
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 09 June 2022
Sec. Rheumatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.885801
 Shan-Fu Yu1,2,3
Shan-Fu Yu1,2,3 Jia-Feng Chen1
Jia-Feng Chen1 Ying-Chou Chen1,3
Ying-Chou Chen1,3 Yu-Wei Wang1
Yu-Wei Wang1 Chung-Yuan Hsu1
Chung-Yuan Hsu1 Han-Ming Lai1
Han-Ming Lai1 Hsiao-Ru He1
Hsiao-Ru He1 Chi-Hua Ko1
Chi-Hua Ko1 Wen-Chan Chiu1
Wen-Chan Chiu1 Tien-Tsai Cheng1,3*
Tien-Tsai Cheng1,3*Objective: To explore the impact of seropositivity on systemic bone loss in rheumatoid arthritis (RA).
Methods: We conducted an interim analysis of the RA registry. Patients were examined with dual-energy X-ray absorptiometry at baseline and again 3 years later. Participants were grouped into seropositive (SPRA) and seronegative (SNRA) based on the presence or absence of rheumatoid factor (RF) and/or anti-cyclic citrullinated peptide antibodies (ACPA). After matching (1:2) for age and sex, SNRA and SPRA patients were divided into groups A and B. Each matched group (A or B) was further subdivided according to the number of antibodies present (0, group I; 1, group II; 2, group III). Multiple ordinary least squares regression was used with the dependent variables to develop a model to predict bone mineral density (BMD) change.
Results: A total of 477 participants who completed a 3-year observation period were included. After matching, 312 participants were enrolled (group A, 104; group B, 208). Three years later, group B had significant BMD reduction in the femoral neck (FN) (p < 0.001), total hip (TH) (p = 0.001), and first through fourth lumbar vertebrae (L1–4) (p = 0.006), while group A had bone loss only at FN (p = 0.002). Groups I, II, and III included 104, 52, and 156 participants, respectively. Compared to baseline, BMD decreased significantly at FN (p = 0.002) in group I, FN (p < 0.001) in group II, and FN (p < 0.001), TH (p = 0.002), and L1–4 (p = 0.016) in group III. In terms of regression-adjusted percent change in BMD, more significantly negative changes were found at all measured sites in group B (p < 0.001, all) and at TH and L1–4 within groups I-III (p for trend < 0.001 and < 0.001, respectively). Regardless of antibodies, anti-osteoporotic therapy can preserve bone density in RA patients.
Conclusion: After 3 years, SPRA patients lost more bone density than SNRA patients. More attention should be paid to SPRA patients, especially those with double-positive antibodies, including a vigorous evaluation of BMD and fracture risk. Anti-osteoporotic therapy can prevent BMD loss irrespective of autoantibodies.
Rheumatoid arthritis (RA) is a chronic systemic disease that can lead to local bone erosion and generalized osteoporosis. Rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) are the two most notable autoantibodies commonly used in diagnosing or classifying RA and providing a variety of clinical and pathophysiological information (1). Patients positive for ACPA and/or RF may be labeled together as having “seropositive” RA (SPRA) and compose approximately 50–80% of the RA population (1). Several studies have indicated that SPRA patients experience greater disease severity in terms of disease activity, functional impairment, increased mortality over time (2, 3), and might show poor response to treatment compared to seronegative RA (SNRA) patients (4). Although both SPRA and SNRA fulfill the 1987 American College of Rheumatology (ACR) revised classification criteria (5) or the 2010 ACR/European League Against Rheumatism (EULAR) classification criteria (6), their clinical manifestations, courses, and prognostic features are distinctive.
It has been reported that the annual rate of bone loss in patients with active RA ranges between 5.5 and 10% (7). Meanwhile, RF and/or ACPA are associated with juxta-articular osteoporosis, erosions, and generalized bone loss (8, 9). Several studies have shown that ACPAs and RF synergize to promote RA-associated inflammation, disease activity, and clinical onset (10, 11) and can be associated with the bone erosion and structural damage seen in RA (12). The combination of ACPA and RF could predict the therapeutic responses to rituximab and abatacept (13). However, the clinical predictive value of each antibody (singly or in combination) on systemic bone loss has not been well studied.
This study aimed to explore long-term bone mineral density (BMD) changes in patients with SPRA and SNRA and investigate the association between antibody number and systemic bone loss in patients with RA.
Participants’ inclusion criteria and the methods used in this study were the same as those reported previously (14). Study participants enrolled in an RA registry at Kaohsiung Chang Gung Memorial Hospital beginning September 1st, 2014. We enrolled a total of 651 patients who satisfied the 1987 revised ACR criteria for RA (5) or the 2010 ACR/EULAR classification criteria for RA (6) in this study. Demographic characteristics such as age, sex, comorbidities, and body mass index were recorded.
We also recorded disease-specific information, such as the age at diagnosis, disease duration, disease activity measured by erythrocyte sedimentation rate (ESR), C-reactive protein, and the disease activity score-28 joint-ESR (DAS28-ESR), medications, including glucocorticoids (GC) and biological and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs), and the presence/absence of ACPA and RF. Lifestyle, evidence of previous fragility fracture (history or radiographic), and risk factors for fragility fracture on the Fracture Risk Assessment Tool (FRAX ®) were also recorded. The 10-year probabilities of major and hip fractures in each patient, calculated using the FRAX ® with BMD (Taiwan version), were collected. Seropositivity was defined as any value > 15 IU/mL for RF and > 7 IU/mL for ACPA.
Each patient’s BMD was measured at enrollment and the 3-year follow-up using a dual-energy X-ray absorptiometry scanner (Delphi A; Hologic Corp., Waltham, MA, United States) for the femoral neck (FN), total hip (TH), and first through fourth lumbar vertebrae (L1–4). For postmenopausal women and men aged 50 years and older, osteoporosis was defined as a T-score of –2.5 or less at the FN based on the normal reference database for young white females (15, 16). We calculated the percentage change in BMD (ΔBMD%) for each participant as follows: [(second BMD – baseline BMD)/baseline BMD] × 100, comparing between assessments.
