- 1Department of Nuclear Medicine, Ha Noi Medical University, Hanoi, Vietnam
- 2Nuclear Medicine and Oncology Center, Bach Mai Hospital, Hanoi, Vietnam
- 3Department of Examination, Bach Mai Hospital, Hanoi, Vietnam
- 4Pathology and Cytology Center, Bach Mai Hospital, Hanoi, Vietnam
- 5Department of Radiology, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Vietnam
Background: This study evaluated the prognostic ability of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) in patients with stage IV adenocarcinoma lung cancer to detect protein death-ligand 1 (PD-L1) expression levels.
Methods: In total, 86 patients with stage IV adenocarcinoma lung cancer underwent 18F-FDG PET/CT imaging and PD-L1 expression evaluation before treatment from February 2019 to November 2020 at Bach Mai Hospital, Hanoi, Vietnam. The assessed patient characteristics in this study included sex, age, smoking status, epidermal growth factor receptor (EGFR) mutation, PD-L1 expression level, survival status, tumor, node, and metastasis (TNM) stage, and metastasis locations.
Results: The average age was 62.23 ± 9.51 years, and men and women represented 67.4% and 32.6% of the population, respectively. The EGFR mutation rate was 36%. PD-L1 expression was negative (detected in <1% of the tumor) in 40.7% of cases and positive in 59.3% of cases (detected in 1–49% of the tumor in 32.6%; detected in ≥50% of the tumor in 26.7%). The mean maximum standardized uptake value (SUVmax) was 11.09 ± 3.94. SUVmax was significantly higher in PD-L1–positive tumors than in PD-L1–negative tumors (12.24 ± 4.01 and 9.43 ± 3.22, respectively; p = 0.001). Receiver operating characteristic curve analysis revealed an area under the curve of SUVmax was 0.681 (95% confidence interval 0.570–0.793, p = 0.004). Compared with PD-L1–negative cases, SUVmax was significantly different in all PD-L1–positive cases (p = 0.001), weakly PD-L1–positive cases (1–49%, p = 0.005), and strongly PD-L1–positive cases (≥50%, p = 0.003). PD-L1 expression levels were significantly associated with SUVmax (p = 0.001), tumor size (p = 0.022), and EGFR mutation status (p = 0.045).
Conclusions: SUVmax in the primary lesions was able to predict PD-L1 expression and may play a role in predicting PD-L1 immunotherapy efficacy in patients with stage IV lung adenocarcinoma.
Introduction
Lung cancer is associated with high morbidity and mortality rates. According to GLOBOCAN 2020, lung cancer is the second-most frequent cancer type worldwide (11.4%), accounting for over 2.3 million new cases each year. Lung cancer is estimated to be the leading cause of cancer-related death in both sexes (1) and is the leading cause of cancer-related death in most developed countries (2).
Lung cancer is highly aggressive, rapidly developing, and has poor prognosis, with a 5-year survival rate of only 15% for both sexes (2). However, the majority of individuals are diagnosed at stage III, and late-stage diagnosis (stage IV) is particularly common in developing countries. In recent years, immunotherapy have become increasingly popular treatment options for late stage lung cancer. Testing for programmed death-ligand 1 (PD-L1) expression level should be routinely performed routinely to choose the most suitable treatment approach for patients with non-small cell lung cancer (NSCLC) (3).
Positron emission tomography (PET)/computed tomography (CT) is a nuclear medicine diagnostic tool that enables early detection, provides comprehensive information regarding disease stage, and has prognostic value in patients with NSCLC. Monoclonal antibodies have been developed that target PD-L1, a critical immune system checkpoint. The binding of programmed cell death protein 1 (PD-1) with PD-L1 induces T-lymphocyte depletion or death. The inhibition of this signaling pathway has been shown to increase T cell activity, boost antitumor immunity, and prevent tumor cells from evading host immune responses, representing a viable technique for successful tumor immunotherapy (4).
Numerous studies have been conducted worldwide to ascertain the relationship between the maximum standard uptake value (SUVmax) and PD-L1 expression. Our published research has not established any relationships between PD-L1 expression levels and SUVmax in patients with NSCLC. PD-L1 expression is higher in solid tumors, such as lung cancer, breast cancer, colorectal cancer, and liver cancer, than in other tumor types (5–9).
