- 1Department of Biomedical Engineering, Amity School of Engineering and Technology, Amity University, Noida, Uttar Pradesh, India
- 2Tissue Engineering and Regenerative Medicine Research Lab, Department of Biomedical Engineering, Amity School of Engineering and Technology, Amity University, Noida, Uttar Pradesh, India
- 3Tissue Engineering and Regenerative Medicine Research Lab, Department of Biomedical Engineering, Amity School of Engineering and Technology, Amity University, Gurgaon, Haryana, India
- 4Institute of Nanomedical Sciences, University of Delhi, Delhi, India
Significant research revealed the preocular tear film composition and regulations that remain vital for maintaining Ocular surface functional integrity. Inflammation triggered by many factors is the hallmark of Ocular surface disorders or dry eyes syndrome (DES). The tear deficiencies may lead to ocular surface desiccation, corneal ulceration and/or perforation, higher rates of infectious disease, and the risk of severe visual impairment and blindness. Clinical management remains largely supportive, palliative, and frequent, lifelong use of different lubricating agents. However, few advancements such as punctal plugs, non-steroidal anti-inflammatory drugs, and salivary gland autografts are of limited use. Cell-based therapies, tissue engineering, and regenerative medicine, have recently evolved as long-term cures for many diseases, including ophthalmic diseases. The present article focuses on the different regenerative medicine and reconstruction/bioengineered lacrimal gland formation strategies reported so far, along with their limiting factors and feasibility as an effective cure in future.
Introduction
The human ocular surface health essentially depends on a stable thin layer of tears (tear film) acting as a protective barrier against the external environment, thus keeping the surface moistened and maintaining ocular epithelial surface homeostasis. Multiple components are responsible for the regular function of the Lacrimal Functional Unit (LFU), including the lacrimal gland, the eye surface (cornea, conjunctiva, and meibomian gland), and related sensor and motor neurons (1–4). The LFU regularly create tear film to maintain corneal transparency, and image quality projected onto the retina because it is the central regulator of the secretion of the major components of the tear film. Aqueous layer secretions, as well as basal and reflex tearing in response to sensations from the outer surface, are controlled by the outer lipid, middle aqueous, and inner mucin layers of the mammalian tear film (Figure 1). These LG are found beneath the orbital's upper temporal compartment in the lacrimal fossa in orbital cavities (4). Many factors, such as advancing age, autoimmune diseases, orbital radiotherapeutics, and a low androgen pool, can cause Lacrimal gland dysfunction, commonly referred to as Dry Eye Syndrome (DES) or Dry Eye Disorders (DEDs) (Table 1: Causing agents). The dry eye syndrome (DES) is defined as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles” (at TFOS/DEWS II) (8). Different types of DES have been defined and classified based on the causing agents (9). Other complicated factors such as ·Non-aqueous Sjögren's deficiency (e.g., age-related) ·Dry eyes are caused by a lack of aqueous humor, such as Sjögren's syndrome. The global prevalence of dry eye disorders (DEDs) is between 11 and 22%, but it is estimated to be between 18.4 and 20% in the Indian subcontinent.
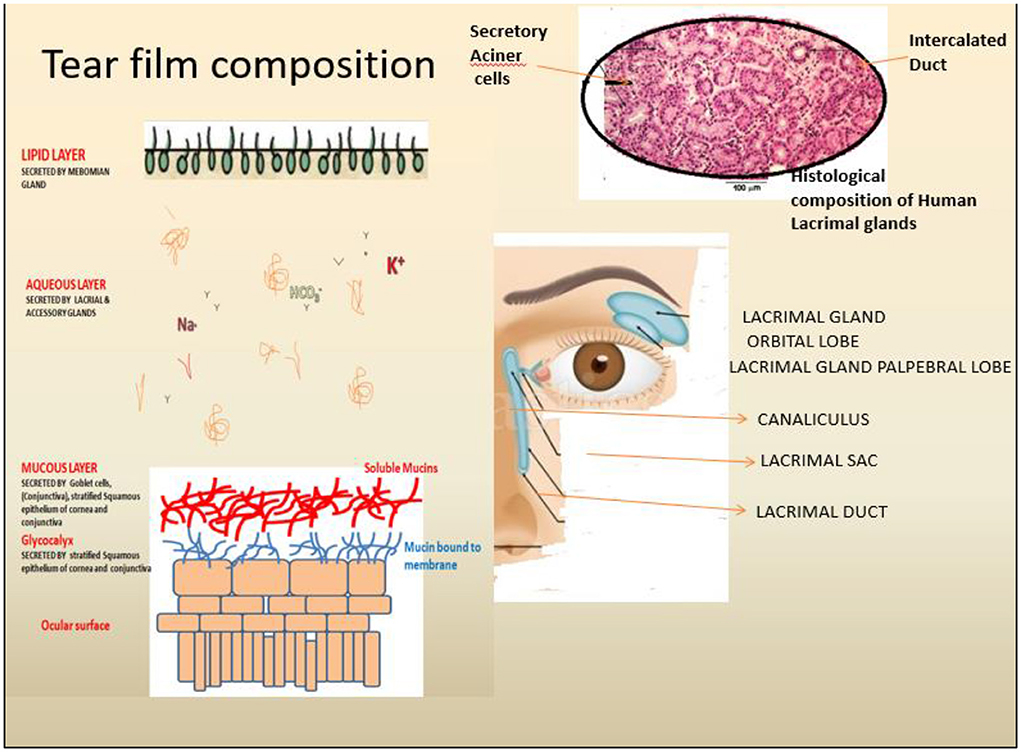
Figure 1. Anatomical and physiological depiction of the human lacrimal gland and associated components. Tears are secreted by Lacrimal gland function units, including the lacrimal gland (LG) (Blue), the ocular surface (cornea, conjunctiva, and the meibomian gland) and the associated sensory and motor nerves. The tear film comprises three significant layers, i.e., Outer lipid, middle Aqueous and inner mucin-rich layers secreted by (Left) secreted by the meibomian gland, lacrimal gland and ocular surface goblet cells as depicted.
The LG (serous tubuloacinar lacrimal gland) secretes fluids transported to the ocular surface via a small channel or duct system (1). The LG is also supported by two accessory secretory glands, the Krause and Wolfring glands (2). Tear fluid is a complex mixture of proteins, inorganic salts, and immunoglobulins such as IgA that can be studied (10, 11). LG parenchymal cells produce these components, which include acinar, duct, and myoepithelial cell types in a specialized structural arrangement forming distinct tubules of acinar, duct, and myoepithelial cells (12). Acinar cells (>80%) are the primary producers of lacrimal fluid, while duct cells (10–12%) play a minor role in the further modification of tear fluid compositions (13). Macrophages, plasma cells, lymphocytes, B cells, helper and suppressor T cells, dendritic cells, and mast cells are just a few of the cell types that reside in the interstitial space of the LG parenchyma. The tear film (secreted by meibomian or tarsal glands) consists of three layers: mucous (innermost layer, secreted by conjunctival goblet cells), intermediate aqueous layers (secreted by LG), and an outermost lipid layer (secreted by LG) (2–4). Tear film functions include lubrication and smoothing of the corneal epithelial layers, preservation of optic properties, moistening of the corneal/conjunctival epithelium layers, participation in metabolic activity, cleaning of the ocular surface by removing dust and debris, and prevention of microbial infections. Due to a lack of corneal epithelial stem cells complete loss of the aqueous layer might result in keratinization of the ocular surface epithelial cells and keratinocyte invasion from the skin (14, 15). A similar study has demonstrated the critical nature of tear film stability and the essential nature of preserving their complex compositions. LG is tightly regulated by the nerve systems (parasympathetic and sympathetic); as DES is produced by even modest changes in the composition and stability of the tear film (13).
There has been significant research for defining the various causing factors and Characterization of the molecular mechanism of different factors causing LG dysfunction and resulting in DES in human and animal models. In addition, other treatment strategies are also being developed by studying animal models and some human clinical data. Here we review the current information available about the various treatment modalities, animal models and human clinical data to treat and restore ocular surface disorders.
Molecular mechanism of DES in animal models and humans
Factors causing tear deficiency
Lacrimal gland impairment and consequent declined tear secretions are the most prominent reasons for DES. Studies in animal/humans derived information about the reasons showing age-dependent hyposecretion, inflammatory reactions causing LG destructions, conjunctival inflammation/scarring causing duct closures, enervation dysfunction because of congenital defects and trauma, any sort of pathogenic infection (bacterial/viral) causing LG dysfunctions. Aging and female gender are related to the higher risk for causing dry eyes in humans (female, age < 50, risk potential of dry eyes 5.7%; age > 45, risk potential 9.5%) (3). LG shows various age-dependent characteristics, including atrophy in acinar cells, fibrosis, obstruction of ducts, and lymphocyte infiltrations, are shown to be displayed by LG.
Age-dependent development of DES
Age-dependent DES is characterized by atrophic acini, fibrosis, ductal blockage, and an increasing prevalence of lymphocytic infiltration (6). Functional alterations in older mice, such as decreased triggered protein production, were found.
Inflammation
Both acute and chronic inflammation are shown to be the most common causative agent (>80%) for severe DES (Figure 2) (2). One of the major causing agents has been Graft vs. host diseases (GVHD) (40–76%) following the transplantations, and the severity of GVHD is directly reflected in the severity of DES (16). GVHD could induce lymphocytic infiltration of both accessory and lacrimal glands, followed by fibrosis of acini and ducts, declined aqueous tear component secretion due to accumulation of amorphous materials, cell debris along with normal-appearing granules (associated with age-associated tear deficiency markers) in the acini and ducts (17). On the other side, inflammation can result in a variety of consequences, including GVHD, other autoimmune disorders, including Sjögren's syndrome Dry Eye (SSDE), and non-syndrome Sjögren's Dry Eye (non-SSDE) (3, 4, 9, 18). Histological examinations of animal and human lacrimal gland biopsy samples (DES lacrimal gland) revealed the normal duct arrangement breakdown in LG, as evidenced by reduced lobular patterns, lymph follicle buildup, fibrosis, and atrophied acinar cells (Figure 2) (19). CD4+ T cells dominated the lymphocytic infiltration, with a lesser proportion of CD8+ T cells and B cells (20, 21). However, immunohistochemical studies revealed additional details about several lymphocytic activation markers, including HLAs, lymphocyte function-associated antigen, and very late antigen-4, as well as an increased level of IFN-ɤ (Interferon- ɤ), cell adhesion molecules (e.g., intracellular adhesion, vascular cellular adhesion molecule) on acinar, ductal, and epithelial cells in LG and conjunctiva (5, 21, 22). Proinflammatory cytokines such as IL-1 and IL-1 have been found in high concentrations in the tear fluid of DES patients. In some cases, they are also associated with the early stage of leukocyte invasion and corneal injury (23). Thus, DES may be described as a multifactorial condition resulting from trauma, age-related degeneration, inflammatory agents, and any pathogenic microbial infection of the LG, corneal surface, or other structures (24, 25). The complex tear system is tightly regulated by a delicate balance of these various factors. Slight changes in any one of them (altered tear fluid composition, decreased blinking frequency, resulting in decreased ocular surface lubrication) can trigger systemic inflammatory responses, which can result in inflammation of the lacrimal gland or other components of the LFU (24, 25).