We defined a new incident fracture as any symptomatic non-traumatic fracture, including the forearm, hip, pelvis, and humerus, or an asymptomatic morphometric vertebral fracture. Morphometric fractures were assessed on lateral radiographs of the lumbar spine according to Genant et al.’s semiquantitative assessment of vertebral fractures (17). An independent radiologist assessed the evidence of morphometric vertebral compression fractures at enrollment and subsequently on an as-needed basis, with follow-up spinal radiographs during the 3-year observation period and at the end of the study. The local Institutional Review Board of Chang Gung Memorial Hospital approved the study (104-3530B, 201901054B0), which was performed according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Patients who were positive for RF or ACPA were grouped into the SPRA group, while those who were negative were grouped as SNRA. The participants were sub-grouped into A and B after matching for age and sex. Finally, the matched group was sub-grouped according to the number of antibodies present (0, group I; 1, group II; 2, group III).
The data were checked for normality, which demonstrated that baseline characteristics had a skewed distribution; therefore, they were analyzed using non-parametric methods. An independent two-sample t-test and one-way analysis of variance (ANOVA) test were used to compare continuous variables with normal distribution and expressed as mean ± standard deviation (SD). We used the Mann-Whitney U and Kruskal-Wallis tests to compare continuous variables with skewed distribution; they were expressed as a median (interquartile range, IQR). The chi-square or Fisher’s exact test was used to assess the association between categorical variables.
The intra-and inter-group BMD changes from enrollment to 3 years later were compared with a linear mixed model. Trend analyses were performed using ANOVA for each category (number of antibodies). Multiple ordinary least squares regression was used with the dependent variables, controlling for disease duration, baseline DAS28-ESR, glucocorticoid, and b/tsDMARDs therapy. From this, we calculated the predicted value of the BMD changes. All statistical analyses were performed using IBM SPSS version 22 software (IBM Co., Armonk, NY, United States). A p-value of < 0.05 was considered significant in all analyses.
The participants’ disposition is given in Figure 1. A total of 651 participants were registered for the RA osteoporosis/fracture study, which started on September 1st, 2014; 477 participants completed the 3-year observation period. To compare BMD changes in SNRA and SPRA, taking the mutual interference of age and sex into account, we controlled for these two factors. We obtained 312 matched participants, of whom 104 were allocated to group A and 208 to group B (Figure 1). The baseline DAS28-ESR and ESR were significantly higher in group B than in group A (p = 0.002 and p = 0.001, respectively) (Table 1, right column). The proportion of patients using GC and b/tsDMARDs in group B was higher than in group A (p = 0.017 and p = 0.004, respectively). No significant difference was found between the two groups in terms of the history of previous fractures (Table 1, right column). There were 134 patients receiving anti-osteoporotic therapy (AOT), of which 115 (85.8%) patients were treated with bisphosphonates, and 7 (5.2%) patients were treated with denosumab (Table 1, right column). The proportion of patients using bisphosphonates in group B was higher than in group A (p = 0.015). The mean treatment duration of bisphosphonates in group A and group B was 17.7 ± 30.3 and 15.5 ± 25.3 months, respectively (p = 0.697). The mean treatment duration of denosumab in group A and group B was 28.0 ± 33.0 and 22.5 ± 19.2 months, respectively (p = 0.790).
Participants’ demographics and clinical characteristics are presented in Table 2. There were 104, 52, and 156 participants in groups I, II, and III, respectively. The baseline comorbidity (p = 0.010), DAS28-ESR (p = 0.006), ESR (p < 0.001), BMD at FN (p = 0.010), FRAX score (hip) (p = 0.049), proportion of osteoporosis (p = 0.011), proportion of b/tsDMARD use (p = 0.025), and proportion of bisphosphonates use (p = 0.041) were significantly different between groups. The mean treatment duration of bisphosphonates or denosumab was not significant in groups I–III.
Group B had a significantly higher proportion of patients with osteoporosis (p = 0.003) and a higher 10-year probability of hip fracture (p = 0.018) but had lower BMD at FN and TH (p = 0.004, p = 0.005) at enrollment, compared to group A (Table 1, right column). Three years later, the BMDs at FN, TH, and L1–4 were significantly decreased from baseline in group B (p < 0.001, p = 0.001, p = 0.006), respectively (Figure 2A). In group A, a significant decrease was found only in FN (p = 0.002) (Figure 2A). Intergroup differences over the three intervening years were significant at the FN and TH (p = 0.003 and p = 0.005, respectively), but were insignificant at L1–4 (p = 0.105) (Figure 2A). The difference in the rate of new incident fractures was not obvious between groups A and B over time (Table 1, right column). The most common types of new incident fractures were vertebral fractures (n = 61), followed by non-hip, non-vertebral fractures (n = 24), hip fractures (n = 8), and wrist fractures (n = 3) in all groups.
