- 1Department of Internal Medicine, Division of Nephrology, Pikeville Medical Center, Pikeville, KY, United States
- 2Department of Internal Medicine, Pikeville Medical Center, Pikeville, KY, United States
Nephrotoxicity is one of the major limiting factors for vancomycin use. The most common histological patterns of kidney injury are acute tubulointerstitial nephritis and acute tubular necrosis. Patients who develop acute tubulointerstitial nephritis are prone to develop acute kidney injury with vancomycin rechallenge and, in most cases, present alone or as a part of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). The purpose of the review study is to identify biopsy-proven vancomycin-associated-tubulointerstitial nephritis in literature, determine possible underlying pathophysiology and identify the consequences of vancomycin rechallenge in such patients.
Introduction
Vancomycin, famously known as “Mississippi Mud,” was first approved for use by the Food and Drug Administration in 1958 (1). It is the antibiotic of choice for gram-positive infections, mainly methicillin-resistant Staphylococcus aureus (MRSA). Despite significant nephrotoxicity associated with vancomycin, it still has widespread use, being the drug of choice in the emergency department/acute setting for initial broader coverage. Vancomycin-associated tubulointerstitial nephritis (VAIN) is a recognizable histopathological pattern observed in patients receiving vancomycin. We present a patient with biopsy proven vancomycin-associated tubulointerstitial nephritis who was re-introduced to the drug after a year of developing maculopapular rash and mild acute kidney injury. We identified 14 case reports with VAIN, nine of which were biopsy-proven. We found that the kidney injury was self-limiting and improved by discontinuing vancomycin; however, steroids were used in some cases. Only three studies showed rapid recurrence of maculopapular rash and/or acute oligo-anuric kidney injury with vancomycin rechallenge.
Case Presentation
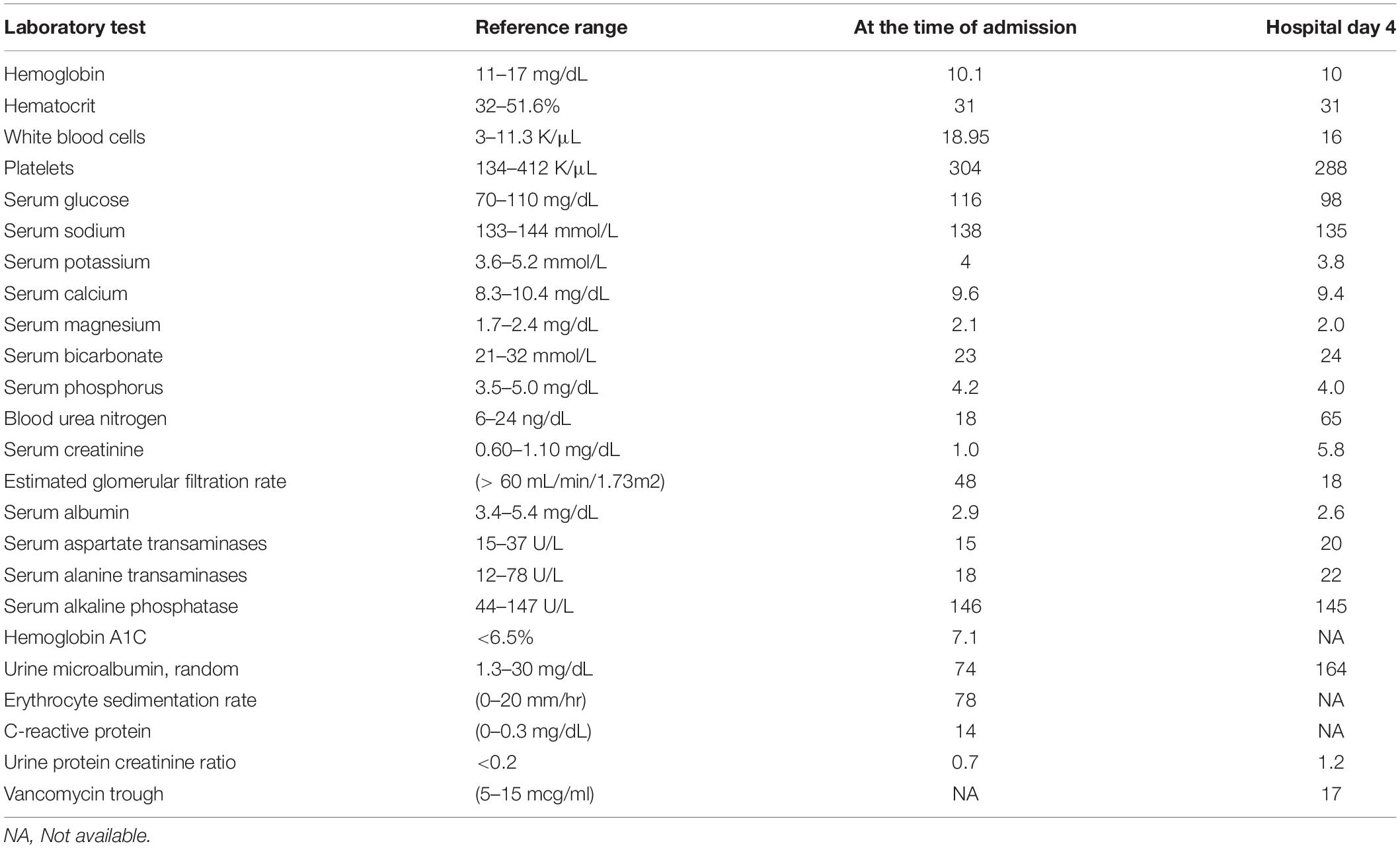
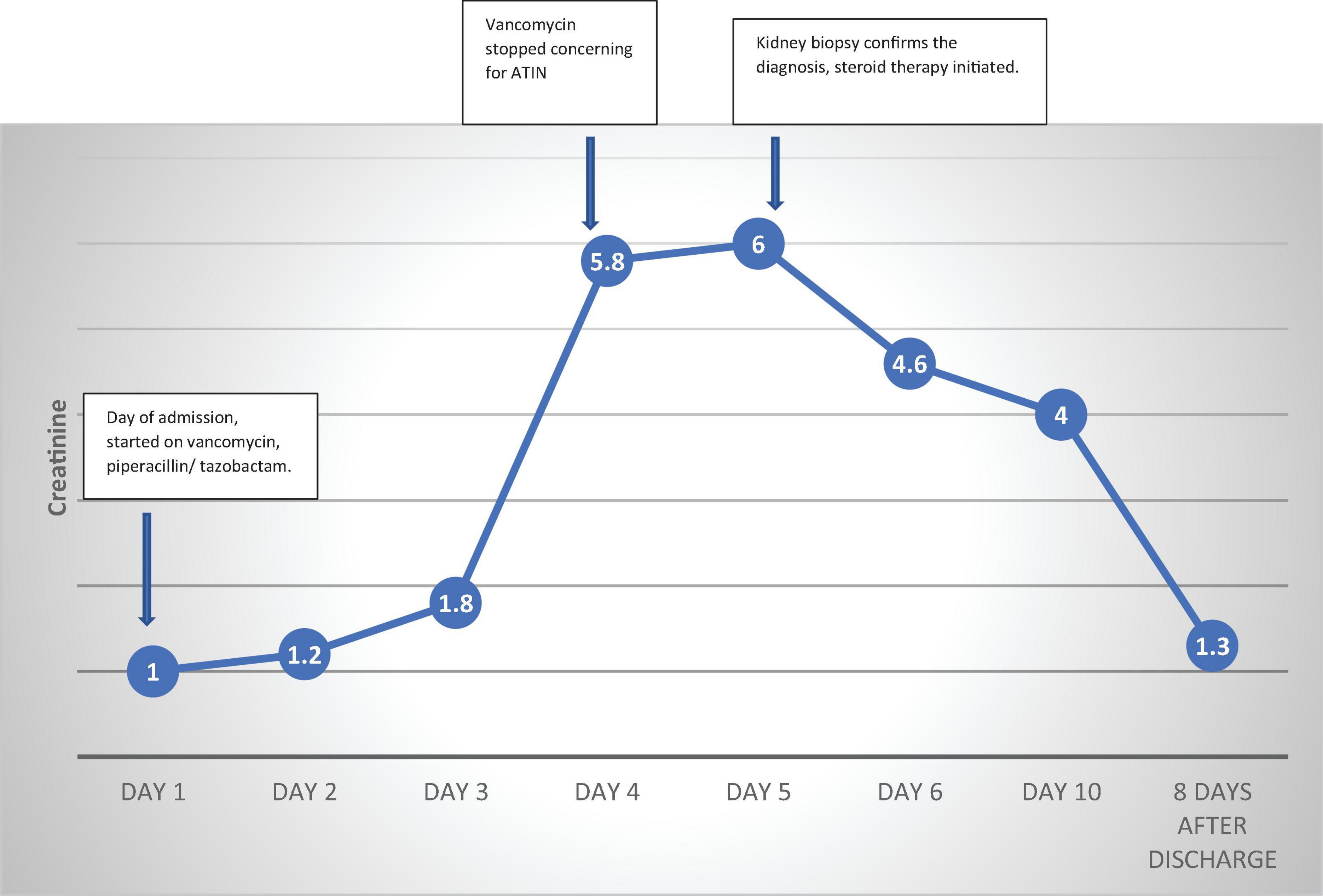
We present the case of a 56-year-old man with hypertension, poorly controlled type II diabetes mellitus (DM) and hyperlipidemia who presented to the Emergency Room with generalized fatigue, fever and an open draining ulcer on his right heel. Laboratory work up showed elevated white blood cell count of 18.95 K/μL (3–11.3 K/μL), initial blood urea nitrogen of 18 mg/dL (6–24 mg/dL) and creatinine of 1.0 mg/dL (0.6–1.10 mg/dL) and rest of the blood work as shown in Table 1. His blood pressure was controlled with lisinopril 40 mg daily, hyperlipidemia with atorvastatin 40mg daily and his DM with insulin therapy. The patient was started on initial broad-spectrum antibiotics