
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 15 June 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.897936
This article is part of the Research TopicTherapeutic Advances in Inflammatory Bowel Disease: Current Therapies and Future DirectionsView all 12 articles
 Jan Marsal1,2*
Jan Marsal1,2* Manuel Barreiro-de Acosta3
Manuel Barreiro-de Acosta3 Irina Blumenstein4
Irina Blumenstein4 Maria Cappello5
Maria Cappello5 Thomas Bazin6
Thomas Bazin6 Shaji Sebastian7
Shaji Sebastian7Anti-tumor necrosis factor (anti-TNF) therapy has been successfully used as first-line biologic treatment for moderate-to-severe inflammatory bowel disease (IBD), in both “step-up” and “top-down” approaches, and has become a cornerstone of IBD management. However, in a proportion of patients the effectiveness of anti-TNF therapy is sub-optimal. Either patients do not achieve adequate initial response (primary non-response) or they lose response after initial success (loss of response). Therapeutic drug monitoring determines drug serum concentrations and the presence of anti-drug antibodies (ADAbs) and can help guide treatment optimization to improve patient outcomes. For patients with low drug concentrations who are ADAb-negative or display low levels of ADAbs, dose escalation is recommended. Should response remain unchanged following dose optimization the question whether to switch within class (anti-TNF) or out of class (different mechanism of action) arises. If ADAb levels are high and the patient has previously benefited from anti-TNF therapy, then switching within class is a viable option as ADAbs are molecule specific. Addition of an immunomodulator may lead to a decrease in ADAbs and a regaining of response in a proportion of patients. If a patient does not achieve a robust therapeutic response with an initial anti-TNF despite adequate drug levels, then switching out of class is appropriate. In conjunction with the guidance above, other factors including patient preference, age, comorbidities, disease phenotype, extra-intestinal manifestations, and treatment costs need to be factored into the treatment decision. In this review we discuss current evidence in this field and provide guidance on therapeutic decision-making in clinical situations.
Inflammatory bowel disease (IBD), broadly comprising Crohn’s disease (CD) and ulcerative colitis (UC), is a lifelong, debilitating condition necessitating a tailored and cost-effective approach to its management. Overarching therapeutic goals are to eliminate symptoms, avoid disease complications and optimize the patient’s quality of life (QoL) (1–5). By reaching certain therapeutic targets (the “treat-to-target” approach) it is believed that the chances of achieving these therapeutic goals are markedly improved. Recently, these therapeutic targets have evolved beyond symptomatic control to the normalization of objective markers of inflammation and endoscopic healing with the aim of modifying the disease course (5, 6).
There are two main strategies for the management of IBD. The “step up” approach is used for patients with mild-to-moderate disease without poor prognosis factors starting with conventional therapies (e.g., 5-ASA, azathioprine, methotrexate) before moving on to newer and more expensive biologic or small molecule treatments, all of which have specific side effects that need to be taken into account when choosing a therapy (7). An accelerated version of the step-up approach involves moving quickly upwards through traditional therapies, driven by predefined time-points for therapeutic evaluation with prespecified criteria for therapeutic targets. If these are not reached one goes quickly to the next level of therapy with the aim of avoiding prolonged periods of under-treatment, but still following the step-up approach (8). The “top down” approach has been proposed for patients with severe disease and a high risk for disease-related complications. It uses the most potent treatments available, including biologics and immunomodulators in combination, earlier in the disease course with the aim of inducing remission and maintaining corticosteroid-free remission (7, 9–11). Over the past two decades, anti-tumor necrosis factor (anti-TNF) therapy has been successfully used as first-line biologic treatment to treat moderate-to-severe IBD in both “step up” and “top down” approaches. In addition, the more recent introduction of vedolizumab, ustekinumab, and tofacitinib provide alternative first-line treatment options, although their use may be limited by regulatory and reimbursement constraints in some countries (1–4, 12–14). A summary of available treatments for IBD is shown in Figure 1.
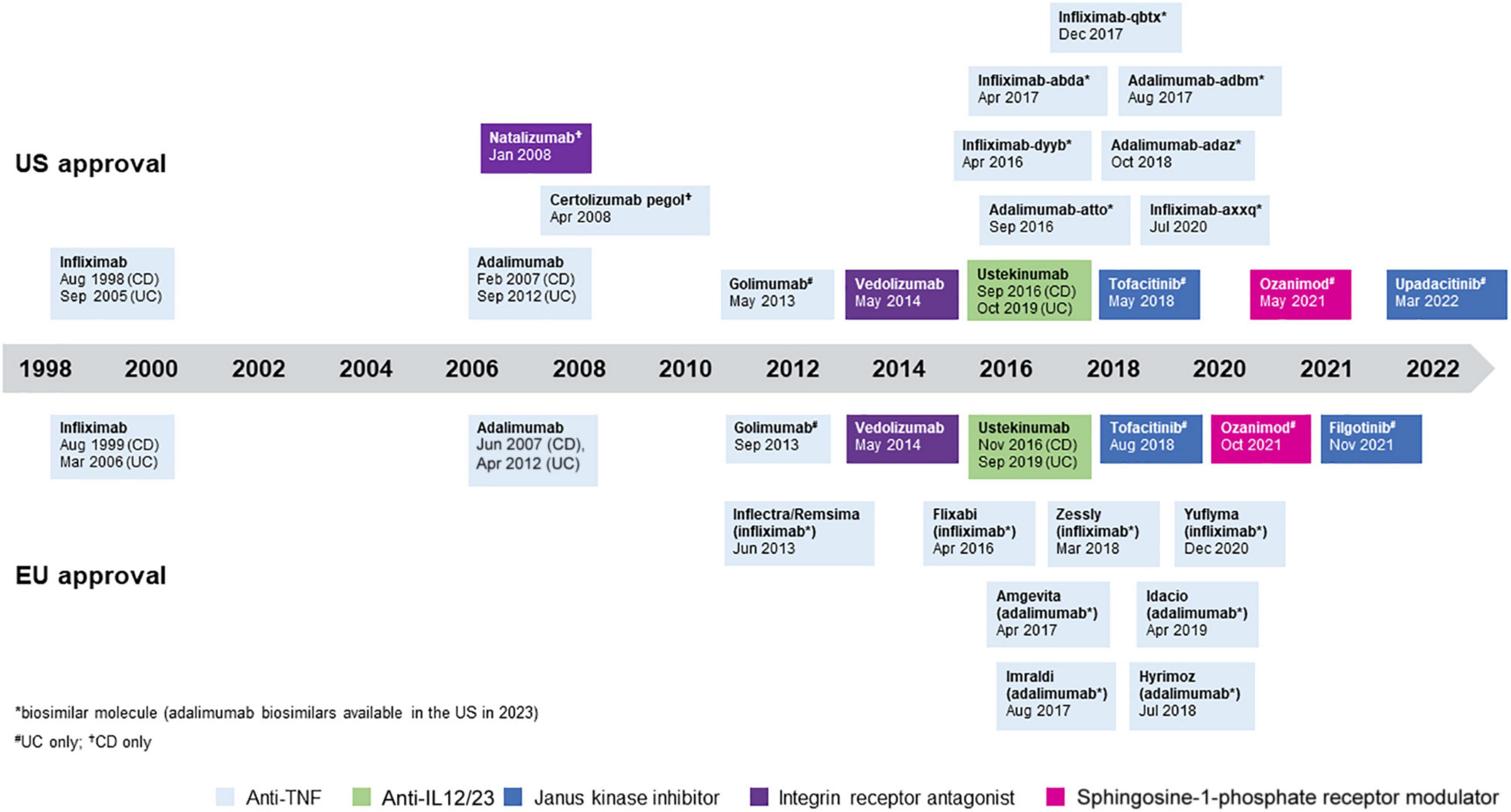
Figure 1. Approved treatments for inflammatory bowel disease (IBD). All treatments are approved for Crohn’s disease (CD) and ulcerative colitis (UC) unless otherwise specified.
The use of anti-TNFs has been shown to improve clinical symptoms, promote endoscopic healing, improve QoL and reduce hospitalizations and surgeries in patients with IBD (15, 16), benefits that can be increased by use early in the disease course, at least in CD (10, 15, 17). While anti-TNFs usually follow initial treatment in a step-up approach, in some patients with moderate-to-severe IBD and prognostic factors of unfavorable outcome (i.e., young age at diagnosis, perianal disease, penetrating disease in CD, and extensive disease) early anti-TNF and immunomodulator combination therapy may be beneficial (18–23).
Unfortunately, failure of anti-TNF therapy can occur and questions that naturally arise are whether regaining response with the current drug or drug class is possible and/or what the patient should be treated with next. This review explores the management of treatment options for IBD patients with a primary non-response (PNR) or loss of response (LOR) to anti-TNF therapy.
While there is no consensus definition of PNR it has been suggested to mean the failure to achieve a clinical response within 14 weeks of initiating treatment (1–4, 13). It has been reported that PNR to anti-TNFs occurs in 10–40% of patients with IBD (24–26). Primary non-response to anti-TNFs may be caused by a number of pharmacokinetic (drug concentrations) or pharmacodynamic (mechanistic) factors (6). Pharmacokinetic PNR is due to increased drug clearance, which may be immune mediated or non-immune mediated. It has also been shown that a proportion of administered anti-TNF is lost from the intestines of UC patients with active disease and that PNR is associated with the highest levels of anti-TNF observed in the feces (27). In contrast, pharmacodynamic PNR occurs when active disease persists despite therapeutic biologic drug levels, which implies that the binding of the drug to TNF is blocked or the presence of a non-inflammatory complication such as stenosis, abscess or a superimposed infection that has not been recognized; or that the underlying disease pathophysiology is primarily driven by inflammatory mediators other than TNF. Low albumin levels have been consistently associated with low infliximab levels and correlate with diminished clinical response, particularly in the setting of severe IBD such as in acute UC (28, 29).
Loss of response refers to those situations where patients respond to initial treatment with anti-TNFs but then subsequently and progressively lose this response. It has been reported that up to 50% of patients experience LOR over time and that the annual rate is ∼5–20% (30–33). The wide range of frequencies reported for LOR between studies can be explained by the differing definitions that have been used. These include those based on a worsening of symptoms, the need for dose escalation, an increased level of inflammation, stopping the drug, as well as differences depending on which anti-TNF agent is being studied (30). Loss of response to anti-TNFs may be related to low trough serum drug concentrations and/or the potential presence of anti-drug antibodies (ADAbs), which result in suboptimal drug concentrations (34) or a reduction in TNF-binding capacity (35). However, in some cases, other mechanisms such as the disease transitioning to other cytokine pathways are thought to cause LOR (12, 34).
