- 1Department of Clinical Prevention and Control of STD, Institute of Dermatology, Chinese Academy of Medical Science and Peking Union Medical College, National Center for STD Control, China Centers for Disease Control and Prevention, Nanjing, China
- 2Department of Dermatology, The Fifth People's Hospital of Suzhou, Suzhou, China
- 3Department of Dermatology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, China
Considered the increased threaten of neurosyphilis in China, a review on cases reported in the literature to describe the clinical epidemiological characteristics of neurosyphilis cases, may be beneficial to the early detection and management strategies of neurosyphilis for clinicians. We searched the literature on Chinese neurosyphilis cases published from January 1, 2009 to December 31, 2021, described their clinical epidemiological characteristics and calculated the prevalence of neurosyphilis amongst other associated diseases, according to the individual study criteria. A total of 284 studies including 7,486 neurosyphilis cases were included. No meta-analysis was performed due to the heterogeneity of the data. Among 149 case reports and 93 retrospective case series studies, the main clinical manifestation of 3,507 neurosyphilis cases was cerebral parenchymal syphilis (57.3%), followed by asymptomatic neurosyphilis (16.7%), meningovascular syphilis (13.6%), meningitis syphilis (7.7%) and ocular syphilis (2.8%), etc. In addition, the initial diagnosis was incorrect in 53.2% patients, and the most frequent misdiagnoses were mental disorders (31.0%), stroke (15.9%), cognitive impairment (9.0%), etc. The positive or abnormal rates of cerebrospinal fluid non-treponemal and treponemal tests, white blood cell counts and protein concentrations were 74.2%, 96.2%, 61.5%, and 60.9%, respectively. Aqueous penicillin was the first choice for treatment in 88.3% cases, and 81.7% and 50.0% patients had response in the improvement of symptoms and serological effective in CSF, respectively. Among 26 studies on neurosyphilis patients amongst other associated diseases, the prevalence of neurosyphilis amongst central nervous system infectious diseases, syphilis-associated neurological symptoms, serofast status, coinfected with human immunodeficiency virus were 10.6%–30.1%, 23.2%–35.5%, 9.8%–56.1%, and 8.9%, respectively. In summary, the lack of early detection of neurosyphilis cases remains a clinical challenge. The high rate of misdiagnosis and high prevalence of neurosyphilis amongst associated diseases strongly remind clinicians to focus on the early detection among suspected cases. Besides, the standard treatment regimen and long-term follow-up, which complied with guideline should be provided. Further prospective studies are urgent to better delineate the clinical epidemiological characteristics of neurosyphilis in China.
Introduction
Neurosyphilis, historically caused by Treponema pallidum (T. pallidum) infection, was reported increasing with the expansion of syphilis screening in China. T. pallidum invades the central nervous system (CNS) and may cause severe and irreversible neurologic sequelae in patients if left untreated (1). According to the latest report, the number of newly reported cases of syphilis was 438,199 (32.2 per 100,000) in 2016 and increased by an annual average of 8.6% from 2007 to 2016 in China; moreover, the number of reported cases of tertiary syphilis increased by 8.0% annually from 2007 to 2016 (2). Previous studies showed that the epidemiology of neurosyphilis largely paralleled that of syphilis (3, 4), and most tertiary syphilis cases were diagnosed as neurosyphilis (5), which indicated an increasing incidence of neurosyphilis in China.
Neurosyphilis has puzzled dermatologists, neurologists and psychiatrists in clinical settings for over two centuries because of its atypical symptoms and lack of a golden criteria of diagnosis. Early injury to CNS in neurosyphilis patients affects the mesenchyma, such as the meninges and blood vessels, manifesting within months to the several years after primary infection as meningismus, blindness, stroke, etc., while late injury affects the brain and spinal cord parenchyma within years to decades and presents as general paresis and tabes dorsalis (6, 7). Therefore, the rates of misdiagnosis and missed diagnosis in clinical settings are relatively high because of the diverse and atypical symptoms (8, 9). It is necessary for clinicians to be aware of the most common misdiagnosed diseases and specific clinical features of neurosyphilis when making a differential diagnosis.
Clinical recognition of neurosyphilis depends on the comprehensive assessment of clinical characteristics and cerebrospinal fluid (CSF) findings. However, no single specific and sensitive test for neurosyphilis exists. CSF pleocytosis and elevated protein concentrations are frequently observed in patients with neurosyphilis. Reactive CSF serologic tests are required for the diagnosis of neurosyphilis, and the Venereal Disease Research Laboratory (VDRL) test for CSF is thought to be the gold standard for specificity in the absence of blood contamination, but its sensitivity is still debated (10). The rapid plasma reagin (RPR) test, toluidine red unheated serum test (TRUST), fluorescent treponemal antibody adsorption (FTA-ABS) test and treponema pallidum particle agglutination (TPPA) test for CSF have all been assessed to have variable sensitivity and specificity in diagnosing neurosyphilis (11–13). Besides, no VDRL kits in China gets the approved of State Food and Drug Administration (SFDA) to date. Together the above all, the RPR and TRUST were recommended as the alternative tests by the ‘China National Guidelines for the Diagnosis and Treatment of Syphilis, Gonorrhea and Chlamydia Trachomatis Infection (2020)' (14). The diagnostic criteria for neurosyphilis differed between studies due to the lack of a gold standard test. Consequently, the methodological quality of neurosyphilis diagnosis among studies is unknown and need to be evaluated.
Although many cases of neurosyphilis in China were reported, their clinical features and management information have not reviewed comprehensively to date. In this paper, all cases of neurosyphilis reported in China in the past 13 years were reviewed, and their clinical epidemiological characteristics were presented, which will be helpful to clinicians to early detection and management of neurosyphilis.
Materials and Methods
The research and reporting methods of this review were consistent with the preferred reporting items for systematic reviews (PRISMA) (Supplementary Table S1).
Search Strategy
We searched the PubMed, EMBASE, and some Chinese Journal databases including China national knowledge infrastructure (CNKI) and WanFang databases for studies on neurosyphilis in China and limited the search to studies published between 1 January 2009 to 31 December 2021.We searched for ((“CSF” OR “lumbar puncture” OR “meningitis” OR “meningovascular” OR “stroke”) AND “syphilis”) OR (“neurosyphilis” OR “tabes dorsalis” OR “general paresis”) AND (“China”) in the PubMed and EMBASE databases and searched for “neurosyphilis” in the CNKI and WanFang databases (15). No language restrictions were set.
Inclusion/Exclusion Criteria
We selected unduplicated references and excluded reviews if the studies did not address neurosyphilis, did not describe the clinical features of neurosyphilis patients, reported patients already described in a different paper, did not include Chinese patients, did not report new primary material or could not be downloaded. We did not limit inclusion based on the diagnostic criteria used for neurosyphilis.