with vancomycin 1 g every 12 h (70-kilogram male) and piperacillin- tazobactam 4.5 g every 8 h after drawing blood cultures. On Hospital Day 1, his blood culture was positive for gram positive cocci, so Piperacillin was stopped. Two days later, he developed pitting pedal edema, reduced urine output (250 ml/day) and serum creatinine increased up to 5.8 mg/dL as shown in Table 1 and creatinine trend shown in Figure 1. His vancomycin trough was 17 mcg/ml. Urinalysis showed moderate hematuria with 10–20 red blood cells and 3 + protein. Microscopic examination of the sediment showed a single white blood cell cast. Given concern for acute tubulointerstitial nephritis, vancomycin was immediately stopped, kidney biopsy was obtained which confirmed the presence of eosinophils in the interstitium and interstitial cells within the tubules as shown in Figure 2. After this event, his previous hospital records were reviewed and it was discovered that the patient was hospitalized a year back with a diabetic foot ulcer and at that time, he had received vancomycin which resulted in him developing maculopapular rash and mild acute kidney injury. Vancomycin was stopped due to concern for an allergic reaction and his symptoms had resolved with a short course of prednisone (500 mg of intravenous methylprednisolone for 3 days followed by oral prednisone at 1 mg/kg tapered over 14 days).
