
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 04 July 2022
Sec. Pathology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.898483
This article is part of the Research TopicBig Data Research in Otorhinolaryngology – Head and Neck DiseasesView all 4 articles
 Wendu Pang1†
Wendu Pang1† Yaxin Luo2†
Yaxin Luo2† Junhong Li1†
Junhong Li1† Danni Cheng1
Danni Cheng1 Yufang Rao1
Yufang Rao1 Minzi Mao1
Minzi Mao1 Ke Qiu1
Ke Qiu1 Yijun Dong1
Yijun Dong1 Jun Liu1
Jun Liu1 Jian Zou1
Jian Zou1 Haiyang Wang1*
Haiyang Wang1* Fei Chen1*
Fei Chen1*Background: The current American Joint Committee on Cancer (AJCC) system only considered the importance of the size and laterality of lymph nodes while not the positive lymph node number (PLNN) for hypopharyngeal squamous cell carcinoma (HPSCC).
Methods: A total of 973 patients with HPSCC from the Surveillance, Epidemiology, and End Results database (2004–2015) were identified. Univariate and multivariate Cox regression analyses were used to evaluate the prognostic effects. We applied six Cox regression models to compare the survival prognostic values of PLNN and AJCC systems.
Results: Positive lymph node number showed a significant association with overall survival (OS) and cancer-specific survival (CSS) (P < 0.001) in univariate and multivariable analyses. The increased PLNN of HPSCC gave rise to poor OS and CSS. The survival model incorporating a composite of PLNN and TNM classification (C-index for OS:0.682, C-index for CSS:0.702) performed better than other models.
Conclusions: A positive lymph node number could serve as a survival predictor for patients with HPSCC and a complement to enhance the prognostic assessment effects of TNM cancer staging systems.
Hypopharyngeal squamous cell carcinoma (HPSCC) is one of the most malignant head and neck squamous cell carcinomas (HNSCC), accounting for 5–15% of HNSCC, with a 5-year survival rate of <40% (1). As early symptoms are relatively insidious, tumors often progress to advanced stages when discovered (2). Lymph node metastasis (LNM) is closely related to poor prognosis (3). However, the current American Joint Committee on Cancer (AJCC) staging system only placed weight on the size and laterality of lymph nodes (LNs) for HPSCC, while not considering positive lymph node number (PLNN). Several studies have suggested that PLNN is a clinicopathological risk factor and potential prognostic determinant for patients with thyroid cancer (4), nasopharyngeal carcinoma (5), and oral cavity squamous cell carcinoma (6), while quantification of the prognostic effects of PLNN has not been adequately performed by a substantial HPSCC cohort.
Therefore, we hypothesized that PLNN could serve as a supplement to the AJCC tumor, lymph node, and metastasis (TNM) staging system of HPSCC to assist in better treatment guidance. In this study, we investigated the prognostic effect of PLNN on patients with HPSCC, developed several survival prediction models based on PLNN, and compared their prognostic prediction values to the 6th and 7th AJCC cancer staging systems.
Patient data between 2004 and 2015 (year of diagnosis) were obtained from the Surveillance, Epidemiology, and End Results (SEER) database, an authoritative data source of the National Cancer Institute (7) via the SEER Stat software (https://seer.cancer.gov). This study was approved by our institutional review board (No. 2019.357), and patient consent was not applicable as SEER data are publicly available.
Pathologic diagnosis of HPSCC was based on the primary site using the International Classification of Diseases for Oncology, third edition, including the codes C129, C130, C131, C132, C138, and C139. Patients with the first primary cancer of HPSCC in the SEER database between 2004 and 2015 were identified (n = 12,136, Figure 1). The inclusion criteria were as follows: (1) histology of squamous cell carcinoma, including malignancy and carcinoma in situ, (2) HPSCC was reported as the first primary tumor, and (3) patients underwent neck dissection (8). Patients lacking the number of regional LNs or lacking complete information on clinical and pathological characteristics (such as primary site, marital status, age, chemotherapy, and radiation sequence with surgery, which were significant survival predictors proved by univariate analyses) were excluded from this study. After the exclusion of ineligible patients, the remaining HPSCC cases (n = 973) were divided into two cohorts: (1) training cohort, consisting of patients with complete TNM staging data (n = 465); (2) validation cohort, consisting of the remaining 508 patients without TNM stage information, to verify the prognostic ability of the training model.