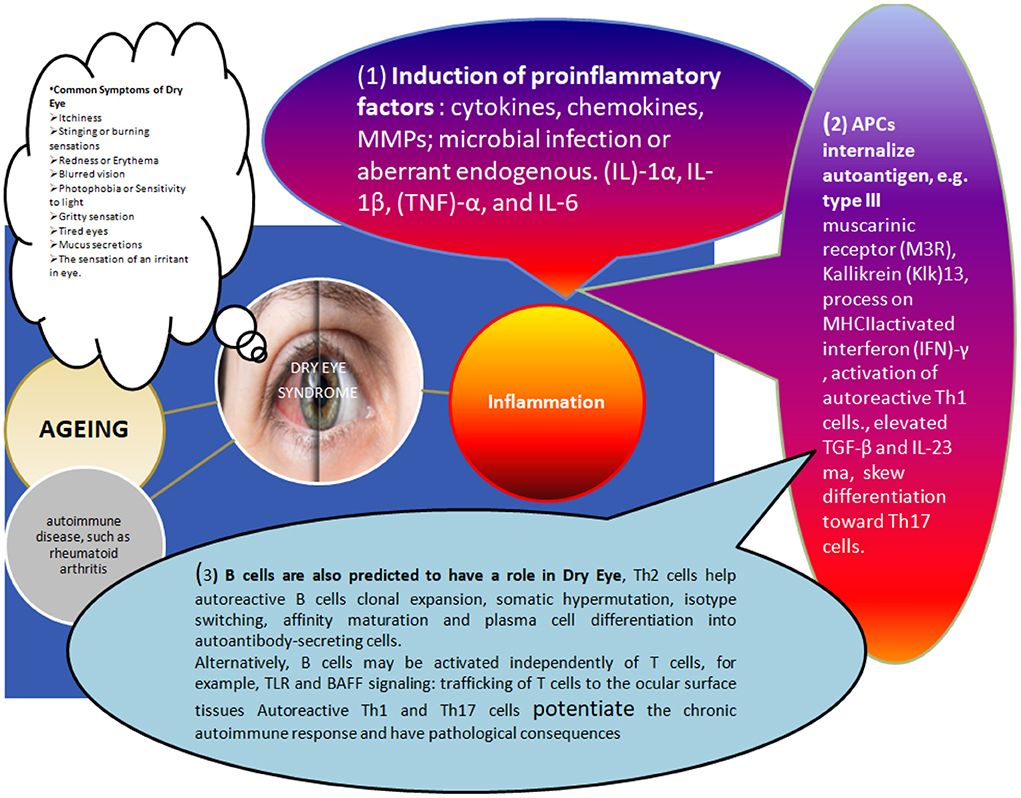
Figure 2. Different factors causing dry eye associated changes in the human ocular surface and disease manifestations: The immunopathogenesis of Dry Eye disease involves many causing agents, including age-related changes, Hormonal imbalance, and infections. Still, most of these agents predominantly display their impact through violation of the ocular surface's delicate immune-regulatory mechanism. The inflammatory response caused by various factors triggering these immune changes can be described as (1) Initiation of Induction of proinflammatory factors: as shown in the figure, cytokines, chemokines, and matrix metalloproteinases (these initial signals can trigger MMPs). This might involve microbial infection-induced downstream signaling [e.g., Toll-like receptor (TLR) signaling]. Various acute inflammatory cytokines (e.g., IL-1α, IL-1, tumor necrosis factor TNF- α, IL-6, etc.) are reported in higher concentrations in dry eye conditions and can amplify inflammatory reactions through APCs activation. In addition, APCs secreted IL-12 and INF-γ eventually activates the differentiation of autoreactive T-cells (e.g., Th1 cells). (2) Secondly, A DIFFERENT COMBINATION OF CYTOKINE AND THEIR ELEVATED CONC (e.g., IL-6, TGF-β, IL-23) can skew toward Th17 cells. Thirdly, B cells also contribute to all these inflammatory changes Related to dry eyes. For example, autoreactive B cells are triggered by Th2 cells helping their clonal expansion and activation of somatic hypermutation isotype switching, affinity maturation and ultimately differentiation of plasma cells into autoantibody-secreting cells (in addition, independent activation mechanisms also do exist, e.g., TLR and BAFF signaling pathways). Migration of autoreactive T cells: Adhesion molecules (e.g., LFA-1, VLA-4), chemokine receptors (e.g., CCR5 and CXCR3) and their interaction with the cognate receptors on ocular cell surface cells (e.g., ICAM-1, CCL5, and CXCL10) can direct the migration of these autoreactive T-cells. Further, Autoreactive Th1 and Th17 cells support chronic immunoresposes causing immunopathological consequences.
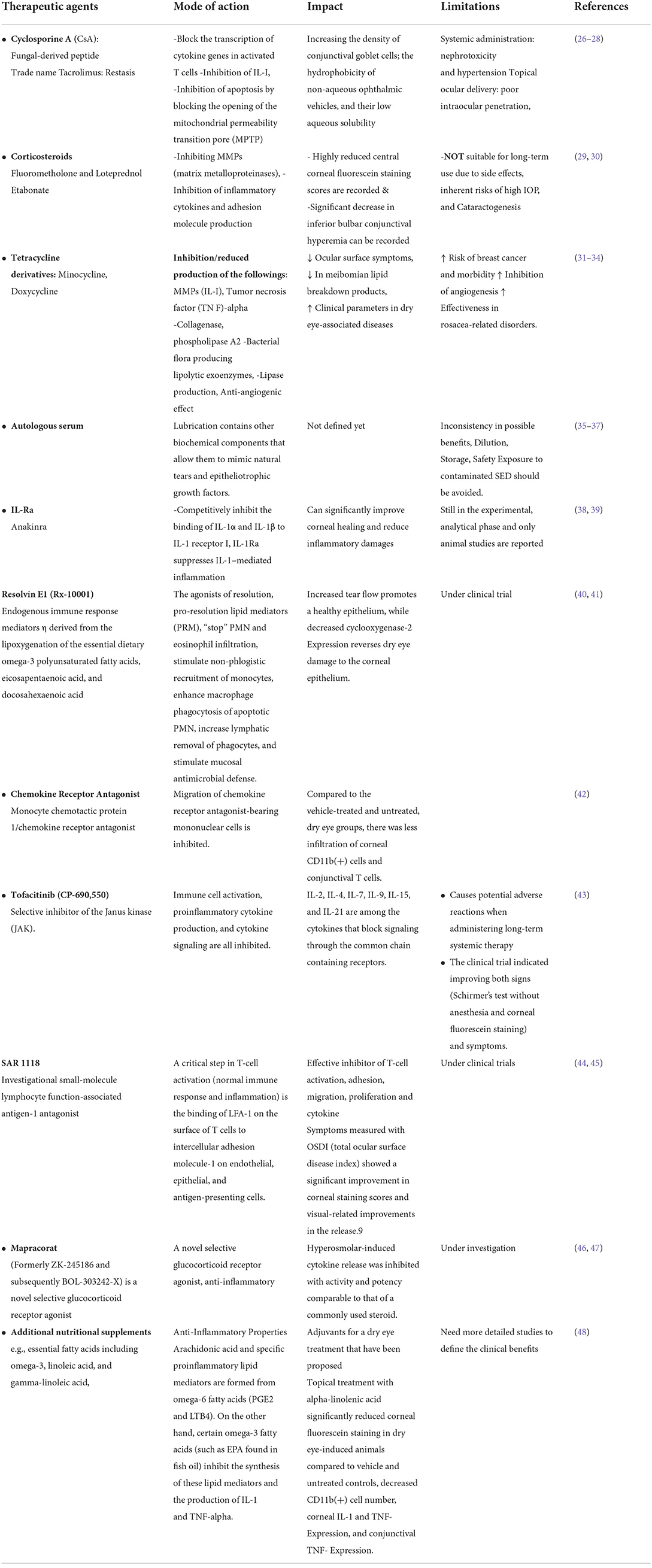
Modern treatment/management modalities for DES and related ocular complications
Most current clinical care techniques are palliative in nature. They remain reliant on therapeutic agents such as lubricating agents, tear substitutes, and tear retention, as well as the use of anti-inflammatory drugs to alleviate ocular surface inflammations (Table 2) (49). Additional techniques, such as partial or complete submandibular salivary gland transplantation, have demonstrated significant alleviation in a small number of patients (50–53). While the distinctive Schirmer test scores and extended tear breakup time (TBUT) are usually used to describe the severity of DES, the whole process does not affect tear film function or visual acuity in patients. One such explanation stems from the fact that the content of tears differs significantly from that of LG gland secretions. Salivary gland transplantation produces hypoosmolar liquids that cause microcystic corneal oedema and epithelial abnormalities (50). Additionally, xenotransplantation of swine lacrimal glands with architectural similarity to human lacrimal glands is presented as a viable therapy option with substantial rejection and retroviral infection risks (54–58).
Lacrimal gland regeneration modalities
In the absence of any effective cure for most DES patients, alternative approaches such as regeneration of the lacrimal glands or transplantation of bioengineered lacrimal gland tissue are being explored by various researchers. They have been demonstrated to have high clinical potential. First, the lacrimal functional unit components' damage or loss of function, e.g., lacrimal gland, can be categorized as either partial or complete. In the “partial damaged” conditions, an obvious treatment strategy could be regenerative measures for the residual cells or tissue. These approaches are non-invasive, and the in-situ microenvironment may provide superior support than any intro culture settings or artificial growth conditions. In the second category, if no residual cell is available for regeneration, in-vitro reconstruction of lacrimal glands or bioengineered lacrimal gland is the only option which is being developed using the most advanced tissue engineering techniques.
Stimulation of the regeneration of existing tissue
These approaches should restore tear volume, accurate tear composition, and anti-inflammatory qualities to protect lacrimal gland cells from additional harm, allowing gland tissue regeneration, remodeling, and functional recovery. No immunogenic problems should be associated with these techniques, indicating a possible role for autologous implants or the availability of HLA-matched donors. Additionally, the administration of these therapies should be sufficiently efficacious to be completed in a single try, reducing the need for repeated intervention. Clinical use can be ensured by adhering to simple and fast GMP-compliant processes. Additionally, these approaches must be highly efficient, which means that if cells are to be delivered to recipients, they must be capable of homing in significant numbers. This would ensure success and cost-effectiveness while also being free of harmful consequences.
Numerous techniques are being investigated to accomplish these goals, which can be classified as (i) Drugs and (ii) Cell-Based Therapies.
Drugs or pharmacological agents for regenerative approaches
All previously known drugs for the Treatment of quantitative DES are only met for palliative relief and anti-inflammatory activities to reduce further damage to the ocular surface. For example, cyclosporine A, tacrolimus, and corticosteroids are routinely prescribed for reducing lacrimal gland inflammation (49). Another range of pharmacological agnates which can improve tear flow rates, such as lacritin, lactoferrin, quercetin, and systemic pilocarpine, are being investigated without showing any regenerative potential (59–65).
There are several intriguing reports describing the use of “autologous platelet-rich plasma” (PRP) injections and “tumor necrosis factor-stimulated gene/protein 6 (TSG-6)” for lacrimal gland regeneration (66, 67). PRP injection close to the lacrimal gland improved tear volume, increased the TBUT, and decreased ocular surface staining in DES patients. Numerous growth factors are contained in PRP, including epidermal growth factor, platelet-derived growth factor, insulin-like growth factor, and neural growth factor. In addition, VEGF is a vascular endothelial growth factor (68) that promotes cell migration, adhesion, proliferation, and differentiation in various cell types. Apart from its use in the treatment of a variety of diseases, such as wound healing, corneal ulcers, chemical burns, fibroblast proliferation, and extracellular matrix remodeling via collagen remodeling (69–73), PRP is an effective epithelial recovery of the cornea following laser-assisted in situ Although the mechanisms underlying PRP's regenerative effects are unknown, one possibility is that it induces epithelial-mesenchymal transition (EMT) cells, which are well-known for their critical roles in tissue repair, remodeling, and regeneration in a variety of glandular tissues, including mammary glands, liver, and lacrimal gland (67, 74). TSG-6's anti-inflammatory properties are further demonstrated in studies examining the regenerative and immunomodulatory functions of MSCs in several glandular tissues, including the skin, kidney, and liver, which identified TSG-6 secretion as a mechanism by which MSCs manifest their protective properties (63, 75, 76). Although the molecular mechanism and additional detailed investigations are required to establish their clinical utility, all of these reports showed a relatively easy and adaptable technology that might be developed for DES regenerative medicine.
