- 1Department of Ophthalmology, Eye Ear Nose and Throat Hospital of Fudan University, Shanghai, China
- 2Department of Neurology, Shanghai Deji Hospital, Shanghai, China
- 3Department of Neurosurgery, Shanghai Deji Hospital, Shanghai, China
- 4Department of Rheumatology, Huashan Hospital, Fudan University, Shanghai, China
- 5State Key Laboratory of Medical Neurobiology, Institutes of Brain Science, Fudan University, Shanghai, China
Purpose: To evaluate demographic and clinical characteristics of a Chinese population with giant cell arteritis using multimodal imaging focusing on ophthalmic examinations.
Design: Retrospective observational case series.
Materials and Methods: In the neuro-ophthalmology division of the Eye, Ear, Nose, and Throat Hospital, Shanghai, we evaluated the demographic and clinical characteristics of patients diagnosed with giant cell arteritis between January 2016 and June 2021. Results of routine ophthalmic examinations including fundus examination, optical coherence tomography, color duplex ultrasonography of ocular and superficial temporal arteries, orbital magnetic resonance imaging, and superficial temporal artery biopsy were evaluated.
Results: A total of 15 patients (22 eyes; ten male and five female) were evaluated with a mean age of 77.0 ± 8.5 years. Among them, seven had bilateral involvement that occurred simultaneously or sequentially. Twelve patients presented with arteritic anterior ischemic optic neuropathy, two with arteritic anterior ischemic optic neuropathy combined with cilioretinal artery occlusion, and one with cotton-wool spots. In acute stages of optic neuropathy and retinopathy, optical coherence tomography revealed optic disc edema, thickening of the inner retinal nerve fiber layer and ganglion cell layer, and loss of layer structure. In late stages, optical coherence tomography revealed diffuse atrophy of the inner retina. The “halo” sign was observed in 12 patients in the superficial temporal artery ultrasound, and seven out of eight patients who underwent biopsy demonstrated classic giant cell arteritis pathological changes. Most patients having poor visual acuity but ability to perceive light; 10/22 eyes had permanent vision loss.
Conclusion: Although rare in Asians, giant cell arteritis may be underdiagnosed among elderly Chinese patients presenting with anterior ischemic optic neuropathy. Non-invasive superficial temporal artery ultrasound detecting inflammatory thickening of the intima as the “halo” sign combined with routine elevated erythrocyte sedimentation rate and C-reactive protein may be helpful in diagnosing patients with a high probability of having giant cell arteritis.
Introduction
Giant cell arteritis (GCA) is a type of systemic vasculitis that mainly affects medium-sized and large arteries. The incidence of GCA in Asians is 20 times less than that in Caucasians (1). Although rare, an increasing number of Asian GCA cases, with typical clinical features that are commonly observed among Caucasians, has been reported (2–6); however, only one nationwide survey has addressed that the prevalence of GCA in patients aged > 50 years in 1997 was 1.47 per 100,000 persons in Japan (7).
In 2018, we reported the case of a Chinese patient with biopsy-proven GCA, presenting with simultaneous anterior ischemic optic neuropathy (AION) with no light perception (6). To our knowledge, this is the first Chinese case report on the ophthalmic involvement in this disease. In the subsequent years, >15 patients presenting with AION and profound visual loss accompanied with headache have been diagnosed in our neuro-ophthalmology clinic, which indicates that GCA might be underdiagnosed among elderly Asian patients. The permanent visual loss associated with this neuro-ophthalmic condition requires careful vigilance.
In this case series, we utilized multimodal imaging techniques, especially non-invasive color duplex ultrasonography (CDUS), in elderly patients with ocular artery ischemic conditions who were at a high risk of GCA and for whom biopsy was not straightforward or available.
Materials and Methods
Patients
Between January 2016 and June 2021, patients with newly diagnosed GCA were admitted to the neuro-ophthalmology division of the Eye, Ear, Nose, and Throat Hospital in Shanghai, China.
The criteria for diagnosing GCA were as follows (8): (1) age of onset, >50 years; (2) new onset headache; (3) temporal artery abnormality on examination (tenderness or reduced pulsation); (4) elevated erythrocyte sedimentation rate (ESR) (> 50 mm/h); and (5) abnormal temporal artery biopsy, revealing necrotizing vasculitis with predominant mononuclear cell infiltration or granulomatous inflammation. Patients who presented with at least three of these five criteria were enrolled.