In patients with a suspicion of PNR or LOR to anti-TNF therapy, guidelines suggest detailed assessment to determine the possible cause as this will guide therapeutic management options (1–4, 13). The first step is to determine whether the increase in symptoms is caused by a true increase in IBD activity or something else. Alternative causes for an increase in symptoms that should be ruled out include gastrointestinal infections, irritable bowel disease, bacterial overgrowth, and bile acid malabsorption (the latter being typically seen in patients with CD that have extensive ileal disease or have undergone ileal resection). The second step is to assess the level of disease-associated inflammatory activity present. A summary of various tools that can be used to assess inflammation is shown in Table 1.
Given the still limited number of available therapies for IBD in 2021, early optimization of a patient’s current treatment and maintenance of clinical response/remission is important to avoid a rapid progression through therapeutic options. A key factor in this is assessment of adherence to treatment as this remains a critical factor in achieving and sustaining remission in IBD (36, 37). Patient-related factors that have been shown to be associated with poorer adherence to treatment include male sex, shorter IBD duration, and clinic non-attendances, Conversely, patients’ preferences have been shown to be important to consider to optimize adherence (36, 38).
Therapeutic drug monitoring (TDM) to determine drug trough serum concentrations and anti-drug antibodies (ADAbs) can help guide treatment optimization, improve outcomes of patients receiving anti-TNFs, and enhance cost efficiency (39–42). Treatment decisions where TDM may offer guidance include dose escalation, de-escalation or stopping, adding an immunomodulator, or switching to an alternative anti-TNF agent (switch within class) or a drug with a different mechanism of action (switch out of class). Such decisions can be made empirically but studies have shown that the use of TDM as a support for decision making is more cost-effective and provides better outcomes (43). An algorithm to guide the optimization of IBD therapy using TDM is shown in Figure 2 (42).
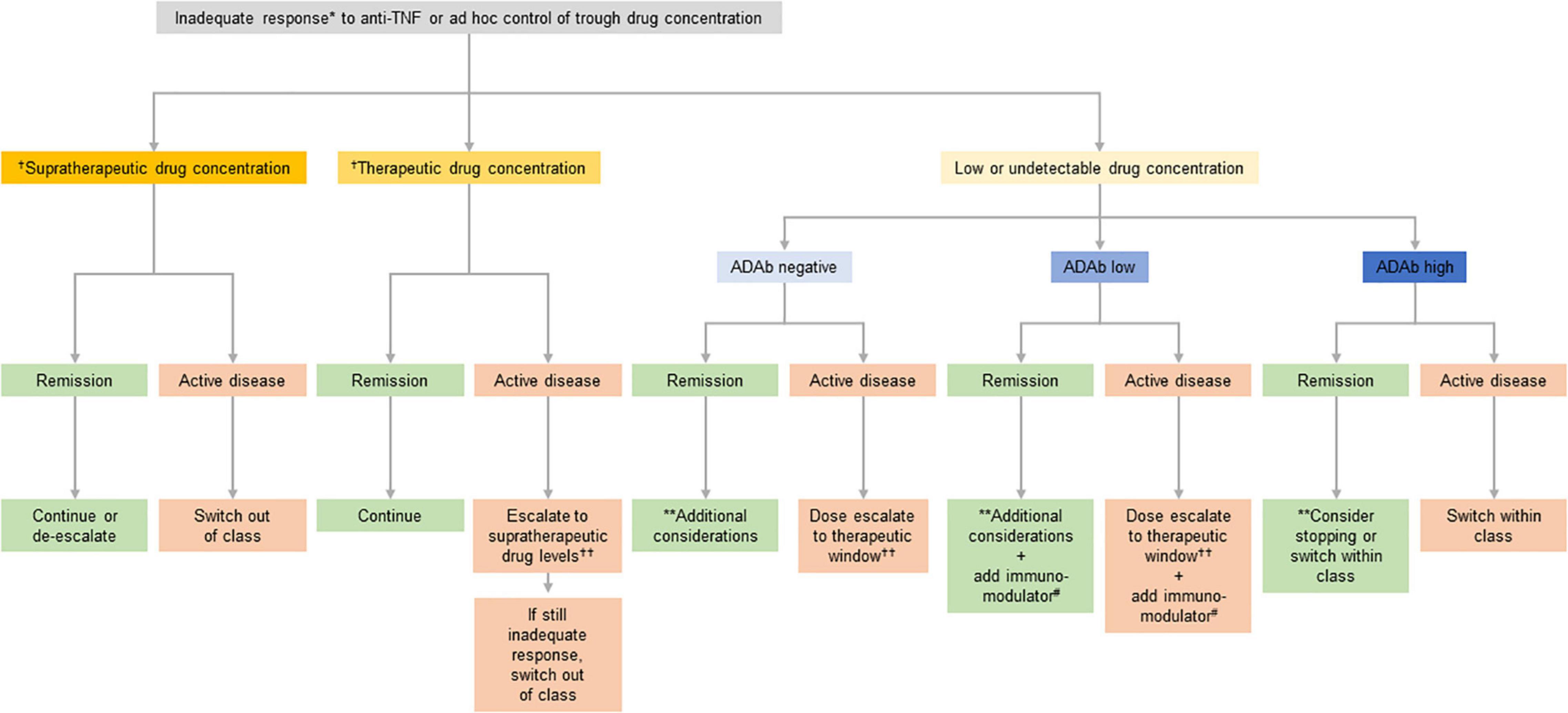
Figure 2. Suggested clinical therapeutic drug monitoring (TDM)-based algorithm for optimizing anti-tumor necrosis factor (anti-TNF) therapy. *If disease activity is defined by symptoms confirm inflammatory activity and/or rule out potential non-inflammatory causes. Potential non-inflammatory causes of increased symptoms include fibrotic stricture, gastrointestinal infection, irritable bowel syndrome, bacterial overgrowth, bile salt diarrhea, colorectal cancer, and andamyloidosis. **This situation may be interpreted either as: (A) the patient being in remission despite not having any relevant anti-TNF activity (low/undetectable drug concentration) and thus it may be stopped; or (B) the patient is in the first step toward a potential relapse according to the multi-step hypothesis suggesting that the first step toward a relapse is a decline in drug concentration, the second step an increase in subclinical inflammation, and the final step a clinical relapse, and thus the drug concentration should be brought back to the therapeutic window. Deciding on which of the two is most likely involves taking several aspects into account including the patient’s disease history, comorbidities, and concomitant medications. †See Table 2 for suggested supratherapeutic and therapeutic drug concentrations. ††Both increase in dose (at standard doses) and increase in frequency are appropriate but maintaining the dose interval saves on nurse/infusion-related resources. #Immunomodulator defined as azathioprine or methotrexate.
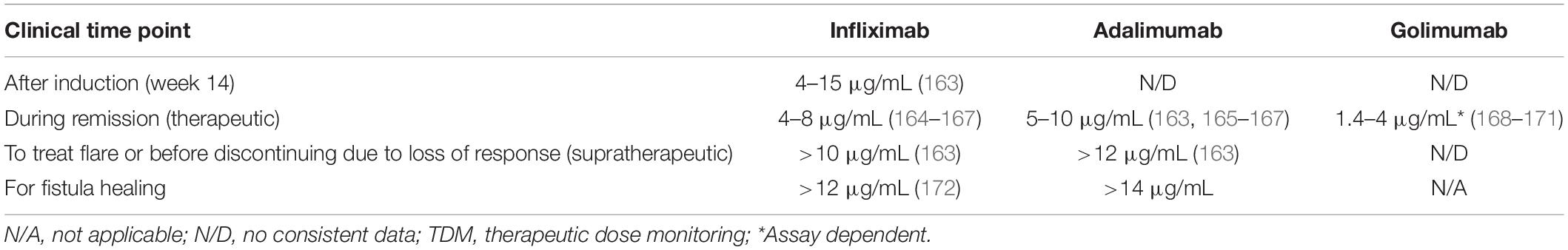
Table 2. Proposed target levels of anti-tumor necrosis factors (anti-TNFs) for clinical decision making based on published data and expert opinion.
TDM can be either reactive (occurs in response to treatment failure to guide therapy) or proactive [occurs at prescheduled time-points irrespective of disease activity to prevent LOR (39, 43)]. As this review discusses management options following failure of first-line anti-TNF, TDM here refers primarily to the reactive version.
TDM is recommended for patients suspected of experiencing PNR to anti-TNFs (41, 44). However, the results of TDM need to be reviewed alongside other factors to ensure that the patient is having a true PNR and that drug levels are not low due to other causes, including poor adherence. For primary non-responders to anti-TNF therapy with low drug concentrations and who are ADAb negative or low ADAb positive (as defined by the method used), dose escalation is recommended in an attempt to optimize symptom and inflammation management (Figure 2; 1–4, 12, 13).
TDM is also recommended for patients suspected of having LOR to anti-TNFs (41, 44), as treatment optimization can be guided by TDM in a similar way as in the event of PNR (Figure 2). Dose escalation may reduce or even reverse the loss of therapeutic response to anti-TNF therapy (37, 45–47). Billioud et al. (45) reported that while one fifth of patients with CD experience a LOR after initiation of adalimumab therapy, dose escalation resulted in response recovery in the majority of patients. Similarly, adalimumab dose escalation enabled recovery of response in nearly half of patients with UC that had experienced LOR (47). In patients with CD with LOR to a standard infliximab dose, shortening the dosing interval from 8 to 6 weeks was at least as effective as doubling the dose (46). On balance, published data suggest that there is no increased risk of infections or other complications with increased doses or serum concentrations of anti-TNFs (47–53).
A number of studies based on small numbers of patients suggest that the addition of an immunomodulator can reverse ADAb formation and LOR, with some studies reporting that response could be regained in over half of all patients treated with anti-TNFs (54–59). The effects of immunomodulation can impact outcomes as early as 4 weeks after the addition of the immunomodulator (56), but on occasion they can take 2–3 months to achieve the full therapeutic effect. A course of steroids used as a bridge until the immunomodulator becomes effective may be an option for these patients.