Limited data is available for late-stage NSCLC, especially stage IV. Additionally, little research has been conducted in Vietnam examining PD-L1 expression in NSCLC patients, and no existing studies in Vietnam have demonstrated a relationship between PET/CT values and PD-L1 expression levels. Therefore, this study evaluated the prognostic significance of 18F-fluorodeoxyglucose (18F-FDG) PET/CT values to predict different PD-L1 expression levels in patients with stage IV adenocarcinoma lung cancer.
Materials and Methods
This cross-sectional study was conducted in patients with stage IV adenocarcinoma lung cancer treated at the Nuclear Medicine and Oncology Center and Pathology and Cytology Center, Bach Mai Hospital, from February 2019 to November 2020.
This study was approved by the Ethics Committee of Hanoi Medical University (accession No. NCS02/HMU-IRB), and written informed consent was obtained from all included patients. We included all patients with stage IV NSCLC with an adenocarcinoma histologic subtype treated at our hospital during the study period. Before treatment, patients underwent 18F-FDG PET/CT and were tested for PD-L1 expression and EGFR mutations. The normal functions of the liver, kidney, and bone marrow were recorded. All patients who agreed to participate in this study provided a complete medical record for study use. Exclusion criteria included lung cancer types and stages other than stage IV adenocarcinoma, no histologic sample from the primary tumor, or lack of EGFR mutation or PD-L1 testing. Patients whose primary tumors were not defined on 18F-FDG PET/CT were also excluded.
The patient characteristics assessed in this study included sex, age, smoking status, EGFR mutation, PD-L1 expression level, CEA level, Cyrfra 21-1 level, survival status, tumor, node, and metastasis (TNM) stage, and metastasis locations. Patients were asked to fast for 4 h prior to intravenous administration of 0.15 mCi/kg bodyweight 18F-FDG. PET testing was performed 45 min after 18F-FDG administration. 18F-FDG PET/CT was performed using an ECAT ACCEL (Siemens). Image slices were obtained and analyzed by Syngo Via software from the skull to midthigh vertex. The following variables were assessed using the PET/CT results: primary tumor size, TNM stage, and tumor characteristics. A region of interest (ROI) was manually placed on the lung lesion detected in PET/CT images, and SUVmax was obtained. Figure 1 shows one representative patient in our study with TNM staging according to PET/CT.
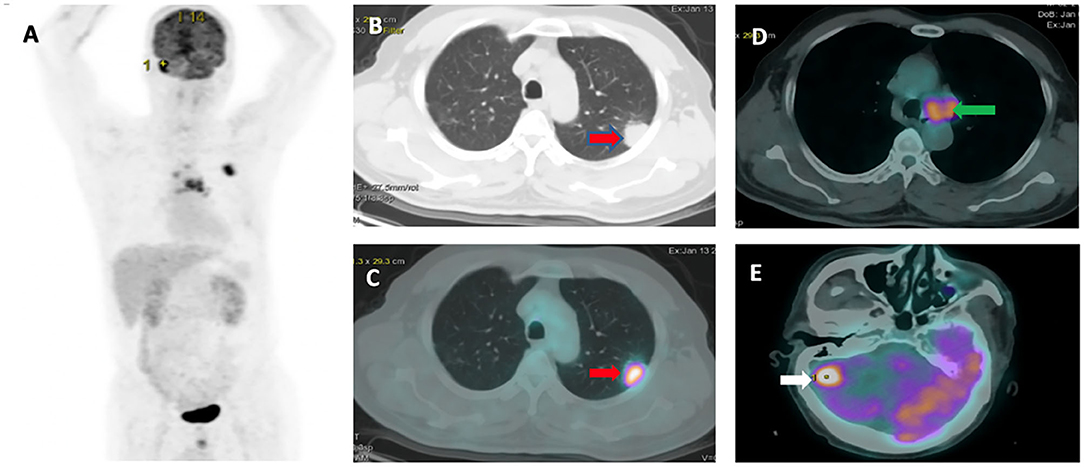
Figure 1. Representative 18F-FDG PET/CT images: (A) PET image. (B,C) Pleural tumor invasion was detected in the upper left lung with SUVmax or 10.17 (red arrow). (D) Mediastinal node metastasis (green arrow). (E) Brain metastasis (white arrow).