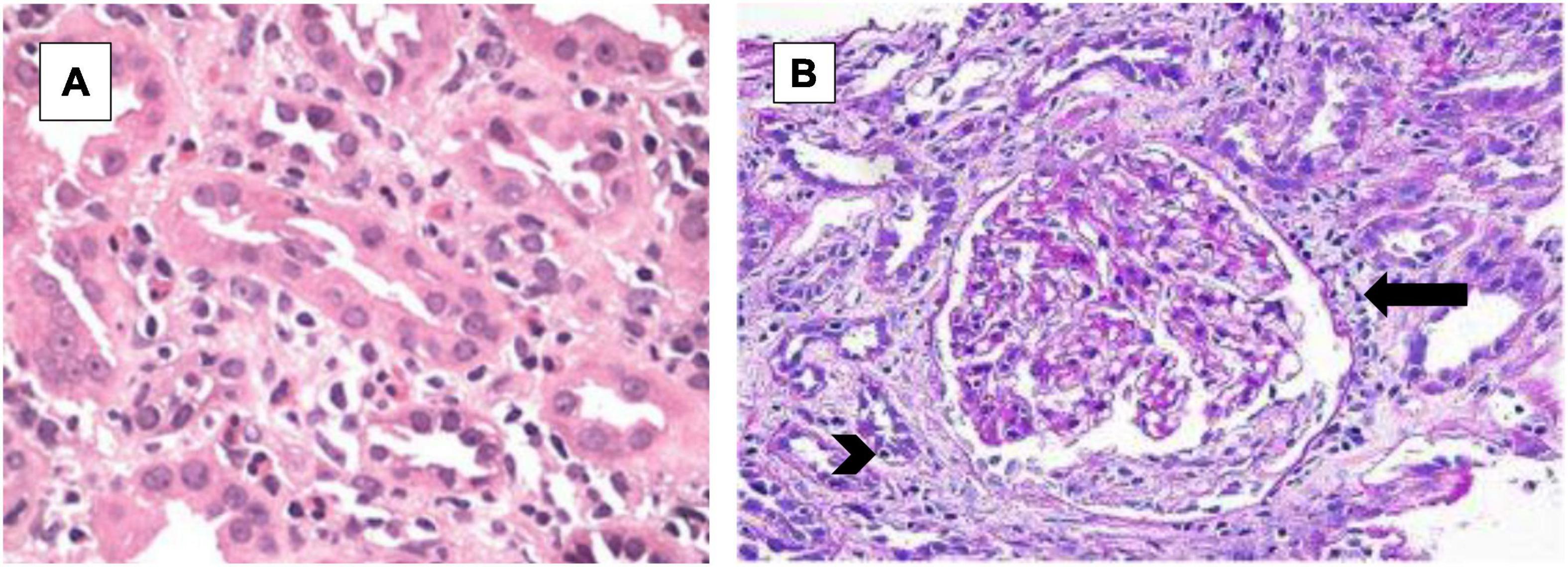
Figure 2. Hematoxylin and eosin (H&E) stain. (A) Low power- diffuse cellular infiltrate within the interstitium with inflammatory cells including eosinophils and lymphocytes. (B) High power- the tissue sample shows the presence of inflammatory cell infiltrate within the tubular wall (arrowhead) and numerous eosinophils in the interstitium (arrow).
Discussion
One of the limiting factors of vancomycin use is kidney injury. Vancomycin related acute kidney injury (AKI) is defined as at least two or three consecutive elevations in serum creatinine by 0.5 mg/dl or at least a 50% increase from the baseline, whichever is greater; the increase must be documented after several days of vancomycin therapy, and no alternative explanation for the impairment in glomerular filtration rate is identified (2).
Vancomycin can cause varied immune-mediated reactions or hypersensitivity reactions (HSR)-immediate, type I IgE mediated HSR, type II antibody-mediated HSR, and type IV cell-mediated delayed HSR. These HSR include Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), IgA bullous dermatosis, and Steven-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN). Acute tubulointerstitial nephritis (AIN) is a part of type II antibody-mediated HSR.