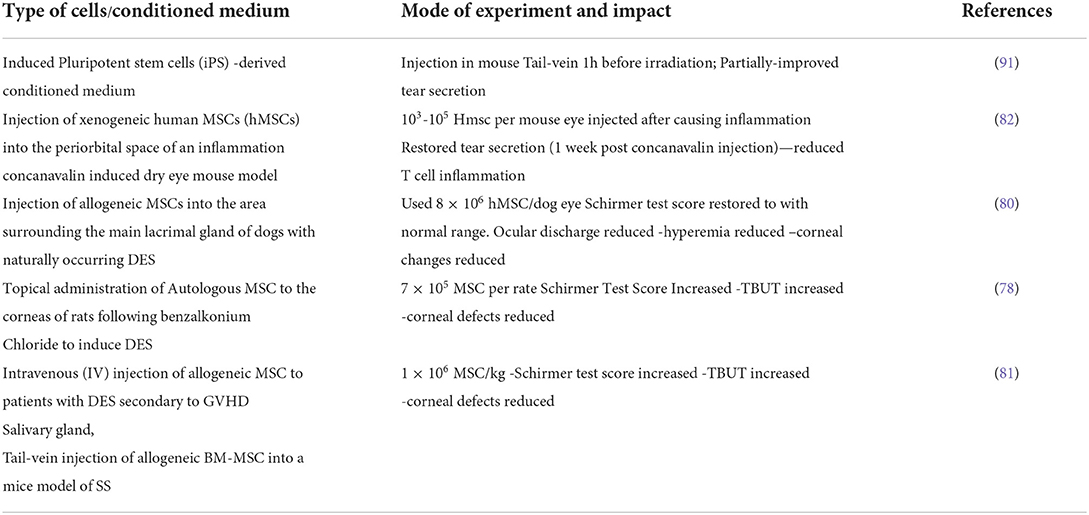
Cell-based regenerative approaches
A few findings highlight the importance of cell-based techniques as a potentially beneficial therapeutic strategy, as evidenced by research in humans with GVHD/SSDE and in animal models (77–83), Most of these research proved the utilization of mesenchymal stem cells to regenerate lacrimal or salivary glands. Freudenstein and colleagues described the de novo formation of osteogenic foci employing a bone marrow (BM) derived cell population termed MSCs for the first time in mice model experiments (84). Significant data has been generated since then defining MSCs surface identification markers (e.g., CD29, CD73, and CD105 positive; CD34 and CD45 negative), isolation procedures and culture standardizations, and their potential therapeutic roles in a variety of tissue types in different animal models and humans (85–87). MSCs, for example, can be classified as dormant multipotent or adult stem cells that exist in an undifferentiated condition in most adult organs and tissues, alongside fully differentiated cells (88).
MSCs have been shown to differentiate into mature muscular (85), corneal epithelial (86), salivary acinar (89), pancreatic and neural cells (85, 86).
MSCs are derived from various sources and have been demonstrated to possess anti-inflammatory capabilities. MSCs suppressed the maturation/proliferation of a variety of immune cells [e.g., T-cell, B-cell, natural killer (NK) cell, monocyte, macrophage, and dendritic cell]; they can impair B-cell Ig synthesis and reduce NK cell cytokine release (90). MSC's anti-inflammatory characteristics suggested they could also be used therapeutically in chronic inflammatory ocular surface illnesses.
MSCs have been explored for their therapeutic potential in salivary and lacrimal gland damage models (Table 3). For example, injection of IL-1α directly into the mouse lacrimal gland has been shown to elicit acinar cell death, resulting in a transient drop of tears and a subsequent regenerative response (92). Additional studies demonstrated the presence of MSC-like cells (nestin- and vimentin-positive cells) that may be responsible for these regenerations in IL-1-treated animals (74, 92, 93). These indirect shreds of evidence, combined with their adipogenic differentiation properties and ability to survive in vitro beyond 14 days, indicated that IL-1 injection may have triggered the destruction of the lacrimal gland, which could be recovered by MSC or other stem progenitor cells in these animal model studies.
Subsequent research has bolstered the putative role of MSC in IL-1 or other injury-induced tear insufficiency models. Numerous gene expression markers, including Nestin, Musashi-1, ABCG2, Pax6, Chx10, and Sox2, putative stem cell markers nestin and α-smooth muscle actin (SMA), or ΔNp6, Nanog, Oct4, Sox2, and Pax6, transcription factors (CMyc and Kruppel-like factor-4), and early lineage markers for endodermal (GATA4, GATA6) and mesodermal (bone morphogenic proteins-4 and−7), have been reported (86, 94).
MSCs-based therapies and in vitro studies
MSCs' therapeutic activities have also been established in various in vitro investigations conducted by diverse research organizations. MSCs' well-known ability to secrete growth factors [e.g., epidermal growth factor (EGF), transforming growth factor (TGF), and vascular endothelial growth factor] is thought to support their growth-inducing effect in a variety of tissues such as the corneal limbus, kidney, lung, and skin. Growth factors [e.g., epidermal growth factor (EGF), transforming growth factor (TGF), and vascular endothelial growth factor] Caplan et al. (95), Chen et al. (96), Akram et al. (97), Hu et al. (98), Moghadasali et al. (99), and Walter et al. (100). Similarly, MSC-conditioned media obtained from mouse lacrimal glands enhances the migration and proliferation of porcine lacrimal gland epithelial cells in vitro (101). Numerous animal models studied for DES demonstrated beneficial effects of MSC transplantation or topical application, including the mouse model, the rat model (benzalkonium derived chloride), dogs, and other canine models, resulting in improved Schirmer test scores and TBUT increment, decreased corneal defects, sustained decreased ocular discharge, hyperemia, and corneal changes over time (77, 79, 80, 83).
Although direct evidence for MSC-derived therapy in human dry eye patients is scarce, some studies exist. For example, a small study (22 patients with GVHD-related dry eyes) indicated that intravenous infusion of human MSCs (BM-derived) improved Schirmer test scores and decreased ocular surface disease index scores in some recipients (81). Although elevated levels of IL-2 and IFN-γ were observed in individuals who had beneficial benefits following MSC treatment in these investigations, this finding contradicts previously documented MSC involvement in lowering these proinflammatory cytokines in dye eye models.
It shows that MSCs are potentially valuable for developing lacrimal gland regenerative strategies. Still, the complex relationship between different immunogenic environments and their effect on MSC-derived therapeutic needs to be defined at a deeper molecular level. Second, identification and culturing methods to obtain large amounts of MSC for these clinical uses will also be another area of research.
Other alternatives: Human amniotic membrane (hAM) epithelial cells and induced pluripotent stem (iPS) cells in cell therapies for dry eyes
Both human amniotic membrane (hAM) epithelial cells and iPSCs are being investigated for salivary gland regeneration in animal models, indicating the possibility of using them for lacrimal gland regeneration as well, owing to the structural and functional similarities between the salivary and lacrimal glands in mammals (102). This is further corroborated by results demonstrating that hAM epithelial cells can be transdifferentiated into acinar cells in an in vitro double-chamber system research (103). Additionally, iPSCs may be of enormous regenerative value in techniques for lacrimal gland regeneration, but no direct trial evaluating their potency has been reported so far. However, studios that use iPS cell-conditioned media to regenerate defective mouse lacrimal glands are available, demonstrating their potential anti-inflammatory qualities that may be useful in lacrimal gland regeneration (91). However, numerous elements of their effects, efficacy, and manufacturing for clinical use have yet to be rigorously explored to establish an acceptable regular treatment alternative for dry eye syndrome.
Gene therapy for DES
This is another promising approach for several incurable disorders, including regenerative medicine. The target molecules (i.e., DNA, mRNA, mi RNA, siRNA, antisense oligonucleotides etc., for any gene sequence) are delivered through various delivery approaches such as viral and non-viral methods and analyzed for the desired impact to achieve potential therapeutic effects. The major obstacle remains the use of optimal delivery approaches in clinics. While viral particle-based strategies are effective and specific, they have a high risk of viral infection. While non-viral vectors are safer than viral vectors, they currently have low delivery efficiency (104).
Nevertheless, in vitro and in vivo investigations have revealed the possibility of creating anti-inflammatory medicinal solutions for lacrimal glands. For example, in an autoimmune rabbit model, blocking tumor necrosis factor-α by transferring an inhibitor gene resulted in normal tear formation, increased TBUT and rose bengal scores, and decreased lymphocyte infiltration (T-cell, CD18+cells) (105). Thomas et al. demonstrated similar results employing adeno-associated virus (AAV) vector-mediated viral (v) IL-10 gene expression in the lacrimal gland (LG) immunopathology and ocular surface disease (rabbit model) (106).
In vitro manufacture of lacrimal gland tissue and transplantation strategies
Another important approach is being explored when there are no resident lacrimal gland cells, as discussed above, that remain to be triggered for regeneration. In these situations, ex vivo construction of the lacrimal glands using tissue engineering techniques could be the only solution, as discussed here. To ensure the functionality of these ex vivo generated tissue, several complex approaches would be required to define the set of parameters, including, 1. Tear volume and composition. 2. Heterogeneity of the cells in tissue-cultured, e.g., acinar epithelial, endothelial, and duct cells. 3. No adverse effects and non-immunogenicity. 4. Mimic a niche environment to sustain the long-term functionality of fully differentiated cell types in the transplant. 5. Three-dimensional or native-like the structural organization of the implant. 6. Optimal vascularization. 7. Optimal Innervation and cannulation to ensure secretion and supply of lacrimal fluid in response to various cues and their transport to the ocular surface for tear film generation. 8. A broad range of criteria applicable to their in vitro mechanical handling, Quality assessment, biomaterials used for easy approval from regulatory authorities and cost-effectiveness (Figure 3).
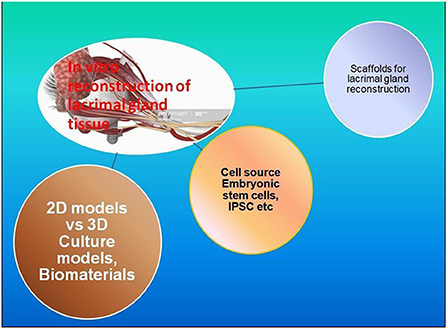
Figure 3. Components of in vitro reconstruction of Lacrimal glands: there are many efforts reported to develop strategies for the in vitro culture of clinical-grade lacrimal glands/tissue. The whole idea revolves around (1) Cell sources and Types; Adult stem cells, ESCs, IPSCs, or MSCs; (2) Biomaterials and their suitability for tissue reconstruction assessment through 2D/3D in vitro culture models; (3) Scaffolds architecture, fabrications and culture associated biochemical, physical and functional parameter assessment.
Cell source for bioengineered lacrimal gland generation approaches
Various cell sources have been successfully demonstrated in ex vivo tissue engineering approaches, such as Embryonic stem cells, iPSC or Epithelial progenitor cells. The lacrimal gland is a complex organ where different cells such as lacrimal acinar cells, myoepithelial cells, and duct cells are required to coexist in a functional interconnected architecture to ensure their functional roles in tear production secretion and transport. As discussed in the following sections, different cells from Allogeneic, autologous or Xenogenic sources are being explored for the regular GMP-compliant lacrimal gland implants. may be xeno-, allo-, or autologous; embryonic stem (ES) cells; or induced pluripotent stem (iPS) cells.
Scaffolds and biomaterials for ex vivo bioengineered lacrimal gland reconstruction
Scaffolds play essential roles in various organs' ex vivo tissue engineering approaches. They offer several benefits in achieving the goal of organ construction and their implantations. For instance, firstly, the Total number of cells permitted for the transplantations, i.e., a large number of viable cells should be transplanted to restore complete functions of the glands or other organs. Scaffolds facilitate this by providing a suitable platform for many cells to grow simultaneously in a controlled microenvironment. Secondly, Scaffold may help in the process by providing a surface on which each cell type can grow and in situ guided growing location for each cell type may be dictated to help achieve functionality. In addition, the precisely selected physicochemical properties of biomaterials (regulating mechanical strength or degradation rates, etc.) may be chosen and designed to enhance the specific application of functions of the maturing tissue, e.g., lacrimal gland.
Biomaterials are essential for constructing these scaffolds, and various of them have been described with variable properties. Therefore, one can select between Natural or synthetic biomaterials depending upon the priorities. Natural biomaterials are advantageous due to biomimicry but are immunogenic and might not be available in sufficient quantities for some time. On the other hand, biosynthetic biomaterials are a rapidly evolving field inheriting several advantages and can be used easily to develop Good Manufacturing Practice (GMP)-compliant products/methods.