PD-L1 testing was performed at our institution by an expert pathologist. Samples were obtained from the primary tumor.
PD-L1 Expression
We followed the instructions provided in the “PD-L1 Immunohistochemistry Testing in Lung Cancer” manual distributed by the International Association for the Study of Lung Cancer (IASLC). Briefly, pathologists counted PD-L1–positive tumor cells, defined as complete circumferential or partial cell membrane staining. Cytoplasmic staining and tumor-associated immune cells, such as macrophages, were excluded from scoring. The tumor proportion score (TPS) was calculated as follows:
TPS (%) = (PD-L1–positive tumor cells / Total number of tumor cells) × 100. (https://www.accessdata.fda.gov/cdrh_docs/pdf16/p160046c.pdf).
The TPS was used to categorize PD-L1 expression status as follows: <1% (negative staining), 1%−49% (weakly positive staining), and ≥50% (highly positive staining). All tumors with TPS ≥1% were considered PD-L1–positive. Figure 2 shows representative images of the three PD-L1 categories.
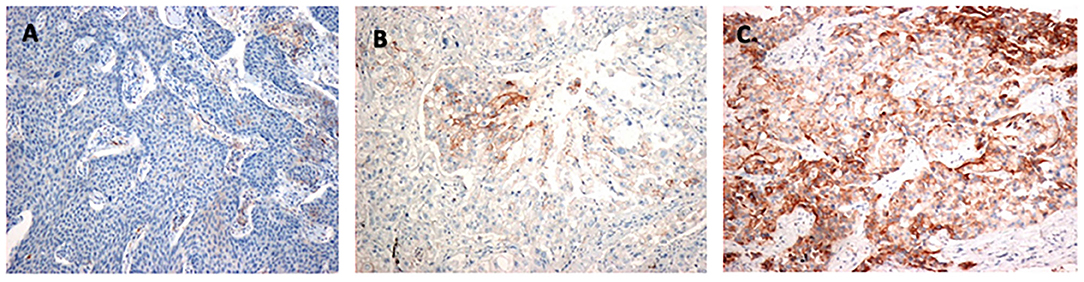
Figure 2. Identification of PD-L1–positive tumor cells: (A) Negative staining (TPS < 1%). (B) Weakly positive staining (TPS 1–49%). (C) Highly positive staining (TPS ≥ 50%). PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
Statistical Analysis
Statistical analyses were performed using SPSS, version 20.0 (SPSS Inc., Chicago, IL, USA). Data are presented as the number and frequency or the mean ± standard deviation (SD). The association between two continuous variables was analyzed by the Mann–Whitney U test or t-test. The association between two categorical variables was evaluated by the Chi-square test. Significance was established at p < 0.05.