Basic demographic information such as gender, age, marital status, tumor primary site, T classification, N classification, M classification, cancer stage, tumor grade, and treatment modalities was considered. PLNN was recorded as the exact number of regional nodes examined by a pathologist, who confirmed tumor infiltration. Owing to the similarity of the sixth and seventh AJCC cancer staging systems for patients with HPSCC in the SEER database, we applied both editions to define the TNM classifications and cancer stages (the sixth and seventh AJCC editions included patients from 2004 to 2009 and 2010 to 2015, respectively).
Continuous variables were presented as the median and maximum/minimum value and categorical variables were presented as frequencies and percentages. We conducted univariate Cox regression analyses using the Wald test to identify the confounding variables. The backward selection algorithm was used for model selection, and non-significant factors were excluded from further multivariable analyses. Multivariable Cox proportional hazards regression analyses were conducted to calculate the hazard ratio (HR) and 95% CI (Confidence Interval), which were used to analyze whether the PLNN could serve as a potential factor in predicting overall survival (OS) and cause-specific survival (CSS). OS was defined from the date of diagnosis to the date of death or last follow-up, with death as an event. Due to the fact that cancer patients may die from complications or other unexpected reasons, we applied CSS as the survival outcome which was defined as the date of diagnosis to the date of death due to HPSCC.
The cut-offs for PLNN were determined by comparing the HR of exact positive node numbers (1, 2, 3, 4, 5, 6, 7, and >7) after adjusting for gender, age, race, and marital status. Six models were built based on different combinations of PLNNs, cancer stages, and T, N, and M classifications. The concordance index (C-index) was processed to evaluate and compare the performance of each model with a value ranging from 0 to 1, where 0.5 corresponds to random chance and 1 corresponds to perfect discriminative ability.
All analyses were performed using R 3.6.3 (R Development Core Team, Vienna, Austria), and P < 0.05 were considered statistically significant.
Characteristics of patients with HPSCC after the exclusion, including the training cohort (n = 465) and validation cohort (n = 508), were summarized in Table 1 and Supplementary Table 1.
In univariate analyses (Supplementary Table 2), patients who had more advanced TNM stages or more PLNN, with tumor size >2 cm or primary tumor site located in overlapping lesions of hypopharynx had a poor prognosis (HR > 1, p < 0.01). The primary site, age, marital status, the primary site of surgery, chemotherapy, and radiation/surgery sequence were further selected (backward selection) for multivariable Cox regression analyses.
Our multivariate analysis revealed that increased PLNN was independently associated with decreased OS and CSS (Table 2). When the PLNN increased from 5 to 6, the HR of OS dramatically increased from 1.14 to 2.34 (95% CI: 1.75–3.14), therefore, we divided PLNN into three groups (PLNN 0, PLNN 1–5, and PLNN > 5) for further analysis. The survival curves of patients in the PLNN 0, PLNN 1–5, and PLNN > 5 groups showed significant differences, demonstrating the good discriminatory ability of the PLNN cut-offs (Figure 2, Supplementary Table 3), and consistent trends were also observed in the subgroups of age and gender (Supplementary Figure 1). Compared to HPSCC patients without LN metastases, patients whose PLNNs were between one and five (PLNN 1–5, HR for OS: 1.27, 95% CI: 1.09–1.47, p = 0.002; HR for CSS: 1.46, 95% CI: 1.2–1.78, p < 0.001) and PLNNs were over five (PLNN > 5, HR for OS: 2.29; 95% CI: 1.92–2.74, p < 0.001; HR for CSS: 3, 95% CI: 2.41–3.75, p < 0.001) had a higher risk of death. Patient characteristics shown by different PLNN cut-offs are presented in Supplementary Table 1.
