- 1Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 2Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 3Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 4Department of Laboratory Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 5Doctoral Degree Program of Toxicology, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan
- 6Department of Radiology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 7Department of Radiology, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 8Department of Preventive Medicine, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 9Department of Occupational & Environmental Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
Background: Fracture Risk Assessment Tool (FRAX) and bone turnover markers (BTMs) predict fractures in the general population. However, the role of FRAX and BTMs in predicting mortality remains uncertain in hemodialysis (HD) patients.
Methods: One hundred and sixty-four HD patients stratified by low or high risk of 10-year fracture probability using FRAX. High risk of fracture was defined as 10-year probability of hip fracture ≥3% or major osteoporotic fracture ≥20%. The association of high risk of fracture and BTMs with all-cause mortality and cardiovascular (CV) mortality were evaluated using multivariate-adjusted Cox regression analysis.
Results: Eighty-five (51.8%) patients were classified as high risk of fracture based on FRAX among 164 HD patients. During a mean follow-up period of 3.5 ± 1.0 years, there were 39 all-cause deaths and 23 CV deaths. In multivariate-adjusted Cox regression, high risk of fracture based on FRAX was independently associated with all-cause mortality [hazard ratio (HR): 2.493, 95% confidence interval (CI): 1.026–6.056, p = 0.044) but not with CV mortality (HR: 2.129, 95% CI: 0.677–6.700, p = 0.196). There were no associations between BTMs and mortality risk. Furthermore, lower geriatric nutritional risk index (GNRI) was significantly associated with increased CV mortality (HR: 0.888, 95% CI: 0.802–0.983, p = 0.022) after adjusting by confounding variables.
Conclusion: High risk of fracture using FRAX was an independent predictor of all-cause mortality in patients undergoing HD. FRAX, rather than BTMs, has an important role of prognostic significance in HD patients.
Introduction
Osteoporotic fractures can lead to physical dysfunction and decreased quality of life, and link to increased mortality and health cost in the general population (1–3). Accumulating evidence indicates that the fracture risk increases steadily with the loss of renal function in patients with chronic kidney disease (CKD) (4, 5), and the risk becomes four times higher in hemodialysis (HD) patients in comparison with healthy controls (6). Mineral and bone disorders, disturbed calcium and phosphate balance, secondary hyperparathyroidism, as well as vitamin D deficiency in chronic kidney disease contribute to the derangements in bone mineralization and turnover (7). Moreover, chronic diseases in CKD could aggravate frailty. Uremic toxin, inflammation, fluid overload, anemia, and malnutrition are involved in the process of muscle mass loss, cognitive impairment, and finally generate frailty in CKD (8, 9). Of note, frailty links to increased risk of fracture and mortality in dialysis-dependent patients (10, 11).
The Fracture Risk Assessment Tool (FRAX) is an online calculated tool, and is able to predict 10-year fracture risk based on clinical risk factors and bone mineral density (BMD) in the general population. Furthermore, FRAX helps doctors make treatment strategies for osteoporosis in clinical practice (12). Although the risk factors for major bone fractures in CKD are complex, FRAX has been demonstrated to stratify fracture risk in non-dialysis CKD patients and in HD patients (13–15). Bone turnover markers (BTMs) are series of biomarkers released during the process of bone remodeling. BTMs include markers of bone resorption and bone formation, and provide a non-invasive approach for studying bone turnover. Previous studies have reported a positive association between BTMs and fracture risk (16, 17). In patients with end-stage kidney disease (ESKD), BTMs were correlated with bone loss and low bone density (18, 19). However, the association between BTMs and fractures were conflicting in patients with CKD (19, 20).
Even though FRAX and BTMs might reflect the severity of low bone density and an increased risk of osteoporotic fractures, the associations of FRAX and BTMs with mortality risk remains unclear in patients with CKD or with ESKD. There are limited studies to examine their role in predicting mortality among this patient population. Hence, the aims of this study are to investigate the relationship between FRAX and BTMs, and further to evaluate the role of FRAX and BTMs in association with all-cause mortality and cardiovascular (CV) mortality in HD patients.
Methods
Study Participants
From March 2017 to December 2017, this interventional cohort study recruited 178 patients on thrice-weekly HD >3 months at the outpatient HD center of a regional hospital in Taiwan. All patients were ≥20 years of age and their each HD treatment lasted for 3.5–4.5 h based on the Kidney Disease Outcomes Quality Initiative clinical practice guideline (21). Patients who refused to undergo dual energy x-ray absorptiometry (DXA) scan (n = 6), patients with bilateral below knee amputation (n = 3), and those who were hospitalized 4 weeks prior the study enrollment (n = 5) were excluded from the study. Finally, a total of 164 maintenance HD patients (mean age 60.1 ± 10.6 years, 54.9% men) were included. The ethics review committee and Institutional Review Board of Kaohsiung Medical University Hospital approved the study protocol (KMUHIRB-F(I)-20150074). All patients provided their written informed consent.
Demographic, Medical and Biochemical Information
Through the interviews and electronic medical records, the information of patients' demographic characteristics and their medical history were obtained. Fasting blood sample was collected for measurement of biochemical markers using an automated chemistry analyzer TBA-c16000 (Toshiba, Tokyo, Japan). The intact parathyroid hormone (PTH) assay was performed using the analyzer Immulite 2000 (Siemens Healthcare Diagnostics, Munich, Germany). Serum 25-hydroxyvitamin D concentration was analyzed using chemiluminescent microparticle immunoassay on an automated Abbott Architect i2000 (Abbott Laboratories, IL, USA). The geriatric nutritional risk index (GNRI), reliable indicator for nutritional status in maintenance HD patients (22), was calculated as GNRI = [14.89 × albumin (g/dl)] + [41.7 × (body weight/ideal body weight)] (23). The ideal body weight was defined as the value calculated from the height and a body mass index of 22 (24). If the patient's body weight was greater than the ideal body weight, body weight/ideal body weight was set to 1 (25).