The use of TDM is helpful in the decision to switch within class to another anti-TNF. If the patient has developed ADAbs, and has previously benefited from an anti-TNF, then using another anti-TNF is a viable option as antibodies are specific for a given therapeutic molecule (a biosimilar is considered as the same anti-TNF in this specific context). This can be an effective alternative treatment strategy for patients with PNR or LOR if they have subtherapeutic drug concentrations and high levels of ADAbs. Based on published data and the authors’ clinical experience, Table 2 proposes levels of anti-TNFs that can be used to make clinical decisions at various clinical situations. Of note, some occurrences of PNR within 14 weeks from start of treatment may actually be a rapid LOR. Supplementary Table 1 provides a summary of selected relevant studies. Overall, available data suggest that switching within class to another anti-TNF following LOR is a viable strategy for a sub-group of patients and that TDM may help identify these patients (60). In addition, some small studies have reported clinical effectiveness following the use of a third, and even fourth, anti-TNF in some patients with CD following failure of two or more previous anti-TNFs (61–63). However, with the arrival of agents with alternative mechanisms of action, this option is not commonly used and may be reserved for certain patients with extra-intestinal manifestations (EIMs).
If a patient does not achieve an adequate therapeutic response with an anti-TNF agent and has therapeutic or supratherapeutic drug levels (Table 3), then selecting an agent from a different treatment class is an appropriate approach. Treatments with alternative mechanisms of action, such as vedolizumab, ustekinumab, and tofacitinib (the latter has only been approved for UC), may be considered (Supplementary Table 2; 1–4, 12, 13).
The degree of efficacy following switching appears to vary by treatment type and previous therapy received. Singh et al. (64) reported that patients with PNR to anti-TNF agents were less likely to respond to second-line non-TNF biologics, as compared with patients who discontinued therapy due to intolerance. In addition, patients with PNR were less likely to respond to second-line ustekinumab than patients with LOR, but there was no difference between patients treated with vedolizumab. These findings may be attributed to the pharmacokinetics and pharmacodynamics of anti-TNFs in patients with PNR.
Some data suggest that biologic-naïve patients respond better to therapy than anti-TNF experienced patients. For example, post-hoc analyses of efficacy data from the GEMINI 2 and GEMINI 3 studies reported rates of response and remission to be numerically higher in patients with CD receiving vedolizumab as a first biologic than in patients who had previously experienced an inadequate response with anti-TNFs (65); clinical efficacy of vedolizumab appeared similar between the different types and number of anti-TNFs previously used. A meta-analysis based upon the CERTIFI and UNITI-1 clinical trials demonstrated that use of ustekinumab resulted in significantly higher responses than placebo in patients with LOR to anti-TNFs, those who had previously received ≥ 2 anti-TNFs, and in intolerant patients, but not in the case of PNR (66). Similar data have been published for patients with UC. A retrospective, observational cohort study of 722 patients with UC showed that vedolizumab-treated patients were more likely to achieve deep clinical remission than those treated with anti-TNFs and that this response was blunted by prior exposure to anti-TNFs (67). For ustekinumab, while an extensive literature review of clinical trials and real-world evidence noted that the efficacy of ustekinumab appears to be blunted by increased use of anti-TNF agents (68), an analysis of data from 95 UC patients from the ENEIDA registry found that number of previous biologic treatments did not affect the response to ustekinumab (69). Finally, exposure to anti-TNFs does not seem to affect the response to tofacitinib (70). Recently, ozanimod has been approved for the treatment of UC. Data from the phase III trial indicated that while treatment effect sizes for ozanimod were not different between anti-TNF naïve and experienced patients, rates of clinical response and clinical remission tended to favor the anti-TNF naïve group, mirroring what has been observed with vedolizumab and ustekinumab (71–73). Thus, while switching out of class can be an effective strategy for some patients, the reason for switching and the patient’s treatment history needs to be considered.
Prior immunogenicity to anti-TNFs does not appear to confer an increased risk of immunogenicity to ustekinumab or vedolizumab (74). The efficacy profiles of non-anti-TNF biologics may also influence treatment choice given that some may additionally treat EIMs of IBD. For example, while ustekinumab may be selected to treat UC or CD, it has also demonstrated efficacy in the treatment of paradoxical psoriasiform skin drug reactions and cutaneous manifestations of IBD (75).
It should also be borne in mind that PNR to anti-TNFs may be representative of a very sick patient who is thus less likely to respond to any biologic that is prescribed.
Understanding different features that contribute to the efficacy of a certain drug may help to predict the therapeutic response in patients with IBD, thus providing the potential for personalized medicine (76, 77). Factors that are important to consider in this context are patient characteristics, comorbidities, disease phenotype, EIMs, the patient’s preferences, results from biomarker analyses, and treatment costs.
Patient-related factors, such as smoking and obesity, may increase the risk of LOR to anti-TNFs, suggesting the need for dose-escalation and alternative therapeutic approaches, such as possible lifestyle changes (34, 78, 79). Kennedy et al. (34) reported the need for dose intensification during induction for at-risk individuals (e.g., patients with obesity and regular smokers) and iterative dose adjustment to achieve target drug concentrations greater than those currently recommended had the potential to improve the durability and effectiveness of anti-TNF therapy in these patients.
The clinical effectiveness of anti-TNF therapy does not seem to differ between older and younger patients [≥60 vs. <60 years (80, 81)]. However, it has been reported that elderly patients had a higher risk of treatment failure with an initial anti-TNF agent compared with younger individuals (81, 82). Furthermore, the risk of serious adverse events and/or serious infections were significantly higher in those ≥60 years, which could be linked to potential comorbidities present (80).
For patients who are, or aim to become, pregnant, available guidelines suggest that all anti-TNFs are safe but could be discontinued at the start of the third trimester in patients with inactive disease (12). For patients with active disease or a high risk of relapse it is recommended to continue this treatment throughout pregnancy. Of note, the potential long-term effects of anti-TNFs on the unborn child throughout pregnancy are still unknown. However, as more data are being collected that are reassuring regarding long-term safety, experts in the field advocate an increasingly lower threshold for maintaining remission-protective treatment throughout pregnancy (83, 84). Although vedolizumab and ustekinumab are recommended to be used with caution, data to suggest that these agents are equally safe as anti-TNF agents are accumulating (85, 86). A recent analysis of 1,490 pregnancies among women with IBD across multiple centers in the US showed that biologic, thiopurine or combination therapy during pregnancy was not associated with increased maternal or fetal outcomes during the first year of life (85). Tofacitinib and ozanimod are thus far contraindicated in pregnancy; patients planning a pregnancy should not start either agent if alternative options are available. However, the data on tofacitinib are continually evolving and as such the decision to continue tofacitinib during pregnancy should be made in discussion with maternal-fetal medicine experts and following full explanation of uncertainty with the patient.
While the presence of comorbidities did not increase the risk of malignancies with anti-TNF use, the presence of cardiovascular disease was independently associated with the occurrence of serious infections (80) and no differences in the clinical effectiveness of anti-TNFs between patients with and without comorbidity with IBD were reported. Thus, patients with cardiovascular disease deemed to be at increased risk of infection may require additional assessment including an overview of the patient’s vaccination status prior to the use of anti-TNFs (see below). In patients with heart disease, such as congestive heart failure and rhythm disturbances, use of anti-TNFs may lead to worsening of cardiac function and alternative agents should be considered.
Patients with IBD may also develop serious infections due to the disease itself or its treatment, including biologic therapies. Increased susceptibility to infections with anti-TNFs, such as tuberculosis, prompts that physicians should try to detect and treat any latent infections and consider the overall risk of opportunistic infections prior to anti-TNF therapy (87, 88); of note, screening does not completely eliminate risk of infection. While the use of vaccinations is country dependent, guidance on opportunistic infections has recently been published by ECCO (89). All patient candidates for treatment with immunomodulators and/or targeted therapies or who are already receiving a targeted therapy should have their vaccine history checked and be provided with influenza and pneumococcal vaccines. While hepatitis B vaccination is usually performed in newborns, immunization status should be assessed and vaccination provided, where seronegative. Patients should be vaccinated for herpes zoster; while the old vaccine had to be administered at least 3 months prior to the initiation of anti-TNFs, the new inactivated vaccine, which is now readily available in many countries, can be given at any time and should therefore be recommended. Availability of the human papillomavirus vaccine varies by country, but should be used, where possible. The use of varicella vaccine should also be considered in those patients without any history of varicella (89). Vaccination for SARS-CoV-2 should also be recommended and this can be administered at any time (90). A recent report suggests that the vaccine response could be blunted by the use of anti-TNFs (91). However, other data suggest that IBD patients become seropositive after two doses of vaccine despite being under treatment with biologics (92) and that anti-TNFs could provide a protective effect against the disease (93). Taken together, booster doses are most likely beneficial for the patients with a blunted SARS-CoV-2 vaccine response, such as those under potent immunomodulatory/targeted therapy including IBD patients (94), and is recommended by local health authorities.
There are conflicting data on the safety of anti-TNFs in patients with active cancer or a history of cancer. In some patients the use of anti-TNFs may be an option in discussion with an experienced oncologist (95–97).
Some treatments may not be suitable for every CD or UC phenotype suggesting the need to select the management approach (e.g., biologics, immunomodulators, steroids and/or surgery) that best targets and addresses the structural complications of the specific patient (15, 98, 99). Importantly, multidisciplinary teams may be needed to support and implement appropriate therapeutic decisions (100).
Available guidelines recommend the use of infliximab for the induction and maintenance of remission in complex perianal fistulae in patients with CD (4, 12). Of note, fistula healing may be more likely in patients with higher infliximab trough levels, suggesting the need for personalized dosing in this setting (4). Adalimumab may also be used to manage complex perianal fistulae (4, 12). There is insufficient evidence regarding the effect of adding immunomodulators to anti-TNFs on fistula healing. In addition, there is currently insufficient evidence to recommend the use of vedolizumab for fistula healing in patients with CD (4, 12, 101). A recent meta-analysis including 198 patients from four studies demonstrated that use of vedolizumab led to the healing of perianal fistulas in approximately one third of patients (102). Finally, recent evidence suggests that ustekinumab may be effective against fistulas (103, 104).
For patients with acute severe UC, guidelines recommend the use of infliximab (1, 2), although no guidance is available regarding the routine use of intensive compared with standard infliximab dosing (1). There are indications that an accelerated dosing regimen could be beneficial (105, 106), however data are scarce and weak in this area thus far.