Advancements in the bioengineering strategies for lacrimal glands by 3D/2D culture of lacrimal cells
Generation of the functionally active lacrimal gland might require many cells (e.g., lacrimal epithelial cells or stem cells etc.) to be seeded in 3D scaffolds or similar 3D culturing approaches. Since 2D culture systems are comparatively more straightforward and may be helpful in the Characterization and assessment of functionally relevant markers, activities etc., they are most often used before the actual 3D environment could be established. In addition, the various quantitative and qualitative parameters studied by 2D culture experiments are further useful in achieving the GMP-compliant 3D culture protocols and their validation before any clinical application could even be hypothesized. There are several 2D culture studies reported using different species (i.e., rat, mouse, rabbit, and human) to demonstrate adult lacrimal epithelial cells isolation, culture methods, Media formulations, and other growth environments using 2D culture methods (107–116). These studies established the requirement of FCS and other growth factors for in vitro culture media formulation, including epidermal growth factor, hepatocyte growth factor, insulin-like growth factor and fibroblast growth factors. However, recently serum-free culture environments such as Hepato-STIM without or reduced FCS concentration have shown clinical suitability (113, 117).
Embryonic stem cells and induced pluripotent stem cells and lacrimal gland generation
Embryonic stem cells are pluripotent stem cells with enormous research and clinical significance. The potential transcriptional factors were identified in a direct differentiation method for the lacrimal gland generated by using human ESC, resulting in differentiation into LG epithelial cell phenotype (118). These studies demonstrated the potential use of Pax6, CK15, AQP5, and lactoferrin as an inducer for lacrimal gland generation. This can be achieved further by developing a stable gene expression model system for these factors and ESC. There is substantial clinical potential for ESC, but its use is restricted due to the associated risk of tumor formations and ethical concerns (119). Hu-iPSC was demonstrated as an alternative to these ESC-associated ethical concerns, and they are being explored for various tissue regeneration and reconstruction strategies. However, no report shows lacrimal gland cell generation using iPSC as source materials. Direct differentiation of iPSC into lacrimal gland cells is still open, offering enormous opportunities for researchers and clinicians to develop effective methods for the large-scale production methods of lacrimal gland dysfunctions in the near future.
Lacrimal gland reconstruction studies and characterization of cell cultures by 2D methods
The simplicity of 2D cell cultures offered the researcher an opportunity to demonstrate various cellular and culture-associated characteristics for the various components that can be useful in defining the qualitative and quantitative parameters of their functional significance in more complex organs or in vivo studies. Many different methods, such as Electron microscopy (both scanning and transmission) (107, 108, 110–114) has been a valuable method to identify showing secretory granules, the polarity of these cells intercellular contacts which can be used to examine the types and nature of these cell-cell interactions in a more complex 3D culture system. Similarly, many immunohistochemical analyses in 2D culture systems could help identify non-epithelial and myoepithelial markers. For instance, the immunohistological examination could recognize the Expression of cytokeratin 3/12, E-cadherin (non-epithelial markers), and distinct cell population expression epithelial cell adhesion molecule, p63,30 pan-cytokeratin (pan-CK) etc. showing their potential for quick assessment approaches (109, 116, 120). In addition, there are other essential markers which could also be determined by these studies, such as aquaporin5 (AQP5, CK822), myoepithelial (α-smooth muscle actin) cell marker lacrimal gland-specific protein secretion (117, 121), Further studies have been done to measure; β-hexosaminidase activity as a result of parasympathetic stimulation of these 2D culture methods (110, 112–114, 122).
Biomaterials and various approaches to culture lacrimal gland cells
Several materials have been tested in 2D/3D culture methods to exemplify the growth conditions for more complex organ constructions in a 3D culture system. These materials might be useful in developing scaffolds, thus providing specific molecular signals or physicochemical cures that can trigger cellular growth and differentiation under controlled environments. Some of the most studied biomaterials may include Polyethersulfone tubing, Poly (L-lactic acid), Polyester, Human amniotic membrane, and Matrigel. Unfortunately, there are few reports about each material and its potential application for developing robust culturing protocols for clinical uses. In the following segment, we discuss a few experiments using these materials and their outcomes to understand their further technical uses.
Approached for developing scaffold using polyethersulfone, poly (L-lactic acid) and polyester
Polyethersulfone (PES) is a well-known synthetic polymeric material for its exceptional biological compatibility (i.e., oxidative, thermal, and hydrolytic stability) and its mechanical and film-forming capabilities. PES membranes are commonly used in biomedical equipment such as blood purifiers (hemodialysis, hemofiltration, and hemodiafiltration), plasma separation (123), and hemodiafiltration (124–128). These widespread applications demonstrated the materials' good blood compatibility, and efforts might be made to construct immune-protective devices utilizing hollow fiber membranes containing xenologous pancreatic islets for subcutaneous implantation in diabetic rat models (129). Subsequently, it was demonstrated that PES hollow fibers might support the growth of a variety of human cells in vitro as a substrate; for example, human endothelial cells kept their morphological and functional properties when grown on fibronectin-coated PES hollow fibers (130, 131). These studies provided an essential basis for exploring PES-based scaffolds for culturing lacrimal epithelial cells. Using a PES scaffold could support as a substrate for cellular growth and immune protectant by physically prohibiting immune cells of the host cell from reaching the graft/cells growing inside these fibers/tubing but does not inhibit the release of lacrimal fluids from them. For instance, PES tubes (50 mm length × 3 mmdiameter, dead-end) seeded with rat lacrimal gland acinar cells were successfully cultured exhibiting permeability of the small molecular components (e.g., glucose) while non-permeable to large molecules such as IgG antibodies, etc. (108). These studies indicated possibilities for the use of PES based scaffold for further lacrimal gland cell culture. However, their clinical use would only be possible after the rigorous evaluation of all relevant information about the growth and function, such as lacrimal fluid volume and composition in human and animal models.
Poly (L-lactic acid)
It is also well-known material investigated by making scaffolds in thin (20 μm) membrane form, also in collaboration with collagen type I, silicone, and Thermanox coverslips (114, 122). The Scaffold designed by these researchers showed a thriving culture of rabbit lacrimal acinar cells and the excretion of small molecules. Still, it did not allow the permeation of IgG-like giant molecules in culture. However, cells were reported to grow to a sub-confluence level with suboptimal functionality levels as described by these reports (122).
Polyester
It has also been evaluated in the form of microporous polyester cell culture inserts (122). Authors in these studies revealed the relationship between autoimmune disease conditions caused acinar cell dystrophy and hypothesized a possible molecular mechanism for functional quiescence of M3 muscarinic acetylcholine receptors due to agonistic auto-antibodies mediated inhibition of downstream signaling mediators such as Gq and G11 in lacrimal parenchymal cells (132). The report indicated the establishment of a confluent cell monolayer (3–5 days of culture) of acinar epithelial cells on polyester membrane Transwell inserts. This study also described the transepithelial electrophysiological behavior of cultured cells using the classic “Using short-circuit methods.”
The cultured cells were positive for tight junction membrane marker “occludin” and showed similar electrophysiological behavior as indicated by their responsiveness to carbachol stimulation and measured increased β-hexosaminidase activity (released by rabbit lacrimal acinar cells in response to cholinergic stimulation) in these studies. These studies indicated a potential use for this biomaterial in lacrimal gland reconstruction, which would require more intense research in the 3D environment.
Human amniotic membrane as a substrate to culture lacrimal gland cells
The placenta's innermost layer, often named Amniotic membrane Using (AM), has been widely used for various types of cell culture as an excellent extracellular matrix support material. AM can be described as a three-layered membranous structure consisting of the epithelium, basement membrane (T = thick layer), and stroma layer (avascular). Several reports state the supportive role of AM in cell adhesion, migration, proliferation, and differentiation of epithelial cells (133–135). Human AM is already used for ocular surface regeneration showing anti-inflammatory and non-immunogenic qualities. Since it is already demonstrated in human experimentations, it is more attractive from the regulatory issues related point of view in clinical trials (136, 137). Schrader et al. (113) demonstrated small lacrimal cell cluster (2–5 cells) generation containing characteristic intracellular granules with the highest secretion capability after 3 days of in vitro culture (113). The author reported a sustainable culture and formation of a more lacrimal gland-like structure (containing lumen) for the next 2–3 weeks, and the sigh of necrosis and some spindle-shaped cells (de-differentiation indicator) started becoming visible in superficial cell layers. These effects were explained as a result of dilution of growth factors from hAM, and/or there may be a decline in the close physical contacts between cells of lacrimal origin (superficial layer) and basement membrane cell proteins from hAM. These results highlighted the need for close cell-cell and cell-to-substrate contact during in vitro reconstruction protocols. Alternatively, it is proposed that the poorly vascularized structure of hAM may not be sufficient to support neovascularization and may be unable to support acinar growth causing necrosis. Further, the hAM is 2D culture since cells can only be seeded on the superficial surface. Thus, the membrane may not be exemplified as a 3D scaffold culture system requiring many cells to grow and differentiate in a more complex architectural arrangement.
Matrigel for lacrimal gland reconstruction
Matrigel is widely used for various research experiments, including lacrimal gland reconstruction. The material contains many basement membrane proteins (e.g., laminin, collagen IV) and growth factors (e.g., TGF-β, FGF, EGF, IGF) (138, 139). Matrigel has been demonstrated to be an excellent growth medium with collagen type I which can support lacrimal cell viability for up to 30 days (with or without 3T3 cells). The cells start forming clusters quickly on the 2nd day of culture and can form an acinar-like structure with high efficiency (117). Studies using collagen only (with or without 3T3 cells) were not capable of supporting cellular viability similar to Matrigel, showing superior qualities of the later substrate. These excellent growth supportive properties over other substrates such as hAM were also further confirmed for the lacrimal and other glands (120, 140). These studies indicate that selecting individual substrates for large numbers of cells for reconstruction purposes would be a significant step. Using Matrigel would be less problematic as long as pre-expansion of lacrimal cells is concerned. Although the clinical application and approval for that might have to demonstrate the safety issue more rigorously since Matrigel is mouse sarcoma-derived, there are hidden risks such as tumorigenesis, which should be avoided at any cost. Similar human tissue derivatives could emerge in the future, providing excellent clinical applications and acceptability at the medical and ethical levels.
Approaches using simple 3D cultures for lacrimal gland reconstruction
Rotary cell culture systems (RCCS)
The method was originally developed for the assessment of cell and tissue culture possibilities in space or in the absence of gravitational forces (141) and later proven useful in supporting tissue structure development, providing essential cell-cell contacts, and showing a potential culture approach with high mass transfer and low shear forces (142, 143). Schrader et al. (112) demonstrated 28 days of culture of Lacrimal gland acinar cells (rabbits) in an RCCS showing the formation of acinar-like cell conglomerates (spheroids) when assessed by electron microscopy after the 7th day of culture. They were optimally functional till day 14, which was indicated by the appearance of a central lumen structure filled with secretory material. The Electron microscopic analysis of the Cells after 7 days of culture revealed polarity with a nucleus at the base and electron-lucent and electron-dense secretory granules in the apical regions, microvilli, and well-connected apical cell parts through tight junctions or desmosomes. At this stage, the overall size was measured to be ~384.6 μm (111.8) in diameter, and a tiny fraction (24.4%) of the spheroids were shown to have apoptotic cells with a condensed small nucleus. The culture reached an optimal state on the 14th day, {size: 382.4 (92.2) μm.} when Acinus-like structures were frequently visible, and the central luminal area was filled with secretory materials inside the spheroids. However, the size and frequency of viable acinar cells started decreasing after the 21st day [290.7 (62.8) μm], and viable cells were visible only in the peripheral regions. Still, large centers of apoptotic cells appeared in 20% of the spheroids. This apoptotic death continued further and reached up to 41.7% of the spheroids, causing a substantial size reduction [388.9 (179.2) μm] on the 28th day of culture. Nevertheless, viable cells were still visible in the peripheral region of the spheroids.
Throughout these time intervals, acinar cells could be detected secretory in response to carbachol stimulation (a parasympathetic stimulant) reduced over the culture period. The gradual increases in the apoptotic activities in the spheroidal central regions might indicate reduced nutritional supplies. The possible explanation could be their non-vascularization, Posing a limitation of these spheroidal cultures for clinical transplantations. Any improvement in the design to trigger vascularization of these spheroids may be of greater use from a clinical point of view.
