
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 06 July 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.898455
 Xiaoli Yang1,2†
Xiaoli Yang1,2† Hua Yin1,2†
Hua Yin1,2† Deyu Zhang2†
Deyu Zhang2† Lisi Peng2
Lisi Peng2 Keliang Li3
Keliang Li3 Fang Cui2
Fang Cui2 Chuanchao Xia2
Chuanchao Xia2 Zhaoshen Li2*
Zhaoshen Li2* Haojie Huang2*
Haojie Huang2*Cathepsin B (CTSB) is a lysosomal protease implicated in the progression of various diseases. A large number of CTSB-related studies have been conducted to date. However, there is no comprehensive bibliometric analysis on this subject. In our study, we performed quantitative analysis of CTSB-related publications retrieved from the Science Citation Index Expanded (SCIE) of the Web of Science Core Collection (reference period: 2011–2021). A total of 3,062 original articles and reviews were retrieved. The largest number of publications were from USA (n = 847, 27.66%). The research output of each country showed positive correlation with gross domestic product (GDP) (r = 0.9745, P < 0.0001). Active collaborations between countries/regions were also observed. Reinheckel T and Sloane BF were perhaps the most impactful researchers in the research landscape of CTSB. Plos ONE was the most prevalent (119/3,062, 3.89%) and cited journal (3,021 citations). Comprehensive analysis of the top citations, co-citations, and keywords was performed to acquire the theoretical basis and hotspots of CTSB-related research. The main topics included CTSB-related cancers and inflammatory diseases, CTSB-associated cell death pattern, and the applications of CTSB. These results provide comprehensive insights into the current status of global CTSB-related research especially in pancreas, which is worthy of continued follow-up by practitioners and clinicians in this field.
Cathepsin B (EC 3.4.22.1, CTSB), a lysosomal cysteine protease in the Papain family, exhibits both endopeptidase and exopeptidase activity. It can act both extracellularly and as an activator of trypsinogen within the cell (1–3). CTSB is believed to colocalize with trypsinogen in the lysosomes, and subsequently activate the trypsinogen causing acute pancreatitis (4). In a study by Sendler et al., trypsinogen was shown to be activated by endocytosed CTSB in macrophages, promoting pancreatitis in mice (5). Additionally, CTSB may play a role in tumor initiation, proliferation, angiogenesis, and metastasis, and promote carcinogenesis in pancreatic tissue (6, 7).
CTSB gene is located at chromosome 8p22 and contains 13 exons and 11 introns (8, 9). Pre-mature CTSB protein is a 44 kD zymogen, and an intermediate formation is 33 kD with a single chain. The active CTSB protein is formed after maturation processing and has a 27–29 kD heavy chain and a 4–6 kD light chain (8, 10, 11). In 1991, the crystal structure of human CTSB in its two-chain form with 2.15 Å resolution was identified, and it was the first determined crystal structure of human cathepsin (12). It is roughly disc-shaped and 50 Å in diameter with 30 Å thickness and a distinct active site cleft. The polypeptide chain folds into two distinct domains interacting through an extended polar interface that opens to V-shaped active site cleft, similar to the related cysteine protease papain, actinidin, and calotropin D (12). CTSB can act as a peptidyl dipeptidase in neutral pH, which as an exopeptidase removes dipeptides from the C-terminus of proteins and peptides; CTSB can also act as an endopeptidase with relatively broad specificity with slight preference for basic residues in acidic pH to cleave internal peptide bonds (12). The dual activation relies on the occluding loop (the covalently closed circular region between Cys108 and Cys119 residues) which is a unique structural element of CTSB (13). The loop partially blocks the end of the active-site cleft and positions a positively-charged imidazole group of a histidine residue to accept the negative charge at the C-terminus of the substrate, enabling CTSB to act as an exopeptidase; in addition, it has a flexible structure that can adopt a conformation that no longer blocks the binding cleft, allowing the enzyme to act as an endopeptidase (14). However, CTSB is a less effective endopeptidase than some other members of the papain family, probably because of the energy cost of altering the conformation of the occluding loop (15). CTSB locates in the islet endocrine cells and acinar cells in pancreas (16, 17). Changes in the expression and distribution of CTSB is associated with various disease. In the study by Saluja et al., redistribution of CTSB in the acinar cells of pancreas and colocalization with trypsinogen led to the development of acute pancreatitis (16). Moreover, the vesicles staining for CTSB were observed more peripheral in the aggregated cells and the redistribution of CTSB vesicles toward the cell periphery may be induced by the acidic pericellular pH, which facilitates the progression and development of pancreatic cancer (18). However, owing to the complexity of etiology and pathogenesis of CTSB-related diseases, especially the enormous interactions of different signal pathways, further studies are required to unravel the exact role of CTSB especially in pancreas.
Bibliometric analysis is one of the most extensively used approaches for assessing the quantity, quality, reliability, and influence of the existing research achievements. It can provide comprehensive integration, interpretation, and analysis of the evolution and dynamics of scientific information in a specific field. The present bibliometric study encompassed 3,062 publications in the field of CTSB published during the past decade to obtain a global understanding of CTSB-related research. The key aspects analyzed included annual output, most productive countries/regions and their gross domestic product (GDP), the top scientific journals, the number of citations and co-citations, co-occurrence and burst detection of keywords. The potential research hotspots and latest trends identified in this study may provide a valuable reference for researchers interested in the CTSB field.
The Clarivate Analytics SCIE database was searched on October 1, 2020, to obtain CTSB-related publications in the latest decade (2011–2021) with no language preference. The retrieval strategy was as follows: ALL FIELDS: (“cathepsin B”) OR ALL FIELDS: (“CTSB”) AND ALL FIELDS: (pancrea*) Refined by: DOCUMENT TYPES: (ARTICLE OR REVIEW) Timespan: 2011–2021. Indexes: SCI-EXPANDED. The CTSB-relativity screen was performed by three authors independently using the EndNote X9, and the differences were discussed until consensus. Then the CTSB-relativity studies were screened from the CTSB-relativity data with the limitation of keywords to “Pancreatic Cancer”, “Cancer of Pancreas”, “Pancreas Neoplasm” or “pancreatitis”. All targeted data were acquired in text format on October 1, 2020, and formatted into Prism software (version 8.3.0). The following bibliometrics and visualization tools VOSviewer (1.6.17) and CiteSpace (5.8.R2) were used for analysis.