Up to 50% of patients with IBD experience EIMs (most commonly affecting the joints, skin, hepatobiliary tract, and eyes), which may parallel luminal disease activity or have an independent course (15, 107–109). For EIMs that are typically independent of intestinal disease activity choosing a more systemic therapy such as an anti-TNF, ustekinumab, or tofacitinib is preferred (15), although ustekinumab has not been shown to be effective in the management of axial arthropathies (110). In general, anti-TNFs appear to provide good response rates for cutaneous manifestations, arthritis, and ocular EIMs (100, 109). However, although data are sparse, ustekinumab may be preferred for some (but not all) cutaneous conditions, such as psoriasis or paradoxical psoriasiform drug reactions. Data remain both limited and conflicting for the use of vedolizumab for EIMs, with some suggesting an improvement in EIMs with treatment (111–113), while others suggest an increase in both the development and worsening of EIMs during treatment (114, 115).
Denesh et al. (116) recently reported that most patients with IBD prefer oral treatments. However, those patients who have already experienced biologic agents have a high level of acceptance for both subcutaneous and intravenous forms of medication (116). While oral formulations remain limited to the JAK inhibitors in IBD with regards to targeted therapies, subcutaneous and intravenous formulations of anti-TNFs, and subsequent anti-IL12/23s and integrin receptor antagonists allow additional patient choice which may support both patient empowerment and compliance (117–119). Of note, while many physicians think that patients prefer subcutaneous treatments over intravenous administration, this is not true for all patients (117). Some patients prefer IV administration with reasons given varying from less frequent dosing, convenience, the chance for interaction with hospital staff, and reassurance with medical presence (120).
Clinicians currently lack a valid tool that can predict an individual patient’s response to treatment and support both initial and subsequent therapeutic choices (76). Several candidate genetic, immunological, pharmacokinetic, and microbial biomarkers have been tested but due to low sensitivity and specificity, low practical feasibility and high costs associated with the suggested procedures, they are difficult to use in clinical practice. However, gene expression profiling, molecular imaging, and the microbiome have potential as future predictive factors of therapeutic efficacy (121).
Genetics may play a part in the therapeutic response given genetic risk alleles appear to predict PNR and durable response to anti-TNF therapy in patients with CD (122–124). A genome-wide association study by Sazonovs et al. reported a significant association between allelic variation in the HLA-DQA1 gene (HLA-DQA1*05 allele) and the development of ADAbs against anti-TNF agents. Thus, HLA-DQ1A*05 may serve as a useful biomarker of immunogenicity risk and testing for this variant might help physicians to decide whether they should receive anti-TNFs in combination with immunomodulator therapy (124). In addition, pharmacogenetic testing has the potential to support improved patient stratification, optimize treatment selection/dose, and to minimize harm caused by adverse drug reactions (125). Arijs et al. (126) reported a 100% accurate predictive gene signature for (non) response to infliximab in patients with Crohn’s colitis, although no such a predictive gene set could be identified for those with Crohn’s ileitis. Finally, Lee et al. (123) showed that the presence of a gene expression signature associated with CD8+ T cells was significantly associated with an increased risk of LOR in patients with CD.
The relationship between the gut microbiota and drugs used in the treatment of IBD may prove to be a source of future biomarkers (127). Aden et al. (128) suggest that metabolic network reconstruction and assessment of metabolic profiles of fecal samples could be used to identify patients with IBD likely to achieve clinical remission following anti-TNF therapy. Other studies suggest that low levels of Faecalibacterium prausnitzii and Bacteroides in the gut may predict relapse after discontinuation of anti-TNF therapy (129), and differences in gut microbiome may be able to differentiate between responders and non-responders (130–132).
While biomarkers predictive of efficacy constitute a promising area of research, their use is currently not recommended in clinical practice.
Cost may also play a role in a physician’s choice of treatment in IBD (4), motivating the use of dose optimization or switching within class instead of switching out of class when no other factors influence treatment choice (Figure 2). Biologic drugs are associated with a high cost (133, 134) which may limit access and result in non-optimized initiation and duration of therapy (135). Due to the chronic nature of IBD and associated high clinical, economic and societal burden, an efficacious, yet cost-effective, approach to its long-term management needs to be considered (136–139). Clinical trials, analytical models and systematic reviews have consistently found TDM-guided strategies for the treatment of IBD to be cost-saving or cost-effective compared with standard treatment without TDM (140–144). The introduction of less costly biosimilar anti-TNF drugs has also been associated with significant cost reductions and has expanded access to biologics in countries, including low-income countries (145–147). The safety and effectiveness of biosimilars within IBD have been established in an increasing body of evidence since the introduction of the first infliximab biosimilar in 2013 (12, 148, 149). As such, anti-TNF biosimilars are strongly recommended as first-line therapy by regulatory authorities. The increasing availability of subcutaneous forms of biologics, such as infliximab (CT-P13), adalimumab, ustekinumab and vedolizumab, are also expected to affect cost considerations (150–152), and the relationship between cost and subcutaneous administration should be clarified.
Several factors need to be considered when deciding upon the best treatment following PNR or LOR to anti-TNF therapy. Here we have presented evidence and experience-based decision-making factors that may help clinicians when deciding to switch within class or to switch out of class to a treatment with a different mechanism of action. Prior to switching treatment, it is critical to understand the reason as to why a patient is not responding, since this can affect management decisions and treatment choices. Switching within class should be considered in those patients with LOR due to high levels of ADAbs and/or where dose escalation has failed. The addition of an immunomodulator may also be considered, if ADAb-levels are low. Switching out of class appears to be an appropriate strategy in true PNR and those patients with a LOR with adequate serum trough drug levels. However, there is no consensus on the standardization of cut-off values for anti-TNF serum concentrations and some patients who are within a “therapeutic window” may still benefit from increased dosing. Treatment decisions also need to incorporate factors that may favor switching within, or out of, class including patient characteristics, disease phenotypes, comorbidities, EIMs, patient preference, and cost. Hopefully the guidance contained within this review will assist physicians in making informed treatment choices resulting in optimal long-term outcomes for their patients.
All authors made substantial contributions to the concept and design, analysis and interpretation of data, drafting of the manuscript or revising it critically for important intellectual content, and provided final approval of the manuscript.
Funding for editorial assistance with this manuscript was provided by Biogen International GmbH, Baar, Switzerland. The funder was not involved in the writing of this article or the decision to submit it for publication.
MB-A has received financial support for traveling and educational activities from or has served as an advisory board member for Pfizer, MSD, Takeda, Abbvie, Kern, Janssen, Fresenius Kabi, Biogen, Ferring, Faes Farma, Shire Pharmaceuticals, Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceuticals, and Tillotts Pharma. TB has received financial support for traveling and educational activities from or has served as an advisory board member for Takeda, Janssen, and Tillotts Pharma. IB has served as an advisory board member for Pfizer, MSD, Takeda, Abbvie, Galapagos, Amgen, Arena Pharma, BMS, Janssen, Fresenius Kabi, Biogen, Ferring, Dr. Falk Pharma, and Tillotts Pharma. MC has received lecture fees and has served as advisory board member for Takeda, Janssen, Shire, MSD, Abbvie, Ferring, Fresenius, and Biogen. JM has served as a speaker, consultant or advisory board member for AbbVie, Bayer, Biogen, Bristol-Myers Squibb, Ferring, Hospira, Janssen, MSD, Otsuka, Pfizer, Sandoz, Svar, Takeda, Tillotts, and UCB, and has received grant support from AbbVie, Ferring, Fresenius Kabi, Pfizer, and Takeda. SS has received personal fees from Janssen, Takeda, Galapagos, Celltrion, Falk Pharma, Tillots pharma, Cellgene, Pfizer, and Pharmacocosmos, and has received grant support from Takeda, Abbvie, Amgen, Tillots Pharm, and Biogen.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Editorial assistance in the preparation of this manuscript was provided by Matthew Joynson and Iain Bartlett of Springer Healthcare Ltd.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.897936/full#supplementary-material
1. Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S, et al. Aga clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. (2020) 158:1450–61. doi: 10.1053/j.gastro.2020.01.006
2. Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. (2017) 11:769–84. doi: 10.1093/ecco-jcc/jjx009
3. Terdiman JP, Gruss CB, Heidelbaugh JJ, Sultan S, Falck-Ytter YT, Practice AGAIC, et al. American gastroenterological association institute guideline on the use of thiopurines, methotrexate, and anti-Tnf-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s Disease. Gastroenterology. (2013) 145:1459–63. doi: 10.1053/j.gastro.2013.10.047
4. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. Ecco guidelines on therapeutics in Crohn’s Disease: medical treatment. J Crohns Colitis. (2020) 14:4–22. doi: 10.1093/ecco-jcc/jjz180
5. Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, et al. Stride-Ii: an update on the selecting therapeutic targets in inflammatory bowel disease (Stride) initiative of the international organization for the study of Ibd (Ioibd): determining therapeutic goals for treat-to-target strategies in Ibd. Gastroenterology. (2021) 160:1570–83. doi: 10.1053/j.gastro.2020.12.031
6. Sparrow MP, Papamichael K, Ward MG, Riviere P, Laharie D, Paul S, et al. Therapeutic drug monitoring of biologics during induction to prevent primary non-response. J Crohns Colitis. (2020) 14:542–56. doi: 10.1093/ecco-jcc/jjz162
7. Tsui JJ, Huynh HQ. Is top-down therapy a more effective alternative to conventional step-up therapy for crohn’s disease? Ann Gastroenterol. (2018) 31:413–24. doi: 10.20524/aog.2018.0253
8. Ordas I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or tnf antagonists for the treatment of Crohn’s Disease: time for a change. Gut. (2011) 60:1754–63. doi: 10.1136/gutjnl-2011-300934
9. D’Haens GR. Top-down therapy for Ibd: rationale and requisite evidence. Nat Rev Gastroenterol Hepatol. (2010) 7:86–92. doi: 10.1038/nrgastro.2009.222
10. Berg DR, Colombel JF, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis. (2019) 25:1896–905. doi: 10.1093/ibd/izz059
11. Salahudeen MS. A review of current evidence allied to step-up and top-down medication therapy in inflammatory bowel disease. Drugs Today. (2019) 55:385–405. doi: 10.1358/dot.2019.55.6.2969816
12. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. (2019) 68:s1–106. doi: 10.1136/gutjnl-2019-318484
13. Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. (2017) 11:649–70. doi: 10.1093/ecco-jcc/jjx008
14. Sandborn WJ, Feagan BG, Hanauer SB, Lichtenstein GR. The guide to guidelines in ulcerative colitis: interpretation and appropriate use in clinical practice. Gastroenterol Hepatol (2021) 17:3–13.