Results
Patient Population
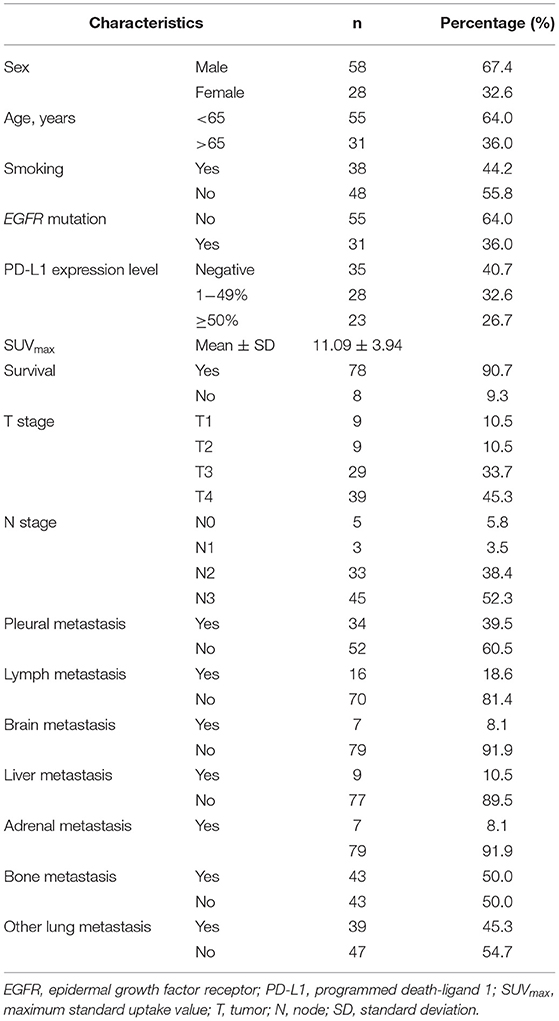
The general characteristics of the study population are shown in Table 1. The average age was 62.23 ± 9.51 years, and 64% of patients were younger than 65 years. Men represented 67.4% of the study population. Smoking was reported by 44.2% of patients. EGFR mutations were detected in 36% of the study population. PD-L1 expression was negative in 40.7% of cases and positive in 59.3% of cases, including 32.6% with weak PD-L1 expression (1–49% of cells) and 26.7% with strong PD-L1 expression (≥50% of cells). The mean SUVmax was 11.09 ± 3.94. Other characteristics are summarized in Table 1.
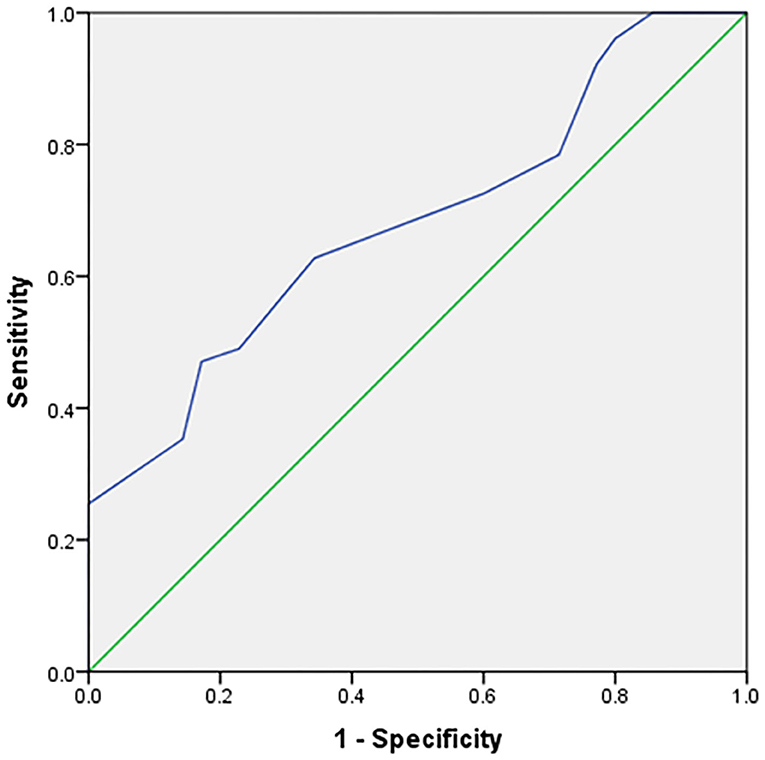
The expression of PD-L1 was evaluated in 86 tumor samples by immunohistochemical analysis. A positive association was identified between SUVmax from 18F-FDG PET/CT imaging and PD-L1 expression. SUVmax was significantly higher in PD-L1–positive tumors than in PD-L1–negative tumors (12.24 ± 4.01 vs. 9.43 ± 3.22, respectively; p = 0.001). The ability of SUVmax to predict PD-L1 expression was determined (Figure 3) by performing receiver operating characteristic curve analysis, which showed revealed an area under the curve (AUC) of 0.681 (95% confidence interval [95% CI] = 0.570–0.793, p = 0.004).