Prism software (version 8.3.0) was used to conduct the histogram, the correlation analysis of top 20 productive countries/regions and their GDPs (deadline: October 2021). The collaborations of countries/regions or authors, top citation journals and key references were visualized by VOSviewer. VOSviewer plots show different bubbles representing elements such as authors, journals, references, and keywords. The area of the bubbles represents the frequency of target elements. The line and its thickness reflect the collaborations and the strength of the relationship between two bubbles (19, 20). In the present study, settings of the VOSviewer were: full counting with threshold (T) based on corresponding-elements. The Biblioshiny program of R-bibliometrix was utilized to identify the most impactful of authors.
A total of 3062 publications related to CTSB in the study reference period were retrieved from the SCIE database. The data used for the subsequent analysis were obtained from the search results of this database. “Original research articles” and “review articles” accounted for 2,784 (90.92%) and 278 (9.08%) publications, respectively. English was the predominant language of publication in this field, accounting for 94.68% (3,049/3,062) of all publications. The other languages were Chinese (4/3,062, 0.13%), Japanese (4, 0.13%), and French, German, Hungarian, Polish and Portuguese (one article each) (Figure 1A). Figure 1B shows the annual output of publications in the field of CTSB; a relatively increasing trend was observed in the study reference period.
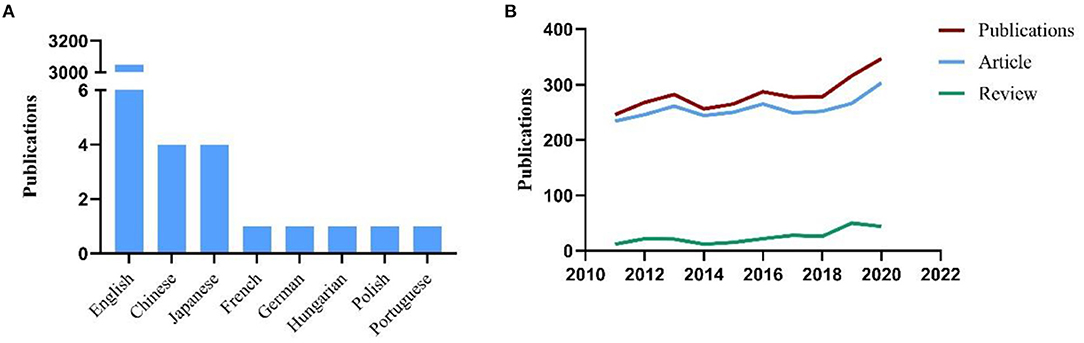
Figure 1. (A) The languages of publications in CTSB field; (B) Number of publications by year (2011–2020).
The contribution of various countries/regions was estimated by the number of publications. The top 20 productive countries/regions acquired from SCIE database are shown in Figure 2A. USA and China accounted for the highest number of papers (n = 847 and 833, respectively), followed by Germany (n = 254), Japan (n = 208), South Korea (n = 145), England (n = 137), Spain (135), India (n = 127), Canada (n = 124), Brazil (n = 118), and France (n = 113). The network map (T = 46) shows the collaboration between these top countries/regions (Figure 2B). The size of the bubbles represents the number of publications of each country/region. The colors represent different clusters, the cluster represents the similar research topic in the CTSB field. The line and its thickness reflect the collaboration and the strength of relationship between two countries/regions. Active collaborations were seen among various countries; for instance, USA cooperated closely with China, Germany, England, Japan, Australia, South Korea and India. Moreover, the relationship between research output and economic development was evaluated for the 20 most productive countries using GraphPad Prism; the GDP data of countries were retrieved from the World Bank Open Data [October 1, 2021(21)]. The results showed a distinct positive relationship between research output and GDP of the top 20 countries (r = 0.9745, P < 0.0001; Figure 2C).

Figure 2. (A) The top 20 most productive countries for CTSB research; (B) Network map showing distribution of collaboration between Countries/Regions; (C) Correlation between total articles published and GDP of 15 highest-output countries.
The influential authors were comprehensively appraised based on four parameters: the total number of their publications, the total citation count, h-index, and g-index. The influence of scientists and their papers was evaluated by citation count. The h-index, which is a well-known metric to determine the quality of a scientist, is calculated by the citation count of papers. The g-index was introduced as an improvement of the h-index (22, 23). Reinheckel T was found to be the most prolific author with a total of 32 published articles (32/3,062, 1.05%), followed by Li Y (30, 0.98%) and Li Y (28, 0.91%) (Figure 3A). According to the number of citations in this field, Sloane BF ranked first (1,572 citations), while Jacobson MP (1,114 citations) and Jaattela M (1,109 citations) ranked second and third, respectively. Barber DL, Chimenti M, and Webb BA, coauthors of the most cited article, showed equal contributions with 1,080 citations each (Figure 3B). Publications by Reinheckel T had the highest h-index (17), followed by those of Sloane BF (16), Wang J (15), Liu Y (13), Bogyo M (13), Li J (13), Turk B (13), and Kos J (13) (Figure 3C). The g-index of publications by Reinheckel T (24) also ranked first, followed by those from Wang J and Liu Y (24, each), then Li J, Li Y and Zhang Y (23 each) (Figure 3D).