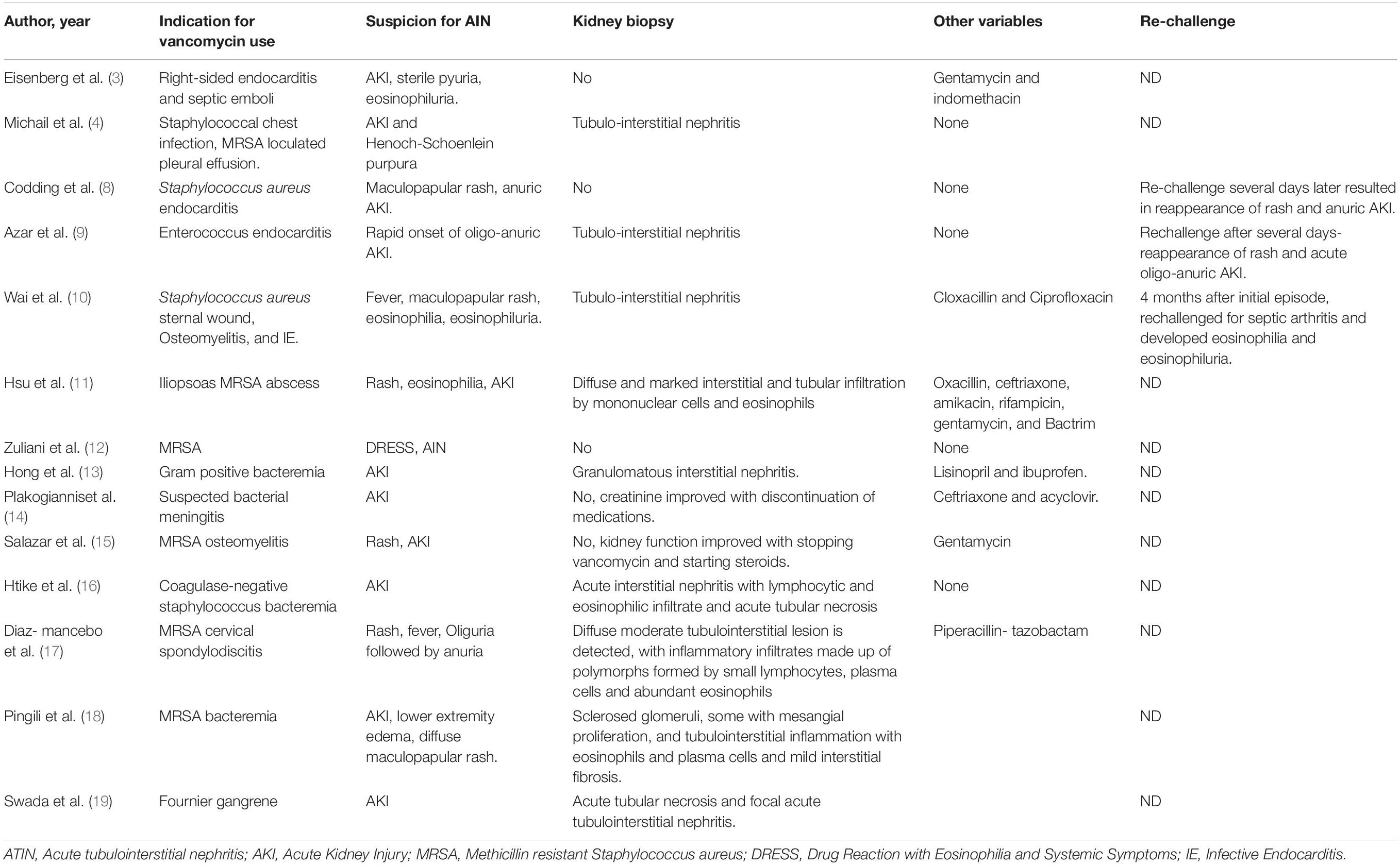
The first case of vancomycin-associated tubulointerstitial nephritis was published in 1981 (3), but it was not until 1988 that the first biopsy-proven acute tubulointerstitial nephritis was described (4) as shown in Table 2. Histologically, vancomycin-associated tubulointerstitial nephritis is identified by the presence of features like tubulitis (57.9%), followed by eosinophilic infiltration (42.1%), epithelial sloughing (42.1%), tubular casts (42.1%), and interstitial fibrosis (36.8%) (5).
Another histological pattern seen with vancomycin is ATN- Animal studies demonstrated that vancomycin leads to tubular damage and interstitial inflammation related to oxidative stress in the proximal tubular cells and vasoconstriction by free oxygen radicals (6). It can also alter mitochondrial function. Oxidative phosphorylation by vancomycin induces free oxygen radicals, thereby reducing the activity of defensive antioxidative enzymes, including superoxide dismutase and catalases. Vancomycin superoxide production causes mitochondrial membrane potential depolarization with the release of cytochrome C and subsequent activation of caspases 9 and 3, leading to apoptotic cell death.
Vancomycin, in addition, causes immunological injury by acting as an exogenous antigen that can be directly trapped within the interstitium or circulate as an immune complex that gets deposited subsequently within the interstitium. Alternatively, it can bind to tubular antigens and act as haptens or perform molecular mimicry of the tubules and/or interstitium. Both cell-mediated and humoral immunity can be involved in the process, but it is initiated when an antigen-presenting lymphocyte is activated. This results in the formation of activated T-cells that promote differentiation and proliferation of other T-cells resulting in cytokine and chemokine production, including TGF-β, PDGF-β, EGF-β, and fibroblast growth factor-2. Over time, tubulointerstitial inflammation and edema transition to fibrosis and tubular loss (7).