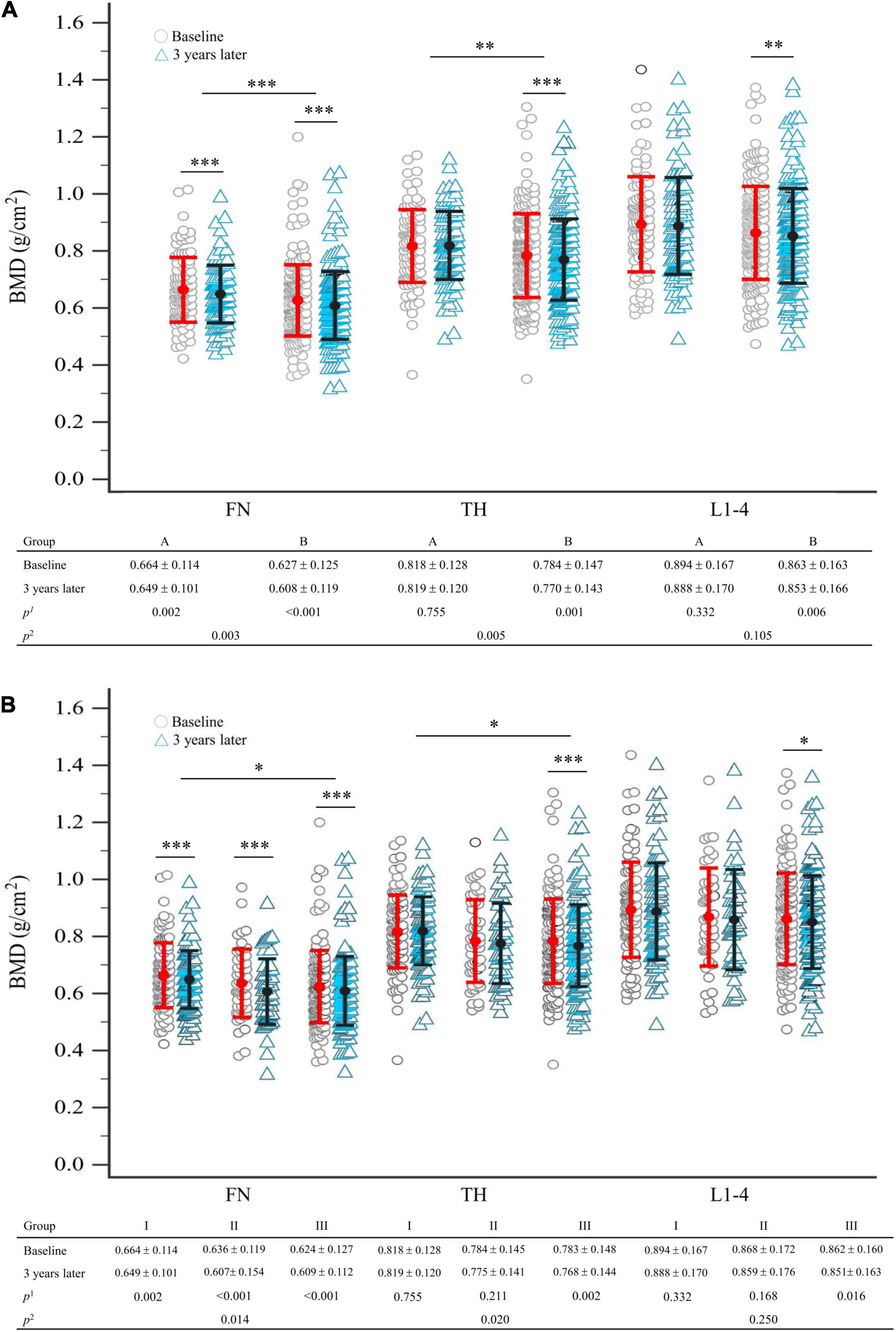
Figure 2. (A) Comparison of BMD between baseline and 3 years later between seronegative (group A) and seropositive (group B) rheumatoid arthritis, after matching; (B) comparison of BMD between baseline and 3 years later in total groups stratified by the number of antibodies presented, after matching. Statistical analysis using the linear mixed model; p1-value refers to intra-group comparison; p2-value refers to inter-group comparison. The error bar represents the standard deviation for the means. Each symbol represents a single data point. BMD, bone mineral density; FN, femoral neck; L1–4, first through fourth lumbar vertebra; TH, total hip. *p < 0.05; **p < 0.01; ***p < 0.005.
After 3 years, compared to baseline, significant BMD reductions were seen in group III participants at FN, TH, and L1–4 (p < 0.001, p = 0.002, p = 0.016, respectively). However, we demonstrated that groups I and II had significant BMD reductions only at FN (p = 0.002, p < 0.001, respectively) and not at TH or L1–4 (Figure 2B). Intergroup BMD differences over 3 years were significant at FN and TH (p = 0.014, p = 0.020), but not at L1–4 (p = 0.250) (Figure 2B).
After 3 years, percent changes in BMD (ΔBMD%) at FN and L1–4 were not significantly different among groups except at TH (p = 0.044) after matching in group A and group B participants (Table 3 and Figure 3A). ΔBMD% at FN and L1–4 were not obviously different among group A and group B participants with AOT (p = 0.121, 0.970, respectively) or without (p = 0.908, 0.650, respectively) (Table 3). However, ΔBMD% at TH in group B were significantly different from group A participants with AOT (p = 0.009). In all participant groups, patients with an increasing number of autoantibodies were associated with a trend toward more negative ΔBMD% at TH (p for trend 0.021, Table 4 and Figure 4A). Among participants with AOT, patients with a higher number of antibodies had more negative ΔBMD% at TH compared with patients with few autoantibodies (p for trend 0.002, Table 4 and Figure 4A).