Selection Process
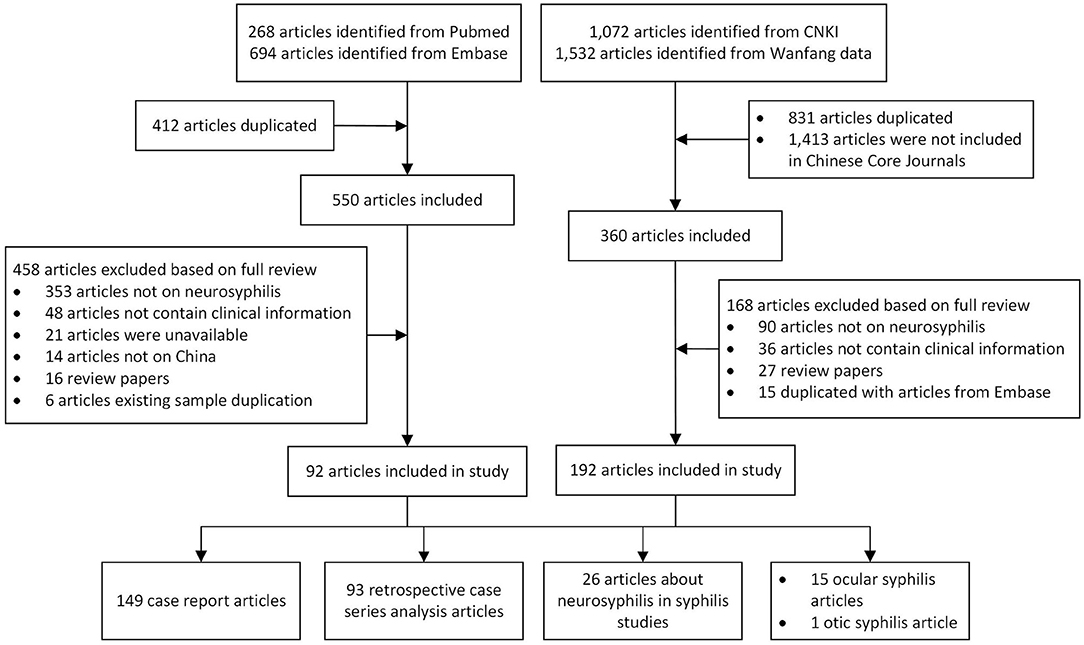
The selection was conducted by two independent reviewers working in parallel, first excluded duplication literature, then screening for title and abstract followed by the full text according to the inclusion/exclusion criteria. After the screening of 50 references, a validation of the screening process was conducted by comparing the screening results and discussing them within the team. Throughout the screening process, discrepancies were discussed and a third reviewer was consulted if consensus was not reached. The reasons for exclusion were documented only for full text publications (Figure 1).
Data Extraction
We extracted variables including available demographic information of neurosyphilis patients, duration of the study, inclusion criteria, diagnostic criteria for neurosyphilis, clinical syndromes and misdiagnosis, neuroimaging findings, and the number of human immunodeficiency virus (HIV) -infected patients in each study. We also extracted information on the treatment and follow-up of patients in these studies.
Statistical Analysis
For case reports and retrospective case series, we analyzed the sex ratio, age distribution, clinical spectrum, misdiagnosis rate, treatment, and the proportion of anomalies of CSF tests and neuroimaging findings. For studies on neurosyphilis in patients with syphilis or HIV, we calculated the proportion of study participants who were diagnosed with neurosyphilis according to the individual study criteria. No meta-analysis was performed due to the heterogeneity of the data.
The chi-squared test was used to compare categorical variables. The Wilcoxon signed-rank test was used to compare related continuous variables with skewed distributions. A P-value of < 0.05 was considered statistically significant. All statistical analyses were conducted in SPSS 21.0 (IBM Corp, Armonk, NY).
Results
A total of 962 articles were identified in the PubMed and EMBASE databases, and 412 duplicated articles were excluded. Among the remaining 550 articles, 458 articles were excluded based on full-text reviews. A total of 2,604 articles were identified from the CNKI and Wanfang databases, and 831 duplicated articles and 1,413 articles that were not included in Chinese Core Journals were excluded. Among the remaining 360 articles, 192 articles were excluded based on full-text reviews. Finally, 284 articles that met our inclusion criteria were selected (Figure 1).
Clinical Characteristics of Cases
The 149 case reports included 180 patients, consisting of 128 males and 52 females. The average age was 47.7 ± 12.9 (range from 12 to 79 years old), and the age of most patients ranged from 41 to 60 (56.7%, 102/180). The 93 retrospective case series included 3,327 patients (2,520 males, 783 females and 24 unknown), and neurosyphilis was more likely to occur in males than in females (|Z| = 8.24, P < 0.001) (Supplementary Table S2).
As shown in Table 1, among the 3,200 patients with clearly defined clinical subtypes, 533 (16.7%) has asymptomatic neurosyphilis, 726 (22.7%) had mesenchymal syphilis (245 (7.7%) had meningitis syphilis, 435 (13.6%) had meningovascular syphilis and 46 had undefined syphilis), 1,832 (57.3%) had parenchymal neurosyphilis (1,537 (48.0%) had general paresis, 115 (3.6%) had tabes dorsalis, 54 (1.7%) had syphilitic gumma and 126 had undefined syphilis), 90 (2.8%) had ocular syphilis, 42 (0.6%) had mixed-type syphilis, and 210 had undefined syphilis. The initial diagnosis was correct in 774 (46.8%) patients and incorrect in 847 (53.2%) patients among 173 studies, the correct diagnostic rate ranged from 17.0 to 100% (95%CI, 59.1–76.2%). Among the 847 misdiagnosed patients, 578 patients showed the misdiagnosed diseases, including mental disorders (179 patients, 31.0%), stroke (92 patients, 15.9%), cognitive impairment (52 patients, 9.0%), encephalitis (46 patients, 8.0%), ophthalmic diseases (40 patients, 6.9%), Alzheimer's disease (AD) (22 patients, 3.8%), brain tumors (20 patients, 3.5%), epilepsy (13 patients, 2.3%), myeleterosis (12 patients, 2.1%), peripheral neuropathy (11 patients, 1.9%), demyelinating disease or multiple sclerosis (10 patients, 1.7%), Parkinson's disease (PD) (nine patients, 1.6%), hydrocephalus (six patients, 1.0%), ataxiaor menopausal symptom (four patients, 0.7%, respectively), encephalatrophy (three patients, 0.5%), and other diseases (55 patients, 9.5%).
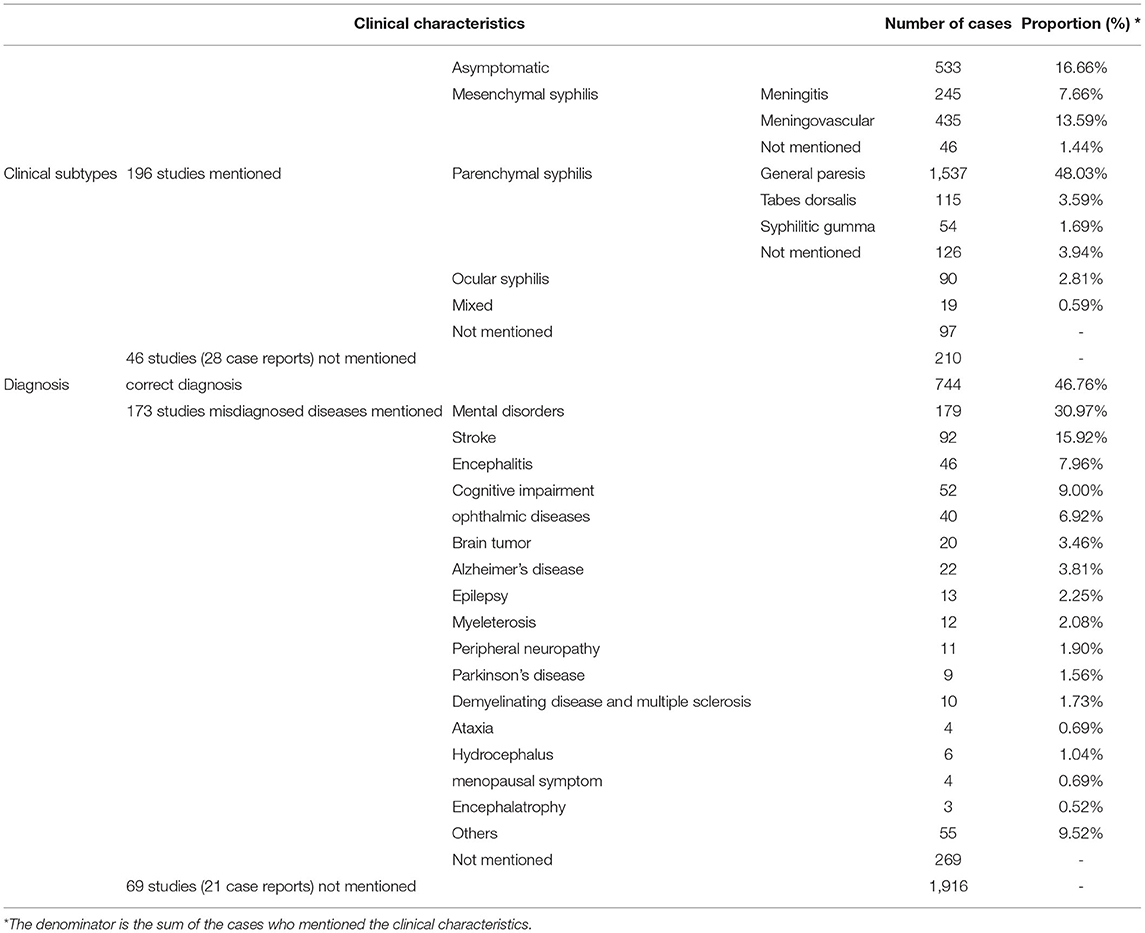
Table 1. Clinical characteristics of neurosyphilis cases from 149 case reports and 93 retrospective case series.
