
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 21 September 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.894541
This article is part of the Research TopicAddressing the Unmet Needs of Cataract Patients: When Quality of Vision Can Make the Difference in Quality of LifeView all 10 articles
 Saif Aldeen AlRyalat1*
Saif Aldeen AlRyalat1* Duha Atieh2
Duha Atieh2 Ayed AlHabashneh2
Ayed AlHabashneh2 Mariam Hassouneh2
Mariam Hassouneh2 Rama Toukan2
Rama Toukan2 Renad Alawamleh2
Renad Alawamleh2 Taher Alshammari3
Taher Alshammari3 Mohammed Abu-Ameerh1
Mohammed Abu-Ameerh1Purpose: This study aimed to assess preoperative predictors of visual outcome after phacoemulsification cataract surgery in Jordan, a Middle Eastern country.
Methods: This was a retrospective longitudinal study of adult patients who underwent phacoemulsification cataract surgery from January 2019 to July 2021. For each patient, we included only the first operated eye. We obtained pre-operative ocular history, cataract surgery complication risk based on a predesigned score, visual acuity, best correction, and best corrected visual acuity. We recorded intraoperative complications. We also obtained postoperative best corrected visual acuity and refractive error for correction after 1–3 months.
Results: A total of 1,370 patients were included in this study, with a mean age of 66.39 (± 9.48). 48.4% of patients achieved visual acuity ≥ 0.8, and 72.7% achieved visual acuity ≥ 0.5. The mean visual acuity improvement after phacoemulsification cataract surgery was 0.33 (95% CI 0.31–0.35). In the regression model, significant predictors that affected visual acuity improvement included the presence of diabetic retinopathy, glaucoma, and complication risk factors (i.e., high-risk surgery).
Conclusion: Predictors of visual acuity improvement vary between studies. This study was conducted in a developing country; we defined predictors of visual acuity improvement. We also provided a new preoperative phacoemulsification cataract surgery complication risk score.
Cataract extraction is considered one of the most beneficial procedures in medicine, with its outcome rapidly observed subjectively and objectively (1). According to the Global Health Commission on Global Eye Health report (2), cataract extraction is considered a “highly cost-effective vision-restoring intervention” in modern medicine. Cataract extraction via phacoemulsification surgery largely replaced older techniques with a high safety profile (3). Its main outcome is primarily measured by visual acuity improvement, which is translated by considerable gains in real-life activities and emotional and social life components (4). Despite the provided visual acuity improvement after phacoemulsification surgery, such improvement might not be sufficient to improve the quality of life of certain populations (5). Several studies tried to predict visual acuity improvement after phacoemulsification surgery and to provide preoperative risk factors for poor visual acuity improvement, which varied for different populations and countries and were generally of low-quality evidence (6–8). Most such studies were performed in developed countries, where surgical training and available technologies are more advanced than in developing countries. Studies from developing countries, including Jordan, are generally limited to small-size studies and cross-sectional designs (9), despite the high volume of cataract surgery performed. In this study, we aimed to analyze predictors of visual acuity gain after phacoemulsification cataract surgery in the major referral center in Jordan. This was the first study from Jordan to assess the outcome of phacoemulsification cataract surgery, where we included a relatively homogenous sample from Jordan’s largest tertiary referral center. We assessed preoperative predictors of visual outcome after phacoemulsification cataract surgery in a large cohort from the largest referral center in Jordan.
This was a retrospective longitudinal study for patients who underwent phacoemulsification cataract surgery at Jordan University Hospital, the largest tertiary referral hospital in Jordan. The patients were followed up for at least 3 months after surgery. We obtained institutional review board (IRB) committee approval from Jordan University Hospital IRB (IRB 5439/2021/67). Due to the retrospective data collection method, patients’ consent was waived, and the data were analyzed anonymously. The study was conducted in accordance with the latest declaration of Helsinki.
We reviewed all phacoemulsification surgeries performed at Jordan’s largest tertiary referral center for 31 months, from January 1st, 2019, to July 30th, 2021. We included the first operated eye for patients who had both eyes operated on in the specified period to avoid correlated data analysis bias (10). We excluded patients with congenital cataracts or aged below 40 years (36 patients) and cataract surgeries done as part of pars plana vitrectomy (24 patients).