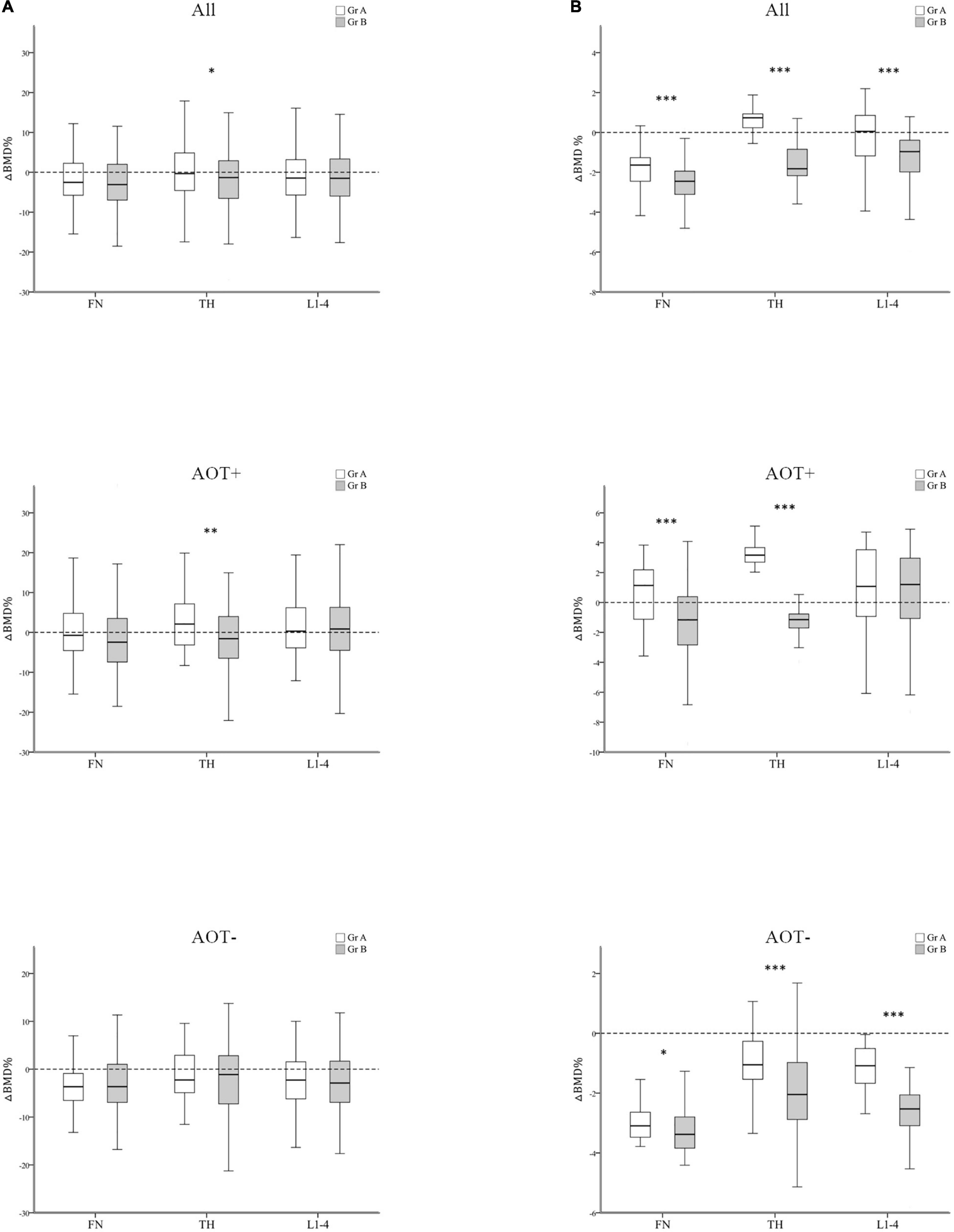
Figure 3. Regression-adjusted percentage change in BMD (ΔBMD%) between group A and group B, after matching in all participants and participants with or without anti-osteoporotic therapy (AOT). Unadjusted (A) and regression-adjusted (B) percentage of change in BMD at the femoral neck (FN), total hip (TH), and first through fourth lumbar vertebra (L1–4) after 3 years in group A and group B, combined with AOT presence. Box-and-whisker plots showed the median, interquartile range, and extreme values. *p < 0.05; **p < 0.01; ***p < 0.005.
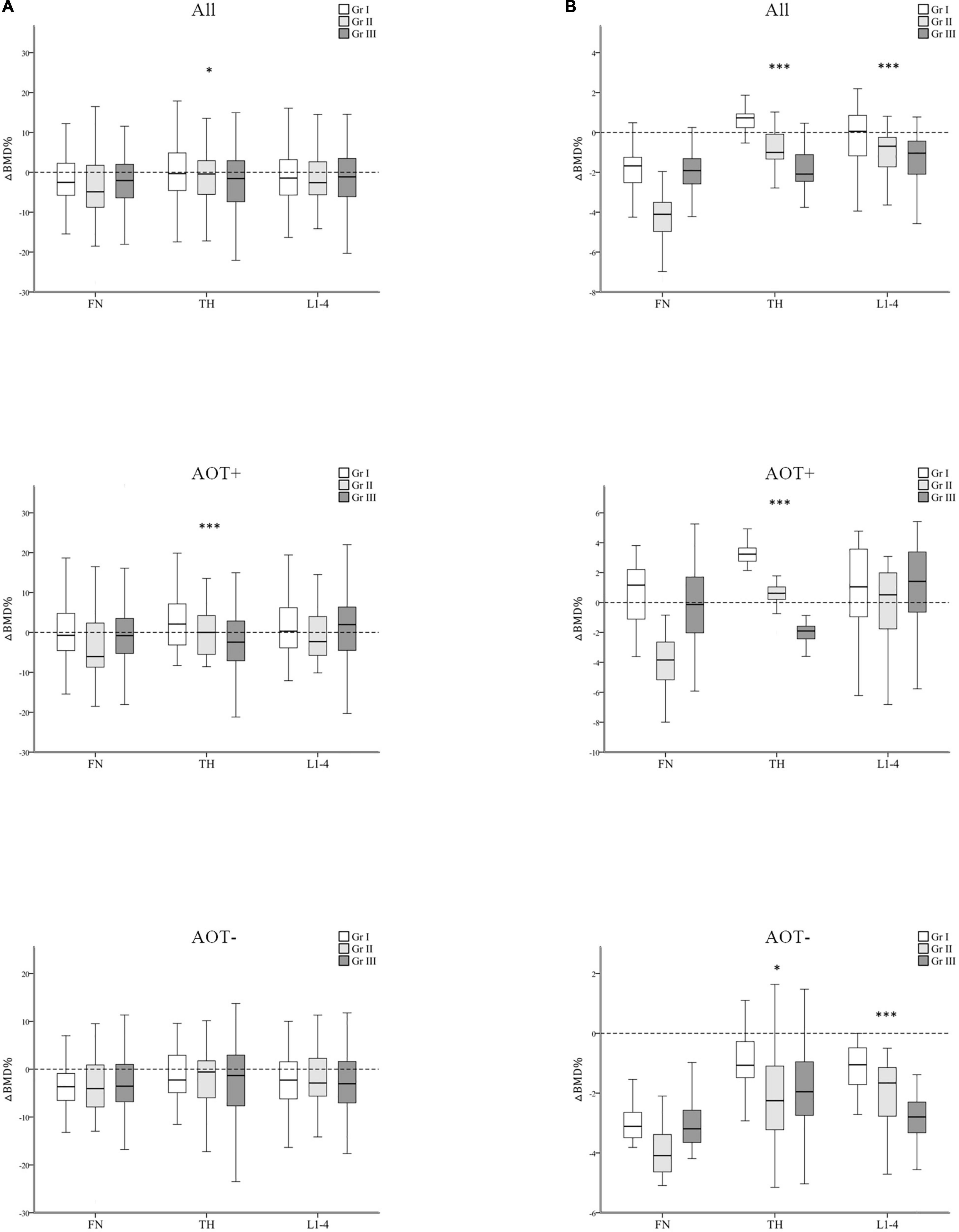
Figure 4. Regression-adjusted percentage change in BMD (ΔBMD%) from baseline in groups I–III, after matching in all participants and participants with or without anti-osteoporotic therapy (AOT). Unadjusted (A) and regression-adjusted (B) percentage of change in BMD at the femoral neck (FN), total hip (TH), and first through fourth lumbar vertebra (L1–4) after 3 years in groups I–III, combined with AOT presence. Box-and-whisker plots showed the median, interquartile range, and extreme values. *p for trend < 0.05; ***p for trend < 0.005.
Next, we calculated the predicted BMD change by multiple regression analysis after adjusting for disease duration, baseline DAS28-ESR, glucocorticoid, and b/tsDMARDs therapy (Tables 3, 4 and Figures 3, 4). In all participant groups, regression-adjusted ΔBMD% at all measured sites in group B were significantly different from those of group A (all p < 0.001) (Table 3 and Figure 3B). Regression-adjusted ΔBMD% at FN and TH in group B were significantly different from group A participants with AOT (p < 0.001, < 0.001, respectively). Among participants without AOT, regression-adjusted ΔBMD% at all measured sites in group B were significantly different from those of group A (all p < 0.005).
In all participant groups, patients with an increasing number of autoantibodies were associated with a trend toward more negative regression-adjusted ΔBMD% at TH and L1-4 (p for trend < 0.001, < 0.001, respectively, Table 4 and Figure 4B). Among participants with AOT, patients with a higher number of antibodies had more negative adjusted ΔBMD% at TH compared with patients with few autoantibodies (p for trend < 0.001). Among participants without AOT, patients with a higher number of antibodies were more negative adjusted ΔBMD% at TH and L1–4 compared with patients with few autoantibodies (p for trend 0.027, < 0.001, respectively).
Our study provides 3-year follow-up results on the impact of seropositivity and the number of autoantibodies on BMD changes in patients with RA. Compared to baseline, SPRA patients experienced a significant decrease in BMD at three measured sites but not in SNRA at TH and L1–4. Regardless of AOT, autoantibodies present could increase the progression of bone loss at all sites. In patients without AOT, SPRA participants had the most obvious bone loss at all sites. Furthermore, we found that patients with higher numbers of antibodies had more systemic bone loss at TH and L1–4, irrespective of taking AOT therapy or not.