Exclusion criteria included: (1) polyarteritis nodosa or other conditions suspicious of systemic vasculitis (9), (2) inability to cooperate for examination to diagnose GCA, (3) incomplete clinical data, and (4) refusal to sign the consent form.
Demographic Data and Ophthalmic Examinations
Data regarding age, sex, lateral involvement of the involved eye, history of headache, jaw claudication, transient monocular visual loss (TMVL), and diplopia were collected. Neuro-ophthalmologic examinations included dilated fundus examination, standard Snellen visual acuity, visual field test, fundus fluorescein angiography, and optical coherence tomography (OCT). Laboratory tests included complete blood count, liver and renal function tests, ESR, C-reactive protein (CRP), rheumatoid factor, anti-streptomycin antibody, and other rheumatology panel tests such as anti-nuclear antibody, anti-extractable nuclear antibodies, and anti-neutrophil cytoplasmic antibody. An infectious disease panel was also included.
Ultrasound Imaging
Color duplex ultrasonography (CDUS) was performed with MyLab 90 (Esaote, Genova, Italy) to detect ocular arteries [ophthalmic artery (OA), central retinal artery (CRA), and posterior ciliary artery (PCA)]. A high-frequency (6–18 MHz) linear probe was used to detect the superficial cutaneous temporal artery in cooperating patients. The detection site of the superficial cutaneous temporal artery is located in the main trunk of the artery bilaterally in front of the ear.
Temporal Artery Biopsy
Patients scheduled for superficial temporal artery biopsy were referred to a neurosurgeon. The biopsy was performed in the operating room under local anesthesia, and the severely involved side guided by CDUS was chosen for the biopsy. Long-segment arteries > 3 cm were used for the biopsy to prevent skip lesions (10).
Statistical Analysis
Descriptive statistics (e.g., means, percentage) were used to summarize the demographic characteristics and clinical features of this case series.
Ethics Approval and Informed Consent
The Institutional Ethics Review Board of the Eye Ear Nose and Throat Hospital of the Fudan University Shanghai approved all the experimental protocols, and written informed consent was obtained from all participants and/or their legal guardians (KJ-2011-04). The methods were carried out in accordance with the relevant guidelines and regulations. The study was performed in accordance with the Declaration of Helsinki.
Results
Demographic and Clinical Features
A total of 15 patients (22 eyes; 10 male, 5 female) were assessed. The mean age was 77.0 ± 8.5 y. Among them, seven patients had bilateral involvement that occurred simultaneously or sequentially. The presenting ophthalmic manifestations included AION (n = 12), AION combined with cilioretinal artery occlusion (CLAO) (n = 2), and cotton-wool spots (n = 1).
Headache was the most common accompanying systemic symptom (n = 14, 99.3%) and could be the only presenting symptom. TMVL was the common aura attack (n = 13, 86.7%), a detail that was provided by the patient only when asked specifically. Other systemic symptoms included weight loss (n = 8, 53.3%), joint pain (n = 2, 13.3%), scalp tenderness (n = 2), jaw claudication (n = 1, 6.7%), and stroke (n = 1). None of the patients had diplopia in our case series. There was only one patient with no systemic symptoms.
Visual acuity was very poor, except in patient 3, who presented with only headache without ocular ischemic attack. Most patients having poor visual acuity but ability to perceive light. The visual acuity results are shown in Table 1.
Fundus examination of most AION eyes (15/22 eyes) revealed a classic chalky-white disc swollen due to GCA, and some with peripapillary hemorrhage (4/22 eyes), retinal exudates, and cotton-wool spots (4/22 eyes). CLAO was observed in two patients; both cases were combined with AION. Peripapillary atrophy (6/22 eyes) was also common in patients with severe disease and bilateral involvement. Optic atrophy with cupping was observed in cases of late-stage AION or in eyes with history of previous attack (Figure 1). Details of demographic and clinical features are shown in Table 1.