BTMs
Serum procollagen type 1 amino-terminal propeptide (P1NP) and C-terminal cross-linking telopeptide of type I collagen (CTX) were analyzed by an automated Roche electrochemiluminescence system (E411, Roche Diagnostics, Mannheim, Germany). The Unicel DxI 800 immunoassay system (Beckman Coulter Inc., Brea, CA, USA) was used to measure bone-specific alkaline phosphatase (BALP). Serum dickkopf-related protein-1 (DKK1) and sclerostin levels were measured using commercially available enzyme-linked immunosorbent assay (EIAab Science Co.:DKK1; Biomedica: sclerostin), following the manufacturer's protocols.
Cardiothoracic Ratio and Aortic Arch Calcification
All patients' chest X-rays were reviewed by an experienced radiologist for assessment of the cardiothoracic ratio and aortic arch calcification (AoAC). The cardiothoracic ratio was calculated based on the ratio of the transverse diameter of the cardiac shadow to the transverse diameter of the chest on patients' chest X-rays. AoAC was assessed using the scale proposed by Ogawa et al. (26). In brief, the number of section with calcification was counted from the divided 16 sections of the aortic arch on patients' chest X-rays.
Assessment of Fracture Risk Using FRAX
To assess the risk of fractures, FRAX is able to provide the 10-year probability of a major osteoporotic fracture and hip fracture, respectively. FRAX assessment requires the information about age, gender, height, weight, previous fracture, family history of hip fracture, current smoking, use of glucocorticoids, history of rheumatoid arthritis and secondary osteoporosis, amount of daily alcohol consumption, and femoral neck BMD (http://www.shef.ac.uk/FRAX). BMD at femoral neck was measured using a DXA scanner (Hologic Inc., Bedford, MA, USA). High risk of fracture was defined as 10-year probability of hip fracture ≥3% or a major osteoporotic fracture ≥20% (27, 28).
Outcomes of Interest
Two clinical outcomes, all-cause mortality and CV mortality were assessed. CV mortality was defined as fatal myocardial infarction, fatal ventricular arrhythmia, sudden cardiac death, and fatal stroke. Study patients were followed until death, and the remaining patients were followed until July 2021.
Statistical Analysis
Descriptive statistics are presented as percentages, mean ± standard deviation, or median (25th−75th percentile) for the dialysis vintage, AoAC, P1NP, CTX, BALP, DKK1, sclerostin, intact PTH, and high-sensitivity C-reactive protein (hs-CRP). The study patients were stratified into two groups based on low or high risk of fracture assessed by using FRAX. Differences between two groups of patients were analyzed by the chi-square test for categorical variables, and by an independent t-test for continuous variables with approximately normal distribution, or by Mann-Whitney U test for continuous variables with skewed distribution. Survival curves for all-cause mortality and CV mortality were illustrated using the Kaplan-Meier method and compared between above-mentioned groups of patients by the log-rank test. The univariate and multivariate adjusted Cox regression analyses were used to identify the factors associated with all-cause and CV mortality. The continuous variables with a skewed distribution were log-transformed to attain normal distribution in the Cox regression analysis. Significant variables (p < 0.05) in the univariate analysis were selected into the multivariate Cox analysis to identify the factors associated with all-cause and CV mortality. A p < 0.05 was considered statistically significant. All statistical analyses were carried out using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) for Windows.
Results
Clinical Characteristics of Study Patients
A total of 164 HD patients were included in this study. Table 1 lists the study patients' baseline characteristics. The median value of 10-year probability of hip fracture and major osteoporotic fracture were 3.35% and 8.35%, respectively. Eighty-five (51.8%) patients were classified as high risk of fracture based on FRAX. Patients with high risk of fracture were more likely to be older, had lower body mass index, lower GNRI, higher AoAC, lower femoral neck BMD, lower femoral neck T-score, and higher level of hs-CRP, compared to those with low risk of fracture.
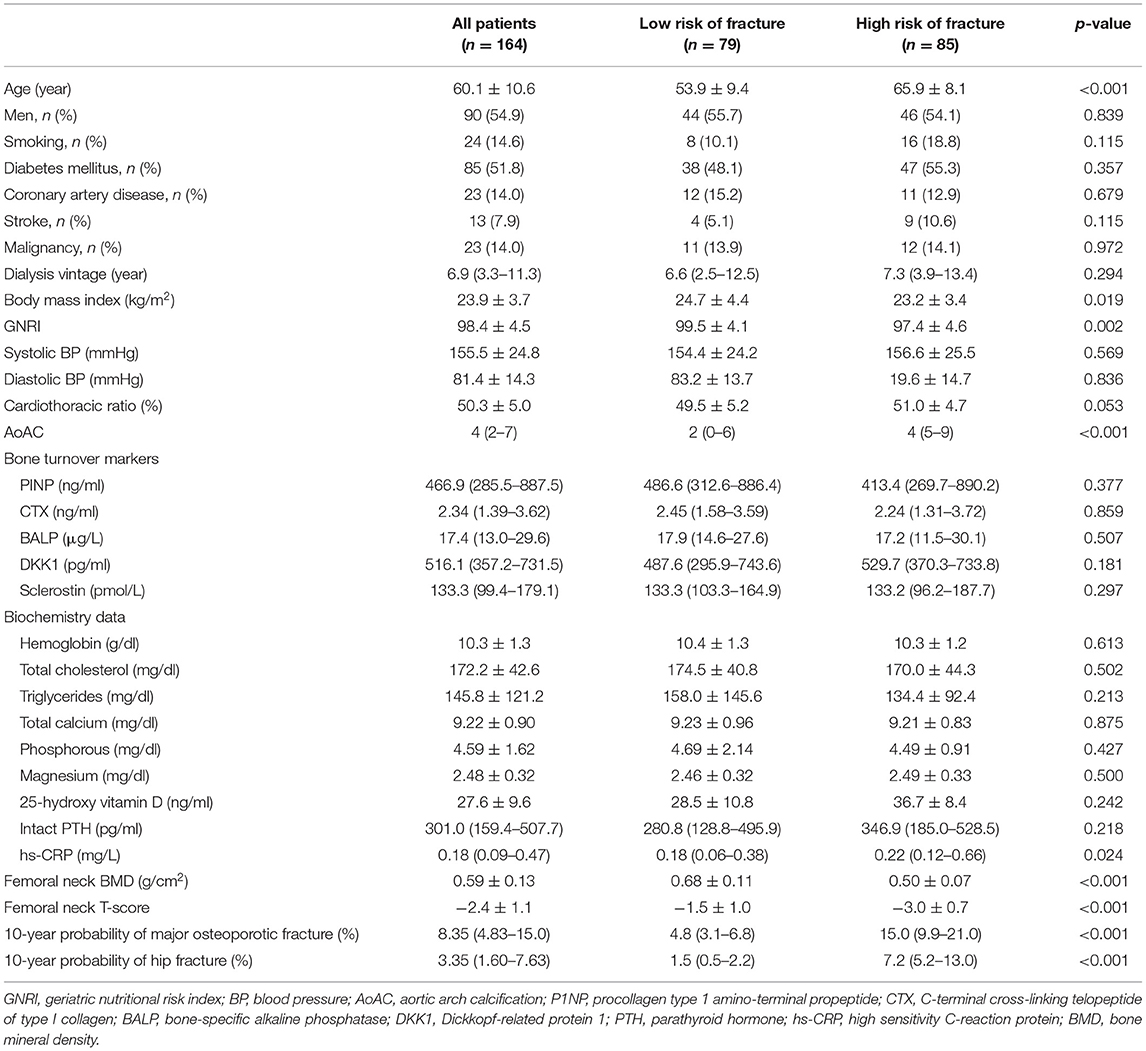
Table 1. Comparison of baseline characteristics between maintenance hemodialysis patients with low or high risk of fracture assessed using FRAX.