It has been well documented that RA patients have a higher risk of osteoporosis and fragility fractures than the general population (18). RA-associated bone loss is not only related to traditional risk factors of osteoporosis (e.g., aging, female) but also to factors related to the disease itself (e.g., disease activity and duration, GC use, and functional disability) (19, 20). Biologics for patients with RA had a protective effect on bone loss. This beneficial effect was also observed in patients who did not exhibit a clinical response (21). However, it is still controversial whether seropositivity affects osteoporosis and BMD changes in RA. Some studies have reported that SNRA patients have a greater chance of developing osteoporosis and lower BMD than SPRA patients (22, 23), but other investigations revealed opposite results (17, 24–26). The aforementioned studies were subjected to a cross-sectional study with a small sample size and no confounding factor adjustment. In terms of the influential factors screened, we conducted a 3-year, longitudinal, observational study with adequate sample size and adjusted the analysis, factoring in confounders. Our study’s results suggest that seropositivity has a detrimental effect on systemic bone loss in RA patients.
Recent investigations have suggested that not only do SPRA patients have distinct genomic backgrounds (27), clinical presentations (28), and treatment responses (29) from SNRA patients, but they also have higher mortality (3). It raises the possibility that SPRA and SNRA are two distinctive disease entities that mediate the different patterns of systemic bone loss seen in these two subtypes of RA (17). Current investigations revealed that RA-related factors and treatment, including baseline DAS28-ESR, ESR, and rate of GC and biologics use, were significantly higher in SPRA than SNRA, which further suggests that these subtypes of RA are different disease entities. RA disease activity (19, 30) and GC use (21, 31) are two of the determinants of RA-related systemic bone loss, suggesting that higher disease activity and greater GC usage are important determinants of greater systemic bone loss and a higher proportion of osteoporosis in SPRA than SNRA in our cohort.
In addition to the indirect effect of different disease patterns on bone loss between SPRA and SNRA, the direct effect of seropositivity on bone loss has been demonstrated. Harre et al. revealed an association between autoantibodies against citrullinated vimentin and serum markers for osteoclast-mediated bone resorption in RA patients (32). RA-associated autoantibodies have recently been found to directly induce differentiation and activation of osteoclasts, which might partly mediate systemic bone loss in RA (33–35). The additive effect of ACPA and RF on the production of the pro-inflammatory cytokine TNF-α, which is among the most potent cytokines to stimulate osteoclastogenesis, has been noted (10, 36, 37). This may explain the antibody-dependent enhancement of systemic bone loss and the concurrence of ACPA and RF in RA patients having the most detrimental effect on BMD in groups I–III. We noticed that more negative regression-adjusted ΔBMD% at TH and L1–4, irrespective of taking AOT therapy, were considered expected factors of the concurrence of RF and ACPA.