Laboratory Characteristics and Auxiliary Examination of Cases
Among 180 patients from 149 case reports studies, nontreponemal tests (VDRL/RPR/TRUSTs) were performed in 83.3% (150/180) of the patients, and 82.0% (123/150) of them patients had positive results. Treponemal tests (TPPA/TPHA) were performed in 78.3% (141/180), and 95.0% (134/141) of them had positive results. As shown in Table 2, patients were more likely to have positive results on the treponemal tests than non-treponemal tests (χ2 = 11.97, P = 0.001). However, there were no differences in the abnormal CSF white blood cell counts (WBC) rate (74.6%, 82/110) and abnormal CSF protein (PRO) rate (78.9%, 86/109) between these two groups (χ2 = 0.58, P = 0.52). We also found that 24 patients were diagnosed with neurosyphilis without reactive CSF serologic tests. (Supplementary Table S1).
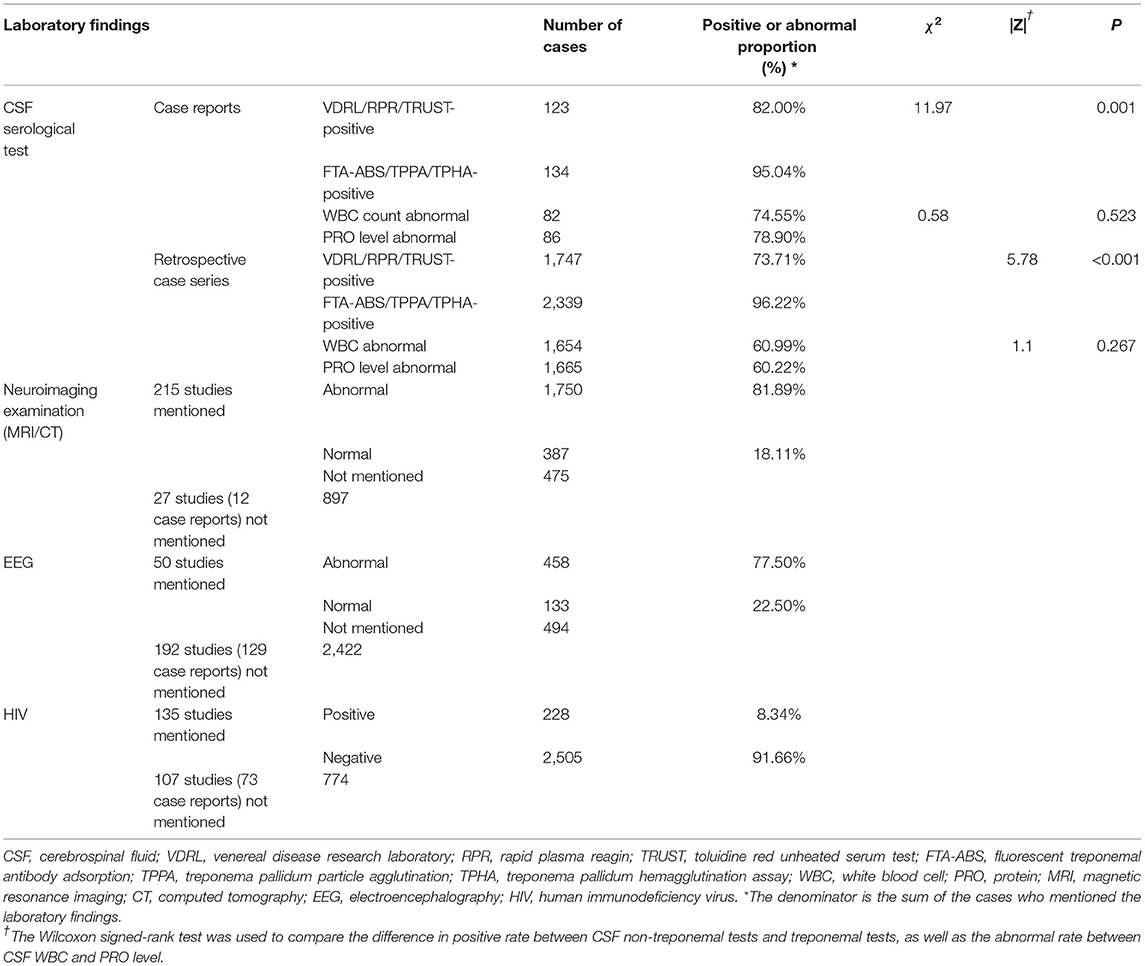
Table 2. Laboratory findings of neurosyphilis cases from 149 case reports and 93 retrospective case series.
Among the 93 retrospective case series studies, non-treponemal tests (VDRL/RPR/TRUST) were performed in 88.8% (2,491/2,805) of the patients, and 73.7% (1,747/2,370) of them had positive results, the positive rates ranged from 0 to 100% (95%CI, 69.5–81.2%). Treponemal tests (FTA-ABS/TPPA/TPHA) were performed in 91.0% (2,552/2,805) of the patients, and 96.2% (2,339/2,431) of them had positive results, the positive rates ranged from 66.7 to 100% (95%CI, 90.8–97.3%). Patients were more likely to have a positive result on a treponemal test than a nontreponemal test (|Z = 5.78, P < 0.001). CSF-WBC were abnormal among 61.0% (1,654/2,712) patients, the abnormal rates ranged from 22.2% to 100% (95%CI, 59.0–69.7%). CSF-PRO test was abnormal among 60.2% (1,665/2,765) patients, the abnormal rates ranged from 11.1 to 100% (95%CI, 65.1–75.8%). There were no differences in the rates of abnormal CSF-WBC and CSF-PRO results among studies (|Z| = 1.1, P = 0.27). The diagnostic criteria for neurosyphilis were not provided in two studies including 51 patients, and the results of CSF serologic tests were not mentioned in 12 studies including 474 patients. (Supplementary Table S3).
The numbers of patients with HIV infection were described in 135 studies, including 2,733 patients, and the coinfection rate was 8.34% (228/2,733). In addition, imaging examination results were presented in 215 studies; 81.8% (2,137/2,612) of the patients underwent neuroimaging examinations (skull/spine/orbital magnetic resonance imaging (MRI) or computed tomography (CT)), and 81.9% (1,750/2,137) of them had abnormal results (Table 2), the abnormal rates ranged from 25.0 to 100% (95%CI, 80.5–88.2%). Forty-two studies presented electroencephalography (EEG) findings; 54.47% (591/1,085) of the patients underwent EEG, and 77.50% (458/591) of them had abnormal results, the abnormal rates ranged from 11.5% to 100% (95%CI, 70.6–87.8%) (Supplementary Table S3).
Treatment and Prognosis
Of the 242 studies, treatment of neurosyphilis was described in 201 studies including 1,738 patients. As shown in Table 3, the treatment drugs were described for 1,634 patients. Aqueous penicillin was the first choice for treatment (88.3%, 1,442/1,634), and ceftriaxone (7.4%, 121/1,634) and doxycycline (0.5%, 8/1,634) were the alternative choices for those who were allergic to penicillin. The remaining 63 (3.9%) patients were not treated with the drugs recommended by the National Guidelines, such as benzathine penicillin (3.2%, 53/1,634), minocycline, azithromycin, traditional Chinese medicine (TCM), etc. Thirty eight patients were referred to other hospitals and 10 patients refused treatment.