Relationship Between BTMs and Clinical Features in Maintenance HD Patients
We further explored the relationships between BTMs and clinical characteristics in HD patients. As shown in Table 2, P1NP, BALP, CTX and sclerostin were not significantly correlated with any of study patients' clinical features. Of note, DKK1 was negatively correlated with hemoglobin, total calcium level and femoral neck T-score. Furthermore, we compared the differences of BTMs in patients with 10-year probability of major osteoporotic fracture <20% or ≥20%. Levels of P1NP, BALP, CTX were lower, while levels of DKK1 and sclerostin were higher in patients with 10-year probability of major osteoporotic fracture ≥20%, but these differences did not achieve statistically significance, as shown in Figure 1A. BTMs demonstrated in a similar fashion in patients with 10-year probability of hip fracture <3% or ≥3% (Figure 1B), and in those with femoral neck T-score ≥-2.5 or < -2.5 (Figure 1C). Specifically, level of DKK1 was significantly higher (p = 0.004) in patients with femoral neck T-score < -2.5 when compared to patients with femoral neck T-score ≥-2.5.
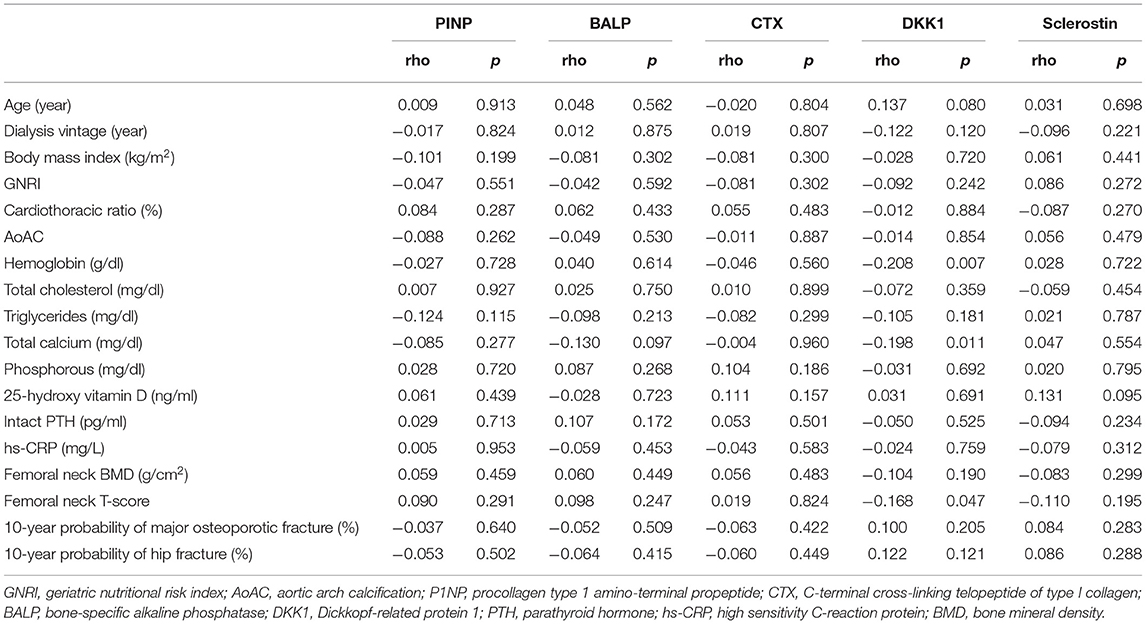
Table 2. Univariate correlations between bone turnover markers and clinical characteristics in maintenance hemodialysis patients.