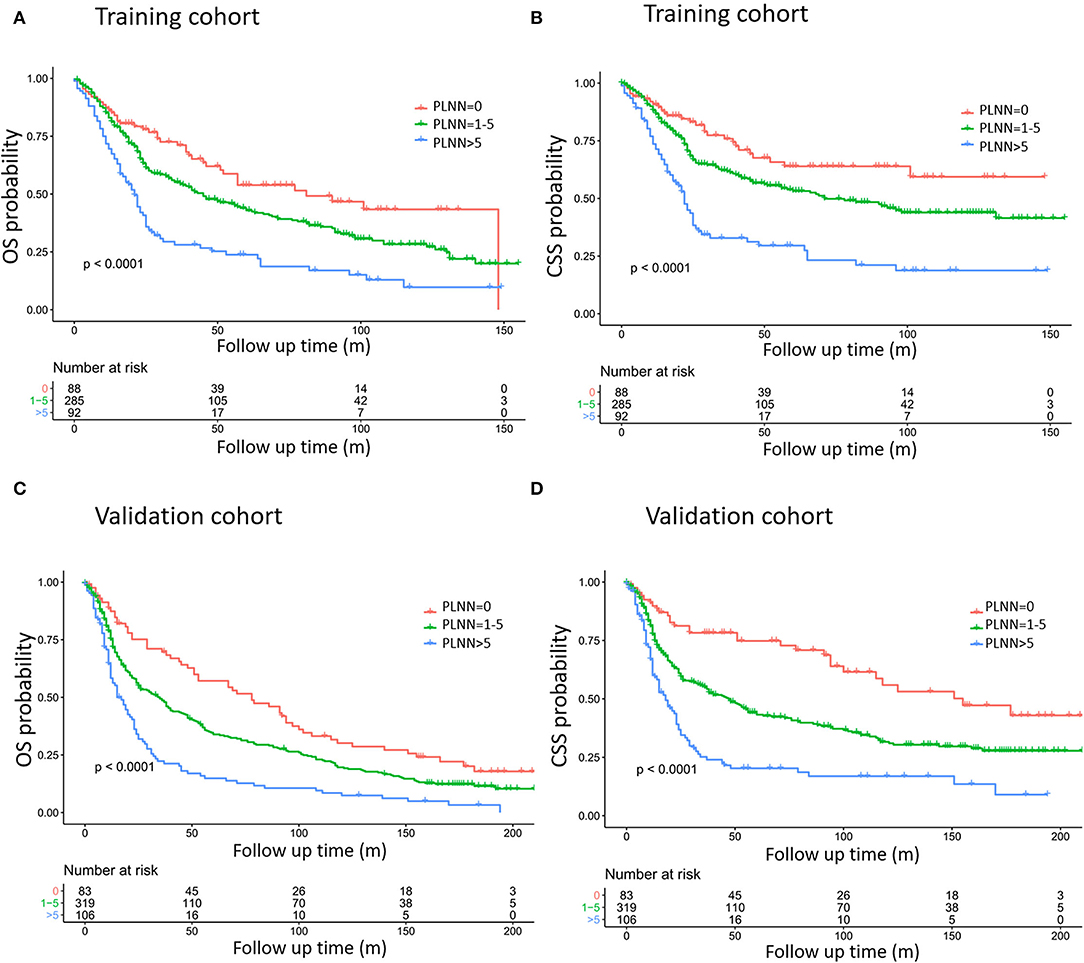
Figure 2. Kaplan–Meier curves estimating overall survival (OS) (A,C) and cause-specific survival (CSS) (B,D) based on training cohort (A,B) and validation cohort (C,D) definition of training cohort and validation cohort referred to Table 1. The exact data of survival rates are referred to in Table 2.
We further conducted six Cox regression models based on different combinations of PLNN (with cut-off values of 1 and 5), T classification (T1–T4), N classification (N0–N3), M classification (M0–M1), and cancer stage (Stage I–IV) to predict survival outcomes. Model 1 (PLNN), model 2 (cancer stages), and model 3 (TNM) were established solely based on PLNN, cancer stage, and TNM classification, respectively. Model 4 (PLNN + cancer stages) was developed by integrating PLNN and cancer stages; model 5 (PLNN + T + M) incorporated PLNN, T, and M classifications, and model 6 (PLNN + TNM) combined PLNN with TNM classification.