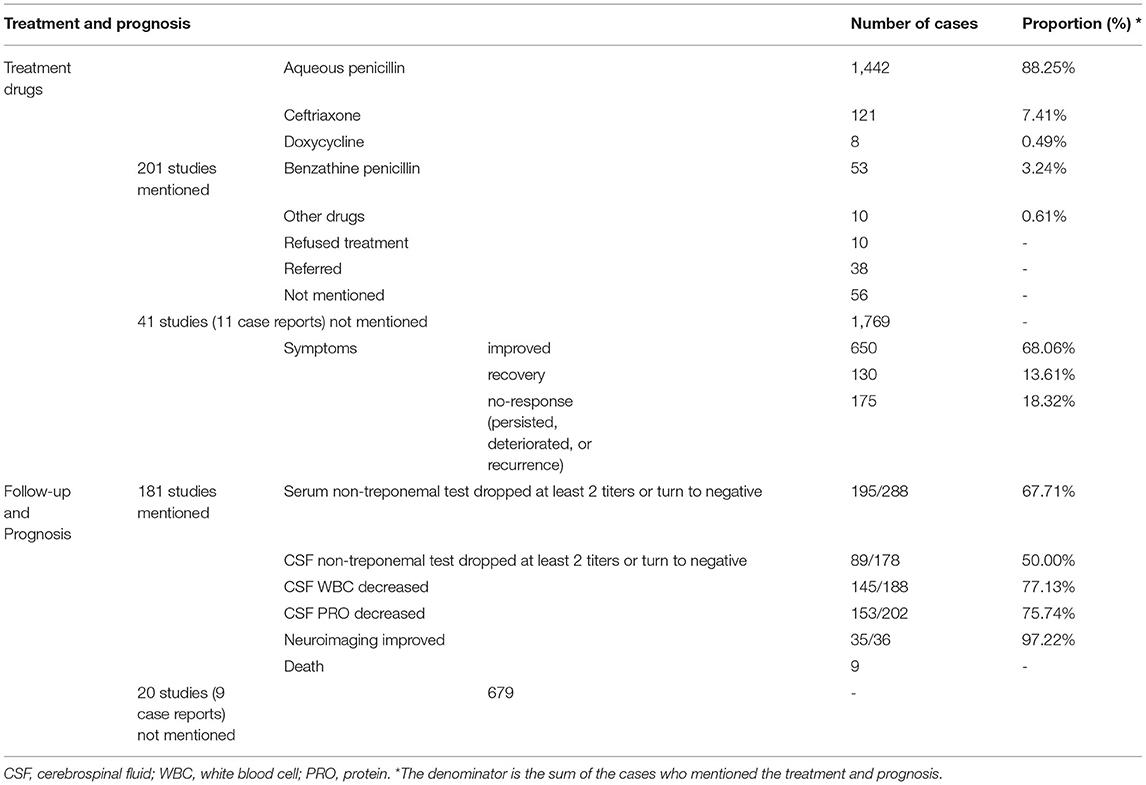
Table 3. The treatment and follow-up of neurosyphilis cases from 149 case reports and 93 retrospective case series.
The follow-up and prognosis of patients were described in 181 studies. The longest follow-up time was 6 years, while the efficacy of treatment among 19.3% (29/150) patients were evaluated before discharge and only 17.3% (26/150) patients follow-up at least 1 year. Most patients (52%, 78/150) follow-up for 3 to 6 months. As shown in Table 3, during the follow-up period, 81.7% (780/955) patients had an improvement or recovery of clinical symptoms (range from 0 to 100% in 47 cases series studies, 95%CI: 75.0–88.3%, Supplementary Table S2). Unfortunately, the persistence, deteriorate or recurrence of symptoms were occurred among 18.3% (175/955) patients. The results of nontreponemal test in serum or CSF turned negative or dropped by at least two titers in 67.7% (195/288) (range from 0 to 100% in 14 cases series studies, 95%CI: 33.0%−73.3%, Supplementary Table S2) and 50% (89/178) (range from 0 to 100% in 10 cases series studies, 95%CI: 23.2–73.7%, Supplementary Table S2) of patients, respectively. The CSF WBC or PRO decreased or return to normal range in 77.1% (145/188) (range from 42.9 to 100% in 12 cases series studies, 95%CI: 64.5–94.8%, Supplementary Table S2) and 75.7% (153/202) (range from 45.5 to 100% in 10 cases series studies, 95%CI: 64.8–93.4%, Supplementary Table S2) of patients, respectively. In addition, the rate of improvement or recovery on neuroimaging findings was 97.2% (35/36) mentioned in 34 case report studies. Nine patients died during follow-up (Supplementary Tables S2, S3).
Among 121 cases report studies, 131 and 13 patients were mentioned treated by using aqueous penicillin or ceftriaxone, and the response to clinical symptoms after treatment were mentioned in 107 and 12 cases, respectively. We found that there is no difference in the rate of improvement or recovery of clinical symptoms between patients treated by using aqueous penicillin or ceftriaxone (87.9% (94/107) vs. 83.3% (10/12), P = 1.0). Data about the syphilitic serological response rate in serum and CSF was too few for comparison.
Neurosyphilis Amongst Patients With Other Associated Diseases
Seven studies reported cases of neurosyphilis amongst CNS diseases (Table 4, Supplementary Table S3). The diagnostic criteria of neurosyphilis were not stated in five studies, and the results of CSF serological tests were presented in only one study. Twenty one studies reported cases of syphilis with neurosyphilis (Table 5). The performance of LP was mentioned in all studies, however, the CSF serologic tests were not performed among 238 patients in three studies.
CNS Diseases
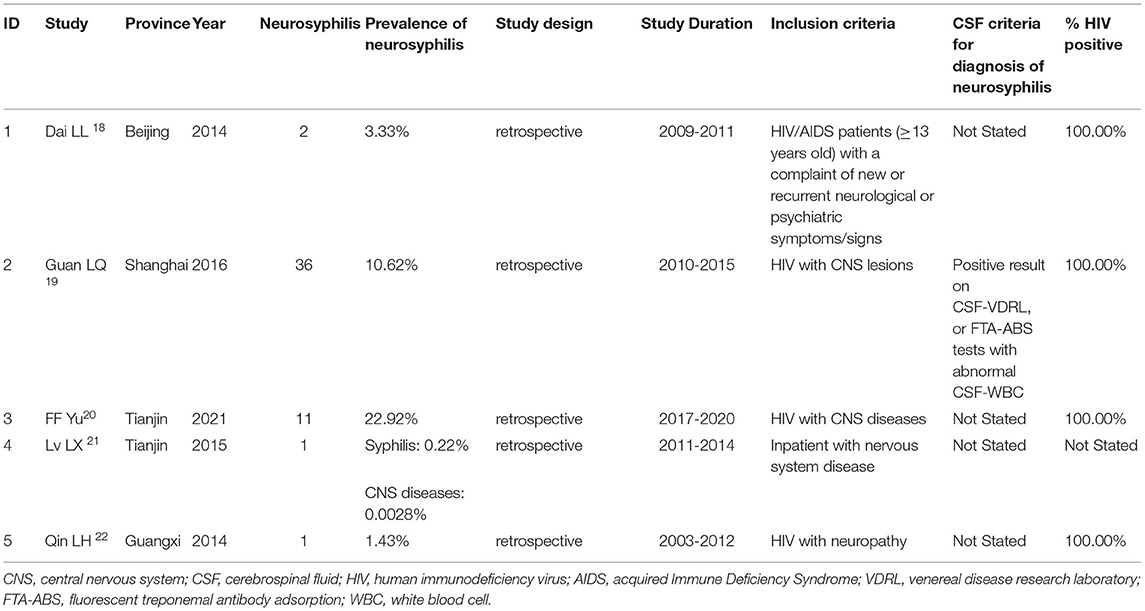
A case-control study in patients with CNS infection showed that 30.1% (84/279) of patients were diagnosed as neurosyphilis with evidence of positive reaction on CSF serological tests (16). Another study in patients with cognitive impairment showed that neurosyphilis was prevalent in 17.1% (6/35) of the patients with CNS infectious diseases; however, the diagnostic criteria of neurosyphilis were not provided (17). Other three studies reported neurosyphilis amongst HIV-infected patients with CNS disorders. There were 60, 339, and 48 patients recruited, and 2, 36 (10.6%) and 11(22.9%) of them were diagnosed with NS, respectively (18–20). However, only one study (19) provided evidence in positive results on the CSF-VDRL test or FTA-ABS test and abnormal CSF-WBC for the diagnosis of neurosyphilis.