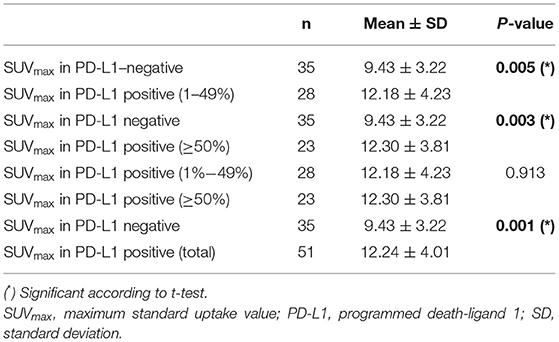
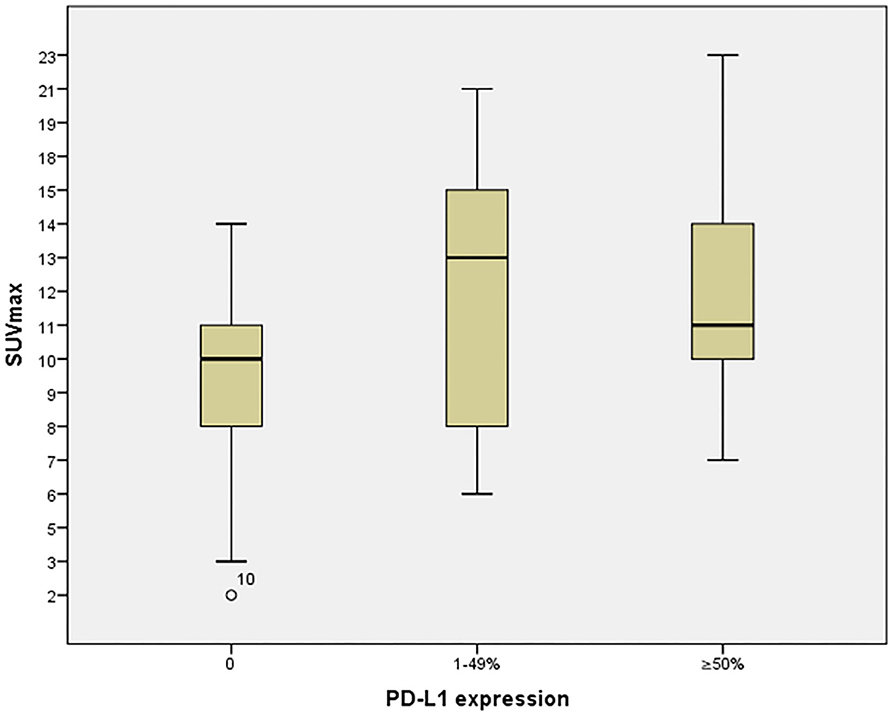
Table 2 shows the relationship between SUVmax and PD-L1 expression. Significance differences were identified between SUVmax in PD-L1–negative and SUVmax for all PD-L1–positive cases (p = 0.001), weakly PD-L1–positive cases (1–49%, p = 0.005), and strongly PD-L1–positive cases (≥50%, p = 0.003). These relationships are displayed graphically in Figure 4.
Table 3 shows all the associations between patient characteristics and PD-L1 expression status. PD-L1 expression was significantly associated with SUVmax (p = 0.001), tumor size (p = 0.022), and EGFR mutation status. However, we found no associations with age, sex, carcinoembryonic antigen (CEA) level, cytokeratin 19 fragment (CYFRA 21-1) level, smoking, survival status, T stage, N stage, or any metastasis location (pleural, lymph, brain, liver, adrenal, bone, or other lung).
Discussion
18F-FDG PET/CT can reveal disease at the molecular level prior to the occurrence of anatomical structural alterations, detected via observing changes in metabolism. SUVmax measured by 18F-FDG PET/CT has excellent reproducibility and is widely available; therefore, this measurement is often used to establish precise diagnoses, perform TNM staging, plan radiation therapy, and monitor therapeutic effects for lung cancers in comparison with other imaging modalities such as computed tomography, magnetic resonance imaging, and scintigraphy (10–12).