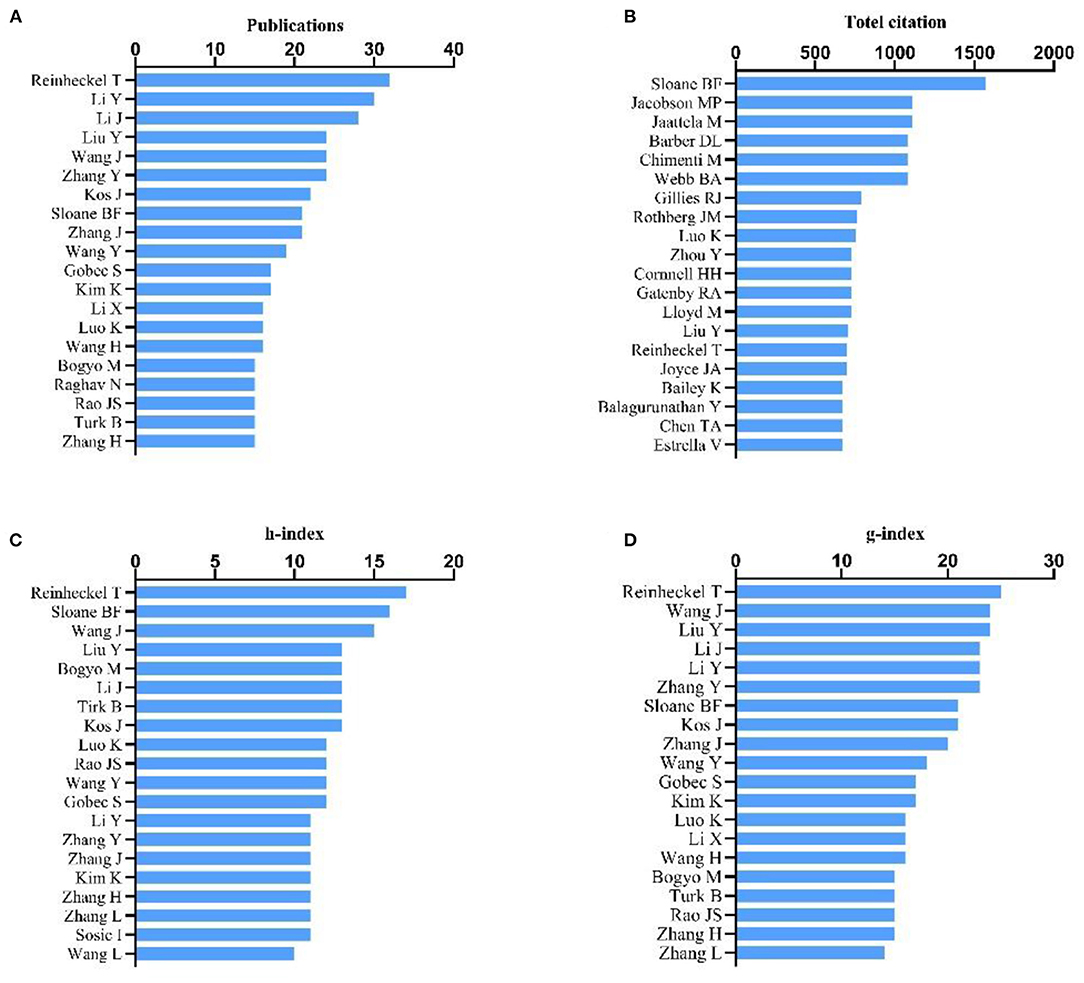
Figure 3. Analysis of authors. (A) Number of publications from different authors; (B) Total citations in the research field from different authors; (C) h-index of publications from different authors; (D) g-index of publications from different authors.
Academic journals publishing CTSB-related research were evaluated by total publications. The top 20 journals for CTSB publications ranked by VOSviewer are presented in Figure 4A. Four journals have published more than 30 papers in this field, of which Plos ONE was way ahead of other journals with 119 publications (119/3,062, 3.89%), followed by Scientific Reports (n = 48, 1.57%), Journal of Biological Chemistry (n = 47, 1.53%), and Cell Death & Disease (n = 33, 1.08%). To visualize the relationships of journals, the citation network map was constructed using journals with ≥7 publications (T = 7) (90/1,068, 8.43%) (Figure 4B). The size of the bubble represents the number of citations per journal. Different colors represent different clusters, the hallmarking of different research topics on the CTSB theme. The line and its thickness reflect the collaboration and the intensity of the mutual citation between two academic journals. As can be seen, Plos ONE, Journal of Biological Chemistry, Cancer Research, Journal of Immunology, ACS Nano, Cell Death & Disease, and Journal of Controlled Release had larger-sized bubbles representing higher journal citations.
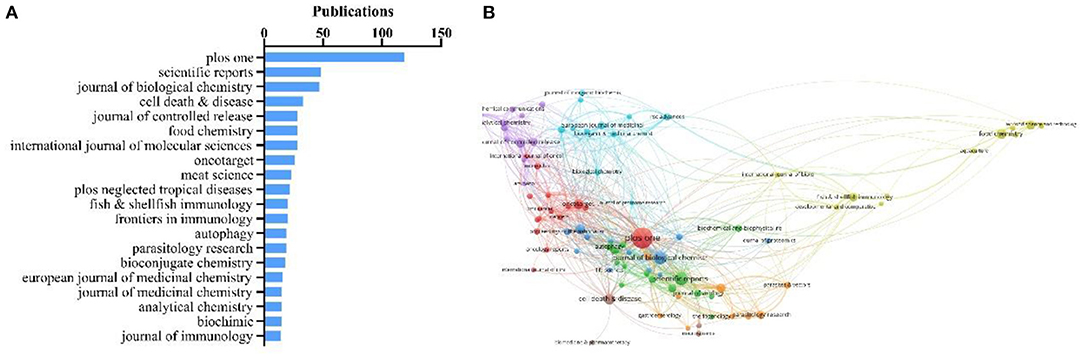
Figure 4. (A) Top 20 journals assessed by number of publications; (B) Network map of scholarly journals (T = 7) (produced by citation).
The top citations of publications were evaluated by VOSviewer. Table 1 displays the top eight papers with the highest citations (T = 310). There were 1,080 citations for “Dysregulated pH: a perfect storm for cancer progression” from Nature Reviews Cancer (25), followed by “Acidity Generated by the Tumor Microenvironment Drives Local Invasion” from Cancer Research (26), with 669 citations. The third most-frequently cited article was “Molecular mechanisms regulating NLRP3 inflammasome activation” (24), with 545 citations. The top 8 references with over 100 co-citations are listed in Table 2. The reference with the highest co-citation (n = 205) was from Mohamed MM (32), titled “Cysteine cathepsins: multifunctional enzymes in cancer”, a review that exhaustively described the role of cysteine cathepsins in tumor growth, migration, invasion, angiogenesis and metastasis. This was followed by the reviews from Turk V (33) (n = 155), Gondi CS (35) (n = 113), Barrett AJ (36) (n = 112), Boya P (37) (n = 109) and Aggarwal N (38) (n = 100). The two articles with high co-citations are from Hornung V (34) and Musil D (12). Five of eight top co-cited references are all reviews, which indicates that reviews may be more highly cited than research articles in the co-citation of references. However, these publications no matter reviews or articles encompassed a wide spectrum of CTSB-related research including the properties of cysteine proteases, the molecular mechanisms, the protease related diseases, and biochemical applications.