Matrigel in 3D culture setting for lacrimal glands
The previous section discusses Matrigel as an excellent medium for various cells in 2D culture systems, including lacrimal acinar cells. Matrigel in 3D Scaffold was also demonstrated to have similar growth supporting characteristics in the “raft cultures” approach (110). This method was developed by improvising the previously described use of Matrigel-coated plastic plated methods hypothesizing a more in situ culture averment would be available for lacrimal growth assessment in these studies. The culture was observed for up to 28 days, while acinar cell cultures started appearing on 5–7 days surrounding a central lumen-like structure. The regular culture evaluation for secretory components and prolactin etc., along with other morphological assessment criteria (as mentioned in different sections of the article), indicated a similar growth pattern with optimal growth between 7 and 14 days of culture, which started diminishing beyond 21 days of culture and reached to the minimal level on 28th day of the culture assessment. These studies indicated superior culturing qualities for Matrigel over other culture materials. However, the non-suitability of Matrigel due to its animal origin still makes it less attractive for clinical transplantations.
Decellularized xenogeneic tissue for lacrimal gland reconstruction
Using decellularized tissue for reseeding cells and further regeneration has been extensively studied. It offers many advantages over synthetic materials-based scaffold approaches. It provides precise membrane protein composition, accurate 3D architecture allowing secretion of secretory fluids, adequate vascularization, innervations, and many more benefits. There are reports using decellularized porcine lacrimal gland (3 mm diameter) and lacrimal acinar cells for lacrimal gland regeneration/reconstruction (144). The authors' used domestic pig (Sus Scrofa) derived lacrimal gland tissue and prepared decellularized LG scaffold (1% sodium deoxycholate and DNAse solution) for these studies (145, 146). Since complete decellularization is essential to avoid any immune rejection, as confirmed in these studies, no significant changes were reported in the composition and architecture of the Scaffold (basement membrane) etc. Subsequently, cells were reseeded in the decellularized matrix, and proper epithelial cell growth and cell polarization were evident, showing characteristic acinar-like structures formation by these cells as described in other studies. As demonstrated in these studies, the cells seeded on the decellularized Scaffold formed a multilayer tissue harboring epithelial phenotypic characteristics after the 7th day of reseeding culture. Subsequently, their migration started, and a duct-sphere-like structure was observed.
Further identification of mucous in the cytoplasm and the presence of secretory vesicles confirmed their functionality as a lacrimal gland acinar cell/tissue. In addition, the secretion function of lacrimal gland acinar cells was observed for up to 30 days. The method shows great promise and must be studied further to scale up the number of cells (which would be required for the human recipients). More specific seeding, such as laser-guided reseeding, could further improve by exploring effective and efficient reseeding methods for the ductal and vascular structure to improve the output. Since xenobiotic tissue implantation in humans is already established, this provides a higher opportunity for regulatory agencies' approval for its clinical use (57). Similarly, the bovine ductal matrix (decellularized) is successfully demonstrated to reconstruct a lacrimal gland duct. This further supports the feasibility of using the same xenobiotic strategies for LFU component reconstruction for clinical use (147).
3D co-culture of adult lacrimal gland cells along with progenitor-like cells
Culture in the absence of scaffolds
Lacrimal progenitor cells are evident in normal and injured tissues, which could be exploited to give rise to a functional lacrimal gland under an appropriate 3D culture environment (86, 92, 93, 148, 149). The immature or less differentiated lacrimal gland epithelial cells (human, ABCG2+ and c-kit+ mixed population) can develop into a sphere and duct-like tissue structures when the culture lacks any scaffold (120). Co-culture of mixed differentiated and undifferentiated lacrimal cell cells has been subjected to develop “microspheres,” which could be useful in increasing the potent stem cell/progenitor cells in these in vitro culture procedures for further applications [(150) abstract] (151). This co-culture approach for lacrimal epithelial cells, along with other specific cell types in any 3D culture technique, would better support the in situ microenvironment, and their corresponding interactions might help in mimicking and therefore maintaining similar phenotypic interactions of each cell type present in vivo (152).
Culture with scaffolds
Culturing the mixed lacrimal epithelial cells (both differentiated and undifferentiated progenitor cells) from a rabbit model on a low adherent plastic have been demonstrated to spontaneously generate lacrimal gland cell spheres [(150) abstract]. The Matrigel-coated decellularized lacrimal glands were used as a scaffold, and lacrispheres-derived cells discussed above were used for reseeding them in a 3D culture format. An immunohistochemical assay performed to define their cellular composition afterwards indicated the existence of (both differentiated) acinar and ductal epithelial cells and myoepithelial cells/progenitor-like cells as indicated by the Expression of pan-cytokeratin, c-kit, lactoferrin, and α-smooth muscle actin markers.
The β-hexosaminidase activity was observed as a measure of functional activity, which remains visible up to 17 days after seeding. The parasympathetic functional activities described here were maxed on 7–10 days and diminished after the 17th day of culture. The functionality was also confirmed by assessing mRNA level HEXB gene expression, which was reported to be increasing till day 14 and then started declining, indicating a beneficial impact of the inclusion of undifferentiated epithelial cells since, unlike all previously reported assays, the functional activity remain detectable even after culture assay was timed out.
Summary and conclusion
The tear film is vital for ocular surface health and visual acuity and a variety of components, including lacrimal glands, meibomian glands, goblet glands, accessory secretory Krause and Wolfring glands; conjunctiva epithelial cells and corneal epithelium are collectively responsible for the production, secretion, and regulation of the tears in normal human healthy eyes. Many different causing agents are now defined as damaging factors that perturb delicate homeostasis in human eyes. Most frequently mentioned causing agents and defining markers for ocular surface health are linked to inflammations that may lead to cytological and molecular level changes in the ocular surface tissue.
Despite decades of research and understanding of the disease etiology, clinical management remains palliative, and many different types of lubricating agents/strategies, including artificial tears substitutes, Gels/Ointments, Moisture chamber spectacles etc., are the only available treatment. Advanced treatment approaches may include Anti-Inflammatory Therapy which has been demonstrated to use pharmacological agents such as Cyclosporine, Corticosteroids, Tetracyclines, etc., autologous serum, and similar approaches. However, none of these approaches has eliminated the disease. Most of the time, they are useful in managing the acute inflammatory associated situation and relieving the pain in the patients. On the other hand, cell-based approaches seem to be comparatively new and promising but need to be explored in much detail before they can offer a long-term solution to the problem. One of the crucial methods demonstrated in these years is partial or total submandibular salivary gland transplantation, which has shown remarkable success in some recipients but suffers from many inherent limitations, such as no improvement in the tear film health and visual acuity. Another similar approach that needs attention here could be xenotransplantation of porcine lacrimal glands, which is still in the developmental phase and possesses high risks of retroviral infections and graft rejections.
Most advancements in the medicinal fields include regenerative approaches and tissue engineering strategies which are being developed for ocular surface regeneration and reconstructions by using a variety of cell types and culturing fabrication protocols. Regenerative medicine involves studying the defining markers and procedures to identify the residual stem cells population, which can be triggered to regenerate and recover the tissue's lost functional capabilities, such as lacrimal glands. Regenerative remedies can be defined both as those which are based on ”Pharmacological Drugs” such as cyclosporine A, tacrolimus, corticosteroids, lacritin, lactoferrin, quercetin, systemic pilocarpine, “autologous platelet-rich plasma” (PRP) injection, and “tumor necrosis factor α-stimulated gene/protein 6 (TSG-6),” etc. that are being investigated for their impact on the various clinical symptoms of DES.
On the other hand, “Cells Based Approaches” such as MSCs are the most frequently demonstrated cells due to their immense regenerative potential reported for various other organs and tissue in clinics. MSCs are reported to have remarkable anti-inflammatory properties against immune cells (e.g., T-cell, B-cell, natural killer cell monocyte, macrophage, and dendritic cell). They can decline Ig production and release cytokines from immune cells. This way, MSC can help by reducing inflammatory damages to the tissue and improving its growth/repairing. This is evident in studies defining MSC secret growth factors [e.g., epidermal growth factor (EGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor] that induce cell proliferation and angiogenesis in wound healing. However, any direct evidence of MSC in DES treatments is still awaited. Present information based on MSC in animal models and other in vitro studies are also required to define more elaborated mechanisms related to the issue. In addition, there would be a requirement for a large amount of GMP-compliant cells for the regular clinical use of MSC. That requires an established process of regular, reliable, and reproducible ways of culturing them to yield sufficient numbers of cells.
Similar evidence for human amniotic membrane (hAM) epithelial cells and iPSC shows their curative, regenerative potential through in vitro and animal model-based studies. However, no direct evidence shows any clinical report in humans. Further, the GMP-compliant culturing protocols and other related issues are a rate-limiting step in most cell-based regenerative therapies.
Another promising area of therapeutics being explored includes Gene therapy. This approach may require the identification of specific gene/protein targets, which may be manipulated by altering their Expression or interactions etc., by delivering specific small molecules (i.e., DNA, mRNA, mi RNA, siRNA, antisense oligonucleotides). The success of therapeutics is regulated mainly by the efficacy of the delivery vehicles and their ability to avoid off-target impacts. There is some progress in the in vitro and animal model studies showing therapeutic potential for many anti-inflammatory gene expression regulatory strategies. But the experimentations on human recipients are yet to be defined. Altogether these reports indicate massive progress in lacrimal gland regeneration medicine approaches, and more clinical studies would pave the way to extend these preliminary studies beyond their present state of experiments to the clinics.
When the resident cells with enough reproducing potential cannot be found, “implantation of ex vivo manufactured cells,” tissue or organs are the most probable choice. In vitro manufacturing of lacrimal gland and other ophthalmic tissue are also an expanding area of research. Standard 2D culturing and 3D culture procedures, biomaterials, and fabrication of specific scaffolds are already demonstrated for different lacrimal epithelial cells. As discussed in the main text, various specific parameters such as tear volume, tear compositions, functional efficacy, degree of innervations in implanted tissue, etc., remain crucial while evaluating these strategies, fabrication process, cultivation method, or product itself before it could be implicated in any experimental or clinical settings. Various types of cells, including ESCs, iPSC, and Epithelial progenitor cells, are being explored for manufacturing clinical-grade ophthalmic tissue, bioengineered lacrimal acinar cells/gland, cornea, and many more. ESC and IPSC are well-established for their differential potential in any type of tissue and organ if specific culture conditions/microenvironment are provided along with architectural guidelines to define their structure and shape-related (mechano-physicochemical parameters) details. Defining their mechanochemical and biochemical characteristics might be essential for any growing tissue/organoids such as lacrimal gland, and scaffolds designed by following these characteristics in mind are vital for the successful generation of these implants. Biomaterials are required to manufacture these scaffolds, and natural and synthetic biomaterials are well-studied in 2D/3D culture settings. Since 2D culture systems are comparatively more straightforward and may be helpful in the Characterization and assessment of functionally relevant markers, activities etc., they are most often used before the actual 3D environment could be established. Thus, various growth condition parameters, growth factors (e.g., epidermal growth factor, hepatocyte growth factor, insulin-like growth factor and fibroblast growth factors; w/+ FCS or w/o FCS), and intracellular molecular mechanisms define the regulatory mechanism (e.g., Pax6, CK15, AQP5, and lactoferrin) of this differentiation could be established by using 2D culture methods and ESC/IPSC cells as source materials. Scaffolds can be fabricated by using either naturally occurring biomaterials such as collagen etc. or may be manufactured by using synthetic polymeric chemicals including Polyethersulfone (e.g., tubing), Poly(L-lactic acid), polyester, etc., Human amniotic membrane and most famous Matrigel which is evident to have extraordinary growth supporting properties. Matrigel seems to be the most promising biomaterial from growth and differentiation supporting properties as described through enormous reports. However, there may be some controversies regarding the use of Matrigel in clinical settings due to its animal-based origin. Polyethersulfone (PES) based scaffolds are useful in generating rat lacrimal glands as described in the main text but remain to be demonstrated for their similar efficacy with human cells. PES-scaffolds would also require their rigorous evaluation for generating sufficient tear volume and adequate tear compositions before they could be taken as a standard method of choice for ex vivo lacrimal gland generation. Similar studies using Poly (L-lactic acid) and Polyester-based scaffolds and culturing the animal cells for lacrimal glands have been highly promising. Still, they might require further standardization and optimization with human cells as demanded by PES-scaffolds.