We reviewed the patient’s pre-operative clinic assessment, operative notes, and post-operative clinic visits. Each included patient had a pre-operative assessment visit, where visual acuity, refraction, anterior segment, and fundus exams were performed. Diabetic patients with diabetic retinopathy also underwent macular optical coherence tomography exams to exclude co-existent diabetic macular edema. Phacoemulsification surgery details obtained from each case’s operative note are detailed in the next section. Post-operatively, our institution’s standard regimen includes eye patching until the next day’s morning visit and movement restrictions for 3 days post-operatively. The next day, the eye patch was removed, and the eyes were examined, including visual acuity, wound leak, and intraocular pressure, along with an anterior segment exam. The postoperative regimen included topical fluoroquinolone antibiotics and topical steroid eye drops.
All patients signed informed consent before entering the theater room. The eye undergoing surgery was marked, and dilating eye drops were applied 15 min before surgery. Intraoperatively, patients underwent topical, retrobulbar, or general anesthesia, depending on the patient’s factors. Each operator had an operative technique for dividing the nucleus and cortex aspiration. Stop and chop was the most commonly used technique. Otherwise, other steps were usually performed according to a standard protocol. The standard protocol intraoperatively after draping and scrubbing included paracentesis creation, injection of intracameral adrenaline and lidocaine, the use of trypan blue dye, cohesive viscoelastic to form the anterior chamber, standard up to 3 mm superior limbal clear corneal incision, capsulorhexis creation, nucleus division, aspiration, and cortex aspiration according to the surgeon’s training and preference, acrylic single-piece monofocal intraocular lens (IOL) injection in most patients (the IOL which was covered by insurance), viscoelastic aspiration, wound hydration, followed by subconjunctival moxifloxacin and steroid injection. No intracameral antibiotic is usually given per our institutional protocol.
All surgeries were performed either using R-Evolution Optikon (Italy), Geuder (Germany), or DORC (Germany) phacoemulsification machines.
We performed a literature review on cataract surgery risk for intraoperative complications and their associations with postoperative outcomes. Based on previous literature (5, 11–26), we identified several pre-operative factors that have the potential to increase surgery difficulty and complication risk. Further details about each risk score are provided in Supplementary Table 1. In regard to combining factors for a final risk score, previous studies varied from a dichotomous classification into high and low risk, which can be simpler and advantageous in statistical models; other studies used an ordinal classification scale from no risk, low risk, moderate risk, and high risk.
In our study, we classified cataract surgery complication risk into either high risk or low risk, where high-risk surgeries are those with any of the following pre-operative risk factors: Pseudoexfoliation or phacodenesis; proliferative diabetic retinopathy; previous vitrectomy; a 4 + dense, white, or brunescent cataract; age above 88; central or paracentral corneal opacity; previous penetrating keratoplasty or radial keratotomy; history of uveitis or synechia; and posterior polar cataract; high myopia (above −6); or high hyperopia (above + 3).
We obtained demographic characteristics for each patient, including pre-operative medical history, ocular history, best corrected visual acuity, and refractive error for correction. We also obtained intraoperative data regarding the operator (senior resident or consultant), surgical notes, and any intra-operative complications, including posterior capsular rupture, dropped nucleus, or IOL, and the use of sutures to secure the wound. Finally, we obtained follow-up data for best corrected visual acuity and refractive error for correction after 1–3 months. Based on the operator, we classified surgeries into teaching cases done by senior ophthalmology residents under the supervision of consultants or cases done by consultants alone.
Visual acuities were measured on a standard E-chart at a 6-meter distance, with acuities measured in decimals. For visual acuities worse than 0.05, we converted counting fingers, hand motion, light perception, and no light perception into 0.014, 0.005, 0.0016, and 0.0013, respectively (27). Based on minimal important difference improvement, we further categorized visual acuity improvement into either improved by more than 0.1, 0.1 or less improvement or worsening in visual acuity (28, 29).