Other Nervous System Diseases
One study reported syphilis serological test results among 36,151 patients hospitalized for nervous system disorders; only one neurosyphilis patient was identified among 449 patients with reactive syphilis serological tests (21). Another study recruited 70 HIV-infected patients with nervous system disorders, and only one patient (1.4%) was diagnosed with neurosyphilis (22). These two patients were diagnosed without any evidence on CSF tests.
Syphilis Patients With Neurological Symptoms or Who Received Lumbar Puncture
Three retrospective studies reported neurosyphilis among syphilis patients with neurological symptoms. The prevalence of neurosyphilis was 23.7% (100/422), 33.2% (123/370), and 35.5% (156/440) (23–25), respectively. All neurosyphilis patients were diagnosed according to reactive CSF serological tests. Four retrospective studies reported patients with suspected neurosyphilis who underwent lumbar puncture (LP). The prevalence of neurosyphilis in these studies ranged from 13.6 to 61.2% (26–29), respectively. However, only 67.72% (426/630) of the patients were diagnosed with neurosyphilis according to reactive CSF serological tests; the remaining 204 patients had only abnormal routine CSF test results.
Serofast Status and RPR/TRUST Titer Fluctuations After Treatment
Six retrospective studies reported 1,171 serofast syphilis patients and 44 patients with RPR/TRUST titers that fluctuated continuously (30–35). A total of 405 (33.3%) patients were diagnosed with neurosyphilis according to reactive results in CSF serological tests and/or abnormal results on CSF routine tests. The prevalence of neurosyphilis in these studies ranged from 9.8 to 56.1%. Unfortunately, 99 neurosyphilis patients were diagnosed without CSF serological evidence, and one study did not provide the results of CSF serological tests.
Individuals With HIV and Syphilis Coinfection
A retrospective study recruited 157 patients with HIV and syphilis coinfection, and 8.9% (14/157) of them were diagnosed with neurosyphilis according to reactive CSF-VDRL (seven cases) or CSF-WBC >20 cells/mL (seven cases) (36). Another study recruited 102 syphilis patients coinfected with HIV and with abnormal CSF routine tests, and half of patients were diagnosed with neurosyphilis according to reactive CSF-TPPA with/without reactive CSF-RPR (15 cases) (37).
Malignant Syphilis
A retrospective study reported 26 malignant syphilis patients with typical, serious skin lesions and high non-treponemal tests titers (38). Seven (26.9%) patients were diagnosed with neurosyphilis according to the reactive CSF-VDRL test among these patients. The study found that the proportion of malignant syphilis patients who developed concurrent neurosyphilis was higher than common syphilis patients (13.1%).
Other Studies
A retrospective study reported an 1.3% prevalence of neurosyphilis in 1,927 inpatients with positive syphilis screening results (39). Another study reported an 82.1% prevalence of neurosyphilis in 1,968 tertiary syphilis and only 27.1% of neurosyphilis patients received standard treatment (5). Recently, a study reported a 78.8% prevalence of neurosyphilis in 528 laboratory-confirmed syphilis in department of neurology or HIV infection patients (40). Another two studies reported 13.7% and 23.6% prevalence of neurosyphilis in 73 and 512 patients with serofast status, or coinfection with HIV, or with neurological symptoms, or serum titer fluctuation after treatment, respectively (41, 42).
Ocular Syphilis and Otic Syphilis
Four case reports included 6 ocular syphilis patients, and 11 retrospective case series included 244 patients (Supplementary Table S4) which comprised 163 males and 81 females, indicating that ocular syphilis tended to occur more frequently in males than in females (|Z| = 2.56, P = 0.01). LP was not performed in two case reports and four retrospective case series; 91.9% (136/148) of the patients in the remaining nine studies received LP, and 56.1% (78/139) (ranged from 9.1 to 91.7%) of them were diagnosed with neurosyphilis according to the reactive CSF serological tests.
A retrospective case series analysis reported 6 syphilis patients with recurrent refractory vertigo and sensorineural deafness. Only one patient with CNS symptoms underwent LP and was diagnosed with neurosyphilis according to the reactive CSF serological tests. (Supplementary Table S4).
Diagnostic Criteria
The CSF diagnostic criteria differed among 93 retrospective case series studies and 26 studies on neurosyphilis amongst other diseases (Table 6), 42 of which mentioned that neurosyphilis was diagnosed according to US CDC or/and European guidelines, and 25 of which mentioned diagnosed according to Chinese guidelines. It was worthy to note that most studies (84.0%, 100/119) met diagnostic criteria established by the Chinese National Guidelines (14), which included VDRL/RPR/TRUST and/or the FTA-ABS/TPPA/TPHA positivity for CSF samples, as well as abnormal CSF-WBC or CSF-PRO levels. Unfortunately, eight studies (6.7%) did not present the results of routine CSF tests. Two studies diagnosed neurosyphilis with only TPPA/TPHA positivity in CSF. Three studies defined neurosyphilis as reactive CSF-RPR/TRUST or CSF-TPPA/TPHA or abnormal CSF-WBC or CSF-PRO levels. The diagnostic criteria were not mentioned in six studies.
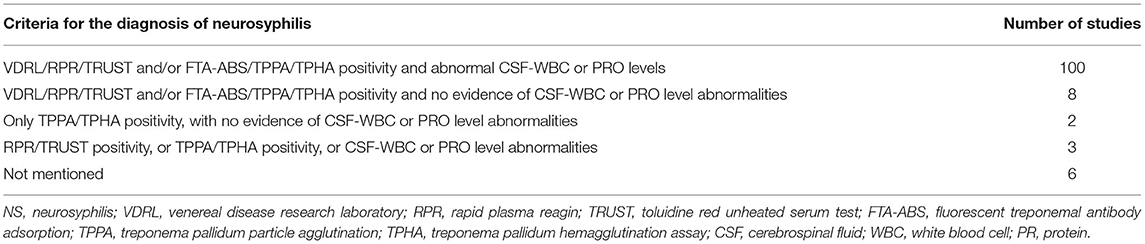
Table 6. Diagnostic criteria in 93 retrospective case series and 26 studies on neurosyphilis in patients with other diseases.
Discussion
This is the first review of the literature on the clinical epidemiological characteristics of neurosyphilis in China from 2009 to 2021, as well as neurosyphilis in patients with other associated diseases. The inconsistent diagnostic criteria in these studies and the heterogeneity among case sources led to limited conclusions.
Although the incidence of neurosyphilis in China is unknown, the number of reported cases increased over 13 years according to the results of this review (Supplementary Figure S1), indicating an increasing health threat requiring neurosyphilis prevention and control. The regional distribution of cases showed a concentration in the eastern coastal areas, including Guangdong, Fujian, Beijing, Zhejiang, Shanghai, etc. (Supplementary Figure S2). YS Tao et al. found that the incidence of early syphilis in inland provinces has increased over time and has been higher than that in eastern coastal provinces since 2010 (2). Considering that the prevalence of neurosyphilis is similar to that of syphilis, more attention probably be paid to the detection and research of neurosyphilis in eastern coastal areas than inland areas. Therefore, more training in the management of neurosyphilis in inland areas is needed in the future.