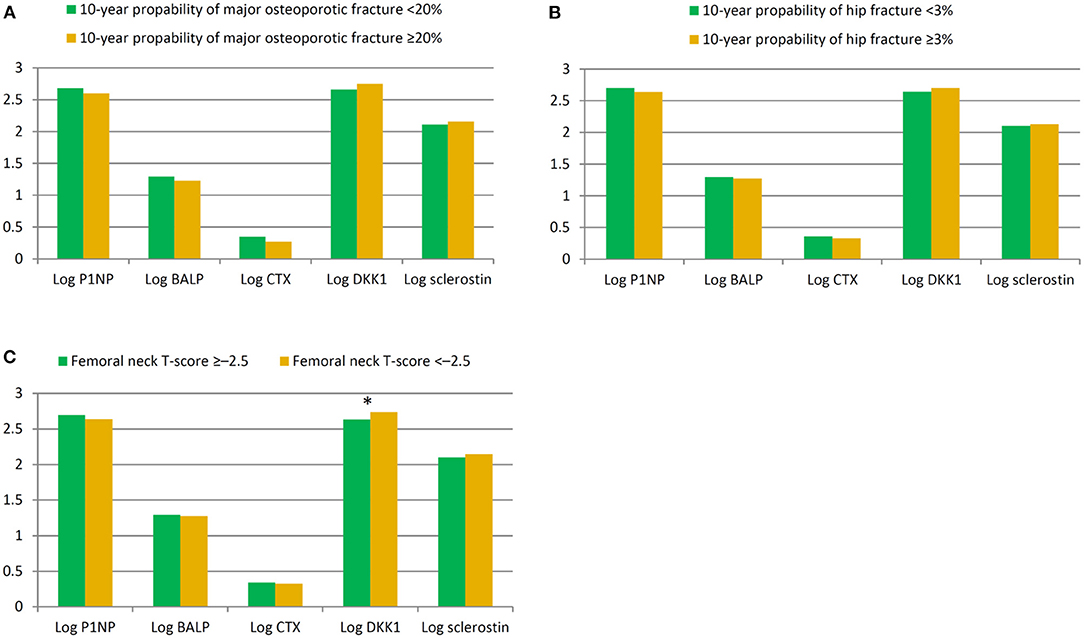
Figure 1. Comparison of bone turnover marks in patients stratified by 10-year probability of major osteoporotic fracture <20% or ≥20% (A), 10-year probability of hip fracture <3% or ≥3% (B), and femoral neck T-score >-2.5 or ≤ -2.5 (C). *p = 0.004.
Factors Associated With All-Cause Mortality Using Cox Proportional Hazard Model
There were 39 (23.8%) deaths during a mean follow-up period of 3.5±1.0 years. The causes of mortality included CV death (n = 23), infectious disease or sepsis (n = 13), massive gastrointestinal bleeding (n = 2), and liver failure (n = 1). Figure 2A illustrates the Kaplan-Meier curves of survival according to low or high risk of fracture. Patients with high risk of fracture had a worse overall survival compare to those with low risk of fracture (Log-rank p < 0.001). In the univariate Cox proportional hazard analysis, high risk of fracture (hazard ratio [HR]: 4.281, 95% confidence interval [CI]: 1.966–9.322, p < 0.001), age ≥60 years, diabetes mellitus, coronary artery disease, higher hs-CRP, higher cardiothoracic ratio, higher AoAC, and lower GNRI were significantly associated with increased risk of all-cause mortality. In multivariate-adjusted Cox analysis, high risk of fracture (HR: 2.493, 95% CI: 1.026–6.056, p = 0.044) was independently associated with increased risk of all-cause mortality (Table 3).
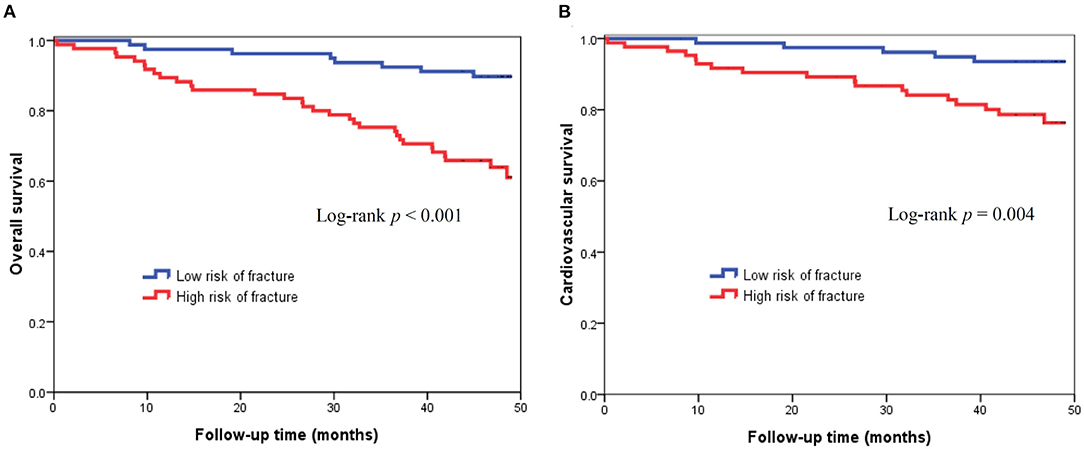
Figure 2. Kaplan-Meier curves of all-cause mortality (log-rank p < 0.001) (A) and cardiovascular mortality (log-rank p = 0.004) (B) according to low or high risk of fracture based on FRAX.