Takada et al. demonstrated that the metabolic features of lung cancers expressing PD-L1 on 18F-FDG PET/CT were associated with other parameters, such as smoking status, pleural invasion, and SUVmax (13). In our study, we discovered no relationships between PD-L1 and smoking status, pleural invasion, or any other invasion type.
In our investigation, 18F-FDG PET/CT measurements were able to predict PD-L1 expression status in stage IV adenocarcinoma lung cancer patients, with an AUC of 0.681. This outcome differs slightly from results reported by Cui Y et al., who studied 73 patients with adenocarcinoma lung cancer and found an AUC of 0.855 for the prediction of PD-L1 expression using SUVmax (14). This difference could be due to differences in the patient populations between these two studies.
SUVmax has been found to be a prognostic indicator for both early and advanced NSCLC (15). A meta-analysis revealed that a high SUVmax is associated with poor OS in patients with NSCLC (16). Although we obtained survival data from the stage IV patients in our study, the OS rate is still being evaluated. Preoperative SUVmax at the primary lesion is a more accurate indicator of nodal metastases when a cutoff of 3 is used (17). Almost all patients in our study had an SUVmax > 3 because they were all in stage IV with metastases. Increased PD-L1 expression is associated with worse prognosis in patients with NSCLC (18), supporting the concept that enhanced PD-L1 expression in tumor cells facilitates the evasion of host immune monitoring, promoting disease progression (19). However, Kerr et al. demonstrated that increased PD-L1 expression was associated with better OS in patients with resected NSCLC (20). Thus, high PD-L1 expression has been associated with both favorable and adverse prognoses (20). PD-L1 expression was associated with poorer OS prognosis in a study examining the relationships between PD-L1 expression and various clinicopathologic factors in 90 resected NSCLC patients, including various adenocarcinoma subtypes (21). The preoperative SUVmax at the primary lesion measured during 18F-FDG PET/CT is a more efficient index of nodal metastasis than tumor size, and SUVmax can predict regional lymph node metastases (22).
In patients with early-stage lung cancer who are suitable for resection, preoperative SUVmax is associated with PD-L1 expression in NSCLC patients (22), as demonstrated in another study (13). An SUVmax of 8.6 is associated with PD-L1 expression (TPS 11%) and is an independent prognostic factor for OS in lung squamous cell carcinoma (23). Additionally, elevated PD-L1 expression and a high SUVmax (>11.2) are both independently associated with poor OS in surgical lung squamous cell carcinoma (24). A significant difference in OS was identified between individuals with lung adenocarcinoma with SUVmax of 2.9 (25).
Although clinical research studies examining the association between SUVmax and PD-L1 are limited, the findings remain controversial. Determining the relationship between PD-L1 expression and SUVmax can determine optimal treatment selection. Previous clinical trials have demonstrated that the expression of immune checkpoints, such as PD-L1, in various patient populations can predict treatment efficacy, including pembrolizumab vs. chemotherapy, pembrolizumab vs. platinum-based chemotherapy for advanced NSCLC (26–28). SUVmax is considerably higher in patients with positive PD-L1 expression than in those with negative PD-L1 expression (13). This finding suggests that combining the evaluation of PD-L1 expression and SUVmax in the primary tumor may help predict stage IV adenocarcinoma lung cancer prognosis.
Immuno-PET imaging may become a routine clinical assessment tool in this field in the near future. By defining tumors using TKI-PET and immuno-PET, we can tailor NSCLC therapy (29). Whole-body PD-L1 PET can also be conducted on NSCLC (30). We could obtain more detailed information on PD-L1 expression by using immune-PET because immune-PET delays the resolution of unresolved issues. Immune-PET can provide more precise information regarding PD-L1 expression while also consistently collecting the SUVmax of the primary site. This study may gain increased significance in the future as this imaging method becomes more regularly used.
Several limitations existed in this study. First, this is a cross-sectional study, which did not allow us to evaluate the response to treatment PD-L1–targeted treatment. Additional studies with larger, externally validated cohorts remain needed to elucidate the value of PD-L1 and SUVmax for evaluating treatment and prognosis. Second, glutamine transporters (GLUT1) and hexokinase II should be included in future investigations. Further studies are also essential to evaluate the value of FDG PET/CT in predicting immunotherapy response.