15. Chang S, Hudesman D. First-line biologics or small molecules in inflammatory bowel disease: a practical guide for the clinician. Curr Gastroenterol Rep. (2020) 22:7. doi: 10.1007/s11894-020-0745-y
16. Hossain A, Lordal M, Olsson AE, Storlahls A, Aleman S, Eberhardson M, et al. Sustained clinical benefit, improved quality of life, and reduced intestinal surgery from maintenance infliximab treatment in inflammatory bowel disease. Scand J Gastroenterol. (2020) 55:178–83. doi: 10.1080/00365521.2020.1722738
17. Frei R, Fournier N, Zeitz J, Scharl M, Morell B, Greuter T, et al. Early initiation of anti-tnf is associated with favourable long-term outcome in crohn’s disease: 10-year-follow-up data from the swiss ibd cohort study. J Crohns Colitis. (2019) 13:1292–301. doi: 10.1093/ecco-jcc/jjz057
18. Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of crohn’s disease. Gastroenterology. (2006) 130:650–6. doi: 10.1053/j.gastro.2005.12.019
19. D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed crohn’s disease: an open randomised trial. Lancet. (2008) 371:660–7. doi: 10.1016/S0140-6736(08)60304-9
20. Dias CC, Rodrigues PP, da Costa-Pereira A, Magro F. Clinical prognostic factors for disabling crohn’s disease: a systematic review and meta-analysis. World J Gastroenterol. (2013) 19:3866–71. doi: 10.3748/wjg.v19.i24.3866
21. Loly C, Belaiche J, Louis E. Predictors of severe crohn’s disease. Scand J Gastroenterol. (2008) 43:948–54. doi: 10.1080/00365520801957149
22. Reinisch W, Reinink AR, Higgins PD. Factors associated with poor outcomes in adults with newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. (2015) 13:635–42. doi: 10.1016/j.cgh.2014.03.037
23. Torres J, Caprioli F, Katsanos KH, Lobaton T, Micic D, Zeroncio M, et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis. (2016) 10:1385–94. doi: 10.1093/ecco-jcc/jjw116
24. Kassouri L, Amiot A, Kirchgesner J, Treton X, Allez M, Bouhnik Y, et al. The outcome of crohn’s disease patients refractory to anti-tnf and either vedolizumab or ustekinumab. Dig Liver Dis. (2020) 52:1148–55. doi: 10.1016/j.dld.2020.07.031
25. Panaccione R, Ghosh S. Optimal use of biologics in the management of crohn’s disease. Therap Adv Gastroenterol. (2010) 3:179–89. doi: 10.1177/1756283X09357579
26. Sabino J, Verstockt B, Vermeire S, Ferrante M. New biologics and small molecules in inflammatory bowel disease: an update. Therap Adv Gastroenterol. (2019) 12:1756284819853208. doi: 10.1177/1756284819853208
27. Brandse JF, van den Brink GR, Wildenberg ME, van der Kleij D, Rispens T, Jansen JM, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. (2015) 149:350–5e2. doi: 10.1053/j.gastro.2015.04.016
28. Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. (2009) 65:1211–28. doi: 10.1007/s00228-009-0718-4
29. Syal G, Robbins L, Kashani A, Bonthala N, Feldman E, Fleshner P, et al. Hypoalbuminemia and bandemia predict failure of infliximab rescue therapy in acute severe ulcerative colitis. Dig Dis Sci. (2021) 66:199–205. doi: 10.1007/s10620-020-06177-7
30. Ben-Horin S, Chowers Y. Review article: loss of response to anti-tnf treatments in crohn’s disease. Aliment Pharmacol Ther. (2011) 33:987–95. doi: 10.1111/j.1365-2036.2011.04612.x
31. Fine S, Papamichael K, Cheifetz AS. Etiology and management of lack or loss of response to anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Gastroenterol Hepatol. (2019) 15:656–65.
32. Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in crohn’s disease: a review. Am J Gastroenterol. (2009) 104:760–7. doi: 10.1038/ajg.2008.88
33. Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-tnfs: definition, epidemiology, and management. Clin Transl Gastroenterol. (2016) 7:e135. doi: 10.1038/ctg.2015.63
34. Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, et al. Predictors of anti-Tnf treatment failure in anti-Tnf-naive patients with active luminal crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. (2019) 4:341–53. doi: 10.1016/S2468-1253(19)30012-3
35. Vande Casteele N, Khanna R, Levesque BG, Stitt L, Zou GY, Singh S, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in crohn’s disease. Gut. (2015) 64:1539–45. doi: 10.1136/gutjnl-2014-307883
36. Tripathi K, Dong J, Mishkin BF, Feuerstein JD. Patient preference and adherence to aminosalicylates for the treatment of ulcerative colitis. Clin Exp Gastroenterol. (2021) 14:343–51. doi: 10.2147/CEG.S237653
37. Khan S, Rupniewska E, Neighbors M, Singer D, Chiarappa J, Obando C. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the united states: a systematic review. J Clin Pharm Ther. (2019) 44:495–507. doi: 10.1111/jcpt.12830
38. Haar GS, Vasudevan A, Curtain CM, van Langenberg DR. Assessing adherence to infusion-based biologic therapies in patients with inflammatory bowel disease. Res Social Adm Pharm. (2021) 17:1420–5. doi: 10.1016/j.sapharm.2020.10.011
39. Argollo M, Kotze PG, Kakkadasam P, D’Haens G. Optimizing biologic therapy in ibd: how essential is therapeutic drug monitoring? Nat Rev Gastroenterol Hepatol. (2020) 17:702–10. doi: 10.1038/s41575-020-0352-2
40. Dreesen E, Bossuyt P, Mulleman D, Gils A, Pascual-Salcedo D. Practical recommendations for the use of therapeutic drug monitoring of biopharmaceuticals in inflammatory diseases. Clin Pharmacol. (2017) 9:101–11. doi: 10.2147/CPAA.S138414
41. Mitrev N, Vande Casteele N, Seow CH, Andrews JM, Connor SJ, Moore GT, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. (2017) 46:1037–53. doi: 10.1111/apt.14368
42. Papamichael K, Vande Casteele N, Ferrante M, Gils A, Cheifetz AS. Therapeutic drug monitoring during induction of anti-tumor necrosis factor therapy in inflammatory bowel disease: defining a therapeutic drug window. Inflamm Bowel Dis. (2017) 23:1510–5. doi: 10.1097/MIB.0000000000001231
43. Papamichael K, Cheifetz AS. Therapeutic drug monitoring in patients on biologics: lessons from gastroenterology. Curr Opin Rheumatol. (2020) 32:371–9. doi: 10.1097/BOR.0000000000000713
44. Papamichael K, Cheifetz AS, Melmed GY, Irving PM, Vande Casteele N, Kozuch PL, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2019) 17:1655–68e3. doi: 10.1016/j.cgh.2019.03.037
45. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in crohn’s disease: a systematic review. Am J Gastroenterol. (2011) 106:674–84. doi: 10.1038/ajg.2011.60
46. Kopylov U, Mantzaris GJ, Katsanos KH, Reenaers C, Ellul P, Rahier JF, et al. The efficacy of shortening the dosing interval to once every six weeks in crohn’s patients losing response to maintenance dose of infliximab. Aliment Pharmacol Ther. (2011) 33:349–57. doi: 10.1111/j.1365-2036.2010.04523.x
47. Taxonera C, Iglesias E, Munoz F, Calvo M, Barreiro-de Acosta M, Busquets D, et al. Adalimumab maintenance treatment in ulcerative colitis: outcomes by prior anti-Tnf use and efficacy of dose escalation. Dig Dis Sci. (2017) 62:481–90. doi: 10.1007/s10620-016-4398-5
48. Taxonera C, Olivares D, Mendoza JL, Diaz-Rubio M, Rey E. Need for infliximab dose intensification in crohn’s disease and ulcerative colitis. World J Gastroenterol. (2014) 20:9170–7. doi: 10.3748/wjg.v20.i27.9170
49. Dumitrescu G, Amiot A, Seksik P, Baudry C, Stefanescu C, Gagniere C, et al. The outcome of infliximab dose doubling in 157 patients with ulcerative colitis after loss of response to infliximab. Aliment Pharmacol Ther. (2015) 42:1192–9. doi: 10.1111/apt.13393
50. Hendler SA, Cohen BL, Colombel JF, Sands BE, Mayer L, Agarwal S. High-dose infliximab therapy in crohn’s disease: clinical experience, safety, and efficacy. J Crohns Colitis. (2015) 9:266–75. doi: 10.1093/ecco-jcc/jju026
51. Landemaine A, Petitcollin A, Brochard C, Miard C, Dewitte M, Le Balc’h E, et al. Cumulative exposure to infliximab, but not trough concentrations, correlates with rate of infection. Clin Gastroenterol Hepatol. (2021) 19:288–95e4. doi: 10.1016/j.cgh.2020.03.018
52. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2005) 353:2462–76. doi: 10.1056/NEJMoa050516
53. Taxonera C, Barreiro-de Acosta M, Calvo M, Saro C, Bastida G, Martin-Arranz MD, et al. Infliximab dose escalation as an effective strategy for managing secondary loss of response in ulcerative colitis. Dig Dis Sci. (2015) 60:3075–84. doi: 10.1007/s10620-015-3735-4
54. Ben-Horin S, Waterman M, Kopylov U, Yavzori M, Picard O, Fudim E, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. (2013) 11:444–7. doi: 10.1016/j.cgh.2012.10.020
55. Bond A, Clark K, Gregg B, Collins P, Dibb M, Probert C, et al. Pth-049 successful reversal of high titre antibodies to infliximab and adalimumab with the addition of immunomodulator therapy. Gut. (2016) 65:A243.