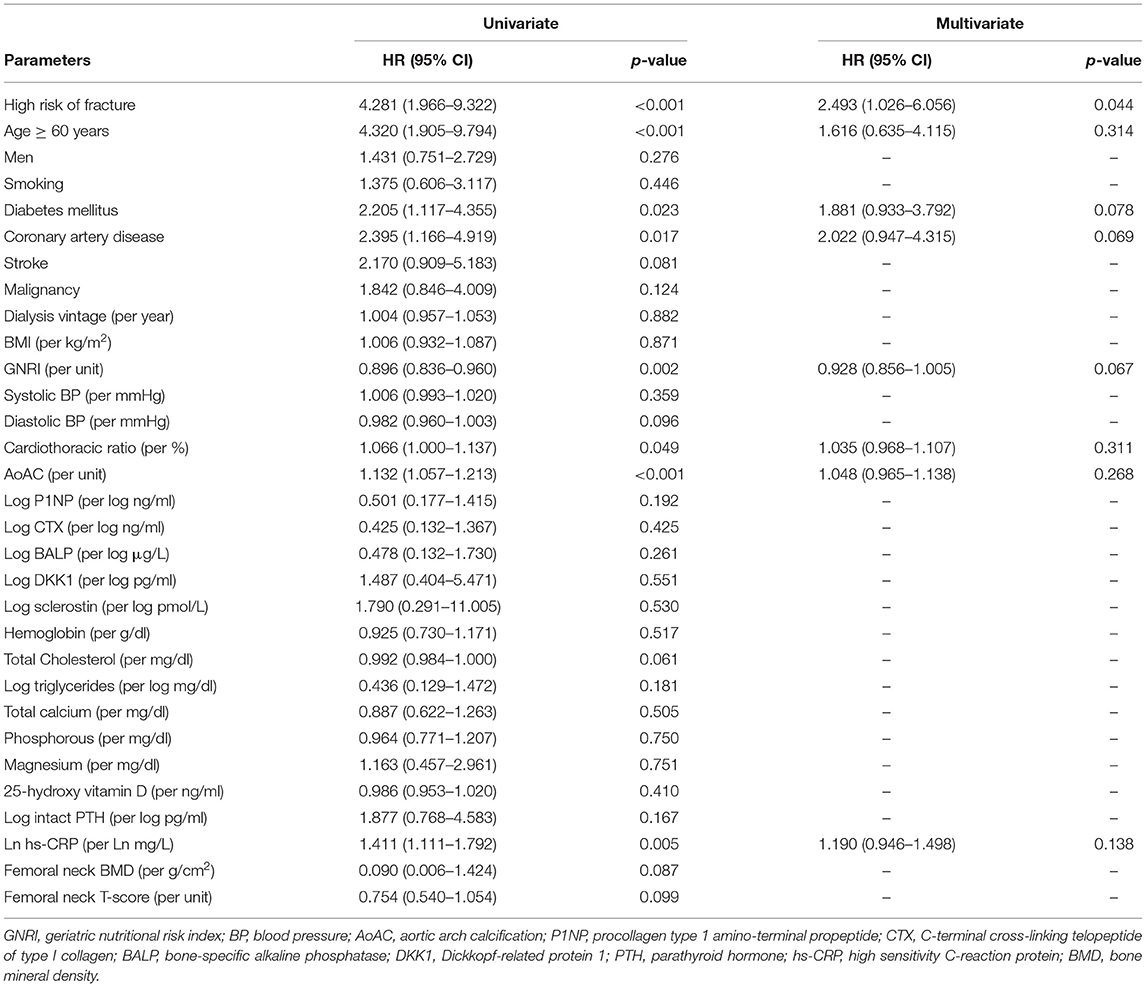
Table 3. Factors associated with all-cause mortality in maintenance hemodialysis patients using Cox proportional hazards model.
Factors Associated With CV Mortality Using Cox Proportional Hazard Model
During the follow-up period, there were 23 CV deaths, including fatal myocardial infarction (n = 4), fatal ventricular arrhythmia (n = 2), sudden cardiac death (n = 13), and fatal stroke (n = 4). Figure 2B displays the Kaplan-Meier curves of CV survival according to low or high risk of fracture. Patients with high risk of fracture had a worse CV survival compare to those with low risk of fracture (Log-rank p = 0.004). In the univariate Cox analysis, high risk of fracture (HR: 3.935, 95% CI: 1.459–10.614, p = 0.007), age ≥60 years, diabetes mellitus, higher AoAC, and lower GNRI were significantly associated with increased risk of CV death. In multivariate-adjusted Cox regression analysis, diabetes mellitus (HR: 2.646, 95% CI: 1.020–6.869, p = 0.046) and lower GNRI (HR: 0.888, 95% CI: 0.802–0.983, p = 0.022) were independent predictors of CV mortality, but high risk of fracture was not associated with CV mortality after adjusting by confounding variables (Table 4).
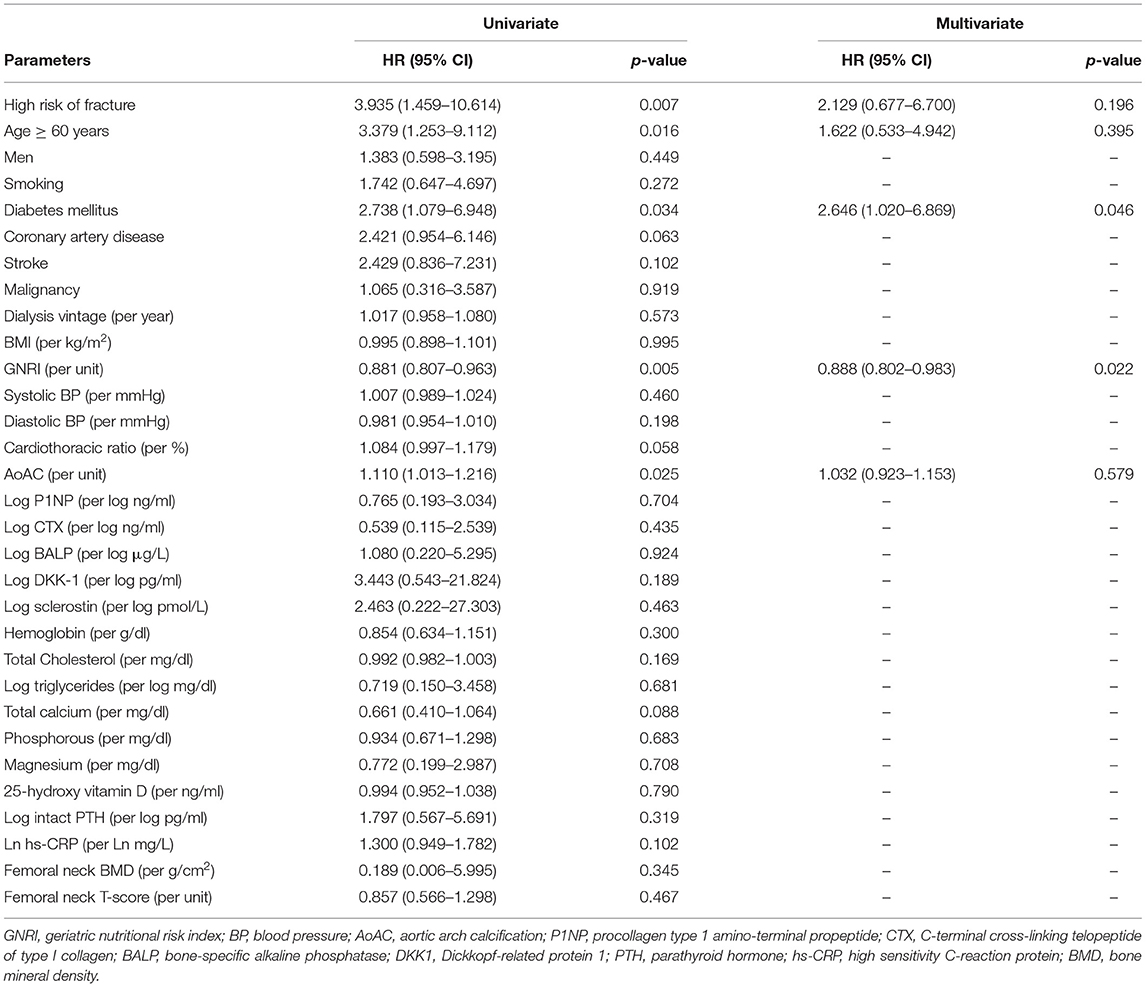
Table 4. Factors associated with cardiovascular mortality in maintenance hemodialysis patients using Cox proportional hazards model.