We used SPSS version 26.0 (Chicago, USA) in our analysis. We used the mean (± standard deviation) to describe continuous variables. We used count (frequency) to describe other nominal variables. We performed linear regression analysis to assess predictors of visual acuity changes between pre-operative and post-operative visual acuity after phacoemulsification cataract surgery. We adopted a model-building strategy, where we first performed a univariate analysis, and then we only included in the regression analysis significant variables from the univariate analysis. For the univariate analysis, we performed an independent sample t-test and one-way ANOVA to analyze the mean difference between visual acuity and each nominal measurement (e.g., gender, operator, risk factors) and presented the data as a mean difference and 95% confidence interval (CI). We performed Pearson correlation to analyze the relationship between visual acuity difference and age, preoperative visual acuity, and refractive error. On univariate analysis, the following pre-operative predictors achieved a significance level above the pre-specified threshold: age (0.001), diabetic retinopathy (<0.001), pre-operative visual acuity (<0.001), spherical pre-operative refractive error (0.001), cylindrical preoperative refractive error (0.038), presence of glaucoma (0.003), history of intravitreal injections (<0.001), age-related macular degeneration (0.019), and cataract surgery complication risk (0.039). However, the following variables did not reach the threshold, including gender (0.666), a teaching case (0.936), laterality (0.789), and cylindrical axis of preoperative refractive error (0.762). We presented regression analysis results in B value and its 95% CI, along with model prediction accuracy, representing the model’s ability to explain the variance in the outcome. All the underlying assumptions were met. We adopted a p-value of 0.05 as a significant threshold.
A total of 1,370 patients were included in this study, with a mean age of 66.39 (± 9.48). They were 673 (49.1%) men and 698 (50.9%) women. Of the total cases, 312 (22.8%) were teaching cases. 48.4% of patients achieved visual acuity of ≥ 0.8, and 72.7% achieved visual acuity of ≥ 0.5. Table 1 details the characteristics of the included sample.
The mean visual acuity improvement after phacoemulsification cataract surgery was 0.33 (95% CI 0.31–0.35), from a mean best corrected visual acuity preoperatively of 0.32 (SD 0.26) to 0.65 (SD 0.32) postoperatively. The regression model predicted 35.7% of the visual acuity change after cataract surgery based on pre-operative characteristics. The significant predictors that affected visual acuity improvement included the presence of diabetic retinopathy, glaucoma, and a complication risk factor (i.e., high-risk surgery). Moreover, increased pre-operative visual acuity, spherical refractive error, or cylindrical refractive error were also significant predictors of decreased visual acuity improvement after cataract surgery (Table 2).
The model building strategy and included variables were detailed in the statistical analysis section.
A total of 350 (25.5%) surgeries were high-risk surgeries. They had a total of 382 risk factors, whereas 39 surgeries had more than one risk factor. The most common risk factor was pseudoexfoliation (23.56%), followed by high myopia (22.25%) and proliferative diabetic retinopathy (19.9%). Figure 1 shows the frequency of each risk factor for cataract surgeries.
We found a significant difference in visual acuity improvement between high-risk and low-risk surgeries (p = 0.039), where the mean visual acuity improvement in low-risk surgeries was 0.355 (SD 0.31), compared to 0.301 (SD 0.33) for high-risk surgeries (mean difference 0.054, 95% CI 0.003–0.105). No significant difference was found in the intra-operative complication rate between both groups (p = 0.523).
Teaching cases operated by senior residents under the supervision of consultants comprised 312 (22.8%) cases. The majority of these cases were of low risk (78.8%), with only 66 (21.2%) cases of high risk compared to 283 (26.8%) non-teaching cases, a frequency that differed significantly (p = 0.025). No significant difference in visual acuity gains after cataract surgery (p = 0.940) or frequency of complications (p = 0.336) between teaching and non-teaching cases. Figure 2 compares consultants and residents who performed surgeries regarding surgery difficulty.