As shown in Table 3, the model of PLNN (model 1, C-index for OS:0.664; C-index for CSS:0.677) showed a comparable prognostic effect when compared with the models utilizing cancer stages (model 2, C-index for OS:0.658; C-index for CSS:0.672) or TNM classification (model 3, C-index for OS:0.674; C-index for CSS:0.691), indicating that PLNN was a reasonable prognostic factor. Additionally, the prognostic prediction effects of PLNN gradually improved when incorporating with cancer stages (model 4, C-index for OS:0.672; C-index for CSS:0.693), TM classifications (model 5, C-index for OS:0.681; C-index for CSS:0.697), or TNM classifications (model 6, C-index for OS:0.682; C-index for CSS:0.702), suggesting that PLNN could serve as a surrogate supplement for TNM classification and cancer stage.
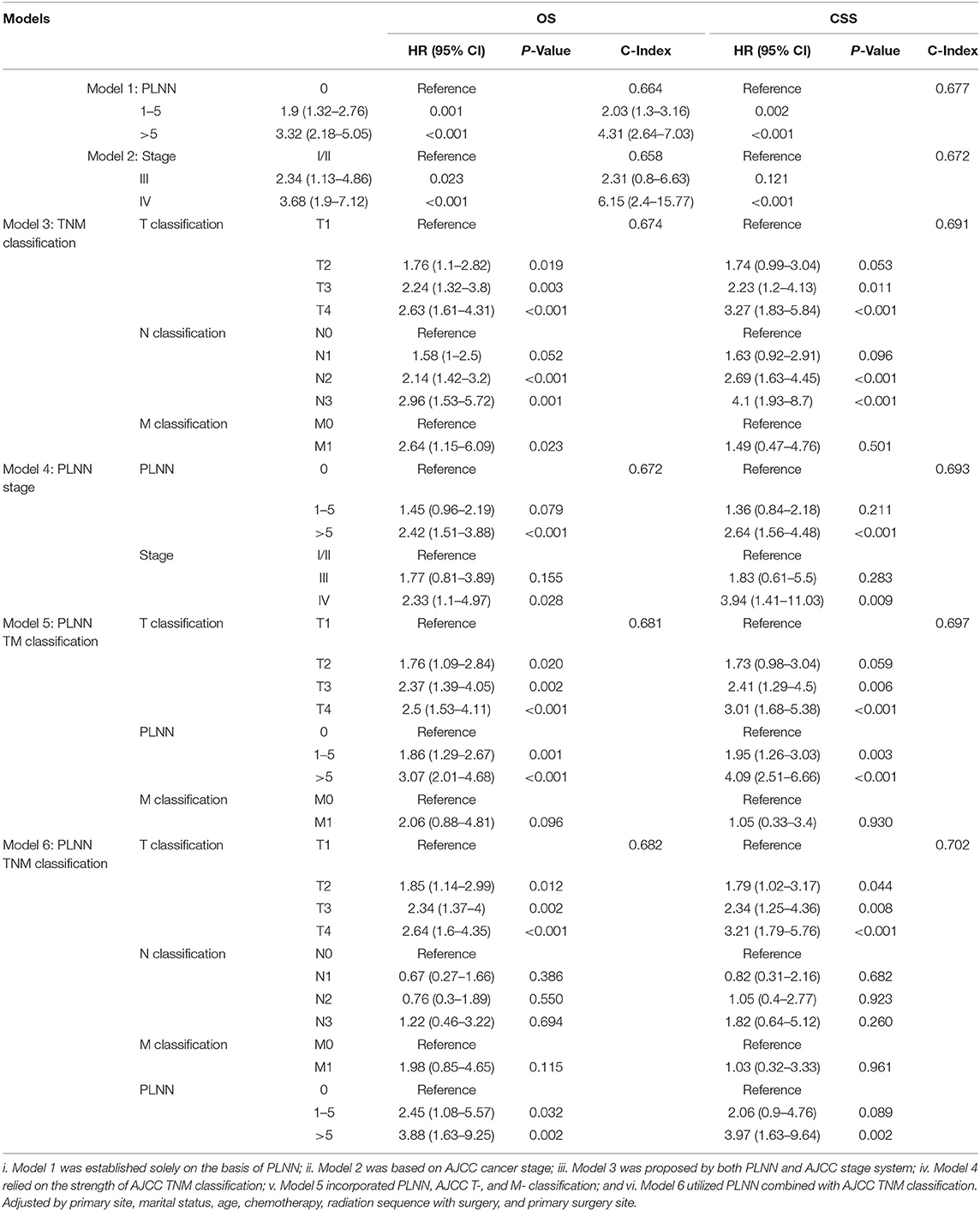
Table 3. Comparison of six survival predicting models based on different permutations and combinations of PLNN (with the cut-off value of 1 and 5), AJCC T- (T1–T4), N- (N0–N3), M- (M0–M1) classification and AJCC cancer stage systems (Stage I–IV).