Among all the studies, we found that neurosyphilis, as well as ocular syphilis, tended to occur most frequently in middle-aged males, consistent with previous reports (27, 43). We also found that parenchymal syphilis was the main manifestation of neurosyphilis, and general paresis with progressively impaired memory, mental abnormalities and occasional seizures was the most common symptom. This clinical spectrum is consistent with those in previous reports in China but is very different from those associated with cases in Western countries (7). In the penicillin era, early forms, such as meningitis and meningovascular, are more common than late forms in Western countries because treatment can effectively prevent the progression of neurosyphilis (44), as indicated in the most recent reports (45). This result suggests that the early detection and treatment of neurosyphilis in China probably be insufficient. In addition, with the rapid expansion of syphilis screening among all populations, an increasing number of patients infected with syphilis have been diagnosed with neurosyphilis, which has probably progressed to the late stage at the time of diagnosis. It is worth noting that many patients present with atypical, ill-defined neurological complaints (46, 47), causing great challenges in the diagnosis of neurosyphilis for clinicians. Unfortunately, the results showed that the misdiagnosis rate of neurosyphilis was more than 50%, and the most common misdiagnosed diseases were neurodegenerative diseases (including stroke, cognitive impairment, Alzheimer's disease, Parkinson's disease, epilepsy, etc.), mental disorders, encephalitis and ophthalmic diseases. Two studies reporting patients with reactive CSF serological tests and diagnosed with neurosyphilis showed that the prevalence rates of neurosyphilis among patients with CNS-associated infectious diseases and CNS disorders and HIV coinfection were 30.1 and 10.6% (16, 19), respectively. In addition, among patients with syphilis infection, the prevalence of neurosyphilis ranged from 23.2 to 35.5% among patients with neurological symptoms (23–25), 13.6 to 67.7% among patients with suspected neurosyphilis who underwent LP (26–29), and 9.8 to 56.1% among syphilis patients with serofast status (30–35); additionally, the rates were 8.9, 26.9 and 43.0% in individuals coinfected with HIV, malignant syphilis and ocular syphilis (36, 37), respectively. The results suggest that clinicians in neurology, psychiatry and ophthalmology departments should pay much attention to detecting suspected neurosyphilis. Serum syphilis tests can be performed in patients with neurological, psychiatric, or ocular symptoms caused by unknown etiologies, especially patients with CNS-associated infectious diseases or HIV infection, to examine the status of syphilis infection. Moreover, CSF serological testing is recommended for syphilis patients with neurological symptoms, serofast status, coinfection with HIV or the presence of serious skin lesions.
Penicillin G has been demonstrated to be effective for the serological and clinical cure of neurosyphilis since 1940 (48, 49), and it has always been the first-line drug for the treatment of neurosyphilis. Ceftriaxone can be used as an alternative regimen in patients with penicillin allergy, and no difference was found in the efficacy of the two drugs in previous studies (50, 51). The data from case reports studies in this paper also showed that there was no difference in the recovery rate of clinical manifestations of patients treated with penicillin or ceftriaxone. It was worthy to note that approximately 4% of patients did not receive treatment regimens recommended by the national guidelines according to the available data in this review, which will be detrimental to preventing the progression of CNS injury. In addition, neurosyphilis patients need long-term follow-up for clinical management according to the recovery of disease, however, the follow-up results within 6 months were reported in more than half of cases. Hence, medical institutions need to strengthening the management of follow-up and increase the compliance, such as call patients regularly to urge return visits, provide higher quality of health care for patients, provide multidisciplinary therapy, psychological guidance, etc. In addition, we found that 81.7% patients had an improvement of symptoms, and 77.1% patients had a decrease of CSF WBC counts during the follow-up period, however, only 66.7% and half of patients had responses to serological tests of syphilis in serum and CSF, respectively. Therefore, more sensitive and specific biomarkers for assessment of prognosis of neurosyphilis are needed, especially if effective biomarkers can be found in serum in patients unwilling to undergo lumbar puncture during follow-up.
From the results of the case reports and retrospective case series, the treponemal test positivity rate was over 95%, which was significantly higher than the non-treponemal test positivity rate (~75%), and abnormal CSF-WBC counts and CSF protein levels were observed in nearly 70% of NS patients, supporting the importance of CSF serological testing for diagnosis (52). Although the CSF-TPPA testis diagnostically sensitive, considering that TP-IgG can cross the intact blood brain barrier (BBB), reactive treponemal testing of CSF samples is not specific for the diagnosis of neurosyphilis (53). The specificity of the CSF-TPPA test has been debated and ranges from 49 to 84% in some studies (54, 55), and its recommendation as a diagnostic indicator varies according to different guidelines (14, 56, 57). Moreover, the cases in most studies were retrospectively analyzed and included reactive treponemal test in the inclusion criteria, which probably biased positivity rate toward 100% (53). Therefore, CSF nontreponemal tests combined with treponemal tests are needed to reduce the possibility of misdiagnosis.
Neurosyphilis patients frequently have abnormal neuroimaging findings due to CNS inflammation and impairment. Neuroimaging may also show progression in patients who have no neurological symptoms or signs and in patients who receive standardized treatment (58). In addition, neurosyphilis patients may have specific EEG signal characteristics that are different from those of non-neurosyphilis patients (59), and the EEG-Lempel-Ziv complexity (LZC) value may be used as a diagnostic index and the reference index to assess neurosyphilis cure (60). According to our review, abnormal neuroimaging or EEG findings were observed in approximately 80% of patients, and the neuroimaging findings of 97% cases improved or return to normal on follow-up, supporting the recommendation of neuroimaging and EEG examinations for the differential clinical diagnosis and neurosyphilis follow-up (61).
Progression to neurosyphilis is more common in patients coinfected with HIV (62). We found that 8.9% of patients with neurosyphilis were infected with HIV, but most studies have used HIV infection as an exclusion or inclusion criterion, resulting in inconsistent rates.
Although the diagnostic criteria in most of the included studies followed international or national guidelines, unlike previous reports from Africa (15), approximately one-tenth of the studies provided insufficient evidence for a neurosyphilis diagnosis. Hence, standardized diagnostic criteria and protocols are urgently needed to ensure accurate diagnostic results and research conclusions.
This review had several limitations. First, there were some missing clinical information extracted from many studies, especially the data of treatment and follow-up, may result in information bias. Second, the great heterogeneity between studies according to the different diagnostic criteria of neurosyphilis may result in limited conclusions. Third, we did not review the literature published before 2009 due to the lack of clinical diagnosis and treatment guidelines for neurosyphilis in China before 2008, may lead to selection bias.