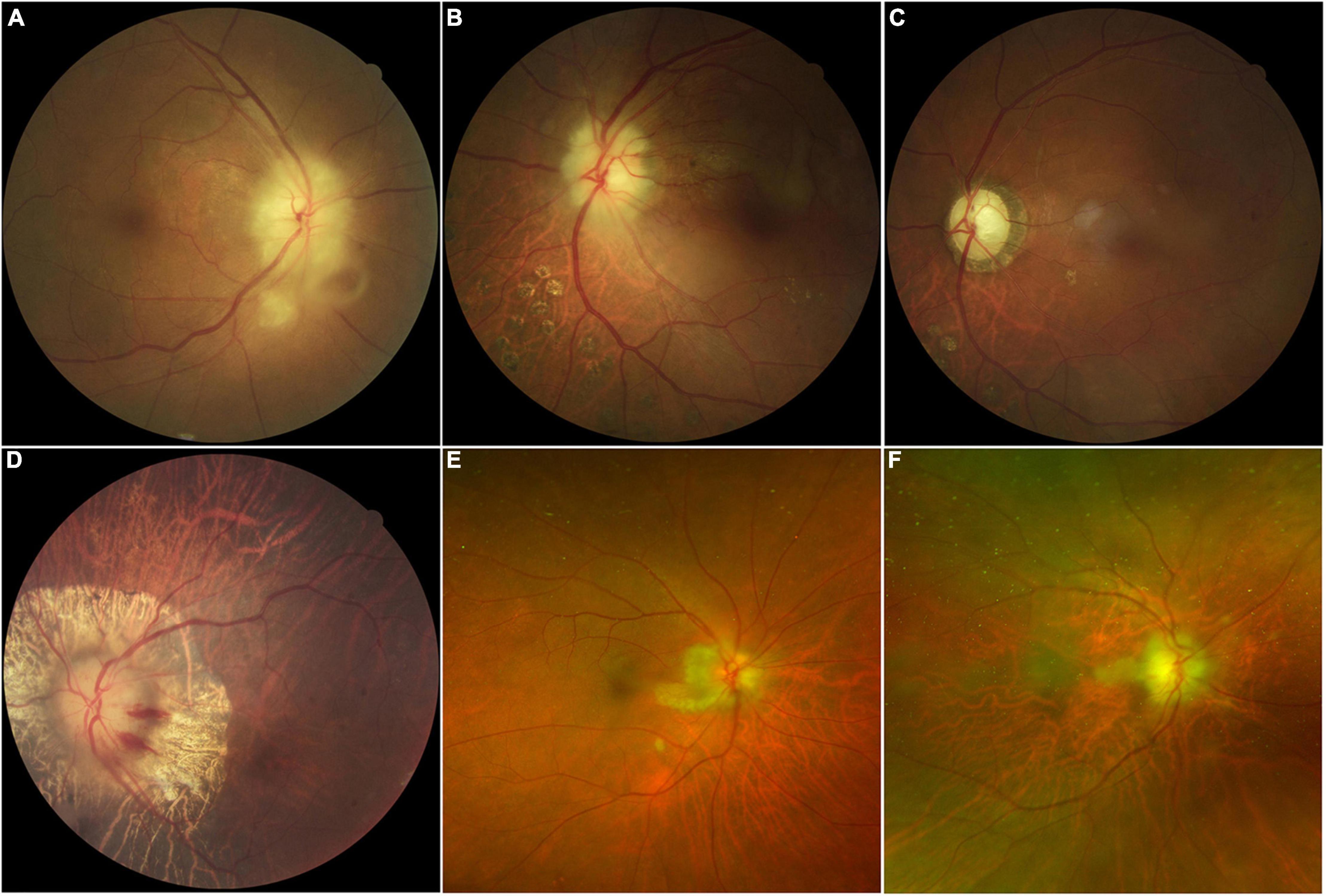
Figure 1. Color fundus photographs in patients with giant cell arteritis showing chalky-white swollen discs (A,B,D) with retina exudate and cotton-wool spots (A), late-stage optic atrophy and cupping (C), peripapillary hemorrhage with atrophy (D), and cilioretinal artery occlusion (B,C,E,F) are the same eye.
The main findings of OCT performed in 11 eyes were ischemic retinopathy or neuropathy. Eight eyes were in the acute stage and showed optic disc edema with a loss of layer structure in the inner retina and extreme edematous thickening of the peripapillary retinal nerve fiber layer and ganglion cell layer (Figures 2A,B). In patients with arteritic AION combined with CLAO, OCT showed edema of the optic disc, thickening of the inner retinal nerve fiber layer and ganglion cell layer, and loss of layer structure (Figure 2C). Patients with late-stage bilateral involvement and no light perception had peripapillary atrophy with extremely thin retinal nerve fiber and ganglion cell layers and epiretinal membranes (Figure 2D). Due to their poor systemic condition, only two patients underwent fundus fluorescein angiography, which revealed a delay in optic disc and choroidal artery perfusion.