Co-occurrence relationship is formed between two keywords that appear in the same publication. Strong co-occurrence relationship of all keywords can more accurately reveal research hotspots than a single keyword. A total of 241 keywords were confirmed as occurring more than 20 times with the full counting method; the visualization map is shown in Figure 5. In the network map, the line is a symbol connecting two keywords. The size of bubbles indicates the number of occurrences, and the color represent keyword clustering (Figure 5A). While, the colors presented in the overlay visualization map indicate the average publication year of the identified keywords (Figure 5B). Three broad categories synthesized from six different clusters showed the protease properties and functions, the CTSB-related diseases, and the applications. Furthermore, keyword burst analysis by CiteSpace was performed based on achievements published since 2011 to 2021 (Figure 6). The blue line indicates the time interval, the red line segment in the blue line indicates that a subject was found to have a burst. As shown in Figure 6, during the period from 2011 to 2021, angiogenesis had the highest burst strength (9.59), followed by proteinase (6.96), and dysfunction (6.7). Angiogenesis had a period of burst between 2011 and 2013, while dysfunction had a period of burst between 2018 and 2021. Of note, both dysfunction and antibody had strong bursts starting in 2018.
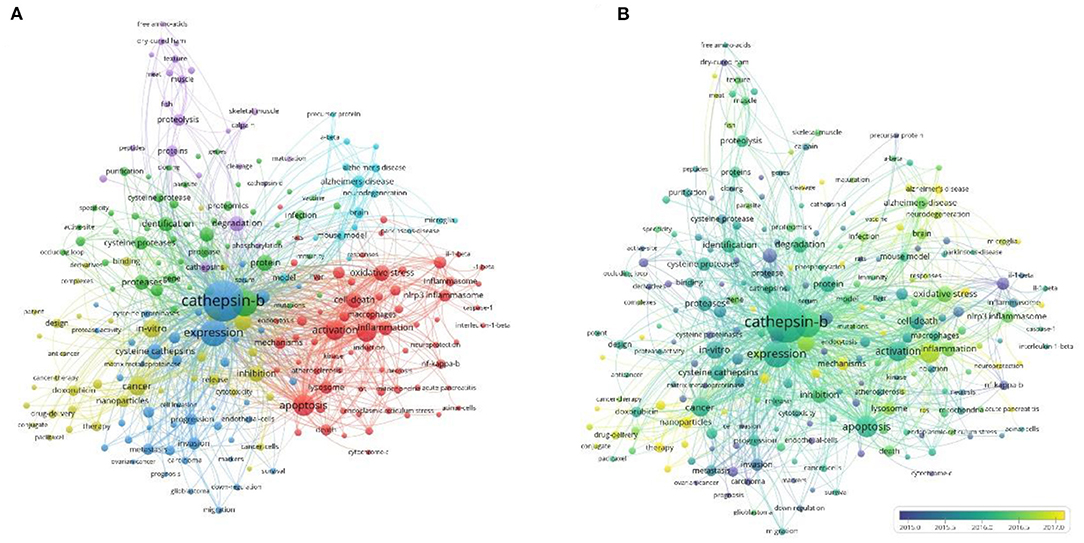
Figure 5. Co-occurrence analysis of keywords. (A) Network visualization map of keywords; (B) Overlay visualization map of keywords.
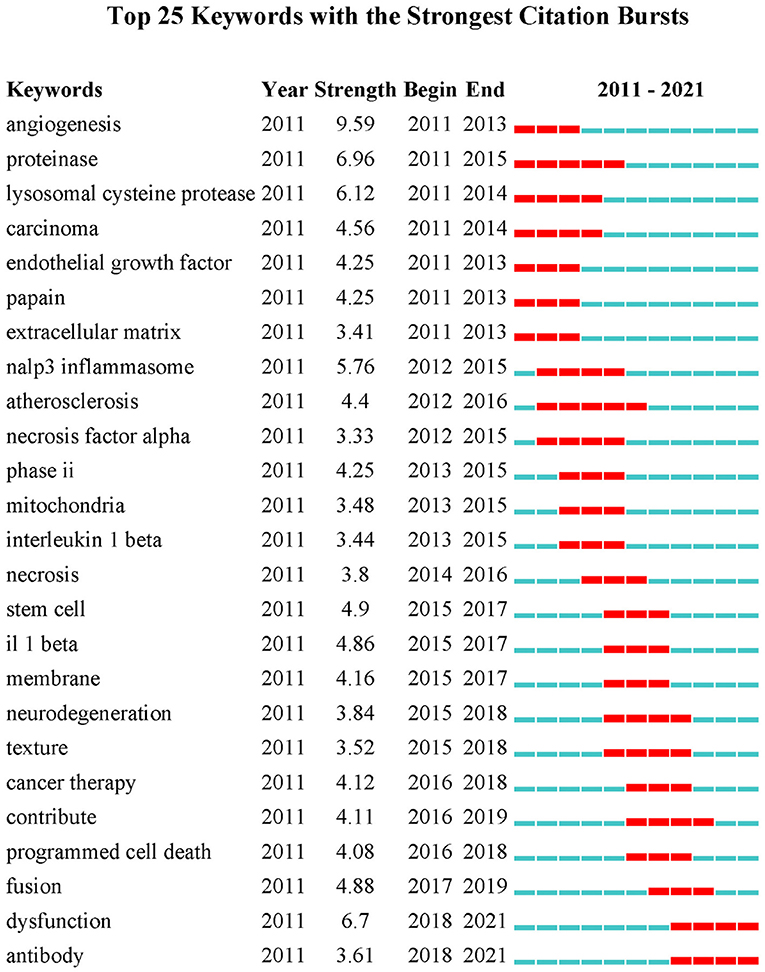
Figure 6. Keywords with the strongest currently ongoing citation bursts (sorted in descending order of Begin).