Conclusions
In summary, through the review of clinical epidemiology data on neurosyphilis in China, we found that the reported cases of neurosyphilis in the literature presented an increasing trend. More than half of the cases were in the late stage of neurosyphilis, suggesting that early detection of neurosyphilis was inadequate. The most common misdiagnosed diseases and the high prevalence of neurosyphilis amongst patients with CNS-associated infectious disease, CNS disease comorbid with HIV, syphilis with neurological symptoms, serofast status, coinfection with HIV or presesntations comprising serious skin lesions should remind clinicians to pay much attention to the early detection of neurosyphilis among these cases. Meanwhile, standardized treatment and long-term follow-up of neurosyphilis according to the national guidelines should be strengthened to promote the recovery of patients. During follow-up, neuroimaging and EEG examinations should be performed, as they play a positive role in auxiliary diagnosis and the observation of curative effects. Moreover, the combination of CSF non-treponemal tests with treponemal tests should be implemented for diagnosis to reduce misdiagnosis. Inadequate diagnostics remain a great obstacle to progress in understanding this disease. Future well-designed prospective studies are needed to better delineate the incidence and clinical epidemiological of neurosyphilis in China.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
F-ZD, Q-QW, and R-LZ wrote the draft and revised it. F-ZD, H-NZ, J-JL, and Z-JZ selected literature. F-ZD and XZ extracted data from literature. F-ZD performed the statistical analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Union Innovation Team Project of the Chinese Academy of Medical Sciences (2016-I2M-3021), the National Natural Science Foundation of China (81772209 and 81601804), and the Nanjing Incubation Program for National Clinical Research Center (2019060001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Many thanks to all participants of the study for their cooperation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.894841/full#supplementary-material
References
1. Gonzalez H, Koralnik IJ, Marra CM. Neurosyphilis. Semin Neurol. (2019) 39:448–55. doi: 10.1212/CON.0000000000000250
2. Tao Y, Chen MY, Tucker JD. A nationwide spatiotemporal analysis of syphilis over 21 years and implications for prevention and control in China. Clin Infect Dis. (2020) 70:136–9. doi: 10.1093/cid/ciz331
3. Berger JR, Dean D. Neurosyphilis. Handb Clin Neuro. (2014) 121:1461–72. doi: 10.1016/B978-0-7020-4088-7.00098-5
4. Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. (2017) 17:e235–e79. doi: 10.1016/S1473-3099(17)30310-9
5. Tang W, Huang S, Chen L, Yang L, Tucker JD, Zheng H, et al. Late neurosyphilis and tertiary syphilis in Guangdong province, china: results from a cross-sectional study. Sci Rep. (2017) 7:45339. doi: 10.1038/srep45339
7. Wu F, Wang QQ. Advances in neurosyphilis. Int J Dermatol Venereol. (2015) 41:268–71. doi: 10.3760/cma.j.issn.1673-4173.2015.04.019
8. Zhang HL, Lin LR, Liu GL, Zeng YL, Wu JY, Zheng WH, et al. Clinical spectrum of neurosyphilis among HIV-negative patients in the modern era. Dermatology. (2013) 226:148–56. doi: 10.1159/000347109
9. Wang YH, Shi HS, Le H, Zhong XM, Chen XR, Ling L, et al. Clinical and neuropsychological characteristics of general paresis misdiagnosed as primary psychiatric disease. BMC Psychiatry. (2016) 16:230–6. doi: 10.1186/s12888-016-0925-3
10. Musher DM. Editorial commentary: polymerase chain reaction for the tpp47 gene: a new test for neurosyphilis. Clin Infect Dis. (2016) 63:1187–8. doi: 10.1093/cid/ciw518
11. Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The Rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. (2012) 39:453–7. doi: 10.1097/OLQ.0b013e31824b1cde
12. Marra CM, Tantalo LC, Maxwell CL, Dougherty K, Wood B. Alternative cerebrospinal fluid tests to diagnose neurosyphilis in HIV-infected individuals. Neurology. (2004) 63:85–8. doi: 10.1212/01.wnl.0000131902.69113.34
13. Marra CM, Maxwell CL, Dunaway SB, Sahi SK, Tantalo LC. Cerebrospinal fluid treponema pallidum particle agglutination assay for neurosyphilis diagnosis. J Clin Microbiol. (2017) 55:1865–70. doi: 10.1128/JCM.00310-17
14. National Center for STD Control China centers for disease control and prevention. guidelines for the diagnosis and treatment of syphilis, gonorrhea and chlamydia trachomatis infection (2020). Chin J Dermatol. (2020) 53:168–79. doi: 10.35541/cjd.20190808
15. Michael Marks, Joseph N Jarvis, William Howlett, Mabey DCW. Neurosyphilis in Africa: a systematic review. PLoS Negl Trop Dis. (2017) 11:e0005880. doi: 10.1371/journal.pntd.0005880
16. Xiao Y, Chen MJ, Shen X, Lin LR, Liu LL, Yang TC, et al. Metabolic disorders in patients with central nervous system infections: associations with neurosyphilis. Eur Neurol. (2019) 81:270–7. doi: 10.1159/000503626
17. Wang S, Zhang J, Liang J, Song H, Ji X. Treatable causes of adult-onset rapid cognitive impairment. Clin Neurol Neurosurg. (2019) 187:105575. doi: 10.1016/j.clineuro.2019.105575
18. Dai LL, Mahajan SD, Guo CP, Zhang T, Wang W, Li T, et al. Spectrum of central nervous system disorders in hospitalized HIV/AIDS patients (2009–2011) at a major HIV/AIDS referral center in Beijing, China. J Neurol Sci. (2014) 342:88–92. doi: 10.1016/j.jns.2014.04.031
19. Guan LQ, Lu HZ, Shen YZ, Liu L, Qi TK, Song W, et al. Spectrum of central nervous system disorders in the first hospitalized HIV/AIDS patient. Chin J AIDS STD. (2016) 22:510–3. doi: 10.13419/j.cnki.aids.2016.07.07
20. Yu FF, Zhang DF, Huang XJ, Zhao SD, Ma P. Clinical analysis of AIDS complicated with central nervous system disease in 48 cases. Shandong Medical Journal. (2021) 61:81–4. doi: 10.3969/j.issn.1002-266X.2021.11.021
21. Lv LX, Kan PC, Zhu Y, Xue J, Zhang B. Analysis of antibody detection of Treponema pallidum in 36,151 inpatients with nervous system diseases. Chin J Infect Dis. (2015) 33:426–7. doi: 10.3760/cma.j.issn.1000-6680.2015.07.013
22. Qin LH, Mo XA, Huang W, Qiu D, Luo WJ, Wu LS, et al. Clinical analysis of 70 cases of AIDS complicated with neuropathy. Chin J Nerv Ment Dis. (2014) 40:109–12. doi: 10.3936/j.issn.1002-0152.2014.02.011
23. Li K, Wang CN, Lu HK, Gu X, Guan Z, Zhou P. Regulatory T cells in peripheral blood and cerebrospinal fluid of syphilis patients with and without neurological involvement. PLoS Negl Trop Dis. (2013) 7:e2528. doi: 10.1371/journal.pntd.0002528
24. Xiao Y, Tong ML, Liu LL, Lin LR, Chen MJ, Zhang HL, et al. Novel predictors of neurosyphilis among HIV-neative syphilis patients with neurological symptoms: an observational study. BMC Infect Dis. (2017) 17:310. doi: 10.1186/s12879-017-2339-3
25. Zhang L, Tian X, Lin LY, Song WZ, Liang YH, Bi C, et al. Analysis on seral and brain spinal fluid positive samples from 2,145 Syphilis case. Chin J Derm Venereol. (2010) 24:939–40.
26. Zhu L, Gu X, Peng RR, Wang CN, Gao ZX, Zhou PY, et al. Comparison of the cerebrospinal fluid (CSF) toluidine red unheated serum test and the CSF rapid plasma reagin test with the CSF venereal disease research laboratory test for diagnosis of neurosyphilis among HIV-negative syphilis patients in China. J Clin Microbiol. (2014) 52:736–40. doi: 10.1128/JCM.02522-13
27. Shi M, Peng RR, Gao Z, Zhang S, Lu H, Guan Z, et al. Risk profiles of neurosyphilis in HIV-negative patients with primary, secondary and latent syphilis: implications for clinical intervention. J Eur Acad Dermatol Venereol. (2016) 30:659–66. doi: 10.1111/jdv.13514
28. Ma CD, Ding KY, Shen HJ, Zhao ZG, Zhang Y. Clinical analysis of 14 cases of syphilis with cerebrospinal fluid abnormality. Chin J Derm Venereol. (2013) 27:166–7.
29. Lin DH, Li SL, Lin HL, Lin ZF, Zhang HL. The Role of serum reagin titer in lumbar puncture of neurosyphilis. Chin J Derm Venereol. (2017) 31:994–7. doi: 10.13735/j.cjdv.1001-7089.201701046
30. Li SL, Lin ZF, Zhang HS, Lin HL, Tong ML, Liu GL, et al. Relationship between syphilis serofast reaction and neurosyphilis. Chin J Nosocomiol Vol. (2012) 22:2235–8.
31. Cai SN, Long J, Chen C, Wan G, Lun WH. Incidence of asymptomatic neurosyphilis in serofast Chinese syphilis patients. Sci Rep. (2017) 7:15456. doi: 10.1038/s41598-017-15641-w
32. Zheng TH, Zeng TS, Feng TJ, Wu XB, Qiu LX. Cost-benefit analysis of neurosyphilis screening in serofast populations. Chin J Heal Statis. (2016) 33:829–32.
33. He WQ, Wang HL, Zhong DQ, Lin LY, Qiu XS, Yang RD. Treponemal antibody in CSF and cellular immunity in peripheral blood of syphilitic patients with persisting positive rapid plasma regain. Int J Clin Exp Pathol. (2015) 8:5775–80.
34. Ye YJ, Liu LF, Xu AE. Epidemiological analysis of 457 cases of refractory syphilis. Chin J Health Lab Tec. (2018) 28:1250–3.
35. Chen XS, Chi FH, Fan RQ, Li HY, Deng JQ. Analysis of clinical, serology and cerebrospinal fluid in 52 cases of syphilis inpafients. Chin J Derm Venereol. (2011) 25:616–7.