Interestingly, we found that not only is substantial bone loss consistent compared to baseline at three measured sites either in SPRA or RA patients with more autoantibodies, but regression-adjusted ΔBMD% over time is also more negative at all sites, especially at TH. This is consistent with previous studies’ findings suggesting that RA patients experienced more bone loss at the hip than the spine (38–40). Orsolini et al. demonstrated that the independent role of ACPA has a negative titer-dependent effect on systemic bone loss, in particular at cortical sites such as the hip (41).
In contrast, results from Bugatti’s analysis indicate that, despite inflammation suppression, spine BMD is sensitive to systemic bone loss in ACPA-positive early RA patients during the first 2 years after treatment onset (42). A Swedish ACPA-positive sub-cohort study demonstrated a trend in reduced BMD at the spine and hip during the first 2 years of treatment (43). Based on the past studies’ findings (38–43), we hypothesized that ACPA and/or RF’s osteoclastogenesis effect on cortical and cancellous bones are different, and the longitudinal assessments of BMD variations in RA are complicated. Further work will hopefully clarify this controversial concern.
The FRAX ® was launched in 2008 to allow health providers to estimate individual 10-year probabilities of fragility fractures (44); it is a free, reliable, and validated tool that is used globally. The current investigation found a significantly higher FRAX score (hip) in SPRA participants regardless of the presence of a single or double-positive antibody. Despite higher FRAX score and greater bone loss in SPRA patients, no significant difference was observed in previous fractures and new incident fractures over time between SPRA and SNRA, and in groups I–III. This suggests that more RA patients and a longer period of observation are needed to determine whether RA seropositivity also has a detrimental effect on incident fracture.
Osteoporosis is characterized by low bone mass, increased bone loss, and micro-architectural disruption, resulting in bone fragility. Based on epidemiological data, an operational definition of osteoporosis was defined as BMD lower than −2.5 SD below the peak bone mass in young adult white women (15, 16). This reference standard can be used to compare the results between studies and assess the accuracy of novel diagnostic tools. In clinical application, the focus lies more on bone quantity which refers to bone mass or density, whereas new technologies target the assessment of bone strength and bone quality information. The current study demonstrated that ACPA and RF were associated with BMD change. Whether these autoantibodies can affect bone strength or bone quality in RA patients warrants further investigation.
As the current study is a real-world investigation, we did not exclude participants who received AOT during the observation period to explore the interaction between autoantibodies and AOT in terms of bone protective effects. Among participants with AOT, there was significant bone loss at FN and TH in SPRA and at TH in patients with higher numbers of antibodies. Respective of antibodies, AOT had a better protective effect against loss of bone density in all sites, especially in the spine. This result echoes Pazianas et al.’s finding that bisphosphonates have a more pronounced effect on trabecular bone than on cortical bone (45).
The strengths of our study are as follows. As a real-world investigation, we thoroughly documented the reported clinical variables that may be associated with osteoporosis or fracture in RA patients to avoid the possible factors related to BMD changes that were not observed in previous investigations. Our initial investigation revealed a significant difference in the distribution of age between SNRA and SPRA groups. As age and sex are two of the most important determinants of BMD, we performed a 1:2 matching for age and sex to exclude the confounding effects of age and sex, which had not been done in previous investigations. Moreover, to adjust for confounding factors of bone loss, we used multiple regression analysis to establish a model for predicting BMD changes. We also explored the additive effects of ACPA and RF on systemic bone loss in RA, which has not been investigated before. Because of the 3-year observation period, we were able to investigate the link between autoantibodies’ presence and BMD at baseline and determine the impact of these autoantibodies on BMD changes over time.
This study had several limitations. The monocentric observational design used to diagnose RA patients allowed us to conclude our data about associations but not about causal relationships. Our patients did not represent an inception cohort; we presented the data at the time of study initiation, not at the time of RA diagnosis. Some of the measurements, including 25(OH) vitamin D and parathyroid hormone, were performed at baseline only (data not shown); serial measurements might be more suitable for exploring these associations. We did not check bone markers throughout the study, which hinders our understanding of the cohort’s pathogenesis of antibodies on systemic bone loss. Finally, RA-associated antibodies have been associated with the occurrence and progression of bone erosions and periarticular bone loss as well as a decreased BMD. Systemic bone loss might increase susceptibility to focal bone erosions in RA patients. We did not collect the erosion scores such as the vdH Sharp score or Larsen score initially and at the 3-year follow-up. Further study is needed to clarify the relationship between autoantibodies to bone density and focal bone damage.
Compared to SNRA patients, SPRA patients had a higher prevalence of osteoporosis, consistent bone loss at all sites, and a higher 10-year probability of hip fracture. We also found that more autoantibodies in RA were associated with more detrimental effects on BMD. We, therefore, suggest that more attention should be paid to osteoporosis and systemic bone loss in SPRA patients, especially double antibody-positive patients. AOT had a better protective effect against BMD loss irrespective of autoantibodies. As for fragility fractures, further investigations are needed to explore the association between RA seropositivity and future fractures in the long term.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Local Institutional Review Board of Chang Gung Memorial Hospital approved the study (104-3530B, 201901054B0). The patients/participants provided their written informed consent to participate in this study.
S-FY, J-FC, and T-TC were responsible for analysis and data interpretation. S-FY and T-TC were responsible for scientific writing. All authors read and approved the final manuscript and were involved with study conceptualization, design, and reporting.