Another critical approach reported the use of microporous polyester-based cell culture inserts, which were impressive while demonstrating the transepithelial electrophysiological behavior of cultured cells, which could be an essential tool in defining the natural-like functionality of manufacturing implants.
In short, manufacturing lacrimal gland tissue could be feasible with the advent of appropriate culturing procedures, Scaffold generating methods, biomaterials, and rigorous biological assessment for various relevant parameters as described herein near future. Both 2D and 3D culturing techniques would remain useful in determining multiple in vitro/in vivo cultures associated and functionally relevant parameters, which would pave the way for the generation of optimal ex vivo manufacturing process and production along with post-implantation assessment would be possible to eradicate the DES associated ocular surface disorders in patients.
Author contributions
VS: conceptualization, writing, drafting, editing, and final decision. PS and UV: editing, drafting, and upgradation. RC: conceptualization and specific discussion suggestions.
Funding
SERB - Empowerment and Equity Opportunities for Excellence in Science, Project No (EEQ/2019/000422).
Acknowledgments
The authors are thankful to the SCIENCE AND ENGINEERING RESEARCH BOARD Government of India for providing essential funds during these studies. We are also grateful toward the Honorable Chancellor, Vice-chancellor, Director ASET and all the associated faculty members for their kind support.
Conflict of interest
Author VS reports grants from SERB during the study: as generated by ICMJE.org.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Venable JH, Grafflin AL. Gross anatomy of the orbital glands in the albino rat. J Mammal. (1940) 21:66–71. doi: 10.2307/1374659
3. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. (2003) 136:318–26. doi: 10.1016/S0002-9394(03)00218-6
5. Stern ME, Gao J, Schwalb TA, Ngo M, Tieu DD, Chan, et al. Conjunctival T-cell subpopulations in Sjögren's and non-Sjögren's patients with dry eye. Invest Ophthalmol Vis Sci. (2002) 43:2609–14.
6. Rocha EM, Alves M, Rios JD, Dartt DA. The ageing lacrimal gland: changes in structure and function. Ocul Surf. (2008) 6:162–74. doi: 10.1016/S1542-0124(12)70177-5
7. Dietrich J, Massie I, Roth M, Geerling G, Mertsch S, Schrader S. Development of causative treatment strategies for lacrimal gland insufficiency by tissue engineering and cell therapy. Part 1: Regeneration of lacrimal gland tissue: can we stimulate lacrimal gland renewal in vivo? Curr Eye Res. (2016) 41:1131–42. doi: 10.3109/02713683.2016.1148741
8. Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. (2017) 15:276–83. doi: 10.1016/j.jtos.2017.05.008
9. Limp MA, Foulks GN. The definition and classification of dry eye disease. Ocul Surf. (2007) 5:75–92. doi: 10.1016/S1542-0124(12)70081-2
10. Janssen P, Van Bijsterveld O. Comparison of electrophoretic techniques for analyzing human tear fluid proteins. Clin Chim Acta. (1981) 114:207–18. doi: 10.1016/0009-8981(81)90393-4
11. Zhou L, Beuerman RW, Foo Y, Liu S, Ang LP, Tan DT. Characterization of human tear proteins using high-resolution mass spectrometry. Ann Acad Med Singapore. (2006) 35:400.
12. Scott BL, Pease DC. Electron microscopy of the salivary and lacrimal glands of the rat. Am J Anat. (1959) 104:115–61. doi: 10.1002/aja.1001040106
13. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. (2009) 28:155–77. doi: 10.1016/j.preteyeres.2009.04.003
14. Tseng SC. Concept and application of limbal stem cells. Eye. (1989) 3:141–57. doi: 10.1038/eye.1989.22
15. Thoft RA, Friend J. The X Y. Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. (1983) 24:1442–3.
16. Tuchocka-Piotrowska A, Puszczewicz M, Kolczewska A, Majewski D. Graft-versus-host disease as the cause of symptoms mimicking Sjogren's syndrome [in Polish]. Ann Acad Med Stetin. (2006) 52(Suppl 2):89–93.
17. Kawai M, Ogawa Y, Shimmura S, Ohta S, Suzuki T, Kawamura N, et al. Expression and localization of ageing markers in the lacrimal gland of chronic graft-versus-host disease. Sci Rep. (2013) 3:2455. doi: 10.1038/srep02455
19. Williamson J, Gibson A, Wilson T, Forrester J, Whaley K, Dick W. Histology of the lacrimal gland in keratoconjunctivitis sicca. Br J Ophthalmol. (1973) 57:852. doi: 10.1136/bjo.57.11.852
20. Matsumoto I, Tsubota K, Satake Y, Kita Y, Matsumura R, Murata H, et al. Common T cell receptor clonotype in lacrimal glands and labial salivary glands from patients with Sjögren's syndrome. J Clin Invest. (1996) 97:1969. doi: 10.1172/JCI118629
21. Saito I, Terauchi K, Shimuta M, Nishimura S, Yoshino K, Takeuchi T, et al. Expression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren's syndrome. J Clin Lab Anal. (1993) 7:180–7. doi: 10.1002/jcla.1860070309
22. Kunert KS, Tisdale AS, Stern ME, Smith J, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. (2000) 118:1489–96. doi: 10.1001/archopht.118.11.1489
23. Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. (2001) 42:2283–92.
24. Nakamura S, Kinoshita S, Yokoi N, Ogawa Y, Shibuya M, Nakashima H, et al. Lacrimal hypofunction as a new mechanism of dry eye in visual display terminal users. PLoS ONE. (2010) 5:e11119. doi: 10.1371/journal.pone.0011119
25. Xiao B., Wang, Y., Reinach, P. S., Ren, Y., Li, J., Hua, S., et al. (2015). Dynamic ocular surface and lacrimal gland changes induced in experimental murine dry eye. PLoS One 10, e0115333.
26. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. (2002) 120:330–7. doi: 10.1001/archopht.120.3.330
27. Mihatsch MJ, Kyo M, Morozumi K, Yamaguchi Y, Nickeleit V, Ryffel B. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol. (1998) 49:356–63.
28. Lallemand F, Felt-Baeyens O, Besseghir K, Behar-Cohen F, Gurny R. Cyclosporine A delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm. (2003) 56:307–18. doi: 10.1016/S0939-6411(03)00138-3
29. Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. (2004) 137:337–42. doi: 10.1016/j.ajo.2003.10.036
30. Marsh P, Pflugfelder SC. Topical non-preserved methylprednisolone therapy of keratoconjunctivitis sicca in Sjogren's syndrome. Ophthalmology. (1999) 106:811–6. doi: 10.1016/S0161-6420(99)90171-9
31. Frucht-Pery J, Sagi E, Hemo I, Ever-Hadani P. Efficacy of doxycycline and tetracycline in ocular rosacea. Am J Ophthalmol. (1993) 116:88–92. doi: 10.1016/S0002-9394(14)71750-7
32. Akpek EK, Merchant A, Pinar V, Foster CS. Ocular rosacea: patient characteristics and follow-up. Ophthalmology. (1997) 104:1863–7. doi: 10.1016/S0161-6420(97)30015-3
33. Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, et al. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci USA. (1996) 93:14014–9. doi: 10.1073/pnas.93.24.14014
34. Shlopov BV, Smith GN, Cole AA, Hasty KA. Differential patterns of response Shlopov et al. 1999 to doxycycline and transforming growth factor beta1 in the down-regulation of collagenases in osteoarthritic and normal human chondrocytes. Arthritis Rheum. (1999) 42:719–27. doi: 10.1002/1529-0131(199904)42:4<719::AID-ANR15>3.0.CO;2-T
35. Beylerian M, Lazaro M, Magalon J, Veran J, Darque A, Grimaud F, et al. Collyres de sérum autologue: traitement à long-terme dans le syndrome sec oculaire [Autologous serum tears: long-term treatment in dry eye syndrome]. J Fr Ophtalmol. (2018) 41:246–54. doi: 10.1016/j.jfo.2017.11.008
36. Pan Q, Angelina A, Zambrano A, Marrone M, Stark WJ, Heflin T, et al. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. (2013) 8:CD009327. doi: 10.1002/14651858.CD009327.pub2
37. Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K. Autologous serum eye drops for dry eye after LASIK. J Refract Surg. (2006) 22:61–6. doi: 10.3928/1081-597X-20060101-13
38. Yamada J, Zhu SN, Streilein JW, Dana MR. Interleukin-1 receptor antagonist therapy and induction of anterior chamber-associated immune deviation-type tolerance after corneal transplantation. Invest Ophthalmol Vis Sci. (2000) 41:4203–8.
39. Yamada J., Dana, M. R., and Sotozono, C. (2003). Kinoshita Local suppression of IL-1 by receptor antagonists in the rat model of corneal alkali injury. Exp. Eye Res. 76, 161–167.
40. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. (2008) 8:349–61. doi: 10.1038/nri2294
41. Serhan J, Dana MR, Sotozono C, Kinoshita S. Local suppression of IL-1 by receptor antagonist in the rat model of corneal alkali injury. Exp Eye Res. (2003) 76:161–7. doi: 10.1016/S0014-4835(02)00293-2
42. Goyal S, Chauhan SK, Zhang S, Dana R. Amelioration of murine dry eye disease by topical antagonist to chemokine receptor 2. Arch Ophthalmol. (2009) 127:882–7. doi: 10.1001/archophthalmol.2009.125
43. Huang JF, Yafawi R, Zhang M, McDowell M, Rittenhouse KD, Sace F, et al. Immunomodulatory effect of the topical ophthalmic Janus kinase inhibitor tofacitinib (CP-690,550) in patients with dry eye disease. Ophthalmology. (2012) 119:e43–50. doi: 10.1016/j.ophtha.2012.03.017
44. Murphy CJ, Bentley E, Miller PE, McIntyre K, Leatherberry G, Dubielzig R, et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest Ophthalmol Vis Sci. (2011) 52:3174–80. doi: 10.1167/iovs.09-5078
45. Semba CP, Torkildsen GL, Lonsdale JD, McLaurin EB, Geffin JA, Mundorf TK, et al. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am J Ophthalmol. (2012) 153:1050–60.e1. doi: 10.1016/j.ajo.2011.11.003
46. Cavet ME, Harrington KL, Ward KW, Zhang JZ. Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells. J Molecular Vision. (2010) 16:1791–800.
47. Shafiee A, Bucolo C, Budzynski E, Ward KW, López FJ. In vivo ocular efficacy profile of mapracorat, a novel selective glucocorticoid receptor agonist, in rabbit models of ocular disease. Invest Ophthalmol Vis Sci. (2011) 52:1422–30. doi: 10.1167/iovs.10-5598
48. Paiva CS, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arquivos Brasileiros de Oftalmologia. (2008) 71(Suppl 6):89–95. doi: 10.1590/S0004-27492008000700017
49. Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. (2014) 9:240–50.
50. Borrelli M, Schroder C, Dart JK, Collin JR, Sieg P, Cree IA, et al. Long-term follow-up after submandibular gland transplantation in severe dry eyes secondary to cicatrizing conjunctivitis. Am J Ophthalmol. (2010) 150:894–904. doi: 10.1016/j.ajo.2010.05.010
51. Qin J, Zhang L, Cai ZG, Mao C, Liu XJ, Lv L, et al. Microvascular autologous transplantation of partial submandibular gland for severe keratoconjunctivitis sicca. Br J Ophthalmol. (2013) 97:1123–8. doi: 10.1136/bjophthalmol-2013-303280
52. Schroder C, Sieg P, Framme C, Honicke K, Hakim SG, Geerling G. Transplantation of the submandibular gland in absolute dry eyes. Effect on the ocular surface. KlinMonblAugenheilkd. (2002) 219:494–501. doi: 10.1055/s-2002-33582
53. Sieg P, Geerling G, Kosmehl H, Lauer I, Warnecke K, von Domarus. H. Microvascular submandibular gland transfer for severe cases of keratoconjunctivitis sicca. Plas Reconstr Surg. (2000) 106:554–60. doi: 10.1097/00006534-200009010-00004
54. Samy K. P., Martin, B. M., Turgeon, N. A., and Kirk, A. D. (2014). Islet cell xenotransplantation: a serious look toward the clinic. Xenotransplantation 21, 221–229.