36. Wang YJ, Chi CY, Chou CH, Ho CM, Lin PC, Liao CH, et al. Syphilis and neurosyphilis in human immunodeficiency virus-infected patients: a retrospective study at a teaching hospital in Taiwan. J Microbiol Immunol Infect. (2012) 45:337–42. doi: 10.1016/j.jmii.2011.12.011
37. Sun XX, Xu HQ, Liu XL, Tian F. Clinical analysis of 102 syphilis cases coinfected with HIV and with abnormal cerebrospinal fluid. J Clin Derm. (2020) 49:200–2. doi: 10.16761/j.cnki.1000-4963.2020.04.003
38. Zhu L, Shi M, Peng RR, Gu X, Guan Z, Xu H, et al. Neurosyphilis is more common in malignant syphilis: a case series and review of the literature. Int J STD AIDS. (2019) 30:779–85. doi: 10.1177/0956462419826710
39. Wang H, Zhang HW, Li DY. Analysis of syphilis infection in inpatients of general hospital. Chin J Derm Venereol. (2011) 25:618–20.
40. Yan J, Luo L, Han J, Yan D, Zhang B, Zhang Z, et al. Comparing non-invasive predictors of neurosyphilis among syphilis patients with and without HIV co-infection based on the real-world diagnostic criteria: a single-center, retrospective cohort study in China. AIDS Res Hum Retroviruses. (2021). doi: 10.1089/AID.2021.0085. [Epub ahead of print].
41. Cao J, Zhang LJ, Fan JW, Palida ABLZ, Ayiguli YSP. Analysis of clinical characteristics, serology and cerebrospinal fluid of 73 hospitalized patients with syphilis. J Clin Derm. (2021) 50:725–7. doi: 10.16761/j.cnki.1000-4963.2021.12.006
42. Hau YH, Li ZH, Yu XY, Yan N. Clinical analysis of 121 neurosyphilis patients. J Clin Derm. (2021) 50:648–50. doi: 10.16761/j.cnki.1000-4963.2021.11.003
43. Vadboncoeur J, Labbé AC, Fortin C, Serhir B, Rabia Y, Najem K, et al. Ocular syphilis: case series (2000–2015) from two tertiary care centres in Montreal, Canada. Can J Ophthalmol. (2020) 55:30–7. doi: 10.1016/j.jcjo.2019.05.009
44. Chilver-Stainer L, Fischer U, Hauf M, Fux CA, Sturzenegger M. Syphilitic myelitis: rare, non-specific, but treatable. Neurology. (2009) 72:673–5. doi: 10.1212/01.wnl.0000342460.07764.5c
45. Borges CR, Almeida SM, Sue K, Koslyk JLA, Sato MT, Shiokawa N, et al. Neurosyphilis and ocular syphilis clinical and cerebrospinal fluid characteristics: a case series. Arq Neuropsiquiatr. (2018) 76:373–80. doi: 10.1590/0004-282X20180054
46. Hooshmand H, Escobar MR, Kopf SW. Neurosyphilis. A study of 241 patients. JAMA. (1972) 219:726–9. doi: 10.1001/jama.219.6.726
47. Joyce-Clarke N, Molteno AC. Modified neurosyphilis in the Cape Peninsula. S Afr Med J. (1978) 53:10–14
48. Mahoney JF, Arnold RC, Sterner BL, Harris A, Zwally MR. Landmark article Sept 9, 1944: Penicillin treatment of early syphilis: II. By JF Mahoney, RC Arnold, BL Sterner, A Harris and MR Zwally. JAMA. (1984) 251:2005–10. doi: 10.1001/jama.251.15.2005
49. Ghanem Kgreview. Neurosyphilis: a historical perspective and review. CNS Neurosci Ther. (2010) 16:e157–68. doi: 10.1111/j.1755-5949.2010.00183.x
50. Buitrago-Garcia D, Martí-Carvajal AJ, Jimenez A, Conterno LO, Pardo R. Antibiotic therapy for adults with neurosyphilis. Cochrane Database Syst Rev. (2019) 5:CD011399. doi: 10.1002/14651858.CD011399.pub2
51. Bettuzzi T, Jourdes A, Robineau O, Alcaraz I, Manda V, Molina JM, et al. Ceftriaxone compared with benzylpenicillin in the treatment of neurosyphilis in France: a retrospective multicentre study. Lancet Infect Dis. (2021) 21:1441–7. doi: 10.1016/S1473-3099(20)30857-4
52. Wu KQ, Zhang SF, Bao CH, Zou X, Gu X, Wang CN, et al. Circulating microRNAs as potential biomarkers in the diagnosis of neurosyphilis. Int J Dermatol Venereol. (2021) 4:16–25. doi: 10.1097/JD9.0000000000000127
53. Park IU, Tran A, Pereira L, Fakile Y. Sensitivity and specificity of treponemal-specific tests for the diagnosis of syphilis. Clin Infect Dis. (2020) 71:S13–20. doi: 10.1093/cid/ciaa349
54. Dumaresq J, Langevin S, Gagnon S, Serhir B, Deligne B, Tremblay C, et al. Clinical prediction and diagnosis of neurosyphilis in HIV-infected patients with early Syphilis. J Clin Microbiol. (2013) 51:4060–6. doi: 10.1128/JCM.01989-13
55. Lu Y, Ke W, Yang L, Wang Z, Lv P, Gu J, et al. Clinical prediction and diagnosis of neurosyphilis in HIV-negative patients: a case-control study. BMC Infect Dis. (2019) 19:1017. doi: 10.1186/s12879-019-4582-2
56. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. (2018). Available online at: https://www.cdc.gov/std/syphilis/default.htm
57. Janier M, Unemo M, Dupin N, Tiplica GS, Potočnik M, Patel R. 2020 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. (2021) 35:574–88. doi: 10.1111/jdv.16946
58. Shang XJ, He CF, Tang B, Chang XL, Ci C, Sang H. Neuroimaging features, follow-up analyses, and comparisons between asymptomatic and symptomatic neurosyphilis. Dermatol Ther (Heidelb). (2020) 10:273–83. doi: 10.1007/s13555-020-00361-3
59. Zhang XQ, Zhao GM, Su HT. Clinical and imaging findings of neurosyphilis: a case report. Chin Med J Metallurgical Ind. (2009) 26:496.
60. Jiang MJ, Zhang HJ, Li WR, Wu WQ, Huang YM, Xu DM, et al. Analysis of EEG Lemple-Ziv complexity and correlative aspects before and after treatment of anti-syphilis therapy for neurosyphilis. Neurol Res. (2019) 41:199–203. doi: 10.1080/01616412.2018.1520438
61. Liu H, Zhao ZB, You NX. Diversity in clinical manifestations and imaging features of neurosyphilis: obstacles to the diagnosis and treatment (report of three cases). Int J Neurosci. (2018) 128:785–90. doi: 10.1080/00207454.2017.1412963
Keywords: neurosyphilis, clinical epidemiological characteristics, prevalence, systematic review, China
Citation: Du F-Z, Zhang H-N, Li J-J, Zheng Z-J, Zhang X, Zhang R-L and Wang Q-Q (2022) Neurosyphilis in China: A Systematic Review of Cases From 2009–2021. Front. Med. 9:894841. doi: 10.3389/fmed.2022.894841
Received: 26 March 2022; Accepted: 25 April 2022;
Published: 13 May 2022.
Edited by:
Joseph D. Tucker, University of North Carolina at Chapel Hill, United StatesReviewed by:
Aldo Di Carlo, San Gallicano Hospital, ItalyIrina Khamaganova, Pirogov Russian National Research Medical University, Russia
Copyright © 2022 Du, Zhang, Li, Zheng, Zhang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian-Qiu Wang, d2FuZ3FpYW5xaXVuakAxMjYuY29t; Rui-Li Zhang, cmVhbGx5dmljdG9yQDEyNi5jb20=
 Fang-Zhi Du1
Fang-Zhi Du1 Hai-Ni Zhang
Hai-Ni Zhang Qian-Qiu Wang
Qian-Qiu Wang