Conclusion
The SUV measured in the primary lesion was valuable for predicting PD-L1 expression status in stage IV adenocarcinoma lung cancer patients. Therefore, SUVmax may play a role in predicting the efficacy of PD-L1 immunotherapy in patients with stage IV lung adenocarcinoma.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
This study was approved by the Ethics Committee of Hanoi Medical University (accession no. NCS02/HMU-IRB). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
BTC and PCP gave a substantial contribution in acquisition, analysis, and data interpretation. BTC, P-VT, and NMD prepared, drafted, and revised manuscript critically for important intellectual content. All authors gave the final approval of the version to be published and agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
3. Mielgo-Rubio X, Uribelarrea EA, Cortés LQ, Moyano MS. Immunotherapy in non-small cell lung cancer: update and new insights. J Clin Transl Res. (2021) 7:1–21.
4. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. (2002) 99:12293–7. doi: 10.1073/pnas.192461099
5. Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. (2016) 8:328rv4. doi: 10.1126/scitranslmed.aad7118
6. Scheel AH, Ansén S, Schultheis AM, Scheffler M, Fischer RN, Michels S, et al. PD-L1 expression in non-small cell lung cancer: correlations with genetic alterations. Oncoimmunology. (2016) 5:e1131379. doi: 10.1080/2162402X.2015.1131379
7. Li Z, Dong P, Ren M, Song Y, Qian X, Yang Y, et al. PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J Cancer. (2016) 7:784–93. doi: 10.7150/jca.14549
8. Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. (2016) 15:55. doi: 10.1186/s12943-016-0539-x
9. Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. (2016) 64:2038–46. doi: 10.1002/hep.28710
10. Chao F, Zhang H. PET/CT in the staging of the non-small-cell lung cancer. J Biomed Biotechnol. (2012) 2012:783739. doi: 10.1155/2012/783739
11. Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. (2003) 139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013
12. Li J, Xu W, Kong F, Sun X, Zuo X. Meta-analysis: accuracy of 18FDG PET-CT for distant metastasis staging in lung cancer patients. Surg Oncol. (2013) 22:151–5. doi: 10.1016/j.suronc.2013.04.001
13. Takada K, Toyokawa G, Okamoto T, Baba S, Kozuma Y, Matsubara T, et al. Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on 18 F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med. (2017) 6:2552–61. doi: 10.1002/cam4.1215
14. Cui Y, Li X, Du B, Diao Y, Li Y. PD-L1 in lung adenocarcinoma: insights into the role of 18F-FDG PET/CT. Cancer Manag Res. (2020) 12:6385–95. doi: 10.2147/CMAR.S256871
15. Billè A, Okiror L, Skanjeti A, Errico L, Arena V, Penna D, et al. The prognostic significance of maximum standardized uptake value of primary tumor in surgically treated non-small-cell lung cancer patients: analysis of 413 cases. Clin Lung Cancer. (2013) 14:149–56. doi: 10.1016/j.cllc.2012.04.007
16. Liu J, Dong M, Sun X, Li W, Xing L, Yu J. Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS ONE. (2016) 11:e0146195. doi: 10.1371/journal.pone.0146195
17. Nakamura H, Saji H, Marushima H, Kimura H, Tagaya R, Kurimoto N, et al. Standardized uptake values in the primary lesions of non-small-cell lung cancer in FDG-PET/CT can predict regional lymph node metastases. Ann Surg Oncol. (2015) 22 Suppl 3:S1388–93. doi: 10.1245/s10434-015-4564-6
18. Kim H, Kwon HJ, Park SY, Park Y, Park E, Chung JH. Clinicopathological analysis and prognostic significance of programmed cell death-ligand 1 protein and mRNA expression in non-small cell lung cancer. PLoS One. (2018) 13:e0198634. doi: 10.1371/journal.pone.0198634