Bibliometric analysis of the annual output of publications enabled us to characterize the global developments in the field of CTSB. Based on Science Citation Index Expanded database of Web of Science, we performed a bibliometric analysis to characterize the trends in CTSB-related research from 2011 to 2021. We observed a relatively increasing trend of the annual output of publications in CTSB research over the past few years and found that CTSB is still a research hotspot. A vast majority of these studies were published in English, which demonstrated that English is the most popular international language of science and medicine. USA, China, Germany, and Japan were the top four productive countries in the field of CTSB research, indicating their greater contribution to CTSB field. Among the top 20 productive countries, USA and China were way ahead of other countries with more than 800 publications, demonstrating that these two countries may be the potential hubs for research and development in the field of CTSB. Collaborations among the top contributing countries further showed that the CTSB research was a worldwide activity. This kind of cooperation among countries/regions may foster academic sharing and knowledge integration, attract more distinguished scientists to participate in this domain, and accelerate CTSB-related research. Further correlation analysis between GDP and publications showed a tendency of high-GDP countries to gain higher outputs, which demonstrates that these countries pay more attention to scientific research investment, and have sufficient funds to perform advanced research and build advanced research platforms. These elements may accelerate innovation and development of techniques of CTSB research in these countries.
Author analysis helps practitioners to identify the researchers who have the greatest contribution in a particular research field as well as their research level and academic status in this field (39). Analysis of the top 20 most prolific authors was used to screen out active and impactful researchers in this theme. Reinheckel T contributed the largest number of papers with the highest h-index and g-index; Sloane BF had the most citations per paper. These data provide us with the most academically influential and authoritative authors in CTSB field. Research by Reinheckel T was more focused on the CTSB-related diseases, especially pancreatic diseases such as acute pancreatitis and pancreatic ductal adenocarcinoma (2, 40). However, Sloane BF was more focused on the tumors and molecular mechanisms of involvement of CTSB (38, 41, 42). Both Reinheckel T and Sloane BF were the most impactful authors in the CTSB field over the recent decade. Reviewing and analyzing the work of these outstanding academic leaders before starting a new CTSB research would facilitate a better understanding of the basic information in this field. Additionally, analysis of the main scientific journals and their collaborations showed the most prevalent journals and their connections with each other in the CTSB field. This may provide a valuable reference for beginners in this field and make it easier to find the related documents and submit novel discoveries in this field.
The number of citations of a study indicates the relevance and importance of a study in the specific field, and the number of co-cited references exhibits the frequency of two publications being cited together by other publications (19). In our current study, we filtered the publications and references with citations and co-citations from 2011 to 2021 by using the VOSviewer. By combining the top cited publications, co-citation references, and the subsequent analysis of the keywords using network map as well as burst detection, we summarized the basic information and obtained the hotspots of CTSB research field, including the CTSB-related cancer and inflammatory diseases, CTSB-associated cell death pattern, and the applications of CTSB.
Since the first identification of the crystal structure of human CTSB, a multitude of three-dimensional structures of CTSB and their compounds have been identified. All the studies about the structure of CTSB have provided basic information for researchers to design specific targeting inhibitors and develop safe drugs for treatment of CTSB-related diseases. The role of CTSB in carcinogenesis was first identified decades ago (43). It was shown to play an important part in the process of pancreatic carcinogenesis, including cancer-related angiogenesis, malignant invasion, and cancer metastasis (30, 33, 38, 40). Researchers have identified high expression of CTSB in human pancreatic ductal adenocarcinomas and pancreatic cancer stem cells, and demonstrated its association with poor survival and surgical outcomes (44, 45). CTSB exhibits optimal activity in a slightly acidic pH (46, 47). Webb BA and Estrella V identified that acidic pH in the tumor microenvironment may drive malignant transformation and cancer progression (25, 26). The potential underlying mechanism is that pericellular pH leads to the redistribution of CTSB to the surface of malignant cells and accelerates the secretion of active CTSB, which may facilitate invasive growth of these cells (18). CTSB can degrade the extracellular matrix, such as collagen, matrix fiber and proteoglycans, to cause dissolution of the tumor matrix and the basement membrane (48–50), promoting invasion and metastasis. Kim verified overexpression of CTSB in tumor samples and identified its association with increased risk of lymph node metastasis (51). Additionally, CTSB is a well-known candidate activator of trypsinogen in pancreatic acinar cells (52).
Sendler M and Fortunato F also found an association between high expression of CTSB and increased severity of pancreatitis (5, 53). However, enteropeptidase (enterokinase), which is the known physiological activator of trypsinogen and has high catalytic activity for trypsinogen, is involved in the progression of acute pancreatitis (54, 55). To date, there is inadequate evidence to suggest that the role of CTSB precedes that of enteropeptidase or that there is an interaction between the two in the occurrence and development of acute pancreatitis. This may be worthy of future research. Nonetheless, inhibiting the overexpression of CTSB and maintaining its dynamic balance in organism may help control or alleviate CTSB-related cancer and inflammatory diseases.
Studies have identified the underlying biological mechanism by which CTSB promotes programmed cell death (56–59). The release of active CTSB from the damaged lysosomes induces mitochondrial dysfunction which increases cytosol-induced release of cytochrome c from mitochondria resulting in activation of caspase and cell apoptosis (37, 57, 60). Release of reactive oxygen species from mitochondria may also cause lysosomal membrane injury and CTSB leakage from lysosome into cytoplasm contributing to the occurrence of apoptotic cell death (61, 62). However, owing to its pH-dependent bidirectional interactions, CTSB plays myriad roles. It serves as a pro-oncogenic molecule involved in ECM degradation, angiogenesis and metastatic induction; as an anti-apoptotic molecule suppressing the TLR3 pathway and maintaining a pro-survival state; and as a pro-apoptotic molecule involved in autophagy and immune response (35). Additionally, Wang et al. observed that CTSB released from lysosomes promoted severe acute pancreatitis by activating NLRP3 inflammasome which induced self-cleavage of caspase-1 and its maturation into an activated form; this in turn promoted the maturation and secretion of various proinflammatory cytokines including IL-1β and IL-18, triggering caspase-1-induced pyroptosis (3). Pyroptosis is a newly-identified pathway of programmed cell death, characterized by rapid lytic cell death through caspase-1 activity. Recent studies have revealed other caspases (caspase-11 and its human orthologs caspase-4 and caspase-5, and the apoptotic effector caspase-3) which cleave gasdermins (GSDMs) to trigger pyroptosis and induce altered levels of host metabolites and environmental irritants (56, 63–66). The activated CTSB binds to NLRP3 inflammasome to activate caspase, resulting in the expression of GSDMD-N that oligomerize within the plasma membrane to form pores. Excessive pore formation compromises the integrity of the plasma membrane, causing a lytic form of cell death known as pyroptosis. A recent study has identified that CTSB as an autophagy regulator plays an active role in the degradation of autophagic substrate engaged in ferroptosis (a type of iron-dependent oxidative cell death driven by lipid peroxidation) (67). In addition to apoptosis and pyroptosis, other cell death patterns including necroptosis and alkaliptosis (a pH-dependent form of regulated cell death) have been shown to be caspase-dependent and to participate in the progression and development of pancreatic cancer (68). Whether CTSB plays a key role in these modes of cell death is not clear, especially in the context of pancreatic diseases. Considering the complex nature of interaction among proteases, both upstream and downstream pathways of CTSB may affect each other. Thus, much headway needs to be made before the specific mechanism of participation of CTSB can be unraveled.
