. doi: 10.1007/s00595-005-3078-4
29. Brat K, Tothova Z, Merta Z, Taskova A, Homolka P, Vasakova M, et al. Resting end-tidal carbon dioxide predicts respiratory complications in patients undergoing thoracic surgical procedures. Ann Thorac Surg. (2016) 102:1725–30. doi: 10.1016/j.athoracsur.2016.05.070
30. Miyazaki T, Callister MEJ, Franks K, Dinesh P, Nagayasu T, Brunelli A. Minute ventilation-to-carbon dioxide slope is associated with postoperative survival after anatomical lung resection. Lung Cancer. (2018) 125:218–22. doi: 10.1016/j.lungcan.2018.10.003
31. Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. (2013) 143:e166S−90. doi: 10.1378/chest.12-2395
32. Syrigos K, Kallianos A, Rapti A, Tsimpoukis S, Charpidou A, Ntanos I, et al. Cardiopulmonary exercise testing (CPET) as preoperative test before lung resection. World Allergy Organ J. (2015) 8:A22. doi: 10.1186/1939-4551-8-S1-A22
33. Pritchard A, Burns P, Correia J, Jamieson P, Moxon P, Purvis J, et al. ARTP statement on cardiopulmonary exercise testing 2021. BMJ Open Respir Res. (2021) 8:e001121. doi: 10.1136/bmjresp-2021-001121
34. Levett DZH, Grocott MPW. Cardiopulmonary exercise testing, prehabilitation, and enhanced recovery after surgery (ERAS). Can J Anesth. (2015) 62:131–42. doi: 10.1007/s12630-014-0307-6
35. Levett DZH, Jack S, Swart M, Carlisle J, Wilson J, Snowden C, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. (2018) 120:484–500. doi: 10.1016/j.bja.2017.10.020
36. Pele I, Mihălţan F-D. Cardiopulmonary exercise testing in thoracic surgery. Pneumologia. (2020) 69:3–10. doi: 10.2478/pneum-2020-0001
37. Zahid A, Yip K, Pritchard A, Morgan A. Pre-operative uptake of cardiopulmonary exercise test (CPET) in lung cancer surgery in Wolverhampton. Lung Cancer. (2019) 127:S56–7. doi: 10.1016/S0169-5002(19)30180-1
38. Gao K, Yu P-m, Su J-h, He C-q, Liu L-x, Zhou Y-b, et al. Cardiopulmonary exercise testing screening and pre-operative pulmonary rehabilitation reduce postoperative complications and improve fast-track recovery after lung cancer surgery: a study for 342 cases. Thorac Cancer. (2015) 6:443–9. doi: 10.1111/1759-7714.12199
39. Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J. (2007) 83:675–82. doi: 10.1136/hrt.2007.121558
40. Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. (2017) 6:CD012020. doi: 10.1002/14651858.CD012020.pub2
41. Steffens D, Ismail H, Denehy L, Beckenkamp PR, Solomon M, Koh C, et al. Preoperative cardiopulmonary exercise test associated with postoperative outcomes in patients undergoing cancer surgery: a systematic review and meta-analyses. Ann Surg Oncol. (2021) 28:7120–46. doi: 10.1245/s10434-021-10251-3
Keywords: lung cancer, perioperative cardiopulmonary rehabilitation, cardiopulmonary exercise testing, video-assisted thoracic surgery, postoperative pulmonary complications
Citation: Chao W-H, Tuan S-H, Tang E-K, Tsai Y-J, Chung J-H, Chen G-B and Lin K-L (2022) Effectiveness of Perioperative Cardiopulmonary Rehabilitation in Patients With Lung Cancer Undergoing Video-Assisted Thoracic Surgery. Front. Med. 9:900165. doi: 10.3389/fmed.2022.900165
Received: 20 March 2022; Accepted: 23 May 2022;
Published: 15 June 2022.
Edited by:
Rodrigo Torres-Castro, University of Chile, ChileReviewed by:
Yajung Cheng, National Taiwan University, TaiwanMehmet Oğuzhan Özyurtkan, Medicana International Istanbul Hospital, Turkey
Copyright © 2022 Chao, Tuan, Tang, Tsai, Chung, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ko-Long Lin, a2xsaW52Z2hrc0BnbWFpbC5jb20=
†These authors have contributed equally to this work
 Wei-Hao Chao
Wei-Hao Chao Sheng-Hui Tuan
Sheng-Hui Tuan En-Kuei Tang5,6
En-Kuei Tang5,6 Yi-Ju Tsai
Yi-Ju Tsai Jing-Hui Chung
Jing-Hui Chung Guan-Bo Chen
Guan-Bo Chen Ko-Long Lin
Ko-Long Lin