In the case of vancomycin, the clinical presentation is quite variable. It comes to attention when there is an unexplained rise in serum creatinine after exposure to vancomycin. Nevertheless, the clinical picture is often complicated by multiple processes ongoing during hospitalization or otherwise, such as systemic infection, bouts of hemodynamic instability, and concomitant use of other nephrotoxic medications. Most often, the suspicion for vancomycin-associated tubulointerstitial nephritis is low as symptoms are non-specific and the classic triad of fever, rash, and eosinophilia suggestive of hypersensitivity are present only in a minority of patients (> 5–10%) (7).
Objectively, there is no one laboratory test that can be used to diagnose VAIN. Most commonly observed laboratory abnormalities are eosinophilia, increased serum IgE levels, elevated erythrocyte sedimentation rate, and anemia. Urinalysis can show hematuria, pyuria, and proteinuria on dipstick testing. Urine eosinophils are neither sensitive nor specific for the diagnosis of VAIN as it is seen in cystitis or prostatitis, pyelonephritis, atheroembolic disease, acute tubular necrosis, and rapidly progressive glomerulonephritis. White blood cell casts seen on urine microscopy in a patient with AKI without pyelonephritis are highly suggestive of VAIN.
Since vancomycin-associated tubulointerstitial nephritis represents a hypersensitivity reaction, sometimes with systemic symptoms and dermatological manifestations, a kidney biopsy can provide diagnostic information regarding exact etiology, decision toward discontinuation of the drug, corticosteroid administration, and prediction of long-term prognosis. Nonetheless, it is often difficult to establish causality due to confounders/risk factors as seen from the literature review (including presence of other antibiotics, non-steroidal anti-inflammatory drugs, severe sepsis) and biopsy timing in relation to the use of vancomycin. In patients who cannot undergo kidney biopsy, scanning with 67Ga scintigraphy or positron emission tomography scanning may be utilized to differentiate VAIN from acute tubular necrosis (ATN) (20).
Conclusion
Vancomycin-associated tubulointerstitial nephritis is presumed to be more frequent than we encounter in clinical practice, and hence the reported literature has been regarded as the tip of the iceberg. Survival analysis has linked the presence of acute tubulointerstitial nephritis to a worse prognosis. Hence, performing a timely kidney biopsy is paramount to diagnosing and treating tubulointerstitial nephritis. Furthermore, for biopsy-proven AIN, future rechallenges with vancomycin should be avoided, and such patients should be listed as having had an adverse reaction to vancomycin.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LK: conception, design of the study, review of literature, and analysis of data. RR: drafting of the manuscript and review of the final manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge the education department at Pikeville Medical Center, Pikeville, KY, United States, for providing financial support for the publication of this manuscript.
References
2. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. (2009) 66:82–98. doi: 10.2146/ajhp080434
3. Eisenberg ES, Robbins N, Lenci M. Vancomycin and Interstitial Nephritis. Ann Intern Med. (1981) 95:658. doi: 10.7326/0003-4819-95-5-658_1
4. Michail S, Vaiopoulos G, Nakopoulou L, Revenas C, Aroni K, Karam P, et al. Henoch-schoenlein purpura and acute interstitial nephritis after intravenous vancomycin administration in a patient with a staphylococcal infection: CASE REPORT. Scand J Rheumatol. (1998) 27:233–5. doi: 10.1080/030097498440886
5. Bellos I, Pergialiotis V, Perrea DN. Kidney biopsy findings in vancomycin-induced acute kidney injury: a pooled analysis. Int Urol Nephrol. (2022) 54:137–48. doi: 10.1007/s11255-021-02831-9
6. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. (2016) 7:136–47. doi: 10.1177/2042018816638223
7. Perazella MA. AKI in a Hospitalized Patient with Cellulitis. Clin J Am Soc Nephrol. (2013) 8:658–64. doi: 10.2215/CJN.09370912
8. Codding CE, Ramseyer L, Allon M, Pitha J, Rodriguez M. Tubulointerstitial nephritis due to vancomycin. Am J Kidney Dis. (1989) 14:512–5. doi: 10.1016/S0272-6386(89)80152-0