Owing to the aberrant expression of CTSB in disease conditions, it has been used as a therapeutic target. Wang et al. integrated fibronectin-targeting MR imaging and CTSB-activatable fluorescence imaging for accurate diagnosis and further selective therapy of cancer (69). A drug delivery system targeting the extracellular CTSB through a highly selective CTSB inhibitor (NS-629) conjugated with a highly biocompatible liposomal nanocarrier was shown to target CTSB-overexpressing tumor and stromal cells in the tumor microenvironment, resulting in irreversible and selective inactivation of CTSB, achieving CTSB-specific therapy in cancer (70). DARPins (designed ankyrin repeat proteins) have been shown to block CTSB activity in tumors and have been successfully applied for optical imaging in cancer models. CTSB-selective DARPins represent an attractive theoretical basis for non-invasive diagnostic imaging (71). Although several studies have demonstrated the applications in biochemistry and bioengineering, development of clinical applications such as CTSB-specific inhibitor or antibody still has a long way to go. For instance, the biosafety aspects including the interaction with other drugs, damage to non-targeted organs, drug tolerance, and other related issues need to be further explored by researchers and clinicians.
In present study, we performed a quantitative analysis of the CTSB-related literature using bibliometric methodology. The findings and suggestions may help researchers and clinicians understand the performance and trends of CTSB-related research globally. Nevertheless, due to the inherent limitations of bibliometric approach, we did not examine individual article records to verify the accuracy of indexing. Additionally, we only retrieved publications in a single database and did not search other databases, such as PubMed, Embase, Cochrane Library, etc. This may have led to exclusion of some articles. Furthermore, studies published in earlier years are likely to have higher citations over time, while recently-published studies, even if high-quality, may not have yet acquired comparable visibility. This may have introduced an element of bias and undermined the significance of more recently-published articles. Therefore, researchers should keep abreast of the latest published achievements. Despite these limitations, this research provides a solid global perspective on CTSB-related research over the past decade and highlights the future research direction.
This bibliometric analysis provides a quantitative synthesis of the published achievements related to CTSB that are available in the Web of Science database during the period 2011 to 2021. Research results and recommendations indicate that USA ranks first in terms of productivity, and the research was performed in close cooperation with other countries, such as China, Germany, England, and Japan. The research output of a country showed positive correlation with GDP. Reinheckel and Sloane BF may be the critical researchers in the field of CTSB. “Plos ONE” was the most productive and cited journal. Analysis of the top citations, co-citations literatures, and the keywords by network map and burst detection clarified the theoretical basis and the key research topics. The hotspots identified in this study included CTSB-related cancer and inflammatory diseases, CTSB-associated cell death pattern, and the applications of CTSB. The topic of CTSB is worthy of continued follow-up by researchers.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
HH and ZL designed and supervised this study. XY, HY, and DZ performed the search. LP and KL collected data. DZ, FC, and CX rechecked data. XY and HY performed analysis. XY and LP wrote the manuscript. All authors read and approved the final manuscript.
Funded by the National Natural Science Foundation of China (to HH), No. 81770642, China and research on demonstration application of collaborative network construction in clinical medical research, No. 2015BAI13B08, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.898455/full#supplementary-material
1. Bohley P, Seglen PO. Proteases and proteolysis in the lysosome. Experientia. (1992) 48:151–7. doi: 10.1007/BF01923508
2. Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. (2000) 106:773–81. doi: 10.1172/JCI9411
3. Wang J, Wang L, Zhang X, Xu Y, Chen L, Zhang W, et al. Cathepsin B aggravates acute pancreatitis by activating the NLRP3 inflammasome and promoting the caspase-1-induced pyroptosis. Int Immunopharmacol. (2021) 94:107496. doi: 10.1016/j.intimp.2021.107496
4. Wartmann T, Mayerle J, Kähne T, Sahin-Tóth M, Ruthenbürger M, Matthias R, et al. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology. (2010) 138:726–37. doi: 10.1053/j.gastro.2009.10.048
5. Sendler M, Weiss FU, Golchert J, Homuth G, van den Brandt C, Mahajan UM, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology. (2018) 154:704–718.e710. doi: 10.1053/j.gastro.2017.10.018
6. Dumartin L, Whiteman HJ, Weeks ME, Hariharan D, Dmitrovic B, Iacobuzio-Donahue CA, et al. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. (2011) 71:7091–102. doi: 10.1158/0008-5472.CAN-11-1367
7. Niedergethmann M, Wostbrock B, Sturm JW, Willeke F, Post S, Hildenbrand R. Prognostic impact of cysteine proteases cathepsin B and cathepsin L in pancreatic adenocarcinoma. Pancreas. (2004) 29:204–11. doi: 10.1097/00006676-200410000-00005
8. Uchiyama Y, Waguri S, Sato N, Watanabe T, Ishido K, Kominami E. Cell and tissue distribution of lysosomal cysteine proteinases, cathepsin-B, cathepsin-H, and cathepsin-L, and their biological roles. Acta Histochem Cytochem. (1994) 27:287–308. doi: 10.1267/ahc.27.287
9. Wang X, Chan SJ, Eddy RL, Byers MG, Fukushima Y, Henry WM, et al. Chromosome assignment of cathepsin-B (Ctsb) to 8p22 and cathepsin-H (Ctsh)to 15q24-Q25. Cytogenet Cell Genet. (1987) 46:710–1.