55. Henker R, Scholz M, Gaffling S, Asano N, Hampel U, Garreis F, et al. Morphological features of the porcine lacrimal gland and its compatibility for human lacrimal gland xenografting. PLoS ONE. (2013) 8:e74046. doi: 10.1371/journal.pone.0074046
56. Choi HJ, Kim MK, Lee HJ, Ko JH, Jeong SH, Lee JI, et al. Efficacy of pig-to-rhesus lamellar corneal xenotransplantation. Invest Ophthalmol Vis Sci. (2011) 52:6643–50. doi: 10.1167/iovs.11-7273
57. Lehmann S, Walther T, Kempfert J, Leontjev S, Rastan A, Falk V, et al. Stentless versus conventional xenograft aortic valve replacement: midterm results of a prospectively randomized trial. Ann Thorac Surg. (2007) 84:467–72. doi: 10.1016/j.athoracsur.2007.02.017
58. Kimsa-Dudek M, Strzalka-Mrozik B, Kimsa MW, Blecharz I, Gola J, Skowronek B, et al. Screening pigs for xenotransplantation: expression of porcine endogenous retroviruses in transgenic pig skin. Transgenic Res. (2015) 24:529–36. doi: 10.1007/s11248-015-9871-y
59. Avni I, Garzozi HJ, Barequet IS, Segev F, Varssano D, Sartani G, et al. treatment of dry eye syndrome with orally administered CF101: data from a phase 2 clinical trial. Ophthalmology. (2010) 117:1287–93. doi: 10.1016/j.ophtha.2009.11.029
60. Kawashima M, Kawakita T, Inaba T, Okada N, Ito M, Shimmura S, et al. Dietary lactoferrin alleviates age-related lacrimal gland dysfunction in mice. PLoS ONE. (2012) 7:e33148. doi: 10.1371/journal.pone.0033148
61. Shigeyasu C, Yamada M, Akune Y, Tsubota K. Diquafosol sodium ophthalmic solution for the treatment of dry eye: clinical evaluation and biochemical analysis of tear composition. Jpn J Ophthalmol. (2015) 59:415–20. doi: 10.1007/s10384-015-0408-y
62. Vijmesi T, Chen FY, Bala Subbu S, Gallup M, McKown RL, Laurie GW, et al. Topical administration of lacritin is a novel therapy for aqueous-deficient dry eye disease. Invest Ophthalmol Vis Sci. (2014) 55:5401–9. doi: 10.1167/iovs.14-13924
63. Wang S, Lee JS, Hyun J, Kim J, Kim SU, Cha HJ, et al. Tumor necrosis factor-inducible gene 6 promotes liver regeneration in mice with acute liver injury. Stem Cell Res Ther. (2015) 6:20. doi: 10.1186/s13287-015-0019-z
64. Kawakita T, Shimmura S, Tsubota K. Effect of oral pilocarpine in treating severe dry eye patients with Sjogren's syndrome. Asia Pac J Ophthalmol. (2015) 4:101–5. doi: 10.1097/APO.0000000000000040
65. Papas AS, Sherrer YS, Charney M, Golden HE, Medsger TA Jr, et al. Successful treatment of dry mouth and dry eye symptoms in Sjogren's syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. (2004) 10:169–77. doi: 10.1097/01.rhu.0000135553.08057.21
66. Avila MY. Restoration of human lacrimal function following platelet-rich plasma injection. Cornea. (2014) 33:18–21. doi: 10.1097/ICO.0000000000000016
67. Lee MJ, Kim DH, Ryu JS, Ko AY, Ko JH, Kim MK, et al. Topical TSG-6 administration protects the ocular surface in two mouse models of inflammation-related dry eye. Invest Ophthalmol Vis Sci. (2015) 56:5175–81. doi: 10.1167/iovs.14-16307
68. Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco Í, Corrêa do Amaral RJ, Granjeiro JM, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. (2013) 4:67. doi: 10.1186/scrt218
69. Akhundov K, Pietramaggiori G, Darwiche S, Guerid S, Scaletta C, Hirt-Burri N, et al. Development of a cost-effective method for platelet-rich plasma (PRP) preparation for topical wound healing. Ann Burns Fire Disasters. (2012) 25:207–13.
70. Alio JL, Abad M, Artola A, Rodriguez-Prats JL, Pastor S, Ruiz-Colecha J. Use autologous platelet-rich plasma to treat dormant corneal ulcers. Ophthalmology. (2007) 114:1286–93. doi: 10.1016/j.ophtha.2006.10.044
71. Panda A, Jain M, Vanathi M, Velpandian T, Khokhar S, Dada T. Topical autologous platelet-rich plasma eyedrops for acute corneal chemical injury. Cornea. (2012) 31:989–93. doi: 10.1097/ICO.0b013e3182114661
72. Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, et al. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. (2011) 23:424–31. doi: 10.5021/ad.2011.23.4.424
73. Cho JW, Kim SA, Lee KS. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med. (2012) 29:32–6. doi: 10.3892/ijmm.2011.803
74. You S, Avidan O, Tariq A, Ahluwalia I, Stark PC, Kublin CL, et al. Role of epithelial-mesenchymal transition in the repair of the lacrimal gland after experimentally induced injury. Invest Ophthalmol Vis Sci. (2012) 53:126–35. doi: 10.1167/iovs.11-7893
75. Kato T, Okumi M, Tanemura M, Yazawa K, Kakuta Y, Yamanaka K, et al. Adipose tissue-derived stem cells suppress acute cellular rejection by TSG-6 and CD44 interaction in rat kidney transplantation. Transplantation. (2014) 98:277–84. doi: 10.1097/TP.0000000000000230
76. Qi Y, Jiang D, Sindrilaru A, Stegemann A, Schatz S, Treiber N, et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J Invest Dermatol. (2014) 134:526–37. doi: 10.1038/jid.2013.328
77. Barabino S, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. (2004) 45:1641–6. doi: 10.1167/iovs.03-1055
78. Beyazyildiz E, Pinarli FA, Beyazyildiz O, Hekimoglu ER, Acar U, Demir MN, et al. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. (2014) 2014:250230. doi: 10.1155/2014/250230
79. Park SA, Reilly CM, Wood JA, Chung DJ, Carrade DD, Deremer SL, et al. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy. (2013) 15:1498–510. doi: 10.1016/j.jcyt.2013.06.009
80. Villatoro AJ, Fernandez V, Claros S, Rico-Llanos GA, Becerra J, Andrades JA. Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. Biomed Res Int. (2015) 2015:527926. doi: 10.1155/2015/527926
81. Weng J, He C, Lai P, Luo C, Guo R, Wu S, et al. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol Ther. (2012) 20:2347–54. doi: 10.1038/mt.2012.208
82. Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, et al. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther. (2015) 23:139–46. doi: 10.1038/mt.2014.159
83. Wood J. A., Chung, D. J., Park, S. A., Zwingenberger, A. L., Reilly, C. M., Ly, I., et al. (2012). Periocular and intra-articular injection of canine adipose derived mesenchymal stem cells: an in-vivo imaging and migration study. J. Ocul. Pharmacol. Ther. 28, 307–317.
84. Friedenstein A, Piatetzky-Shapiro I, Petrakova K. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. (1966) 16:381–90. doi: 10.1242/dev.16.3.381
85. Karaoz E, Ayhan S, Gacar G, Aksoy A, Duruksu G, Okçu A, et al. Isolation and characterization of stem cells from pancreatic islet: pluripotency, differentiation potential and ultrastructural characteristics. Cytotherapy. (2010) 12:288–302. doi: 10.3109/14653240903580296
86. Shatos MA, Haugaard-Hedstrom L, Hodges RR, Dartt DA. Isolation and characterization of progenitor cells in uninjured, adult rat lacrimal gland. Invest Ophthalmol Vis Sci. (2012) 53:2749–59. doi: 10.1167/iovs.11-9025
87. Zhang L, Hong T-P, Hu J, Liu Y-N, Wu YH, Li LS. Nestin Positive progenitor cells isolated from human fetal pancreas have phenotypic markers identical to mesenchymal stem cells. World J Gastroenterol. (2005) 11:2906–11. doi: 10.3748/wjg.v11.i19.2906
88. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. (2006) 8:315–7. doi: 10.1080/14653240600855905
89. Jeong J, Baek H, Kim Y-J, Choi Y, Lee H, Lee E, et al. Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Exp Mol Med. (2013) 45:e58. doi: 10.1038/emm.2013.121
90. Bifari F., Lisi, V., Mimiola, E., Pasini, A., and Krampera, M. (2008). Immune modulation by mesenchymal stem cells. Transfus. Med. Hemother. 35, 194–204.
91. Zhang Y, Deng C, Qian J, Zhang M, Li X. Improvement of radiotherapy-induced lacrimal gland injury by induced pluripotent stem cell-derived conditioned medium via MDK and inhibition of the p38/JNK pathway. Int J Mol Sci. (2014) 15:18407–21. doi: 10.3390/ijms151018407
92. Zoukhri D, Macari E, Kublin CL. A single injection of interleukin1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. (2007) 84:894–904. doi: 10.1016/j.exer.2007.01.015
93. Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. (2008) 49:4399–406. doi: 10.1167/iovs.08-1730
94. Ackermann P, Hetz S, Dieckow J, Schicht M, Richter A, Kruse C, et al. Isolation and investigation of presumptive murine lacrimal gland stem cells. Invest Ophthalmol Vis Sci. (2015) 56:4350–63. doi: 10.1167/iovs.15-16475
95. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. (2006) 98:1076–84. doi: 10.1002/jcb.20886
96. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. (2008) 3:e1886. doi: 10.1371/journal.pone.0001886
97. Akram KM, Samad S, Spiteri MA, Forsyth NR. Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Resp Res. (2013) 14:9. doi: 10.1186/1465-9921-14-9
98. Hu N, Zhang YY, Gu HW, Guan HJ. Effects of bone marrow mesenchymal stem cells on cell proliferation and growth factor expression of limbal epithelial cells in vitro. Ophthal Res. (2012) 48:82–8. doi: 10.1159/000331006
99. Moghadasali R, Mutsaers HA, Azarnia M, Aghdami N, Baharvand H, Torensma R, et al. Mesenchymal stem cell-conditioned medium accelerates regeneration of human renal proximal tubule epithelial cells after gentamicin toxicity. Exp Toxicol Pathol. (2013) 65:595–600. doi: 10.1016/j.etp.2012.06.002
100. Walter MN, Wright KT, Fuller HR, MacNeil S, Johnson WE. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. (2010) 316:1271–81. doi: 10.1016/j.yexcr.2010.02.026
101. Roth M, Spaniol K, Kordes C, Schwarz S, Mertsch S, Haussinger D, et al. The influence of oxygen on the proliferative capacity and differentiation potential of lacrimal gland-derived mesenchymal stem cells. Invest Ophthalmol Vis Sci. (2015) 56:4741–52. doi: 10.1167/iovs.14-15475
102. Zhang NN, Huang GL, Han QB, Hu X, Yi J, Yao L, et al. Functional regeneration of irradiated salivary glands with human amniotic epithelial cells transplantation. Int J Clin Exp Pathol. (2013) 6:2039–47.
103. Huang GL, Zhang NN, Wang JS, Yao L, Zhao YJ, Wang YY. Transdifferentiation of human amniotic epithelial cells into acinar cells using a double-chamber system. Cell Reprogram. (2012) 14:377–83. doi: 10.1089/cell.2011.0096
104. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. (2014) 15:541–55. doi: 10.1038/nrg3763
105. Trousdale M. D., Zhu, Z., Stevenson, D., Schechter, J. E., Ritter, T., and Mircheff, A. K. (2005). Expression of TNF inhibitor gene in the lacrimal gland promotes recovery of tear production and tear stability and reduced immunopathology in rabbits with induced autoimmune dacryoadenitis. J. Autoimmune. Dis. 2, 6.
106. Thomas PB, Samant DM, Selvam S, Wei RH, Wang Y, Stevenson D, et al. Adeno-associated virus-mediated IL-10 gene transfer suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. (2010) 51:5137–44. doi: 10.1167/iovs.10-5423
107. Hann LE, Tatro JB, Sullivan DA. Morphology and function of lacrimal gland acinar cells in primary culture. Invest Ophthalmol Vis Sci. (1989) 30:145–58.