56. Macaluso FS, Sapienza C, Ventimiglia M, Renna S, Rizzuto G, Orlando R, et al. The addition of an immunosuppressant after loss of response to anti-tnfalpha monotherapy in inflammatory bowel disease: a 2-year study. Inflamm Bowel Dis. (2018) 24:394–401. doi: 10.1093/ibd/izx010
57. Strik AS, van den Brink GR, Ponsioen C, Mathot R, Lowenberg M, D’Haens GR. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther. (2017) 45:1128–34. doi: 10.1111/apt.13994
58. Ungar B, Kopylov U, Engel T, Yavzori M, Fudim E, Picard O, et al. Addition of an immunomodulator can reverse antibody formation and loss of response in patients treated with adalimumab. Aliment Pharmacol Ther. (2017) 45:276–82. doi: 10.1111/apt.13862
59. Roblin X, Williet N, Boschetti G, Phelip JM, Del Tedesco E, Berger AE, et al. Addition of azathioprine to the switch of anti-Tnf in patients with Ibd in clinical relapse with undetectable anti-Tnf trough levels and antidrug antibodies: a prospective randomised trial. Gut. (2020) 69:1206–12. doi: 10.1136/gutjnl-2019-319758
60. Gisbert JP, Marin AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-Tnf in patients with inflammatory bowel disease whose previous anti-tnf treatment has failed. Aliment Pharmacol Ther. (2015) 41:613–23. doi: 10.1111/apt.13083
61. Allez M, Vermeire S, Mozziconacci N, Michetti P, Laharie D, Louis E, et al. The efficacy and safety of a third anti-tnf monoclonal antibody in crohn’s disease after failure of two other anti-tnf antibodies. Aliment Pharmacol Ther. (2010) 31:92–101. doi: 10.1111/j.1365-2036.2009.04130.x
62. de Silva PS, Nguyen DD, Sauk J, Korzenik J, Yajnik V, Ananthakrishnan AN. Long-term outcome of a third anti-tnf monoclonal antibody after the failure of two prior anti-tnfs in inflammatory bowel disease. Aliment Pharmacol Ther. (2012) 36:459–66. doi: 10.1111/j.1365-2036.2012.05214.x
63. Russi L, Scharl M, Rogler G, Biedermann L. The efficacy and safety of golimumab as third- or fourth-line anti-tnf therapy in patients with refractory crohn’s disease: a case series. Inflamm Intest Dis. (2017) 2:131–8. doi: 10.1159/000481400
64. Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: a systematic review and meta-analysis. J Crohns Colitis. (2018) 12:635–43. doi: 10.1093/ecco-jcc/jjy004
65. Sands BE, Sandborn WJ, Van Assche G, Lukas M, Xu J, James A, et al. Vedolizumab as induction and maintenance therapy for crohn’s disease in patients naive to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. (2017) 23:97–106. doi: 10.1097/MIB.0000000000000979
66. Kawalec P, Mocko P, Malinowska-Lipien I, Brzostek T. Efficacy and safety of ustekinumab in the induction therapy of tnf-alpha-refractory crohn’s disease patients: a systematic review and meta-analysis. J Comp Eff Res. (2017) 6:601–12. doi: 10.2217/cer-2017-0022
67. Lukin D, Faleck D, Xu R, Zhang Y, Weiss A, Aniwan S, et al. Comparative safety and effectiveness of vedolizumab to tumor necrosis factor antagonist therapy for ulcerative colitis. Clin Gastroenterol Hepatol. (2020) 20:126–35. doi: 10.1016/j.cgh.2020.10.003
68. Gutierrez A, Rodriguez-Lago I. How to optimize treatment with ustekinumab in inflammatory bowel disease: lessons learned from clinical trials and real-world data. Front Med. (2021) 8:640813. doi: 10.3389/fmed.2021.640813
69. Chaparro M, Garre A, Iborra M, Sierra M, Barreiro-de Acosta M, Fernandez-Clotet A, et al. Effectiveness and safety of ustekinumab in ulcerative colitis: real-world evidence from the eneida registry. J Crohns Colitis. (2021) 15:1846–51. doi: 10.1093/ecco-jcc/jjab070
70. Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2017) 376:1723–36. doi: 10.1056/NEJMoa1606910
71. Danese S, Feagan B, Hanauer S, Jovanovic I, Ghosh S, Petersen AK, et al. Ozanimod efficacy, safety, and histology in patients with moderate-to-severe ulcerative colitis during maintenance in the phase 3 true north study. Am J Gastroenterol. (2020) 115:030.
72. Sandborn W, D’Haens G, Wolf D, Hanauer S, Jovanovic I, Ghosh S, et al. Ozanimod efficacy, safety, and histology in patients with moderate-to-severe ulcerative colitis during induction in the phase 3 true north study. Am J Gastroenterol. (2020) 115:025.
73. Sandborn WJ, Feagan BG, D’Haens G, Wolf DC, Jovanovic I, Hanauer SB, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2021) 385:1280–91. doi: 10.1056/NEJMoa2033617
74. Costable NJ, Borman ZA, Ji J, Dubinsky MC, Ungaro RC. Prior immunogenicity to anti-tnf biologics is not associated with increased anti-drug antibodies to vedolizumab or ustekinumab. Dig Dis Sci. (2021) [Online ahead of print]. doi: 10.1007/s10620-021-07046-7
75. Wu J, Smogorzewski J. Ustekinumab for the treatment of paradoxical skin reactions and cutaneous manifestations of inflammatory bowel diseases. Dermatol Ther. (2021) 34:e14883. doi: 10.1111/dth.14883
76. Privitera G, Pugliese D, Rapaccini GL, Gasbarrini A, Armuzzi A, Guidi L. Predictors and early markers of response to biological therapies in inflammatory bowel diseases. J Clin Med. (2021) 10:10040853. doi: 10.3390/jcm10040853
77. Volk N, Siegel CA. Defining failure of medical therapy for inflammatory bowel disease. Inflamm Bowel Dis. (2019) 25:74–7. doi: 10.1093/ibd/izy238
78. Danese S, Fiorino G, Reinisch W. Review article: causative factors and the clinical management of patients with crohn’s disease who lose response to anti-tnf-alpha therapy. Aliment Pharmacol Ther. (2011) 34:1–10. doi: 10.1111/j.1365-2036.2011.04679.x
79. Singh S, Facciorusso A, Singh AG, Vande Casteele N, Zarrinpar A, Prokop LJ, et al. Obesity and response to anti-tumor necrosis factor-alpha agents in patients with select immune-mediated inflammatory diseases: a systematic review and meta-analysis. PLoS One. (2018) 13:e0195123. doi: 10.1371/journal.pone.0195123
80. Asscher VER, van der Vliet Q, van der Aalst K, van der Aalst A, Brand EC, van der Meulen-de Jong AE, et al. Anti-tumor necrosis factor therapy in patients with inflammatory bowel disease; comorbidity, not patient age, is a predictor of severe adverse events. Int J Colorectal Dis. (2020) 35:2331–8. doi: 10.1007/s00384-020-03716-6
81. de Jong ME, Smits LJT, van Ruijven B, den Broeder N, Russel M, Romkens TEH, et al. Increased discontinuation rates of anti-tnf therapy in elderly inflammatory bowel disease patients. J Crohns Colitis. (2020) 14:888–95. doi: 10.1093/ecco-jcc/jjaa012
82. Cottone M, Kohn A, Daperno M, Armuzzi A, Guidi L, D’Inca R, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. (2011) 9:30–5. doi: 10.1016/j.cgh.2010.09.026
83. Duricova D, Dvorakova E, Hradsky O, Mitrova K, Durilova M, Kozeluhova J, et al. Safety of anti-tnf-alpha therapy during pregnancy on long-term outcome of exposed children: a controlled, multicenter observation. Inflamm Bowel Dis. (2019) 25:789–96. doi: 10.1093/ibd/izy294
84. Mahadevan U. Overview of pregnancy in patients with inflammatory bowel disease. Gastroenterol Hepatol. (2021) 17:73–5.
85. Mahadevan U, Long MD, Kane SV, Roy A, Dubinsky MC, Sands BE, et al. Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease. Gastroenterology. (2021) 160:1131–9. doi: 10.1053/j.gastro.2020.11.038
86. Wils P, Seksik P, Stefanescu C, Nancey S, Allez M, Pineton de Chambrun G, et al. Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: a multicentre cohort study. Aliment Pharmacol Ther. (2021) 53:460–70. doi: 10.1111/apt.16192
87. Murdaca G, Negrini S, Pellecchio M, Greco M, Schiavi C, Giusti F, et al. Update upon the infection risk in patients receiving tnf alpha inhibitors. Expert Opin Drug Saf. (2019) 18:219–29. doi: 10.1080/14740338.2019.1577817
88. Theis VS, Rhodes JM. Review article: minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment Pharmacol Ther. (2008) 27:19–30. doi: 10.1111/j.1365-2036.2007.03553.x
89. Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, et al. Ecco guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. (2021) 15:879–913. doi: 10.1093/ecco-jcc/jjab052
90. Neurath MF. Covid-19: biologic and immunosuppressive therapy in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. (2021) 18:705–15. doi: 10.1038/s41575-021-00480-y
91. Kennedy NA, Goodhand JR, Bewshea C, Nice R, Chee D, Lin S, et al. Anti-Sars-Cov-2 antibody responses are attenuated in patients with ibd treated with infliximab. Gut. (2021) 70:865–75. doi: 10.1136/gutjnl-2021-324388
92. Wong SY, Dixon R, Martinez Pazos V, Gnjatic S, Colombel JF, Cadwell K, et al. Serologic response to messenger Rna coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology. (2021) 161:715–8e4. doi: 10.1053/j.gastro.2021.04.025
93. Ungaro RC, Brenner EJ, Agrawal M, Zhang X, Kappelman MD, Colombel JF, et al. Impact of medications on Covid-19 outcomes in inflammatory bowel disease: analysis of over 6,000 patients from an international registry. Gastroenterology. (2021) 162:316.e–9.e. doi: 10.1053/j.gastro.2021.09.011
94. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of Bnt162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. (2021) 385:1393–400. doi: 10.1056/NEJMoa2114255
95. Chupin A, Perduca V, Meyer A, Bellanger C, Carbonnel F, Dong C. Systematic review with meta-analysis: comparative risk of lymphoma with anti-tumour necrosis factor agents and/or thiopurines in patients with inflammatory bowel disease. Aliment Pharmacol Ther. (2020) 52:1289–97. doi: 10.1111/apt.16050
96. Dahmus J, Rosario M, Clarke K. Risk of lymphoma associated with anti-tnf therapy in patients with inflammatory bowel disease: implications for therapy. Clin Exp Gastroenterol. (2020) 13:339–50. doi: 10.2147/CEG.S237646