Discussion
In this study, we examined the association among FRAX, BTMs and mortality risk in maintenance HD patients. Our results revealed that high risk of fracture using FRAX independently predicted all-cause mortality in patients on chronic HD. Besides, lower GNRI was also an important predictor of CV death in these patients. However, none of BTMs was associated with mortality risk.
Our study highlights the prognostic significance of FRAX in patients undergoing HD. FRAX has been extensively used to estimate 10-year fracture risk in the general population in various countries, and it helps decision-making process in management of osteoporosis (12, 29). However, the utility of FRAX has been questioned as chronic kidney disease-mineral and bone disorder (CKD-MBD) and multiple comorbidities may drastically affect turnover and remodeling of bones in patients with kidney disease. Over the last couple of years, mounting evidence has indicated that FRAX performs as well in patients with CKD or ESKD as in the general population. Whitlock et al. reported that FRAX was significantly discriminated risk of fractures in nondialysis CKD patients (13). In addition, FRAX and major osteoporotic fractures had a stronger relationship in CKD patients when compared with those with preserved kidney function (13). Przedlacki et al. analyzed 718 HD patients in a 2-year prospective study, which demonstrated that FRAX was the strongest independent risk factor for major bone fractures (14). Thus, FRAX is recommended in assessment of fracture risk and intervention in patients with CKD or ESKD in the updated consensus report (30).
Fracture events would increase the risk of subsequent unfavorable outcomes. In HD patients, the post-fracture risk of death was 3.7-fold higher in HD patients than those without fracture (31). However, few studies have elucidated the association between FRAX and mortality. In a retrospective study, Sezgin et al. showed that high fracture risk category of FRAX was associated with higher one-year mortality rate in 94 elderly patients (32). Hayashi et al. reported that higher major osteoporotic risk using FRAX was a predictor of death among incident HD patients in Japan (33). The findings in the present study were in line with these works. As for peritoneal dialysis patients, study in regard to investigation of the utility or the role of FRAX remains lacking. Furthermore, we also found that lower GNRI was also an independent predictor of CV mortality in this study. In the assessment of nutritional status in chronic HD patients, GNRI is an accurate indicator (34). We included 10-year probability of hip fracture in addition to major osteoporotic fracture using FRAX, and GNRI as a reliable nutritional marker, and CV death for analysis, which were not considered in the work by Hayashi et al. (33). Malnutrition, inflammation, multiple comorbidities, and nutrient loss from dialysis were risk factors of protein energy wasting and associated with frailty in patients with ESKD (8, 35). Frailty was prevalent in ESKD and significantly increased overall mortality risk regardless of age (10, 36). Recently, an association between a higher FRAX-score and frailty has been recognized (37). Overlapped risk factors for frailty and FRAX, including age, female sex, smoking, alcohol intake, multimorbidity and micronutrient deficits, might underlie the association between FRAX and increased mortality risk. Malnutrition was also the risk factor of frailty (38), and this might explain why a lower GNRI was associated with unfavorable outcomes in our study.
The regulation of bone formation and resorption involving in CKD-MBD is complex. Additionally, Wnt/β-catenin pathway increases osetoblastogenesis and decreases osetoclastogenesis, and it also plays an important role in bone remodeling process of CKD-MBD (39). P1NP and BALP are the markers of bone formation, whereas CTX, DKK1, and sclerostin are the markers of bone resorption, and DKK1 and sclerostin can inhibit Wnt/β-catenin pathway (19). The associations among BTMs, fracture, and BMD remain controversial in HD patients. A previous study found BTMs (P1NP, BALP, and CTX) were not associated with fracture, but were negatively associated with hip BMD in ESKD (19). However, Iimori et al. reported that BALP was associated with fracture in HD patients (40). Furthermore, the relationships between sclerostin, DKK1 and bone turnover were contradictory in patients with CKD (41, 42). In our study, only DKK1 was significantly higher in patients with femoral neck T-score < -2.5 and was consistent with a prior study in CKD (42). This result might be related to Wnt/β-catenin pathway inhibition. We also demonstrated that BTMs were not helpful in prediction of mortality in HD patients. Although certain studies indicated the link between serum sclerostin and adverse outcomes (43, 44), a meta-analysis and a recent prospective study showed that it was not associated with overall and CV mortality in HD patients (45, 46). The controversy in prognostic significance of BTMs in maintenance HD patients may be attributed to the differences in method of BTMs measurement, study heterogeneity, and different observation time.
There were several study limitations. First, this study enrolled HD patients in a single hospital with relatively small sample size. Second, we did not investigate all known BTMs and we checked BTMs only once at baseline. Since the BTMs are dynamic markers as changes in bone turnover and mineralization in CKD-MBD, it may be difficult to clearly address the association between BTMs and mortality risk. Third, the association between FRAX and fracture was not assessed because we cannot obtain the fracture events that were diagnosed in other hospitals. Finally, because age is an essential variable in the FRAX algorithm, a large-scale cohort study with age-matched patients is needed to better elucidate the role of FRAX in predicting mortality among chronic HD patients.