10. Eiján AM, Sandes EO, Riveros MD, Thompson S, Pasik L, Mallagrino H, et al. High expression of cathepsin B in transitional bladder carcinoma correlates with tumor invasion. Cancer. (2003) 98:262–8. doi: 10.1002/cncr.11493
11. Mijanović O., Brankovi,ć A., Panin A. N., Savchuk S., Timashev P., Ulasov I., et al. (2019). Cathepsin B: a sellsword of cancer progression. Cancer Lett 449, 207–214. doi: 10.1016/j.canlet.2019.02.035
12. Musil D., Zucic D., Turk D., Engh R. A., Mayr I., Huber R., et al. (1991). The refined 2.15 A X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. Embo J 10, 2321–2330 doi: 10.1002/j.1460-2075.1991.tb07771.x
13. Quraishi O, Nägler DK, Fox T, Sivaraman J, Cygler M, Mort JS, et al. The occluding loop in cathepsin B defines the pH dependence of inhibition by its propeptide. Biochemistry. (1999) 38:5017–23. doi: 10.1021/bi981950o
14. Illy C, Quraishi O, Wang J, Purisima E, Vernet T, Mort JS. Role of the occluding loop in cathepsin B activity. J Biol Chem. (1997) 272:1197–202. doi: 10.1074/jbc.272.2.1197
15. Cygler M, Sivaraman J, Grochulski P, Coulombe R, Storer AC, Mort JS. Structure of rat procathepsin B: model for inhibition of cysteine protease activity by the proregion. Structure. (1996) 4:405–16. doi: 10.1016/S0969-2126(96)00046-9
16. Saluja A, Hashimoto S, Saluja M, Powers RE, Meldolesi J, Steer ML. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol. (1987) 253:G508–516. doi: 10.1152/ajpgi.1987.253.4.G508
17. Watanabe M, Watanabe T, Ishii Y, Matsuba H, Kimura S, Fujita T, et al. Immunocytochemical localization of cathepsins B, H, and their endogenous inhibitor, cystatin beta, in islet endocrine cells of rat pancreas. J Histochem Cytochem. (1988) 36:783–91. doi: 10.1177/36.7.3290333
18. Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. (1994) 54:6517–25.
19. Ke L, Lu C, Shen R, Lu T, Ma B, Hua Y. Knowledge mapping of drug-induced liver injury: a scientometric investigation (2010-2019). Front Pharmacol. (2020) 11:842. doi: 10.3389/fphar.2020.00842
20. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
21. World Bank Open Data. (2021). Available online at: https://data.worldbank.org/.
22. Butun E, Kaya M. Predicting citation count of scientists as a link prediction problem. IEEE Trans Cybern. (2020) 50:4518–29. doi: 10.1109/TCYB.2019.2900495
23. Egghe L. Theory and practise of the g-index. Scientometrics. (2006) 69:131–52. doi: 10.1007/s11192-006-0144-7
24. Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. (2016) 13:148–59. doi: 10.1038/cmi.2015.95
25. Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. (2011) 11:671–7. doi: 10.1038/nrc3110
26. Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. (2013) 73:1524–35. doi: 10.1158/0008-5472.CAN-12-2796
27. Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. (2013) 14:454–60. doi: 10.1038/ni.2550
28. Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. (2013) 19:57–64. doi: 10.1038/nm.2999
29. Yuan Y, Zhang CJ, Gao M, Zhang R, Tang BZ, Liu B. Specific light-up bioprobe with aggregation-induced emission and activatable photoactivity for the targeted and image-guided photodynamic ablation of cancer cells. Angew Chem Int Ed Engl. (2015) 54:1780–6. doi: 10.1002/anie.201408476
30. Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. (2011) 21:228–37. doi: 10.1016/j.tcb.2010.12.002
31. Redecke L, Nass K, DePonte DP, White TA, Rehders D, Barty A, et al. Natively inhibited Trypanosoma brucei cathepsin B structure determined by using an X-ray laser. Science. (2013) 339:227–30. doi: 10.1126/science.1229663
32. Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. (2006) 6:764–75. doi: 10.1038/nrc1949
33. Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. (2012) 68–88. doi: 10.1016/j.bbapap.2011.10.002
34. Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. (2008) 9:847–56. doi: 10.1038/ni.1631
35. Gondi CS, Rao JS. Cathepsin B as a cancer target. Expert Opin Ther Targets. (2013) 17:281–91. doi: 10.1517/14728222.2013.740461
36. Barrett AJ, Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. (1981) 80:535–61. doi: 10.1016/S0076-6879(81)80043-2
37. Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. (2008) 27:6434–51. doi: 10.1038/onc.2008.310
38. Aggarwal N, Sloane BF. Cathepsin B: multiple roles in cancer. Proteomics Clini Applicat. (2014) 8:427–37. doi: 10.1002/prca.201300105
39. Mann S, Mann K. Enterokinase. Proc Soc Exp Biol Med. (1994) 206:114–8. doi: 10.3181/00379727-206-43728
40. Gopinathan A, Denicola GM, Frese KK, Cook N, Karreth FA, Mayerle J, et al. Cathepsin B promotes the progression of pancreatic ductal adenocarcinoma in mice. Gut. (2012) 61:877–84. doi: 10.1136/gutjnl-2011-300850
41. Ji K, Sameni M, Osuala K, Moin K, Mattingly RR, Sloane BF. Spatio-temporal modeling and live-cell imaging of proteolysis in the 4D microenvironment of breast cancer. Cancer Metastasis Rev. (2019) 38:445–54. doi: 10.1007/s10555-019-09810-8
42. Ji K, Mayernik L, Moin K, Sloane BF. Acidosis and proteolysis in the tumor microenvironment. Cancer Metastasis Rev. (2019) 38:103–12. doi: 10.1007/s10555-019-09796-3
43. Sloane BF, Dunn JR, Honn KV. Lysosomal cathepsin B: correlation with metastatic potential. Science. (1981) 212:1151–3. doi: 10.1126/science.7233209
44. Fujimoto T, Tsunedomi R, Matsukuma S, Yoshimura K, Oga A, Fujiwara N, et al. Cathepsin B is highly expressed in pancreatic cancer stem-like cells and is associated with patients' surgical outcomes. Oncol Lett. (2021) 21:30. doi: 10.3892/ol.2020.12291