97. Sebastian S, Neilaj S. Practical guidance for the management of inflammatory bowel disease in patients with cancer. which treatment? Therap Adv Gastroenterol. (2019) 12:1756284818817293. doi: 10.1177/1756284818817293
98. Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-tnf therapy in crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther. (2016) 43:30–51. doi: 10.1111/apt.13445
99. Dubinsky MC, Mei L, Friedman M, Dhere T, Haritunians T, Hakonarson H, et al. Genome wide association (Gwa) predictors of anti-tnfalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. (2010) 16:1357–66. doi: 10.1002/ibd.21174
100. Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. (2016) 10:239–54. doi: 10.1093/ecco-jcc/jjv213
101. Singh S, Proctor D, Scott FI, Falck-Ytter Y, Feuerstein JD. Aga technical review on the medical management of moderate to severe luminal and perianal fistulizing crohn’s disease. Gastroenterology. (2021) 160:2512–56e9. doi: 10.1053/j.gastro.2021.04.023
102. Ayoub F, Odenwald M, Micic D, Dalal SR, Pekow J, Cohen RD, et al. Vedolizumab for perianal fistulizing crohn’s disease: systematic review and meta-analysis. Intest Res. (2022) 20:240–50. doi: 10.5217/ir.2021.00091
103. Attauabi M, Burisch J, Seidelin JB. Efficacy of ustekinumab for active perianal fistulizing crohn disease: a double-center cohort study. Inflamm Bowel Dis. (2021) 27:e37–8. doi: 10.1093/ibd/izaa297
104. Godoy Brewer GM, Salem G, Afzal MA, Limketkai BN, Haq Z, Tajamal M, et al. Ustekinumab Is effective for perianal fistulising crohn’s disease: a real-world experience and systematic review with meta-analysis. BMJ Open Gastroenterol. (2021) 8:702. doi: 10.1136/bmjgast-2021-000702
105. Choy MC, Seah D, Faleck DM, Shah SC, Chao CY, An YK, et al. Systematic review and meta-analysis: optimal salvage therapy in acute severe ulcerative colitis. Inflamm Bowel Dis. (2019) 25:1169–86. doi: 10.1093/ibd/izy383
106. Gibson DJ, Doherty J, McNally M, Campion J, Keegan D, Keogh A, et al. Comparison of medium to long-term outcomes of acute severe ulcerative colitis patients receiving accelerated and standard infliximab induction. Frontline Gastroenterol. (2020) 11:441–7. doi: 10.1136/flgastro-2019-101335
107. Greuter T, Rieder F, Kucharzik T, Peyrin-Biroulet L, Schoepfer AM, Rubin DT, et al. Emerging treatment options for extraintestinal manifestations in Ibd. Gut. (2021) 70:796–802. doi: 10.1136/gutjnl-2020-322129
108. Hanzel J, Ma C, Casteele NV, Khanna R, Jairath V, Feagan BG. Vedolizumab and extraintestinal manifestations in inflammatory bowel disease. Drugs. (2021) 81:333–47. doi: 10.1007/s40265-020-01460-3
109. Hedin CRH, Vavricka SR, Stagg AJ, Schoepfer A, Raine T, Puig L, et al. The pathogenesis of extraintestinal manifestations: implications for Ibd research, diagnosis, and therapy. J Crohns Colitis. (2019) 13:541–54. doi: 10.1093/ecco-jcc/jjy191
110. Deodhar A, Gensler LS, Sieper J, Clark M, Calderon C, Wang Y, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. (2019) 71:258–70. doi: 10.1002/art.40728
111. Feagan BG, Sandborn WJ, Colombel JF, Byrne SO, Khalid JM, Kempf C, et al. Incidence of arthritis/arthralgia in inflammatory bowel disease with long-term vedolizumab treatment: post hoc analyses of the gemini trials. J Crohns Colitis. (2019) 13:50–7. doi: 10.1093/ecco-jcc/jjy125
112. Fleisher M, Marsal J, Lee SD, Frado LE, Parian A, Korelitz BI, et al. Effects of vedolizumab therapy on extraintestinal manifestations in inflammatory bowel disease. Dig Dis Sci. (2018) 63:825–33. doi: 10.1007/s10620-018-4971-1
113. Tadbiri S, Peyrin-Biroulet L, Serrero M, Filippi J, Pariente B, Roblin X, et al. Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the observ-ibd cohort. Aliment Pharmacol Ther. (2018) 47:485–93. doi: 10.1111/apt.14419
114. Dubinsky MC, Cross RK, Sandborn WJ, Long M, Song X, Shi N, et al. Extraintestinal manifestations in vedolizumab and anti-tnf-treated patients with inflammatory bowel disease. Inflamm Bowel Dis. (2018) 24:1876–82. doi: 10.1093/ibd/izy065
115. Ramos GP, Dimopoulos C, McDonald NM, Janssens LP, Hung KW, Proctor D, et al. The impact of vedolizumab on pre-existing extraintestinal manifestations of inflammatory bowel disease: a multicenter study. Inflamm Bowel Dis. (2021) 27:1270–6. doi: 10.1093/ibd/izaa293
116. Denesh D, Carbonell J, Kane JS, Gracie D, Selinger CP. Patients with Inflammatory Bowel Disease (Ibd) prefer oral tablets over other modes of medicine administration. Expert Rev Gastroenterol Hepatol. (2021) 15:1091–6. doi: 10.1080/17474124.2021.1898944
117. Allen PB, Lindsay H, Tham TC. How do patients with inflammatory bowel disease want their biological therapy administered? BMC Gastroenterol. (2010) 10:1. doi: 10.1186/1471-230X-10-1
118. Bell CF, Lau M, Lee M, Poulos C. Insights into the choice between intravenous infusion and subcutaneous injection: physician and patient characteristics driving treatment in Sle. Clin Rheumatol. (2021) 40:581–90. doi: 10.1007/s10067-020-05226-w
119. Liu J, Sylwestrzak G, Ruggieri AP, DeVries A. Intravenous versus subcutaneous anti-tnf-alpha agents for crohn’s disease: a comparison of effectiveness and safety. J Manag Care Spec Pharm. (2015) 21:559–66. doi: 10.18553/jmcp.2015.21.7.559
120. Overton PM, Shalet N, Somers F, Allen JA. Patient preferences for subcutaneous versus intravenous administration of treatment for chronic immune system disorders: a systematic review. Patient Prefer Adherence. (2021) 15:811–34. doi: 10.2147/PPA.S303279
121. Flamant M, Roblin X. Inflammatory bowel disease: towards a personalized medicine. Therap Adv Gastroenterol. (2018) 11:1756283X17745029. doi: 10.1177/1756283X17745029
122. Barber GE, Yajnik V, Khalili H, Giallourakis C, Garber J, Xavier R, et al. Genetic markers predict primary non-response and durable response to anti-tnf biologic therapies in crohn’s disease. Am J Gastroenterol. (2016) 111:1816–22. doi: 10.1038/ajg.2016.408
123. Lee JC, Lyons PA, McKinney EF, Sowerby JM, Carr EJ, Bredin F, et al. Gene expression profiling of cd8+ t cells predicts prognosis in patients with crohn disease and ulcerative colitis. J Clin Invest. (2011) 121:4170–9. doi: 10.1172/JCI59255
124. Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, et al. Hla-Dqa1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with crohn’s disease. Gastroenterology. (2020) 158:189–99. doi: 10.1053/j.gastro.2019.09.041
125. Voskuil MD, Bangma A, Weersma RK, Festen EAM. Predicting (Side) effects for patients with inflammatory bowel disease: the promise of pharmacogenetics. World J Gastroenterol. (2019) 25:2539–48. doi: 10.3748/wjg.v25.i21.2539
126. Arijs I, Quintens R, Van Lommel L, Van Steen K, De Hertogh G, Lemaire K, et al. Predictive value of epithelial gene expression profiles for response to infliximab in crohn’s disease. Inflamm Bowel Dis. (2010) 16:2090–8. doi: 10.1002/ibd.21301
127. Franzin M, Stefancic K, Lucafo M, Decorti G, Stocco G. Microbiota and drug response in inflammatory bowel disease. Pathogens. (2021) 10:10020211. doi: 10.3390/pathogens10020211
128. Aden K, Rehman A, Waschina S, Pan WH, Walker A, Lucio M, et al. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology. (2019) 157:1279–92e11. doi: 10.1053/j.gastro.2019.07.025
129. Rajca S, Grondin V, Louis E, Vernier-Massouille G, Grimaud JC, Bouhnik Y, et al. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in crohn’s disease. Inflamm Bowel Dis. (2014) 20:978–86. doi: 10.1097/MIB.0000000000000036
130. Shaw KA, Bertha M, Hofmekler T, Chopra P, Vatanen T, Srivatsa A, et al. Dysbiosis, inflammation, and response to treatment: a longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med. (2016) 8:75. doi: 10.1186/s13073-016-0331-y
131. Busquets D, Oliver L, Amoedo J, Ramio-Pujol S, Malagon M, Serrano M, et al. Raid prediction: pilot study of fecal microbial signature with capacity to predict response to anti-tnf treatment. Inflamm Bowel Dis. (2021) 27:S63–6. doi: 10.1093/ibd/izab273
132. Ventin-Holmberg R, Eberl A, Saqib S, Korpela K, Virtanen S, Sipponen T, et al. Bacterial and fungal profiles as markers of infliximab drug response in inflammatory bowel disease. J Crohns Colitis. (2021) 15:1019–31. doi: 10.1093/ecco-jcc/jjaa252
133. Gottlieb S. Don’t Give up on Biosimilars—Congress Can Give Them a Boost. New York City: Wall Street Journal (2019). Available line at: www.wsj.com/articles/dont-give-up-on-biosimilarscongress-can-give-them-a-boost-11566755042 (accessed July 19, 2021)
134. IQVIA. Global, European and Belgian Pharmaceutical Market Trends. (2019). Available online at:https://www.cib-pharma.be/uploads/global-european-and-belgian-pharmaceutical-market-trends-2019-final1552840137.pdf (accessed July 19, 2021)
135. Rezk MF, Pieper B. Unlocking the value of anti-tnf biosimilars: reducing disease burden and improving outcomes in chronic immune-mediated inflammatory diseases: a narrative review. Adv Ther. (2020) 37:3732–45. doi: 10.1007/s12325-020-01437-4
136. Armuzzi A, Bouhnik Y, Cummings F, Bettey M, Pieper B, Kang T. Enhancing treatment success in inflammatory bowel disease: optimising the use of anti-tnf agents and utilising their biosimilars in clinical practice. Dig Liver Dis. (2020) 52:1259–65. doi: 10.1016/j.dld.2020.06.008
137. Kim H, Alten R, Avedano L, Dignass A, Gomollon F, Greveson K, et al. The future of biosimilars: maximizing benefits across immune-mediated inflammatory diseases. Drugs. (2020) 80:99–113. doi: 10.1007/s40265-020-01256-5