In conclusion, our study demonstrated that high risk of fracture using FRAX and lower GNRI independently predicted all-cause and CV mortality, respectively, in patients with HD. However, BTMs were not able to predict mortality risk. FRAX, rather than BTMs, has an important role of prognostic significance in maintenance HD patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Kaohsiung Medical University Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
J-CH: conception and design. P-YW and J-CH: writing–original draft. S-CC and J-CH: writing–review and editing and statistical analysis. P-YW, S-CC, Y-CL, P-CC, W-SC, P-HW, Y-CT, and J-CH: acquisition of data. S-CC, Y-CH, and J-CH: analysis of data. P-YW, S-CC, and J-CH: interpretation of data. Y-WC and J-MC: supervision or mentorship. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported partially by a grant from the Ministry of Science and Technology, Taiwan (MOST 110-2622-H-037-001), and by a grant from Kaohsiung Municipal Siaogang Hospital (H-110-007), Kaohsiung Medical University, Kaohsiung, Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yong EL, Ganesan G, Kramer MS, Howe TS, Koh JSB, Thu WP, et al. Risk factors and trends associated with mortality among adults with hip fracture in Singapore. JAMA Netw Open. (2020) 3:e1919706. doi: 10.1001/jamanetworkopen.2019.19706
2. Tarride JE, Burke N, Leslie WD, Morin SN, Adachi JD, Papaioannou A, et al. Loss of health related quality of life following low-trauma fractures in the elderly. BMC Geriatr. (2016) 16:84. doi: 10.1186/s12877-016-0259-5
3. Hopkins RB, Burke N, Von Keyserlingk C, Leslie WD, Morin SN, Adachi JD, et al. The current economic burden of illness of osteoporosis in Canada. Osteoporos Int. (2016) 27:3023–32. doi: 10.1007/s00198-016-3631-6
4. Runesson B, Trevisan M, Iseri K, Qureshi AR, Lindholm B, Barany P, et al. Fractures and their sequelae in non-dialysis-dependent chronic kidney disease: the stockholm creatinine measurement project. Nephrol Dial Transplant. (2020) 35:1908–15. doi: 10.1093/ndt/gfz142
5. Naylor KL, McArthur E, Leslie WD, Fraser LA, Jamal SA, Cadarette SM, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. (2014) 86:810–8. doi: 10.1038/ki.2013.547
6. Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. (2006) 70:1358–66. doi: 10.1038/sj.ki.5001754
7. Jorgensen HS, David K, Salam S, Evenepoel P. European Renal Osteodystrophy (EUROD) workgroup, an initiative of the CKD-MBD working group of the ERA-EDTA. Traditional and Non-Traditional Risk Factors for Osteoporosis in CKD. Calcif Tissue Int. (2021) 108:496–511. doi: 10.1007/s00223-020-00786-0
8. Chao CT, Lin SH. Uremic toxins and frailty in patients with chronic kidney disease: a molecular insight. Int J Mol Sci. (2021) 22:6270. doi: 10.3390/ijms22126270
9. Lorenz EC, Kennedy CC, Rule AD, LeBrasseur NK, Kirkland JL, Hickson LJ. Frailty in CKD and transplantation. Kidney Int Rep. (2021) 6:2270–80. doi: 10.1016/j.ekir.2021.05.025
10. Zhang Q, Ma Y, Lin F, Zhao J, Xiong J. Frailty and mortality among patients with chronic kidney disease and end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. (2020) 52:363–70. doi: 10.1007/s11255-019-02369-x
11. Delgado C, Shieh S, Grimes B, Chertow GM, Dalrymple LS, Kaysen GA, et al. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am J Nephrol. (2015) 42:134–40. doi: 10.1159/000439000
12. Kanis JA, Harvey NC, McCloskey E, Bruyere O, Veronese N, Lorentzon M, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. (2020) 31:1–12. doi: 10.1007/s00198-019-05176-3
13. Whitlock RH, Leslie WD, Shaw J, Rigatto C, Thorlacius L, Komenda P, et al. The Fracture Risk Assessment Tool (Frax®) predicts fracture risk in patients with chronic kidney disease. Kidney Int. (2019) 95:447–54. doi: 10.1016/j.kint.2018.09.022
14. Przedlacki J, Buczynska-Chyl J, Kozminski P, Niemczyk E, Wojtaszek E, Gieglis E, et al. The Utility of Frax® in predicting bone fractures in patients with chronic kidney disease on hemodialysis: a two-year prospective multicenter cohort study. Osteoporos Int. (2018) 29:1105–15. doi: 10.1007/s00198-018-4406-z
15. Jamal SA, West SL, Nickolas TL. The clinical utility of frax to discriminate fracture status in men and women with chronic kidney disease. Osteoporos Int. (2014) 25:71–6. doi: 10.1007/s00198-013-2524-1
16. Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. (2011) 22:391–420. doi: 10.1007/s00198-010-1501-1
17. Vilaca T, Gossiel F, Eastell R. Bone Turnover Markers: Use in Fracture Prediction. J Clin Densitom. (2017) 20:346–52. doi: 10.1016/j.jocd.2017.06.020
18. Malluche HH, Davenport DL, Cantor T, Monier-Faugere MC. Bone Mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin J Am Soc Nephrol. (2014) 9:1254–62. doi: 10.2215/CJN.09470913
19. Jorgensen HS, Winther S, Bottcher M, Hauge EM, Rejnmark L, Svensson M, et al. Bone turnover markers are associated with bone density, but not with fracture in end stage kidney disease: a cross-sectional study. BMC Nephrol. (2017) 18:284. doi: 10.1186/s12882-017-0692-5
20. Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol. (2011) 22:1560–72. doi: 10.1681/ASN.2010121275
21. National Kidney F. Kdoqi clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. (2015) 66:884–930. doi: 10.1053/j.ajkd.2015.07.015
22. Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. (2008) 87:106–13. doi: 10.1093/ajcn/87.1.106
23. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
24. Shah B, Sucher K, Hollenbeck CB. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract. (2006) 21:312–9. doi: 10.1177/0115426506021003312