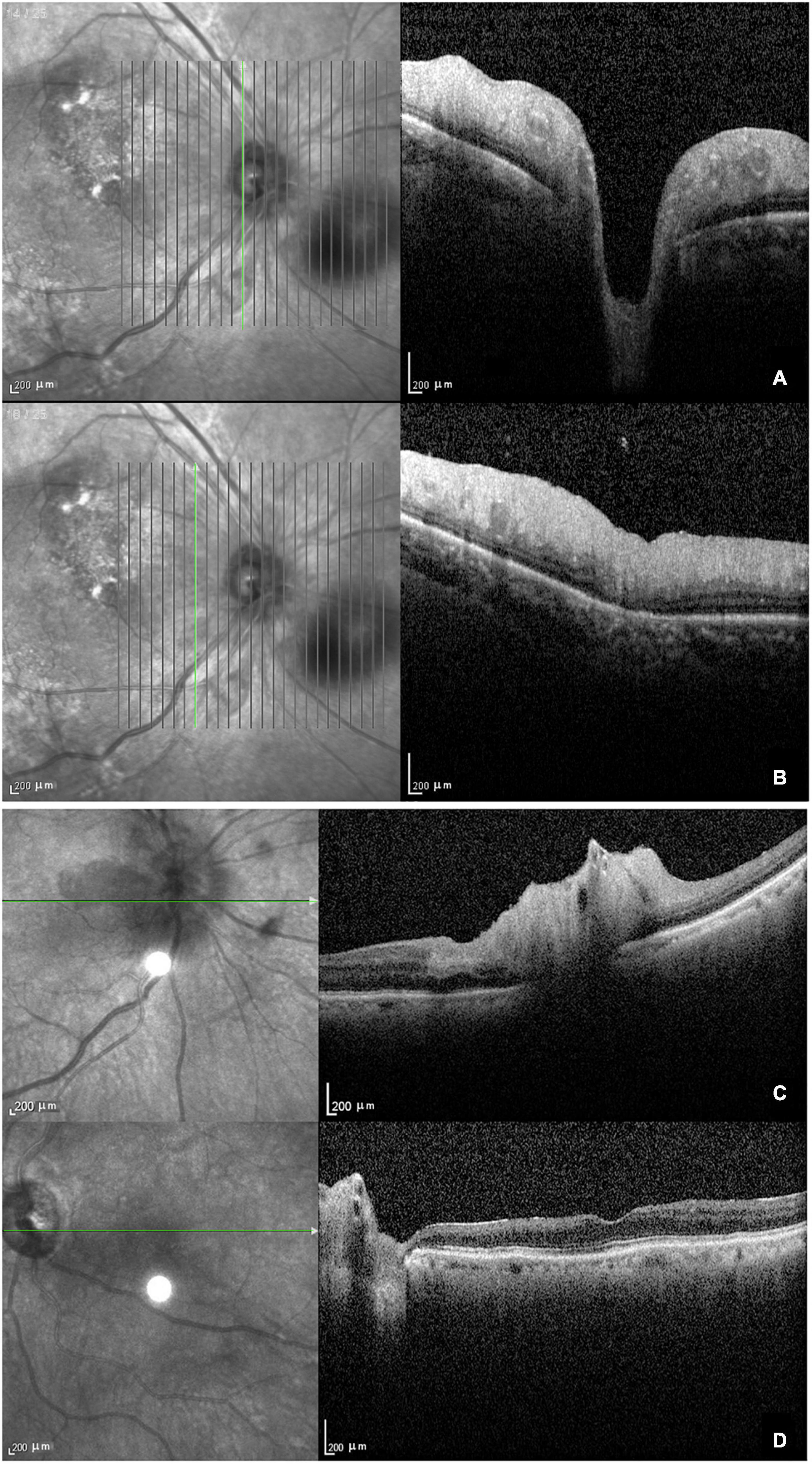
Figure 2. Optical coherence tomography in patients with giant cell arteritis. (A) Optic disc edema with a loss of layer structure in the inner retina; (B) edematous thickening of the peripapillary retinal nerve fiber layer and ganglion cell layer; (C) arteritic anterior ischemic optic neuropathy combined with ciliary vascular obstruction showing edema of the optic disc, thickening, and a loss of layer structure in the inner retina; (D) atrophy and thinning of the retinal nerve fiber layer and ganglion cell layer with epiretinal membrane at the late stage.