138. Park SH, Park JC, Lukas M, Kolar M, Loftus EV. Biosimilars: concept, current status, and future perspectives in inflammatory bowel diseases. Intest Res. (2020) 18:34–44. doi: 10.5217/ir.2019.09147
139. Reinisch W, Gecse K, Halfvarson J, Irving PM, Jahnsen J, Peyrin-Biroulet L, et al. Clinical practice of adalimumab and infliximab biosimilar treatment in adult patients with crohn’s disease. Inflamm Bowel Dis. (2021) 27:106–22. doi: 10.1093/ibd/izaa078
140. Guidi L, Pugliese D, Panici Tonucci T, Berrino A, Tolusso B, Basile M, et al. Therapeutic drug monitoring is more cost-effective than a clinically based approach in the management of loss of response to infliximab in inflammatory bowel disease: an observational multicentre study. J Crohns Colitis. (2018) 12:1079–88. doi: 10.1093/ecco-jcc/jjy076
141. Martelli L, Olivera P, Roblin X, Attar A, Peyrin-Biroulet L. Cost-effectiveness of drug monitoring of anti-tnf therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. J Gastroenterol. (2017) 52:19–25. doi: 10.1007/s00535-016-1266-1
142. Steenholdt C, Brynskov J, Thomsen OO, Munck LK, Fallingborg J, Christensen LA, et al. Individualised therapy is more cost-effective than dose intensification in patients with crohn’s disease who lose response to anti-tnf treatment: a randomised, controlled trial. Gut. (2014) 63:919–27. doi: 10.1136/gutjnl-2013-305279
143. Velayos FS, Kahn JG, Sandborn WJ, Feagan BG. A test-based strategy is more cost effective than empiric dose escalation for patients with crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol. (2013) 11:654–66. doi: 10.1016/j.cgh.2012.12.035
144. Yao J, Jiang X, You JHS. A systematic review on cost-effectiveness analyses of therapeutic drug monitoring for patients with inflammatory bowel disease: from immunosuppressive to anti-tnf therapy. Inflamm Bowel Dis. (2021) 27:275–82. doi: 10.1093/ibd/izaa073
145. Di Giuseppe D, Frisell T, Ernestam S, Forsblad-D’Elia H, Lindqvist E, Lindstrom U, et al. Uptake of rheumatology biosimilars in the absence of forced switching. Expert Opin Biol Ther. (2018) 18:499–504. doi: 10.1080/14712598.2018.1458089
146. Pentek M, Lakatos PL, Oorsprong T, Gulacsi L, Pavlova M, Groot W, et al. Access to biologicals in crohn’s disease in ten European countries. World J Gastroenterol. (2017) 23:6294–305. doi: 10.3748/wjg.v23.i34.6294
147. Razanskaite V, Bettey M, Downey L, Wright J, Callaghan J, Rush M, et al. Biosimilar infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohns Colitis. (2017) 11:690–6. doi: 10.1093/ecco-jcc/jjw216
148. Bergqvist V, Kadivar M, Molin D, Angelison L, Hammarlund P, Olin M, et al. Switching from originator infliximab to the biosimilar Ct-P13 in 313 patients with inflammatory bowel disease. Therap Adv Gastroenterol. (2018) 11:1756284818801244. doi: 10.1177/1756284818801244
149. Jorgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar Ct-P13 compared with maintained treatment with originator infliximab (nor-switch): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. (2017) 389:2304–16. doi: 10.1016/S0140-6736(17)30068-5
150. Armuzzi A, Fiorino G, Variola A, Manetti N, Fries W, Orlando A, et al. The prosit cohort of infliximab biosimilar in ibd: a prolonged follow-up on the effectiveness and safety across Italy. Inflamm Bowel Dis. (2019) 25:568–79. doi: 10.1093/ibd/izy264
151. Ben-Horin S, Leszczyszyn J, Dudkowiak R, Lahat A, Gawdis-Wojnarska B, Pukitis A, et al. Op24 a novel subcutaneous infliximab (Ct-P13): 1-year results including switching results from intravenous infliximab (Ct-P13) in patients with active crohn’s disease and ulcerative colitis. J Crohns Colitis. (2020) 14:S21–2.
152. Sandborn WJ, Baert F, Danese S, Krznaric Z, Kobayashi T, Yao X, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. (2020) 158:562–72e12. doi: 10.1053/j.gastro.2019.08.027
153. Grinman AB, de Souza M, Bouskela E, Carvalho ATP, de Souza HSP. Clinical and laboratory markers associated with anti-tnf-alpha trough levels and anti-drug antibodies in patients with inflammatory bowel diseases. Medicine. (2020) 99:e19359. doi: 10.1097/MD.0000000000019359
154. Cappello M, Morreale GC. The role of laboratory tests in crohn’s disease. Clin Med Insights Gastroenterol. (2016) 9:51–62. doi: 10.4137/CGast.S38203
155. Bressler B, Panaccione R, Fedorak RN, Seidman EG. Clinicians’ guide to the use of fecal calprotectin to identify and monitor disease activity in inflammatory bowel disease. Can J Gastroenterol Hepatol. (2015) 29:369–72. doi: 10.1155/2015/852723
156. Moran CP, Neary B, Doherty GA. Endoscopic evaluation in diagnosis and management of inflammatory bowel disease. World J Gastrointest Endosc. (2016) 8:723–32. doi: 10.4253/wjge.v8.i20.723
157. Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. (2013) 7:827–51. doi: 10.1016/j.crohns.2013.06.001
158. Bruining DH, Zimmermann EM, Loftus EV Jr., Sandborn WJ, Sauer CG, Strong SA, et al. consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel crohn’s disease. Gastroenterology. (2018) 154:1172–94. doi: 10.1053/j.gastro.2017.11.274
159. Calabrese E, Rispo A, Zorzi F, De Cristofaro E, Testa A, Costantino G, et al. Ultrasonography tight control and monitoring in crohn’s disease during different biological therapies: a multicenter study. Clin Gastroenterol Hepatol. (2021) 20:e711–22. doi: 10.1016/j.cgh.2021.03.030
160. Castiglione F, Testa A, Rea M, De Palma GD, Diaferia M, Musto D, et al. Transmural healing evaluated by bowel sonography in patients with crohn’s disease on maintenance treatment with biologics. Inflamm Bowel Dis. (2013) 19:1928–34. doi: 10.1097/MIB.0b013e31829053ce
161. Goodsall TM, Jairath V, Feagan BG, Parker CE, Nguyen TM, Guizzetti L, et al. Standardisation of intestinal ultrasound scoring in clinical trials for luminal crohn’s disease. Aliment Pharmacol Ther. (2021) 53:873–86. doi: 10.1111/apt.16288
162. Maaser C, Petersen F, Helwig U, Fischer I, Roessler A, Rath S, et al. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the trust&Uc study. Gut. (2020) 69:1629–36. doi: 10.1136/gutjnl-2019-319451
163. Cheifetz AS, Abreu MT, Afif W, Cross RK, Dubinsky MC, Loftus EV Jr., et al. A comprehensive literature review and expert consensus statement on therapeutic drug monitoring of biologics in inflammatory bowel disease. Am J Gastroenterol. (2021) 116:2014–25. doi: 10.14309/ajg.0000000000001396
164. Drobne D, Kurent T, Golob S, Svegl P, Rajar P, Terzic S, et al. Success and safety of high infliximab trough levels in inflammatory bowel disease. Scand J Gastroenterol. (2018) 53:940–6. doi: 10.1080/00365521.2018.1486882
165. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American gastroenterological association institute clinical guidelines C. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. (2017) 153:827–34. doi: 10.1053/j.gastro.2017.07.032
166. Khan A, Berahmana AB, Day AS, Barclay ML, Schultz M. New Zealand society of gastroenterology guidelines on therapeutic drug monitoring in inflammatory bowel disease. NZ Med J. (2019) 132:46–62.
167. Ungar B, Levy I, Yavne Y, Yavzori M, Picard O, Fudim E, et al. Optimizing anti-tnf-alpha therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2016) 14:550–7e2. doi: 10.1016/j.cgh.2015.10.025
168. Adedokun OJ, Xu Z, Marano CW, Strauss R, Zhang H, Johanns J, et al. Pharmacokinetics and exposure-response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from phase 2/3 pursuit induction and maintenance studies. J Crohns Colitis. (2017) 11:35–46. doi: 10.1093/ecco-jcc/jjw133
169. Berends SE, Strik AS, Jansen JM, de Boer NK, van Egmond PS, Brandse JF, et al. Pharmacokinetics of golimumab in moderate to severe ulcerative colitis: the go-kinetic study. Scand J Gastroenterol. (2019) 54:700–6. doi: 10.1080/00365521.2019.1619828
170. Magro F, Lopes S, Silva M, Coelho R, Portela F, Branquinho D, et al. Low golimumab trough levels at week 6 are associated with poor clinical, endoscopic and histological outcomes in ulcerative colitis patients: pharmacokinetic and pharmacodynamic sub-analysis of the evolution study. J Crohns Colitis. (2019) 13:1387–93. doi: 10.1093/ecco-jcc/jjz071
171. Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American gastroenterological association institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. (2017) 153:835–57e6. doi: 10.1053/j.gastro.2017.07.031
172. Miranda EF, Nones RB, Kotze PG. Correlation of serum levels of anti-tumor necrosis factor agents with perianal fistula healing in crohn’s disease: a narrative review. Intest Res. (2021) 19:255–64. doi: 10.5217/ir.2020.00029
173. Berends SE, Strik AS, Lowenberg M, D’Haens GR, Mathot RAA. Clinical pharmacokinetic and pharmacodynamic considerations in the treatment of ulcerative colitis. Clin Pharmacokinet. (2019) 58:15–37. doi: 10.1007/s40262-018-0676-z
Keywords: anti-TNF, loss of response, primary non-response, switch out of class, switch within class, therapeutic drug monitoring
Citation: Marsal J, Barreiro-de Acosta M, Blumenstein I, Cappello M, Bazin T and Sebastian S (2022) Management of Non-response and Loss of Response to Anti-tumor Necrosis Factor Therapy in Inflammatory Bowel Disease. Front. Med. 9:897936. doi: 10.3389/fmed.2022.897936
Received: 16 March 2022; Accepted: 25 May 2022;
Published: 15 June 2022.
Edited by:
Enrique Quintero, University of La Laguna, SpainReviewed by:
Fernando Gomollón, University of Zaragoza, SpainCopyright © 2022 Marsal, Barreiro-de Acosta, Blumenstein, Cappello, Bazin and Sebastian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Marsal, amFuLm1hcnNhbEBtZWQubHUuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.