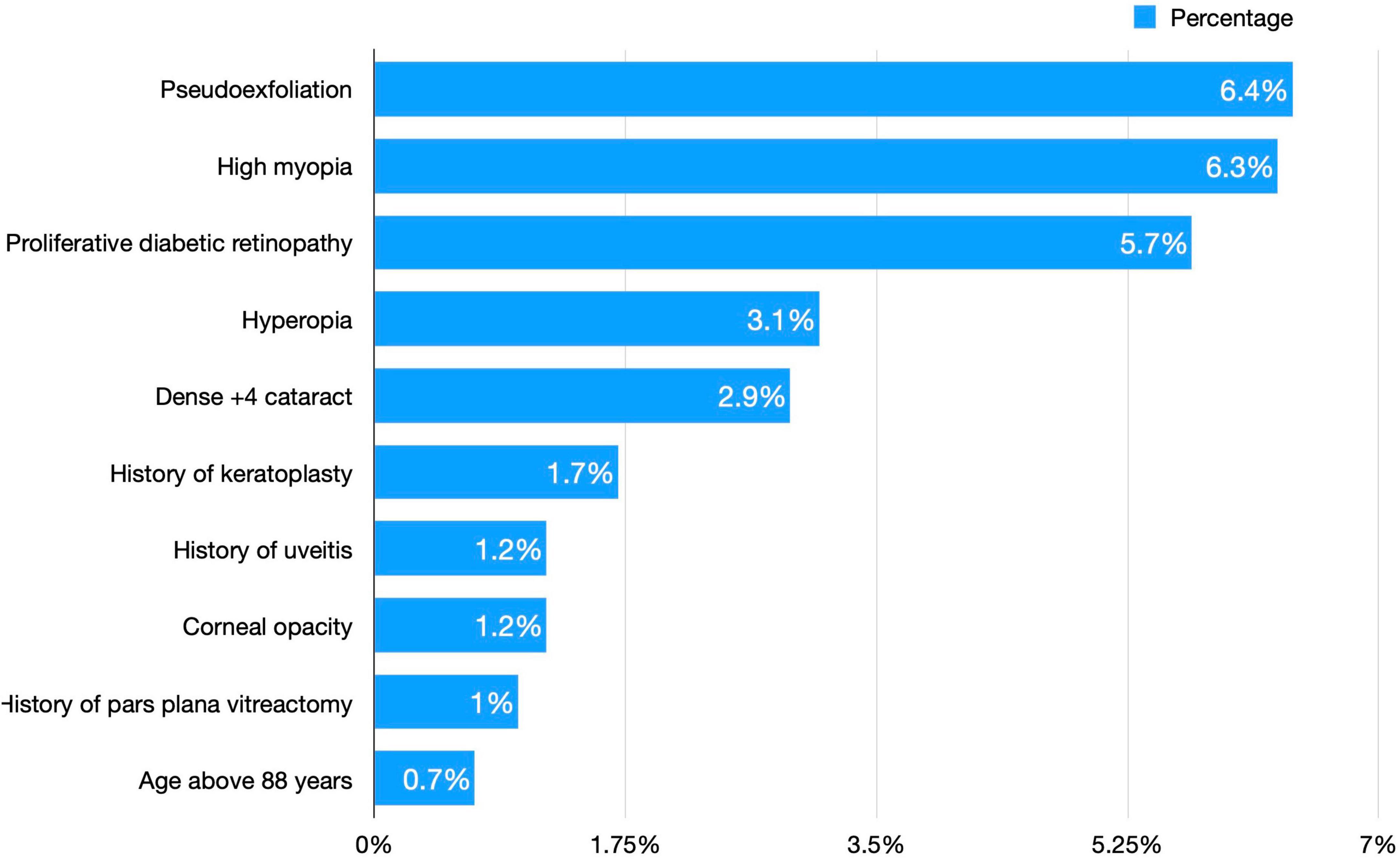
Figure 2. A comparison of surgery difficulty between consultants and residents who performed phacoemulsification surgeries.
Upon comparing refractive error change after cataract surgery, we found a significant difference in spherical refractive error (p < 0.001), with a mean increase in spherical refractive error by a mean of 2.18 (95% CI −2.74 to −1.62). No significant difference was found in cylindrical refractive errors or their axes (Table 3).
After categorizing patients into three categories, we found that most patients had an improvement of > 0.1 in visual acuity (69.4%), while 20% of patients had 0.1 or less visual acuity improvement, and only 10.6% had a worsening in visual acuity. Baseline visual acuity was significantly associated with each category of visual acuity improvement (p < 0.001). In addition, the visual acuity worsening group had a higher cataract surgery complication risk. Table 4 compares the mean baseline visual acuity and complication risk among the three categories.

Table 4. Comparison between best corrected visual acuity (BCVA) improvement by > 0.1, ≤0.1, and worsening in terms of mean baseline visual acuity and complication risk among the three categories.
This study was the largest to define predictors of visual acuity improvement after phacoemulsification cataract surgery. The mean improvement expected after phacoemulsification cataract surgery was 0.33 (95% CI 0.31–0.35); this magnitude of improvement would decrease if the eye had glaucoma, diabetic retinopathy, pre-operative complication risk factors, higher pre-operative visual acuity, or refractive error. We also performed a literature review to find factors that increase the risk of surgical complications, and we classified phacoemulsification into high- and low-risk surgeries accordingly. We found that surgeries classified as high-risk had significantly lower visual acuity improvement compared to low-risk surgeries. Almost 23% of included cases were teaching cases operated by senior ophthalmology residents, and we did not find a higher complication rate or worse visual acuity in teaching cases. Regarding refractive error change after phacoemulsification cataract surgery, we found an improvement in spherical error. A European Registry of Quality Outcomes for Cataract and Refractive Surgery study found that ocular comorbidities were the most important predictor of visual acuity improvement, where ocular comorbidities included macular degeneration, glaucoma, diabetic retinopathy, and amblyopia, among others (7). Another US-based study also found pre-operative comorbidities to be predictors of poor visual acuity, which included diabetes mellitus, chronic pulmonary disease, and age-related macular degeneration (30).