9. Azar R, Bakhache E, Boldron A. [Acute interstitial nephropathy induced by vancomycin]. Nephrologie. (1996) 17:327–8.
10. Wai AO, Lo AM, Abdo A, Marra F. Vancomycin-Induced Acute Interstitial Nephritis. Ann Pharmacother. (1998) 32:1160–4. doi: 10.1345/aph.17448
11. Hong Hsu SI. Biopsy-Proved Acute Tubulointerstitial Nephritis and Toxic Epidermal Necrolysis Associated with Vancomycin. Pharmacotherapy. (2001) 21:1233–9. doi: 10.1592/phco.21.15.1233.33901
12. Zuliani E, Zwahlen H, Gilliet F, Marone C. Vancomycin-induced hypersensitivity reaction with acute renal failure: resolution following cyclosporine treatment. Clin Nephrol. (2005) 64:155–8. doi: 10.5414/CNP64155
13. Hong S, Valderrama E, Mattana J, Shah HH, Wagner JD, Esposito M, et al. Vancomycin-induced acute granulomatous interstitial nephritis: therapeutic options. Am J Med Sci. (2007) 334:296–300. doi: 10.1097/MAJ.0b013e3180a6ec1e
14. Plakogiannis R, Nogid A. Acute interstitial nephritis associated with coadministration of vancomycin and ceftriaxone: case series and review of the literature. Pharmacotherapy. (2007) 27:1456–61. doi: 10.1592/phco.27.10.1456
15. Salazar MN, Matthews M, Posadas A, Ehsan M, Graeber C. Biopsy proven interstitial nephritis following treatment with vancomycin: a case report. Conn Med. (2010) 74:139–41.
16. Htike NL, Santoro J, Gilbert B, Elfenbein IB, Teehan G. Biopsy-proven vancomycin-associated interstitial nephritis and acute tubular necrosis. Clin Exp Nephrol. (2012) 16:320–4. doi: 10.1007/s10157-011-0559-1
17. Díaz-Mancebo R, Costero-Fernández O, Vega-Cabrera C, Olea-Tejero T, Yébenes L, Picazo ML, et al. Dress syndrome and acute tubulointerstitial nephritis after treatment with vancomycin and beta-lactams. Case report and literature review. Nefrologia. (2012) 32:685–7. doi: 10.3265/Nefrologia.pre2012.Jun.11455
18. Pingili CS, Okon EE. Vancomycin-induced leukocytoclastic vasculitis and acute renal failure due to tubulointerstitial nephritis. Am J Case Rep. (2017) 18:1024–7. doi: 10.12659/AJCR.905214
19. Sawada A, Kawanishi K, Morikawa S, Nakano T, Kodama M, Mitobe M, et al. Biopsy-proven vancomycin-induced acute kidney injury: a case report and literature review. BMC Nephrol. (2018) 19:72. doi: 10.1186/s12882-018-0845-1
Keywords: vancomycin, acute interstitial nephritis, nephrotoxicity, MRSA, kidney biopsy
Citation: Kannan L and Raj R (2022) Case Report: Vancomycin-Associated Tubulointerstitial Nephritis in Clinical Practice-Case Report and Review of Literature. Front. Med. 9:899886. doi: 10.3389/fmed.2022.899886
Received: 19 March 2022; Accepted: 22 April 2022;
Published: 31 May 2022.
Edited by:
Sree Bhushan Raju, Nizam’s Institute of Medical Sciences, IndiaReviewed by:
Mark Anthony Perazella, Yale University, United StatesAnushree Shirali, Yale University, United States
Copyright © 2022 Kannan and Raj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lakshmi Kannan, TGFrc2htaS5rYW5uYW5AcGlrZXZpbGxlaG9zcGl0YWwub3Jn
†ORCID: Lakshmi Kannan, orcid.org/0000-0001-8306-6723; Rishi Raj, orcid.org/0000-0002-4151-3246
 Lakshmi Kannan
Lakshmi Kannan Rishi Raj
Rishi Raj