We further conducted two subgroup analyses for survival outcomes by stratifying age and gender (Supplementary Figure 1). The generalizability of the survival prediction model was examined using a validation cohort. All models were adjusted for the primary site, marital status, age, chemotherapy, radiation/surgery sequence, and primary surgery site.
Consistent prognostic prediction performances of the models were observed in the validation cohort (C-index for OS, 0.638; C-index for CSS, 0.656; Table 4). As shown in Table 1, although some of the clinical factors demonstrated statistical differences between the training and validation cohorts, the overall model performance of the validation cohort was consistent with that of the training cohort (C-index: training cohort:0.664 for OS and 0.677 for CSS; validation cohort:0.638 for OS and 0.656 for CSS).
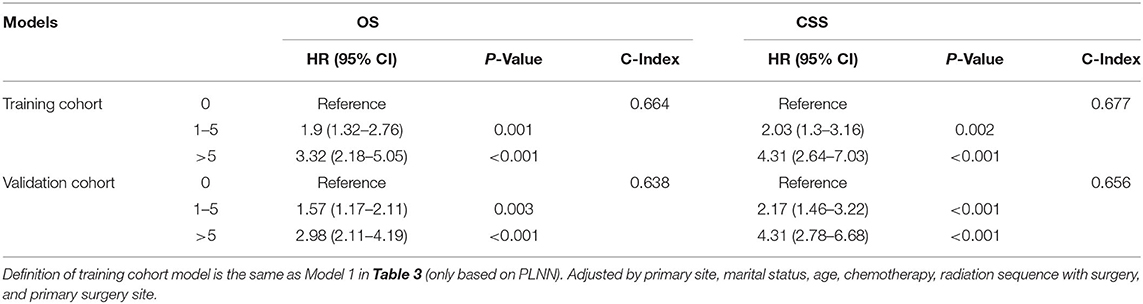
Table 4. Comparison of survival predicting model based on training cohort (N = 461) and validation cohort (N = 365).
Our current study showed the prognostic effects of PLNNs on patients with HPSCC. In continuous multivariable regression models, we observed that successive increasing PLNNs were associated with an increased risk of death, and when patients were classified by PLNN (PLNN 0; PLNN 1–5; PLNN > 5), significant prognostic differences were observed in both the training and validation cohorts. We further confirmed that PLNN was a superior supplement to enhance the prognostic prediction value of the current 6th and 7th AJCC staging system.
Due to early and diffuse submucosal infiltration along with an extensive lymphatic network, HPSCC has a higher rate of LN metastasis than other types of HNSCC, where more than 50% of patients present with positive LNs (9). It has been widely accepted that metastatic LNs are independent prognostic factors for the survival of HNSCC (10). The concept of LN ratio (LNR) or LN density was calculated as the number of positive LNs divided by the number of LNs harvested from neck dissection (11, 12). A recent meta-analysis (13) demonstrated that LNR is an important CSS predictor for patients with HPSCC along with LN metastasis. Retrospective studies demonstrated that patients with hypopharyngeal cancer had a high risk of retropharyngeal LN involvement, and frequently progressed to distant metastasis with dismal outcomes (14, 15).
Some previous studies (16–19) suggested that the prognostic value of PLNN could even surpass other clinical factors such as tumor size and the laterality of LN. However, the prognostic effect of PLNN on patients with HPSCC remains poorly understood. Despite the comprehensiveness of the AJCC TNM staging system that has considered the diameter and location of LNs and distant metastasis information, it did not consider the value of PLNN, which could provide a simpler, more precise, and reliable prognosis reference.