Among the factors that affect the outcome of cataract surgery is the difficulty and complexity of the surgery itself, which can be predicted by preoperative factors (31). The complexity of cataract surgery was one of the most commonly appearing predictors of poorer visual acuity improvement (13, 32). Considering preoperative risk scoring in surgery, decision-making and planning should also be included during the surgery decision-making process (5). Studies used different scores to classify surgeries into high-risk (aka. complex surgery) and low-risk surgeries. In the study by Lundström et al. complex surgery is defined by the presence of previous vitrectomy, previous corneal refractive surgery, miosis, white/brown cataract, corneal opacities, pseudoexfoliation, and others (7). Another negative predictor factor of visual acuity improvement was glaucoma. The relationship between cataract extraction and glaucoma is complex. Although it has been established that cataract extraction has a beneficial intraocular pressure lowering effect and improves the quality of life (33, 34), phacoemulsification cataract extraction surgery might sometimes be challenging in these patients. Patients with glaucoma usually also have other ocular co-morbidities, both diagnosed and undiagnosed, along with frequent topical medication use (35, 36). After surgery, glaucoma patients experience increased intraocular pressure, severe corneal edema, endothelial cell damage, and poor vision (37, 38). A study performed on a European registry of 15 European countries found that preoperative ocular co-morbidity was the strongest negative predictor for visual outcome, where comorbidities included glaucoma and other retinal diseases (7). A previous study in several African developing countries found that pre-operative refractive error was the leading cause of poor visual outcomes (39). Consultants operated at a higher frequency of high-risk surgeries compared to residents, a finding also found in a UK-based national study (40). A recent systematic review found that the previous history of intravitreal injection can be regarded as a risk factor for PCR and should be considered when planning cataract surgery. However, the magnitude of this risk is generally small (41). The complexity of preoperative risk score discussion increases when we consider protective factors that might decrease surgery difficulty or complication rate (42), which should be considered in future studies.
In our study, no significant difference in complication rates was found between teaching cases operated by residents and non-teaching cases operated by specialists. Our results were consistent with previous studies done in other countries, including the USA (43), the UK (40), Canada (44), and Australia (45). On the other hand, a recent study on surgeries performed in Europe found higher complication rates for surgeries performed by residents (46). Higher complication rates for residents were also found in studies done in Hungary (47). It is important to note that these studies differed in settings, countries, and teaching methods. A future review investigating surgical factors and teaching methods might reveal the reason behind these differences. While we did not measure the duration of surgery, a previous study found that the duration of surgery significantly differed according to experience, with the longest duration for trainees and the shortest duration for experienced specialists (48).
Our study is the first in Jordan and the Middle East to assess the visual outcome and predictors of visual acuity in a large cohort; its main limitation is the use of a retrospective design for data collected from university hospital-based ophthalmology clinics. As a result, we could not include certain factors that may be considered pre-operative risk factors due to under-reporting by patients’ records.
In our cohort from Jordan, a developing country, we found that the mean improvement expected after phacoemulsification cataract surgery was 0.33 (95% CI 0.31–0.35), where the mean best corrected visual acuity after cataract surgery was 0.65 (SD 0.32) postoperatively, which is above the limit for driving in most countries. The majority of patients had visual acuity improvement in more than one line. Patients with higher baseline visual acuity would be expected to improve less than patients with lower baseline visual acuity. Poor visual acuity improvement predictors include glaucoma, diabetic retinopathy, pre-operative complication risk factors, higher pre-operative visual acuity, and refractive error. We provided a literature-based new preoperative phacoemulsification cataract surgery complication risk score.
• Phacoemulsification has revolutionized the management of cataracts in recent years. However, there has been wide variation in its outcome and predictors of outcome between different studies in different countries.
• Most such studies were performed in developed countries, where surgical training and available technologies are more advanced than in developing countries.
• Our study is the first in Jordan, a developing country, and the Middle East to assess the visual outcome and predictors of visual acuity in a large cohort.