This work was supported by the grant CMRPG8K0441 from Chang Gung Memorial Hospital (https://www.cgmh.org.tw/), which covered the costs of data collection, input, processing, and publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are indebted to the Special Interest Group of Osteoporosis in the Taiwan Rheumatology Association to guide this study’s progression. We thank Hsin-Yi Chien and Chih-Yun Lin for their assistance with the statistical analysis. We appreciate the support provided for statistics at the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital. This manuscript was edited by the Wordvice Academic Editing.
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. (2016) 388:2023–38. doi: 10.1016/S0140-6736(16)30173-8
2. Katchamart W, Koolvisoot A, Aromdee E, Chiowchanwesawakit P, Muengchan C. Associations of rheumatoid factor and anti-citrullinated peptide antibody with disease progression and treatment outcomes in patients with rheumatoid arthritis. Rheumatol Int. (2015) 35:1693–9. doi: 10.1007/s00296-015-3271-8
3. Alemao E, Bao Y, Weinblatt ME, Shadick N. Association of seropositivity and mortality in rheumatoid arthritis and the impact of treatment with disease-modifying antirheumatic drugs: results from a real-world study. Arthritis Care Res. (2020) 72:176–83. doi: 10.1002/acr.24071
4. Ajeganova S, Huizinga TW. Rheumatoid arthritis: seronegative and seropositive RA: alike but different? Nat Rev Rheumatol. (2015) 11:8–9.
5. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. (1998) 31:315–24. doi: 10.1038/nrrheum.2014.194
6. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 Rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. (2010) 62:2569–81. doi: 10.1002/art.27584
7. Gough AK, Lilley J, Eyre S, Holder RL, Emery P. Generalised bone loss in patients with early rheumatoid arthritis. Lancet. (1994) 344:23–7. doi: 10.1016/s0140-6736(94)91049-9
8. Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. (2012) 8:656–64. doi: 10.1038/nrrheum.2012.153
9. Kocijan R, Harre U, Schett G. ACPA and bone loss in rheumatoid arthritis. Curr Rheumatol Rep. (2013) 15:366.
10. Sokolove J, Johnson DS, Lahey LJ, Wagner CA, Cheng D, Thiele GM, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheum. (2014) 66:813–21. doi: 10.1002/art.38307
11. Rantapää-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. (2003) 48:2741–9. doi: 10.1002/art.11223
12. Hecht C, Englbrecht M, Rech J, Schmidt S, Araujo E, Engelke K, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann Rheum Dis. (2015) 74:2151–6. doi: 10.1136/annrheumdis-2014-205428
13. Martin-Mola E, Balsa A, García-Vicuna R, Gómez-Reino J, González-Gay MA, Sanmartí R, et al. Anti-citrullinated peptide antibodies and their value for predicting responses to biologic agents: a review. Rheumatol Int. (2016) 36:1043–63. doi: 10.1007/s00296-016-3506-3
14. Cheng TT, Yu SF, Su FM, Chen YC, Su BY, Chiu WC, et al. Anti-CCP-positive patients with RA have a higher 10-year probability of fracture evaluated by FRAX ®: a registry study of RA with osteoporosis/fracture. Arthritis Res Ther. (2018) 20:16. doi: 10.1186/s13075-018-1515-1
15. Kanis JA, Adachi JD, Cooper C, Clark P, Cummings SR, Diaz-Curiel M, et al. Standardising the descriptive epidemiology of osteoporosis: recommendations from the epidemiology and quality of life working group of IOF. Osteoporos Int. (2013) 24:2763–4. doi: 10.1007/s00198-013-2413-7
16. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. (2014) 25:2359–81. doi: 10.1007/s00198-014-2794-2
17. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. (1993) 8:1137–48. doi: 10.1002/jbmr.5650080915
18. Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo county rheumatoid arthritis register. Arthritis Rheum. (2000) 43:522–30. doi: 10.1002/1529-0131(200003)43:33.0.CO;2-Y
19. Lodder MC, de Jong Z, Kostense PJ, Molenaar ET, Staal K, Voskuyl AE, et al. Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann Rheum Dis. (2004) 63:1576–80. doi: 10.1136/ard.2003.016253
20. Ma CC, Xu SQ, Gong X, Wu Y, Qi S, Liu W, et al. Prevalence and risk factors associated with glucocorticoid-induced osteoporosis in Chinese patients with rheumatoid arthritis. Arch Osteoporos. (2017) 12:33. doi: 10.1007/s11657-017-0329-0
21. Marotte H, Pallot-Prades B, Grange L, Gaudin P, Alexandre C, Miossec P. A 1-year case-control study in patients with rheumatoid arthritis indicates prevention of loss of bone mineral density in both responders and nonresponders to infliximab. Arthritis Res Ther. (2007) 9:R61. doi: 10.1186/ar2219
22. Sahatciu-Meka V, Rexhepi S, Manxhuka-Kerliu S, Rexhepi M. Extra-articular manifestations of seronegative and seropositive rheumatoid arthritis. Bosn J Basic Med Sci. (2010) 10:26–31. doi: 10.17305/bjbms.2010.2729
23. Arain SR, Riaz A, Nazir L, Umer TP, Rasool T. Low bone mineral density among patients with newly diagnosed rheumatoid arthritis. J Ayub Med Coll Abbottabad. (2016) 28:175–8.