Besides, our results showed that the performance of the model 1–5, except for the model 6 (PLNN + TNM), was not excellent (c-index < 0.7). Consistently, previous studies showed similar inferior prognostic effects of 6th and 7th TNM staging systems on patients with hypopharyngeal cancer, indicating that a supplement for TNM staging system for patients with HPSCC is still necessary.
Although our study showed potential prognostic effects of PLNN on patients with HPSCC, a few limitations still exist. First, the SEER database has limited clinical and treatment information, which restricts our access to relevant data for clinical LN staging via ultrasound or computed tomography, extracapsular spread, and the method and dose of adjuvant radiotherapy. Second, the results of PLNN might be affected by the neck dissection and collection performed by surgeons and the LN examination performed by pathologists. In addition, the SEER database (2004–2015) did not include staging information of the latest AJCC 8th edition. Our study only considered that PLNN can be used as a supplement to help improve the prognostic evaluation effect of the AJCC 6th and 7th staging strategy, while it still needs to be further verified in the HPSCC cohort which was staged by the 8th AJCC edition in the future. Despite these concerns, PLNN is suggested to quantify LN metastasis, which is easier to apply in clinical practice and has the potential to help predict HPSCC prognosis.
Positive lymph node number was a potential prognostic factor associated with OS and CSS in patients with HPSCC. The survival model incorporating PLNN and TNM classification performed better than other models that are based on any single variable. PLNN may serve as a survival predictor for patients with HPSCC and a supplement to enhance the evaluation results of TNM cancer staging systems.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by West China Hospital review board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
FC and WP designed the study. DC and YR analyzed and interpreted data in the included studies. MM and KQ performed quality control of data and algorithms. YL and JLi performed the statistical analysis. YD contributed to the acquisition of data. JZ helped perform the analysis with constructive discussions. WP, YL, and JLi were major contributors for writing the manuscript. FC and HW reviewed the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.898483/full#supplementary-material
SEER, database, the Surveillance, Epidemiology and End Results database; PLNN, positive lymph node number; AJCC, the American Joint Committee on Cancer; TNM, staging system, tumor, lymph node and metastasis staging system; HPSCC, hypopharyngeal squamous cell carcinoma; OS, overall survival; CSS, cancer-specific survival; LNM, lymph nodes metastasis; HR, hazard ratio; CI, confidence interval; LNs, lymph nodes; LNR, lymph nodes ratio.
1. Zhou J, Li Y, Wei D, Qian Y, Li W, Liu D, et al. Overall survival with and without laryngeal function preservation in 580 patients with hypopharyngeal squamous cell carcinoma. Oncol Rep. (2015) 34:3196–202. doi: 10.3892/or.2015.4313
2. Hall SF, Groome PA, Irish J, O'Sullivan B. The natural history of patients with squamous cell carcinoma of the hypopharynx. Laryngoscope. (2008) 118:1362–71. doi: 10.1097/MLG.0b013e318173dc4a
3. Imai T, Ito S, Oikawa T, Asada Y, Matsumoto K, Miyazaki T, et al. Risk factors for cervical lymph node metastasis in endoscopically resected superficial hypopharyngeal cancers. Auris Nasus Larynx. (2019) 46:424–30. doi: 10.1016/j.anl.2018.09.005
4. Amin SN, Shinn JR, Naguib MM, Netterville JL, Rohde SL. Risk factors and outcomes of postoperative recurrent well-differentiated thyroid cancer: a single institution's 15-year experience. Otolaryngol Head Neck Surg. (2020) 162:469–75. doi: 10.1177/0194599820904923