45. Ohta T, Terada T, Nagakawa T, Tajima H, Itoh H, Fonseca L, et al. Pancreatic trypsinogen and cathepsin B in human pancreatic carcinomas and associated metastatic lesions. Br J Cancer. (1994) 69:152–6. doi: 10.1038/bjc.1994.25
46. Khouri HE, Plouffe C, Hasnain S, Hirama T, Storer AC, Ménard R. A model to explain the pH-dependent specificity of cathepsin B-catalysed hydrolyses. Biochem J. (1991) 275:751–7. doi: 10.1042/bj2750751
47. Voisin A, Monville C, Plancheron A, Béré E, Gaillard A, Leveziel N. Cathepsin B pH-dependent activity is involved in lysosomal dysregulation in atrophic age-related macular degeneration. Oxid Med Cell Longev. (2019) 5637075. doi: 10.1155/2019/5637075
48. Guo M, Mathieu PA, Linebaugh B, Sloane BF, Reiners JJ. Phorbol ester activation of a proteolytic cascade capable of activating latent transforming growth factor-betaL a process initiated by the exocytosis of cathepsin B. J Biol Chem. (2002) 277:14829–37. doi: 10.1074/jbc.M108180200
49. Kobayashi H, Moniwa N, Sugimura M, Shinohara H, Ohi H, Terao T. Effects of membrane-associated cathepsin B on the activation of receptor-bound prourokinase and subsequent invasion of reconstituted basement membranes. Biochim Biophys Acta. (1993) 55–62. doi: 10.1016/0167-4889(93)90109-3
50. Steffan JJ, Snider JL, Skalli O, Welbourne T, Cardelli JA. Na+/H+ exchangers and RhoA regulate acidic extracellular pH-induced lysosome trafficking in prostate cancer cells. Traffic. (2009) 10:737–53. doi: 10.1111/j.1600-0854.2009.00904.x
51. Kim EK, Song MJ, Jang HH, Chung YS. Clinicopathologic analysis of cathepsin B as a prognostic marker of thyroid cancer. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21249537
52. Chen JM, Férec C. Chronic pancreatitis: genetics and pathogenesis. Annu Rev Genomics Hum Genet. (2009) 10:63–87. doi: 10.1146/annurev-genom-082908-150009
53. Fortunato F, Bürgers H, Bergmann F, Rieger P, Büchler MW, Kroemer G, et al. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology. (2009) 137:360.e351–355. doi: 10.1053/j.gastro.2009.04.003
54. Hartwig W, Jimenez RE, Werner J, Lewandrowski KB, Warshaw AL, Fernández-del Castillo C. Interstitial trypsinogen release and its relevance to the transformation of mild into necrotizing pancreatitis in rats. Gastroenterology. (1999) 117:717–25. doi: 10.1016/S0016-5085(99)70466-X
55. Light A, Janska H. Enterokinase (enteropeptidase): comparative aspects. Trends Biochem Sci. (1989) 14:110–2. doi: 10.1016/0968-0004(89)90133-3
56. Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. (2019) 26:99–114. doi: 10.1038/s41418-018-0212-6
57. Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. (2000) 106:1127–37. doi: 10.1172/JCI9914
58. Talukdar R, Sareen A, Zhu H, Yuan Z, Dixit A, Cheema H, et al. Release of Cathepsin B in Cytosol Causes Cell Death in Acute Pancreatitis. Gastroenterology. (2016) 151:747–758.e745. doi: 10.1053/j.gastro.2016.06.042
59. Aits S, Jäättelä M. Lysosomal cell death at a glance. J Cell Sci. (2013) 126:1905–12. doi: 10.1242/jcs.091181
60. Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol. (2013) 191:5230–8. doi: 10.4049/jimmunol.1301490
61. Martins WK, Santos NF, Rocha CS, Bacellar IOL, Tsubone TM, Viotto AC, et al. Parallel damage in mitochondria and lysosomes is an efficient way to photoinduce cell death. Autophagy. (2019) 15:259–79. doi: 10.1080/15548627.2018.1515609
62. Zhou J, Li XY, Liu YJ, Feng J, Wu Y, Shen HM, et al. Full-coverage regulations of autophagy by ROS: from induction to maturation. Autophagy. (2021) 1–16. doi: 10.1080/15548627.2021.1984656
63. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. (2011) 479:117–21. doi: 10.1038/nature10558
64. Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. (2017) 8:14128. doi: 10.1038/ncomms14128
65. Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. (2014) 514:187–92. doi: 10.1038/nature13683
66. Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. (2017) 547:99–103. doi: 10.1038/nature22393
67. Zhang L, Qiu J, Shi J, Liu S, Zou H. MicroRNA-140-5p represses chondrocyte pyroptosis and relieves cartilage injury in osteoarthritis by inhibiting cathepsin B/Nod-like receptor protein 3. Bioengineered. (2021) 12. doi: 10.1080/21655979.2021.1985342
68. Chen X, Zeh HJ, Kang R, Kroemer G, Tang D. Cell death in pancreatic cancer: from pathogenesis to therapy. Nat Rev Gastroenterol Hepatol. (2021) 18:804–23. doi: 10.1038/s41575-021-00486-6
69. Wang Y, Jiang L, Zhang Y, Lu Y, Li J, Wang H, et al. Fibronectin-targeting and cathepsin B-activatable theranostic nanoprobe for MR/fluorescence imaging and enhanced photodynamic therapy for triple negative breast cancer. ACS Appl Mater Interfaces. (2020) 12:33564–74. doi: 10.1021/acsami.0c10397
70. Mikhaylov G, Klimpel D, Schaschke N, Mikac U, Vizovisek M, Fonovic M, et al. Selective targeting of tumor and stromal cells by a nanocarrier system displaying lipidated cathepsin B inhibitor. Angew Chem Int Ed Engl. (2014) 53:10077–81. doi: 10.1002/anie.201402305
Keywords: cathepsin B, pancreatic diseases, Research Frontier, bibliometrics, visualization
Citation: Yang X, Yin H, Zhang D, Peng L, Li K, Cui F, Xia C, Li Z and Huang H (2022) Bibliometric Analysis of Cathepsin B Research From 2011 to 2021. Front. Med. 9:898455. doi: 10.3389/fmed.2022.898455
Received: 17 March 2022; Accepted: 17 June 2022;
Published: 06 July 2022.
Edited by:
Rupjyoti Talukdar, Asian Institute of Gastroenterology, IndiaReviewed by:
Kishu Ranjan, Yale University, United StatesCopyright © 2022 Yang, Yin, Zhang, Peng, Li, Cui, Xia, Li and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoshen Li, emhzX2xpQDEyNi5jb20=; Haojie Huang, aHVhbmdoYW9qaWVAc21tdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.