• We also provided a literature-based new preoperative phacoemulsification cataract surgery complication risk score.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by we obtained institutional review board (IRB) committee approval from Jordan University Hospital IRB (IRB 5439/2021/67). Due to the retrospective data collection method, patients’ consent was waived, and the data were analyzed anonymously. The study was conducted in accordance with the latest declaration of Helsinki.
SA, DA, and MA-A contributed to research conception, protocol development, manuscript writing, and data analysis. AA, MH, RT, and RA contributed to data collection and manuscript writing. TA contributed to research conception and manuscript writing. All authors approved final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.894541/full#supplementary-material
2. Burton MJ, Ramke J, Marques AP, Bourne RRA, Congdon N, Jones I, et al. The lancet global health commission on global eye health: vision beyond 2020. Lancet Glob Health. (2021) 9:e489–551. doi: 10.1016/S2214-109X(20)30488-5
3. Hoffman RS, Fine IH, Packer M. New phacoemulsification technology. Curr Opin Ophthalmol. (2005) 16:38–43.
4. Lamoureux EL, Fenwick E, Pesudovs K, Tan D. The impact of cataract surgery on quality of life. Curr Opin Ophthalmol. (2011) 22:19–27. doi: 10.1097/ICU.0b013e3283414284
5. Zheng Y, Qu B, Jin L, Wang C, Zhong Y, He M, et al. Patient-centred and economic effectiveness of a decision aid for patients with age-related cataract in China: study protocol of a randomised controlled trial. BMJ Open. (2020) 10:e032242. doi: 10.1136/bmjopen-2019-032242
6. Norregaard JC, Hindsberger C, Alonso J, Bellan L, Bernth-Petersen P, Black C, et al. Visual outcomes of cataract surgery in the United States, Canada, Denmark, and Spain: report from the international cataract surgery outcomes study. Arch Ophthalmol. (1998) 116:1095–100. doi: 10.1001/archopht.116.8.1095
7. Lundström M, Barry P, Henry Y, Rosen P, Stenevi U. Visual outcome of cataract surgery; study from the European registry of quality outcomes for cataract and refractive surgery. J Cataract Refract Surg. (2013) 39:673–9. doi: 10.1016/j.jcrs.2012.11.026
8. AlRyalat SA, Abukahel A, Elubous KA. Randomized controlled trials in ophthalmology: a bibliometric study. F1000Research. (2019) 8:1718. doi: 10.12688/f1000research.20673.1
9. Al-dolat W, Alqudah NM, Atoum D, Al-Omari R, Khatatbeh M. Preferred surgical and anesthesia techniques for cataract surgery in Jordan. Clin Ophthalmol. (2021) 15:4259–67. doi: 10.2147/OPTH.S334425
10. Ying G-S, Maguire MG, Glynn RJ, Rosner B. Tutorial on biostatistics: longitudinal analysis of correlated continuous eye data. Ophthalmic Epidemiol. (2021) 28:3–20. doi: 10.1080/09286586.2020.1786590
11. Weingessel B, Wahl M, Huf W, Vécsei-Marlovits PV. Decision-making for cataract surgery: changes within 7 years. Acta Ophthalmol. (2019) 97:e139–40. doi: 10.1111/aos.13834
12. Saifee M, Zhu I, Lin Y, Oldenburg CE, Ramanathan S. Effect of full-time vs volunteer faculty supervision on resident cataract surgery complications. J Cataract Refract Surg. (2020) 46:700–4. doi: 10.1097/j.jcrs.0000000000000145
13. Muhtaseb M, Kalhoro A, Ionides A. A system for preoperative stratification of cataract patients according to risk of intraoperative complications: a prospective analysis of 1441 cases. Br J Ophthalmol. (2004) 88:1242. doi: 10.1136/bjo.2004.046003
14. Kaur M, Bhai N, Titiyal JS. Risk factors for complications during phacoemulsification cataract surgery. Expert Rev Ophthalmol. (2020) 15:303–12. doi: 10.1080/17469899.2020.1806715
15. Blomquist PH, Morales ME, Tong L, Ahn C. Risk factors for vitreous complications in resident-performed phacoemulsification surgery. J Cataract Refract Surg. (2012) 38:208–14. doi: 10.1016/j.jcrs.2011.10.001