5. Yeung DCM, Yeung Z, Wong EWY, Vlantis AC, Chan JYK. Neck lymph node status on survival of regionally recurrent or persistent nasopharyngeal carcinoma. Sci Rep. (2020) 10:5622. doi: 10.1038/s41598-020-62625-4
6. Lee H, Roh JL, Cho KJ, Choi SH, Nam SY, Kim SY. Number of positive lymph nodes better predicts survival for oral cavity cancer. J Surg Oncol. (2019) 119:675–82. doi: 10.1002/jso.25386
7. Zhu X, Heng Y, Zhou L, Zhang M, Li W, Tao L. Survival prediction and treatment strategies for patients with advanced laryngeal carcinoma: a population-based study. Int J Clin Oncol. (2020) 25:1483–91. doi: 10.1007/s10147-020-01688-9
8. Lloyd S, Yu JB, Ross DA, Wilson LD, Decker RH. A prognostic index for predicting lymph node metastasis in minor salivary gland cancer. Int J Radiat Oncol Biol Phys. (2010) 76:169–75. doi: 10.1016/j.ijrobp.2009.01.021
9. Hua YH, Hu QY, Piao YF, Tang Q, Fu ZF. Effect of number and ratio of positive lymph nodes in hypopharyngeal cancer. Head Neck. (2015) 37:111–6. doi: 10.1002/hed.23574
10. Jang JY, Kim MJ, Ryu G, Choi N, Ko YH, Jeong HS. Prediction of lymph node metastasis by tumor dimension versus tumor biological properties in head and neck squamous cell carcinomas. Cancer Res Treat. (2016) 48:54–62. doi: 10.4143/crt.2014.332
11. Harada R, Isobe K, Watanabe M, Kobayashi H, Horikoshi T, Motoori K, et al. The incidence and significance of retropharyngeal lymph node metastases in hypopharyngeal cancer. Jpn J Clin Oncol. (2012) 42:794–9. doi: 10.1093/jjco/hys106
12. Kamiyama R, Saikawa M, Kishimoto S. Significance of retropharyngeal lymph node dissection in hypopharyngeal cancer. Jpn J Clin Oncol. (2009) 39:632–7. doi: 10.1093/jjco/hyp080
13. Abdeyrim A, He S, Zhang Y, Mamtali G, Asla A, Yusup M, et al. Prognostic value of lymph node ratio in laryngeal and hypopharyngeal squamous cell carcinoma: a systematic review and meta-analysis. J Otolaryngol Head Neck Surg. (2020) 49:31. doi: 10.1186/s40463-020-00421-w
14. Chen CC, Lin JC, Chen KW. Lymph node ratio as a prognostic factor in head and neck cancer patients. Radiat Oncol. (2015) 10:181. doi: 10.1186/s13014-015-0490-9
15. Wang YL, Feng SH, Zhu J, Zhu GP, Li DS, Wang Y, et al. Impact of lymph node ratio on the survival of patients with hypopharyngeal squamous cell carcinoma: a population-based analysis. PLoS ONE. (2013) 8:e56613. doi: 10.1371/journal.pone.0056613
16. Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS. Association of quantitative metastatic lymph node burden with survival in hypopharyngeal and laryngeal cancer. JAMA Oncol. (2018) 4:985–9. doi: 10.1001/jamaoncol.2017.3852
17. Ho AS, Kim S, Tighiouart M, Gudino C, Mita A, Scher KS, et al. metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol. (2017) 35:3601–9. doi: 10.1200/JCO.2016.71.1176
18. Zumsteg ZS, Luu M, Kim S, Tighiouart M, Mita A, Scher KS, et al. Quantitative lymph node burden as a 'very-high-risk' factor identifying head and neck cancer patients benefiting from postoperative chemoradiation. Ann Oncol. (2019) 30:76–84. doi: 10.1093/annonc/mdy490
Keywords: hypopharyngeal squamous cell carcinoma, positive lymph nodes number, prognosis, prediction models, survival predictive values
Citation: Pang W, Luo Y, Li J, Cheng D, Rao Y, Mao M, Qiu K, Dong Y, Liu J, Zou J, Wang H and Chen F (2022) The Prognostic Prediction Value of Positive Lymph Nodes Numbers for the Hypopharyngeal Squamous Cell Carcinoma. Front. Med. 9:898483. doi: 10.3389/fmed.2022.898483
Received: 17 March 2022; Accepted: 17 May 2022;
Published: 04 July 2022.
Edited by:
Wei Xu, University of Toronto, CanadaReviewed by:
Yao-Te Tsai, Chiayi Chang Gung Memorial Hospital, TaiwanCopyright © 2022 Pang, Luo, Li, Cheng, Rao, Mao, Qiu, Dong, Liu, Zou, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Chen, aHhjaGVuZmVpQDE2My5jb20=; Haiyang Wang, d2FuZ2hhaWtvc0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.