24. Bruno D, Fedele AL, Tolusso B, Barini A, Petricca L, Di Mario C, et al. Systemic bone density at disease onset is associated with joint erosion progression in early naive to treatment rheumatoid arthritis: a prospective 12-month follow-up open-label study. Front Med (Lausanne). (2021) 8:613889. doi: 10.3389/fmed.2021.613889
25. Sargın G, Köse R, Şentürk T. Relationship between bone mineral density and anti-citrullinated protein antibody and rheumatoid factor in patients with rheumatoid arthritis. Eur J Rheumatol. (2019) 6:29–33. doi: 10.5152/eurjrheum.2018.18099
26. Solomon DH, Finkelstein JS, Shadick N, LeBoff MS, Winalski CS, Stedman M, et al. The relationship between focal erosions and generalized osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis Rheum. (2009) 60:1624–31. doi: 10.1002/art.24551
27. Padyukov L, Seielstad M, Ong RT, Ding B, Rönnelid J, Seddighzadeh M, et al. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis. (2011) 70:259–65. doi: 10.1136/ard.2009.126821
28. Gadeholt O, Hausotter K, Eberle H, Klink T, Pfeil A. Differing X-ray patterns in seronegative and seropositive rheumatoid arthritis. Clin Rheumatol. (2019) 38:2403–10. doi: 10.1007/s10067-019-04602-5
29. Nordberg LB, Lillegraven S, Aga AB, Sexton J, Olsen IC, Lie E, et al. Comparing the disease course of patients with seronegative and seropositive rheumatoid arthritis fulfilling the 2010 ACR/EULAR classification criteria in a treat-to-target setting: 2-year data from the ARCTIC trial. RMD Open. (2018) 4:e000752. doi: 10.1136/rmdopen-2018-000752
30. Hsu CY, Chen JF, Su YJ, Chen YC, Lai HM, Yu SF, et al. Time-averaged disease activity of rheumatoid arthritis associated with long-term bone mineral density changes. Ther Adv Chronic Dis. (2020) 11:2040622320981517. doi: 10.1177/2040622320981517
31. Blavnsfeldt AG, de Thurah A, Thomsen MD, Tarp S, Langdahl B, Hauge EM. The effect of glucocorticoids on bone mineral density in patients with rheumatoid arthritis: a systematic review and meta-analysis of randomized, controlled trials. Bone. (2018) 114:172–80. doi: 10.1016/j.bone.2018.06.008
32. Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. (2012) 122:1791–802. doi: 10.1172/JCI60975
33. Krishnamurthy A, Joshua V, Haj Hensvold A, Jin T, Sun M, Vivar N, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. (2016) 75:721–9. doi: 10.1136/annrheumdis-2015-208093
34. Wang X, Sun L, He N, An Z, Yu R, Li C, et al. Increased expression of CXCL2 in ACPA-positive rheumatoid arthritis and its role in osteoclastogenesis. Clin Exp Immunol. (2021) 203:194–208. doi: 10.1111/cei.13527
35. Steffen U, Schett G, Bozec A. How autoantibodies regulate osteoclast induced bone loss in rheumatoid arthritis. Front Immunol. (2019) 10:1483. doi: 10.3389/fimmu.2019.01483
36. Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest. (2005) 115:3418–27. doi: 10.1172/JCI26132
37. Lingampalli N, Sokolove J, Lahey LJ, Edison JD, Gilliland WR, Holers VM, et al. Combination of anti-citrullinated protein antibodies and rheumatoid factor is associated with increased systemic inflammatory mediators and more rapid progression from preclinical to clinical rheumatoid arthritis. Clin Immunol. (2018) 195:119–26. doi: 10.1016/j.clim.2018.05.004
38. Cortet B, Guyot MH, Solau E, Pigny P, Dumoulin F, Flipo RM, et al. Factors influencing bone loss in rheumatoid arthritis: a longitudinal study. Clin Exp Rheumatol. (2000) 18:683–90.
39. Haugeberg G, Ørstavik RE, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone loss in patients with rheumatoid arthritis: results from a population-based cohort of 366 patients followed up for two years. Arthritis Rheum. (2002) 46:1720–8. doi: 10.1002/art.10408
40. Haugeberg G, Helgetveit KB, Førre Ø, Garen T, Sommerseth H, Prøven A. Generalized bone loss in early rheumatoid arthritis patients followed for ten years in the biologic treatment era. BMC Musculoskelet Disord. (2014) 15:289. doi: 10.1186/1471-2474-15-289
41. Orsolini G, Caimmi C, Viapiana O, Idolazzi L, Fracassi E, Gatti D, et al. Titer-dependent effect of anti-citrullinated protein antibodies on systemic bone mass in rheumatoid arthritis patients. Calcif Tissue Int. (2017) 101:17–23. doi: 10.1007/s00223-017-0253-8
42. Bugatti S, Bogliolo L, Manzo A, De Stefano L, Delvino P, Motta F, et al. Impact of anti-citrullinated protein antibodies on progressive systemic bone mineral density loss in patients with early rheumatoid arthritis after two years of treat-to-target. Front Immunol. (2021) 14:701922. doi: 10.3389/fimmu.2021.701922
43. Amkreutz JAMP, de Moel EC, Theander L, Willim M, Heimans L, Nilsson JÅ, et al. Association between bone mineral density and autoantibodies in patients with rheumatoid arthritis. Arthritis Rheumatol. (2021) 73:921–30. doi: 10.1002/art.41623
44. Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. (2008) 19:385–97. doi: 10.1007/s00198-007-0543-5
Keywords: rheumatoid arthritis, bone mineral density, anti-cyclic citrullinated peptide antibodies, rheumatoid factor, fracture
Citation: Yu S-F, Chen J-F, Chen Y-C, Wang Y-W, Hsu C-Y, Lai H-M, He H-R, Ko C-H, Chiu W-C and Cheng T-T (2022) The Impact of Seropositivity on Systemic Bone Loss in Rheumatoid Arthritis—A 3-Year Interim Analysis of a Longitudinal Observational Cohort Study. Front. Med. 9:885801. doi: 10.3389/fmed.2022.885801
Received: 10 March 2022; Accepted: 25 May 2022;
Published: 09 June 2022.
Edited by:
Mihir D. Wechalekar, Flinders Medical Centre, AustraliaReviewed by:
Stephanie Finzel, University of Freiburg, GermanyCopyright © 2022 Yu, Chen, Chen, Wang, Hsu, Lai, He, Ko, Chiu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tien-Tsai Cheng, dGlhbnRzYWkwOTE5QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.