19. Wang J, Yuan R, Song W, Sun J, Liu D, Li Z. PD-1, PD-L1 (B7-H1) and tumor-site immune modulation therapy: the historical perspective. J Hematol Oncol. (2017) 10:34. doi: 10.1186/s13045-017-0403-5
20. Kerr KM, Thunnissen E, Dafni U, Finn SP, Bubendorf L, Soltermann A, et al. A retrospective cohort study of PD-L1 prevalence, molecular associations and clinical outcomes in patients with NSCLC: results from the European Thoracic Oncology Platform (ETOP) lungscape project. Lung Cancer. (2019) 131:95–103. doi: 10.1016/j.lungcan.2019.03.012
21. Miyazawa T, Marushima H, Saji H, Kojima K, Hoshikawa M, Takagi M, et al. PD-L1 expression in non-small-cell lung cancer including various adenocarcinoma subtypes. Ann Thorac Cardiovasc Surg. (2019) 25:1–9. doi: 10.5761/atcs.oa.18-00163
22. Kudo Y, Shimada Y, Saji H, Kato Y, Yoshida K, Matsubayashi J, et al. Prognostic factors for survival after recurrence in patients with completely resected lung adenocarcinoma: important roles of epidermal growth factor receptor mutation status and the current staging system. Clin Lung Cancer. (2015) 16:e213–21. doi: 10.1016/j.cllc.2015.04.005
23. Kasahara N, Kaira K, Bao P, Higuchi T, Arisaka Y, Erkhem-Ochir B, et al. Correlation of tumor-related immunity with 18F-FDG-PET in pulmonary squamous-cell carcinoma. Lung Cancer. (2018) 119:71–7. doi: 10.1016/j.lungcan.2018.03.001
24. Zhang M, Wang D, Sun Q, Pu H, Wang Y, Zhao S, et al. Prognostic significance of PD-L1 expression and 18F-FDG PET/CT in surgical pulmonary squamous cell carcinoma. Oncotarget. (2017) 8:51630–40. doi: 10.18632/oncotarget.18257
25. Kaira K, Shimizu K, Kitahara S, Yajima T, Atsumi J, Kosaka T, et al. 2-Deoxy-2-[fluorine-18] fluoro-d-glucose uptake on positron emission tomography is associated with programmed death ligand-1 expression in patients with pulmonary adenocarcinoma. Eur J Cancer. (2018) 101:181–90. doi: 10.1016/j.ejca.2018.06.022
26. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
27. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. (2019) 37:537–46. doi: 10.1200/JCO.18.00149
28. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
29. Bahce I, Yaqub M, Smit EF, Lammertsma AA, van Dongen GA, Hendrikse NH. Personalizing NSCLC therapy by characterizing tumors using TKI-PET and immuno-PET. Lung Cancer. (2017) 107:1–13. doi: 10.1016/j.lungcan.2016.05.025
Keywords: lung cancer, adenocarcinoma, stage IV, FDG PET/CT, PD-L1, prediction, SUVmax
Citation: Tien Cong B, Cam Phuong P, Thai P-V, Thuong V-L, Quang Hung N, Hang D-T, Anh Tuan H, Minh Khuy D, Tuyen P-V and Minh Duc N (2022) Prognostic Significance of PD-L1 Expression and Standardized Uptake Values in the Primary Lesions of Stage IV Adenocarcinoma Lung Cancer. Front. Med. 9:895401. doi: 10.3389/fmed.2022.895401
Received: 13 March 2022; Accepted: 19 April 2022;
Published: 13 May 2022.
Edited by:
Domenico Albano, University of Brescia, ItalyReviewed by:
Francesco Dondi, Università degli Studi di Brescia, ItalySang Van Nguyen, Thai Nguyen Pharmacy and Medical University, Vietnam
Copyright © 2022 Tien Cong, Cam Phuong, Thai, Thuong, Quang Hung, Hang, Anh Tuan, Minh Khuy, Tuyen and Minh Duc. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pham-Van Thai, cGhhbXZhbnRoYWlAaG11LmVkdS52bg==; Nguyen Minh Duc, YnNuZ3V5ZW5taW5oZHVjQHBudC5lZHUudm4=
†These authors have contributed equally to this work and share first authorship
 Bui Tien Cong1,2†
Bui Tien Cong1,2† Nguyen Minh Duc
Nguyen Minh Duc