16. Mylona I, Dermenoudi M, Glynatsis M, Ziakas N, Tsinopoulos I. Development of a reliable preoperative risk stratification system for phacoemulsification. J Cataract Refract Surg. (2020) 46:1132–7. doi: 10.1097/j.jcrs.0000000000000223
17. Zetterberg M, Kugelberg M, Nilsson I, Lundström M, Behndig A, Montan PA. Composite risk score for capsule complications based on data from the swedish national cataract register: relation to surgery volumes. Ophthalmology. (2021) 128:364–71. doi: 10.1016/j.ophtha.2020.07.033
18. Ergun ŞB, Kocamış SÍ, Çakmak HB, Çağıl N. The evaluation of the risk factors for capsular complications in phacoemulsification. Int Ophthalmol. (2018) 38:1851–61. doi: 10.1007/s10792-017-0667-3
19. Nderitu P, Ursell P. Updated cataract surgery complexity stratification score for trainee ophthalmic surgeons. J Cataract Refract Surg. (2018) 44:709–17. doi: 10.1016/j.jcrs.2018.04.036
20. Hashemi H, Mohammadpour M, Jabbarvand M, Nezamdoost Z, Ghadimi H. Incidence of and risk factors for vitreous loss in resident-performed phacoemulsification surgery. J Cataract Refract Surg. (2013) 39:1377–82. doi: 10.1016/j.jcrs.2013.03.028
21. Rutar T, Porco TC, Naseri A. Risk factors for intraoperative complications in resident-performed phacoemulsification surgery. Ophthalmology. (2009) 116:431–6. doi: 10.1016/j.ophtha.2008.10.028
22. Lomi N, Sharma R, Khokhar S, Dada T, Vanathi M, Agarwal T. Risk factors for intra-operative complications during phacoemulsification performed by residents. Int Ophthalmol. (2016) 36:401–6. doi: 10.1007/s10792-015-0146-7
23. Gharaei H, Sedaghat MR, Banan S. Evaluation of the correspondence between preoperative risk factors and intraoperative complications in resident-performed phacoemulsification. J Patient Saf Qual Improv. (2015) 3:273–6. doi: 10.22038/psj.2015.5243
24. Narendran N, Jaycock P, Johnston RL, Taylor H, Adams M, Tole DM, et al. The cataract national dataset electronic multicentre audit of 55,567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye Lond Engl. (2009) 23:31–7. doi: 10.1038/sj.eye.6703049
25. Habib MS, Bunce CV, Fraser SG. The role of case mix in the relation of volume and outcome in phacoemulsification. Br J Ophthalmol. (2005) 89:1143–6. doi: 10.1136/bjo.2005.070235
26. Najjar DM, Awwad ST. Cataract surgery risk score for residents and beginning surgeons. J Cataract Refract Surg. (2003) 29:2036–7. doi: 10.1016/j.jcrs.2003.08.004
27. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. (2006) 47:1236–40. doi: 10.1167/iovs.05-0981
28. Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. (2007) 114:1804–9. doi: 10.1016/j.ophtha.2007.06.047
29. Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). Trans Am Ophthalmol Soc. (2009) 107:311–24.
30. Greenberg PB, Tseng VL, Wu W-C, Liu J, Jiang L, Chen CK, et al. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. (2011) 118:507–14. doi: 10.1016/j.ophtha.2010.07.023
31. See CW, Iftikhar M, Woreta FA. Preoperative evaluation for cataract surgery. Curr Opin Ophthalmol. (2019) 30:3–8. doi: 10.1097/ICU.0000000000000535
32. Gaskin GL, Pershing S, Cole TS, Shah NH. Predictive modeling of risk factors and complications of cataract surgery. Eur J Ophthalmol. (2016) 26:328–37. doi: 10.5301/ejo.5000706
33. Masis M, Mineault PJ, Phan E, Lin SC. The role of phacoemulsification in glaucoma therapy: a systematic review and meta-analysis. Surv Ophthalmol. (2018) 63:700–10. doi: 10.1016/j.survophthal.2017.08.006
34. Skalicky SE, Martin KR, Fenwick E, Crowston JG, Goldberg I, McCluskey P. Cataract and quality of life in patients with glaucoma. Clin Exp Ophthalmol. (2015) 43:335–41. doi: 10.1111/ceo.12454
35. Shingleton BJ, Heltzer J, O’Donoghue MW. Outcomes of phacoemulsification in patients with and without pseudoexfoliation syndrome. J Cataract Refract Surg. (2003) 29:1080–6. doi: 10.1016/S0886-3350(02)01993-4