25. Chen SC, Chung WS, Wu PY, Huang JC, Chiu YW, Chang JM, et al. Associations among geriatric nutrition risk index, bone mineral density, body composition and handgrip strength in patients receiving hemodialysis. Nutrition. (2019) 65:6–12. doi: 10.1016/j.nut.2019.02.013
26. Ogawa T, Ishida H, Matsuda N, Fujiu A, Matsuda A, Ito K, et al. Simple evaluation of aortic arch calcification by chest radiography in hemodialysis patients. Hemodial Int. (2009) 13:301–6. doi: 10.1111/j.1542-4758.2009.00366.x
27. Dawson-Hughes B, Tosteson AN, Melton LJ 3rd, Baim S, Favus MJ, Khosla S, et al. Implications of Absolute Fracture Risk Assessment for Osteoporosis Practice Guidelines in the USA. Osteoporos Int. (2008) 19:449–58. doi: 10.1007/s00198-008-0559-5
28. Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV, et al. A systematic review of intervention thresholds based on frax: a report prepared for the national osteoporosis guideline group and the international osteoporosis foundation. Arch Osteoporos. (2016) 11:25. doi: 10.1007/s11657-016-0278-z
29. Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos Int. (2019) 30:3–44. doi: 10.1007/s00198-018-4704-5
30. Evenepoel P, Cunningham J, Ferrari S, Haarhaus M, Javaid MK, Lafage-Proust MH, et al. European consensus statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrol Dial Transplant. (2021) 36:42–59. doi: 10.1093/ndt/gfaa192
31. Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. (2014) 85:166–73. doi: 10.1038/ki.2013.279
32. Sezgin EA, Tor AT, Markevičiute V, Širka A, Tarasevičius Š, Raina DB, et al. A combined fracture and mortality risk index useful for treatment stratification in hip fragility fractures. Jt Dis Relat Surg. (2021) 32:583–9. doi: 10.52312/jdrs.2021.382
33. Hayashi T, Joki N, Tanaka Y, Iwasaki M, Kubo S, Asakawa T, et al. The Frax® as a predictor of mortality in Japanese incident hemodialysis patients: an observational, follow-up study. J Bone Miner Metab. (2015) 33:674–83. doi: 10.1007/s00774-014-0631-5
34. Kobayashi I, Ishimura E, Kato Y, Okuno S, Yamamoto T, Yamakawa T, et al. Geriatric nutritional risk index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant. (2010) 25:3361–5. doi: 10.1093/ndt/gfq211
35. Hanna RM, Ghobry L, Wassef O, Rhee CM, Kalantar-Zadeh K A. Practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif. (2020) 49:202–11. doi: 10.1159/000504240
36. Kojima G. Prevalence of frailty in end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. (2017) 49:1989–97. doi: 10.1007/s11255-017-1547-5
37. Tembo MC, Holloway-Kew KL, Mohebbi M, Sui SX, Hosking SM, Brennan-Olsen SL, et al. The association between a fracture risk tool and frailty: geelong osteoporosis study. BMC Geriatr. (2020) 20:196. doi: 10.1186/s12877-020-01595-8
38. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. doi: 10.1016/S0140-6736(19)31786-6
39. Carrillo-Lopez N, Panizo S, Alonso-Montes C, Roman-Garcia P, Rodriguez I, Martinez-Salgado C, et al. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. (2016) 90:77–89. doi: 10.1016/j.kint.2016.01.024
40. Iimori S, Mori Y, Akita W, Kuyama T, Takada S, Asai T, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients–a single-center cohort study. Nephrol Dial Transplant. (2012) 27:345–51. doi: 10.1093/ndt/gfr317
41. Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, et al. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol. (2011) 6:877–82. doi: 10.2215/CJN.06550810
42. Thambiah S, Roplekar R, Manghat P, Fogelman I, Fraser WD, Goldsmith D, et al. Circulating sclerostin and dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int. (2012) 90:473–80. doi: 10.1007/s00223-012-9595-4
43. Stavrinou E, Sarafidis PA. Associations of serum sclerostin and dickkopf-related protein-1 proteins with future cardiovascular events and mortality in haemodialysis patients: a prospective cohort study. Clin Kidney J. (2021) 14:1165–72. doi: 10.1093/ckj/sfaa069
44. Zou Y, Yang M, Wang J, Cui L, Jiang Z, Ding J, et al. Association of sclerostin with cardiovascular events and mortality in dialysis patients. Ren fail. (2020) 42:282–8. doi: 10.1080/0886022X.2020.1741386
45. Kanbay M, Solak Y, Siriopol D, Aslan G, Afsar B, Yazici D, et al. Sclerostin, Cardiovascular disease and mortality: a systematic review and meta-analysis. Int Urol Nephrol. (2016) 48:2029–42. doi: 10.1007/s11255-016-1387-8
Keywords: Fracture Risk Assessment Tool, bone turnover markers, fracture, mortality, cardiovascular, hemodialysis
Citation: Wu P-Y, Chen S-C, Lin Y-C, Chen P-C, Chung W-S, Huang Y-C, Wu P-H, Tsai Y-C, Huang J-C, Chiu Y-W and Chang J-M (2022) Role of Fracture Risk Assessment Tool and Bone Turnover Markers in Predicting All-Cause and Cardiovascular Mortality in Hemodialysis Patients. Front. Med. 9:891363. doi: 10.3389/fmed.2022.891363
Received: 07 March 2022; Accepted: 21 March 2022;
Published: 07 April 2022.
Edited by:
Yoshiyuki Morishita, Jichi Medical University Saitama Medical Center, JapanReviewed by:
Naoki Nakagawa, Asahikawa Medical University, JapanShohei Kaneko, Jichi Medical University Saitama Medical Center, Japan
Copyright © 2022 Wu, Chen, Lin, Chen, Chung, Huang, Wu, Tsai, Huang, Chiu and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiun-Chi Huang, a2FyYWphbjc3QGdtYWlsLmNvbQ==
 Pei-Yu Wu
Pei-Yu Wu Szu-Chia Chen
Szu-Chia Chen Yi-Ching Lin
Yi-Ching Lin Po-Chih Chen4
Po-Chih Chen4 Yi-Chun Tsai
Yi-Chun Tsai Jiun-Chi Huang
Jiun-Chi Huang Yi-Wen Chiu
Yi-Wen Chiu