108. Long L, Liu Z, Wang T, Deng X, Yang K, Li L, et al. Polyethersulfone dead-end tube as a scaffold for artificial lacrimal glands in vitro. J Biomed Mater Res B Appl Biomater. (2006) 78:409–16. doi: 10.1002/jbm.b.30502
109. Vanaken H, Vercaeren I, Claessens F, De Vos R, Dewolf-Peeters C, Vaerman JP, et al. Primary rat lacrimal cells undergo acinar-like morphogenesis on reconstituted basement membrane and express secretory component under androgen stimulation. Exp Cell Res. (1998) 238:377–88. doi: 10.1006/excr.1997.3856
110. Schechter J, Stevenson D, Chang D, Chang N, Pidgeon M, Nakamura T, et al. growth of purified lacrimal acinar cells in Matrigel raft cultures. Exp Eye Res. (2002) 74:349–60. doi: 10.1006/exer.2001.1158
111. Schonthal AH, Warren DW, Stevenson D, Schecter JE, Azzarolo AM, Mircheff AK, et al. proliferation of lacrimal gland acinar cells in primary culture. Stimulation by extracellular matrix, EGF, and DHT. Exp Eye Res. (2000) 70:639–49. doi: 10.1006/exer.2000.0824
112. Schrader S, Kremling C, Klinger M, Laqua H, Geerling G. Cultivation of lacrimal gland acinar cells in a microgravity environment. Br J Ophthalmol. (2009) 93:1121–5. doi: 10.1136/bjo.2008.137927
113. Schrader S, Wedel T, Kremling C, Laqua H, Geerling G. Amniotic membrane as a carrier for lacrimal gland acinar cells. Graefes Arch Clin Exp Ophthalmol. (2007) 245:1699–704. doi: 10.1007/s00417-007-0612-7
114. Selvam S, Chang WV, Nakamura T, Samant DM, Thomas PB, Trousdale MD, et al. Microporous poly(L-lactic acid) membranes fabricated by polyethylene glycol solvent-cast/particulate leaching technique. Tissue Eng Part C Methods. (2009) 15:463–74. doi: 10.1089/ten.tec.2008.0431
115. Kobayashi S, Kawakita T, Kawashima M, Okada N, Mishima K, Saito I, et al. Characterization of cultivated murine lacrimal gland epithelial cells. Mol Vis. (2012) 18:1271–7.
116. Ueda Y, Karasawa Y, Satoh Y, Nishikawa S, Imaki J, Ito M. Purification and characterization of mouse lacrimal gland epithelial cells and reconstruction of an acinarlike structure in three-dimensional culture. Invest Ophthalmol Vis Sci. (2009) 50:1978–87. doi: 10.1167/iovs.08-2503
117. Yoshino K, Tseng SC, Pflugfelder SC. Substrate modulation of morphology, growth, and tear protein production by cultured human lacrimal gland epithelial cells. Exp Cell Res. (1995) 220:138–51. doi: 10.1006/excr.1995.1300
118. Hirayama M, Ogawa M, Oshima M, Sekine Y, Ishida K, Yamashita K, et al. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat Comm. (2013) 4:2497. doi: 10.1038/ncomms3497
119. Shih C. C., Forman, S. J., Chu, P., and Slovak, M. (2007). Human embryonic stem cells are prone to generate primitive, undifferentiated tumors in engrafted human fetal tissues in severe combined immunodeficient mice. Stem Cells Dev. 16, 893–902.
120. Tiwari S, Ali MJ, Balla MM, Naik MN, Honavar SG, Reddy VA, et al. Establishing human lacrimal gland cultures with secretory function. PLoS ONE. (2012) 7:e29458. doi: 10.1371/journal.pone.0029458
121. Tiwari S, Ali MJ, Vemuganti GK. Human lacrimal gland regeneration: perspectives and review of literature. Saudi J Ophthalmol. (2014) 28:12–8. doi: 10.1016/j.sjopt.2013.09.004
122. Selvam S, Thomas PB, Gukasyan HJ, Yu AS, Stevenson D, Trousdale MD, et al. Transepithelial bioelectrical properties of rabbit acinar cell monolayers on polyester membrane scaffolds. Am J Physiol Cell Physiol. (2007) 293:C1412–9. doi: 10.1152/ajpcell.00200.2007
123. David S, Gerra D, De Nitti C, Bussolati B, Teatini U, Longhena GR, et al. Hemodiafiltration and high-flux hemodialysis with polyethersulfone membranes. Contrib Nephrol. (2003) 138:43–5. doi: 10.1159/000067389
124. Moce-Llivina L, Jofre J, Muniesa M. Comparison of polyvinylidene fluoride and polyethersulfone membranes in filtering viral suspensions. J Virol Methods. (2003) 109:99–101. doi: 10.1016/S0166-0934(03)00046-6
125. Paul F, Montag P, Schall C, Vienken J, Bowry SK. Verification of the chemical composition and specifications of haemodialysis membranes by NMR and GPC-FTIR-coupled spectroscopy. Biomaterials. (2002) 23:3131–40. doi: 10.1016/S0142-9612(02)00057-1
126. Hasegawa T, Iwasaki Y, Ishihara K. Preparation of blood compatible hollow fibers from a polymer alloy composed of polysulfone and 2-methacryloyloxyethyl phosphorylcholine polymer. J Biomed Mater Res. (2002) 63:333–41. doi: 10.1002/jbm.10210
127. Zhao CS, Liu T, Lu ZP, Chen LP, Huang J. Evaluation of polyethersulfone hollow fiber plasma separator by animal experiments. Artif Organs. (2001) 25:60–3. doi: 10.1046/j.1525-1594.2001.06655-2.x
128. Fei WL, Chen JQ, Yuan J, Quan DP, Zhou SY. Preliminary study of the effect of FK506 nanospheric-suspension eye drops on the rejection of penetrating keratoplasty. J OculPharmacolTher. (2008) 24:235–44. doi: 10.1089/jop.2007.0059
129. Figliuzzi M, Cornolti R, Plati T, Rajan N, Adobati F, Remuzzi G, et al. Subcutaneous xenotransplantation of bovine pancreatic islets. Biomaterials. (2005) 26:5640–7. doi: 10.1016/j.biomaterials.2005.02.019
130. Unger RE, Huang Q, Peter K, Protzer D, Paulb D, Kirkpatrick CJ. Growth of human cells on polyethersulfone (PES) hollow fiber membranes. Biomaterials. (2005) 26:1877–84. doi: 10.1016/j.biomaterials.2004.05.032
131. Unger RE, Peters K, Huang Q, Funk A, Paul D, Kirkpatrick CJ. Vascularization and gene regulation of human endothelial cells growing on porous Polyethersulfone (PES) hollow fiber membranes. Biomaterials. (2005) 26:3461–9. doi: 10.1016/j.biomaterials.2004.09.047
132. Qian L, Wang Y, Xie J, Rose CM, Yang T, Nakamura T, et al. Biochemical changes contributing to functional quiescence in lacrimal gland acinar cells after chronic ex vivo exposure to a muscarinic agonist. Scand J Immunol. (2003) 58:550–65. doi: 10.1046/j.1365-3083.2003.01343.x
133. Koizumi N, Inatomi T. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea. (2000) 19:65–71. doi: 10.1097/00003226-200001000-00013
134. Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. (1999) 40:878–86.
135. Schwab I. R. (1999). Cultured corneal epithelia for ocular surface disease. Trans. Am. Ophthalmol. Soc. 97, 891–986.
136. Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of anti-angiogenic and anti-inflammatory proteins in the human amniotic membrane. Cornea. (2000) 19:348–52. doi: 10.1097/00003226-200005000-00018
137. Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. (1981) 2:1003–5. doi: 10.1016/S0140-6736(81)91212-5
138. Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. (2005) 15:378–86. doi: 10.1016/j.semcancer.2005.05.004
139. Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. (1992) 202:1–8. doi: 10.1016/0014-4827(92)90397-Q
140. Maria OM, Zeitouni A, Gologan O, Tran SD. Matrigel improves functional properties of primary human salivary gland cells. Tissue Eng Part A. (2011) 17:1229–38. doi: 10.1089/ten.tea.2010.0297
141. Lewis ML, Moriarity DM, Campbell PS. Use microgravity bioreactors to develop an in vitro rat salivary gland cell culture model. J Cell Biochem. (1993) 51:265–73. doi: 10.1002/jcb.240510305
142. Becker JL, Prewett TL, Spaulding GF, Goodwin TJ. Three-dimensional growth and differentiation of ovarian tumour cell line in high aspect rotating-wall vessel: morphologic and embryologic considerations. J Cell Biochem. (1993) 51:283–9. doi: 10.1002/jcb.240510307
143. Goodwin TJ, Jessup JM, Wolf DA. Morphologic differentiation of colon carcinoma cell lines HT-29 and HT-29KM in rotating-wall vessels. In Vitro Cell Dev Biol. (1992) 28A:47–60. doi: 10.1007/BF02631079
144. Spaniol K, Metzger M, Roth M, Greve B, Mertsch S, Geerling G, et al. Engineering of a secretory active three-dimensional lacrimal gland construct on the basis of decellularized lacrimal gland tissue. Tissue Eng Part A. (2015) 21:2605–17. doi: 10.1089/ten.tea.2014.0694
145. Groenen MA, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature. (2012) 491:393. doi: 10.1038/nature11622
146. Schulte JB, Simionescu A, Simionescu DT. The acellular myocardial flap: a novel extracellular matrix scaffold enriched with patent microvascular networks and biocompatible cell niches. Tissue Eng Part C Methods. (2012) 19:518. doi: 10.1089/ten.tec.2012.0536
147. Chen L, Gong B, Wu Z, Jetton J, Chen R, Qu C. A new method uses a xenogeneic acellular dermal matrix to reconstruct lacrimal drainage. Br J Ophthalmol. (2014) 98:1583–7. doi: 10.1136/bjophthalmol-2014-304932
148. You S., Kublin, C. L., Avidan, O., Miyasaki, D., and Zoukhri, D. (2011). Isolation and propagation of mesenchymal stem cells from the lacrimal gland. Invest. Ophthalmol. Vis. Sci. 52, 2087–2094.
149. Zoukhri D. Mechanisms involved in injury and repair of the murine lacrimal gland: role of programmed cell death and mesenchymal stem cells. Ocul Surf. (2010) 8:60–9. doi: 10.1016/S1542-0124(12)70070-8
150. Lin H, Sun G, He H, Botsford B, Li M, Elisseeff JH, et al. Three dimensional culture of functional adult rabbit lacrimal gland epithelial cells on decellularized scaffold. Tissue Eng Part A. (2016) 22:65–74. doi: 10.1089/ten.tea.2015.0286
151. Vemuganti G. K. (2015). Stem-like cells in in-vitro cultures of human lacrimal gland. Invest. Ophthalmol. Vis. Sci. 56, 302.
Keywords: dry eyes, tear film, lacrimal gland, biomaterials, scaffolds, organoids, stem cells, Sjögren's syndrome
Citation: Singh VK, Sharma P, Vaksh UKS and Chandra R (2022) Current approaches for the regeneration and reconstruction of ocular surface in dry eye. Front. Med. 9:885780. doi: 10.3389/fmed.2022.885780
Received: 28 February 2022; Accepted: 18 August 2022;
Published: 23 September 2022.
Edited by:
Sayan Basu, L V Prasad Eye Institute, IndiaReviewed by:
Hua-Tao Xie, Huazhong University of Science and Technology, ChinaMelis Palamar, Ege University, Turkey
Copyright © 2022 Singh, Sharma, Vaksh and Chandra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vimal Kishor Singh, dmtzaW5naEBnZ24uYW1pdHkuZWR1; dmltX2tpc3NvckB5YWhvby5jby5pbg==; dmltYWxraXNob3JzaW5naEBnbWFpbC5jb20=
 Vimal Kishor Singh
Vimal Kishor Singh Pallavi Sharma
Pallavi Sharma Uttkarsh Kumar Sharma Vaksh
Uttkarsh Kumar Sharma Vaksh Ramesh Chandra4
Ramesh Chandra4