36. Poley BJ, Lindstrom RL, Samuelson TW, Schulze R. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. (2009) 35:1946–55. doi: 10.1016/j.jcrs.2009.05.061
37. He L, Cui Y, Tang X, He S, Yao X, Huang Q, et al. Changes in visual function and quality of life in patients with senile cataract following phacoemulsification. Ann Palliat Med. (2020) 9:3802–9. doi: 10.21037/apm-20-1709
38. Li G, Song H, Chen L, Yang W, Nan K, Lu P. TUG1 promotes lens epithelial cell apoptosis by regulating miR-421/caspase-3 axis in age-related cataract. Exp Cell Res. (2017) 356:20–7. doi: 10.1016/j.yexcr.2017.04.002
39. Lindfield R, Kuper H, Polack S, Eusebio C, Mathenge W, Wadud Z, et al. Outcome of cataract surgery at one year in Kenya, the Philippines and Bangladesh. Br J Ophthalmol. (2009) 93:875–80. doi: 10.1136/bjo.2008.152744
40. Day AC, Donachie PHJ, Sparrow JM, Johnston RL. The royal college of ophthalmologists’ national ophthalmology database study of cataract surgery: report 1, visual outcomes and complications. Eye. (2015) 29:552–60. doi: 10.1038/eye.2015.3
41. Bjerager J, van Dijk EHC, Holm LM, Singh A, Subhi Y. Previous intravitreal injection as a risk factor of posterior capsule rupture in cataract surgery: a systematic review and meta-analysis. Acta Ophthalmol. (2022) 100:614–23. doi: 10.1111/aos.15089
42. Dahshan D, Kuzbel J, Verma V. A role for music in cataract surgery: a systematic review. Int Ophthalmol. (2021) 41:4209–15. doi: 10.1007/s10792-021-01986-9
43. Chen X, Zafar S, Sikder S, Srikumaran D, Boland M, Ramanathan S, et al. National survey and outcomes of resident-performed cataract surgery in monocular patients in the United States. J Cataract Refract Surg. (2019) 45:939–45. doi: 10.1016/j.jcrs.2019.02.018
44. Low SAW, Braga-Mele R, Yan DB, El-Defrawy S. Intraoperative complication rates in cataract surgery performed by ophthalmology resident trainees compared to staff surgeons in a Canadian academic center. J Cataract Refract Surg. (2018) 44:1344–9. doi: 10.1016/j.jcrs.2018.07.028
45. Fong CS, Mitchell P, de Loryn T, Rochtchina E, Hong T, Cugati S, et al. Long-term outcomes of phacoemulsification cataract surgery performed by trainees and consultants in an Australian cohort. Clin Experiment Ophthalmol. (2012) 40:597–603. doi: 10.1111/j.1442-9071.2012.02759.x
46. Oliveira-Ferreira C, Leuzinger-Dias M, Tavares Ferreira J, Macedo JP, Falcão-Reis F. Cataract phacoemulsification performed by resident trainees and staff surgeons: intraoperative complications and early postoperative intraocular pressure elevation. J Cataract Refract Surg. (2020) 46:555–61. doi: 10.1097/j.jcrs.0000000000000105
47. Magyar M, Sándor GL, Ujváry L, Nagy ZZ, Tóth G. Intraoperative complication rates in cataract surgery performed by resident trainees and staff surgeons in a tertiary eyecare center in Hungary. Int J Ophthalmol. (2022) 15:586–90. doi: 10.18240/ijo.2022.04.10
Keywords: cataract, phacoemulsification, risk score, visual acuity, developing country
Citation: AlRyalat SA, Atieh D, AlHabashneh A, Hassouneh M, Toukan R, Alawamleh R, Alshammari T and Abu-Ameerh M (2022) Predictors of visual acuity improvement after phacoemulsification cataract surgery. Front. Med. 9:894541. doi: 10.3389/fmed.2022.894541
Received: 11 March 2022; Accepted: 25 August 2022;
Published: 21 September 2022.
Edited by:
Rita Mencucci, University of Florence, ItalyReviewed by:
Paul Nderitu, King’s College London, United KingdomCopyright © 2022 AlRyalat, Atieh, AlHabashneh, Hassouneh, Toukan, Alawamleh, Alshammari and Abu-Ameerh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saif Aldeen AlRyalat, cy5hbHJ5YWxhdEBqdS5lZHUuam8=; c2FpZnJ5YWxhdEB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.