Routine Blood Test
The ESR and CRP levels in all patients are listed in Table 1. According to the American College of Rheumatology 1990 criteria for GCA, the ESR was elevated in 11 patients (73.3%), and the CRP was substantially elevated in all 15 patients. The results of the rheumatologic panel were unremarkable, including the rheumatoid, anti-streptomycin antibody, anti-nuclear antibody, and anti-neutrophil cytoplasmic antibody. The complete blood count showed a considerable increase in white blood cell count (n = 9) and decrease in red blood cell count and hemoglobin (n = 12). The platelet count was elevated in four patients, with increased thrombocytes in nine patients.
Ultrasonography Imaging
Color duplex ultrasonography (CDUS) detected that almost all patients had varying degrees of ophthalmic ischemic conditions associated with the CRA, PCA, and OA. Among the 15 patients, 14 underwent superficial temporal artery ultrasound with a high-frequency linear probe. The results demonstrated bilateral thickening of the vessel wall with the hypoechoic halo sign, consistent with mural inflammation, in 12 (92.9%) patients. The mural showed rough signals with segmental thickening and stenosis, and some had diffuse stenosis or occlusion. Non-specific calcification was also observed in some individuals (Figure 3). The mean thickness of the intima was 0.49 ± 0.12 mm (0.25-0.75 mm), and the mean vessel diameter was 1.36 ± 0.42 mm (0.74-2.5 mm).
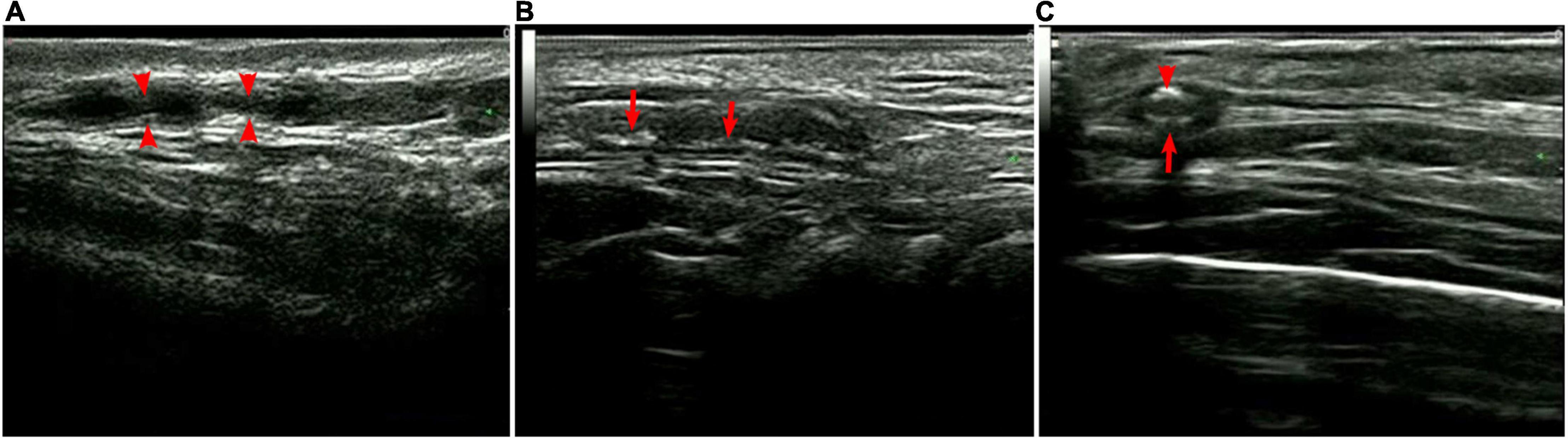
Figure 3. Superficial temporal artery ultrasound. (A) Segmental stenosis (arrows); (B) segmental thickening with calcification (arrows), and (C) hypoechoic halo (large arrow) with calcification (small arrow).
Magnetic Resonance Imaging Orbit/Brain
Fat-suppressed contrast-enhanced T1-weighted magnetic resonance imaging (MRI) of the orbit, available for only nine patients demonstrated bilateral optic nerve sheath enhancement. The cranial vessels, including the internal/external carotid arteries and some scalp arteries, showed enhancement of the thickened intima with luminal stenosis or occlusion (Figure 4). Brain MRI showed non-specific diffuse brain atrophy with enlarged ventricles in 11 patients.
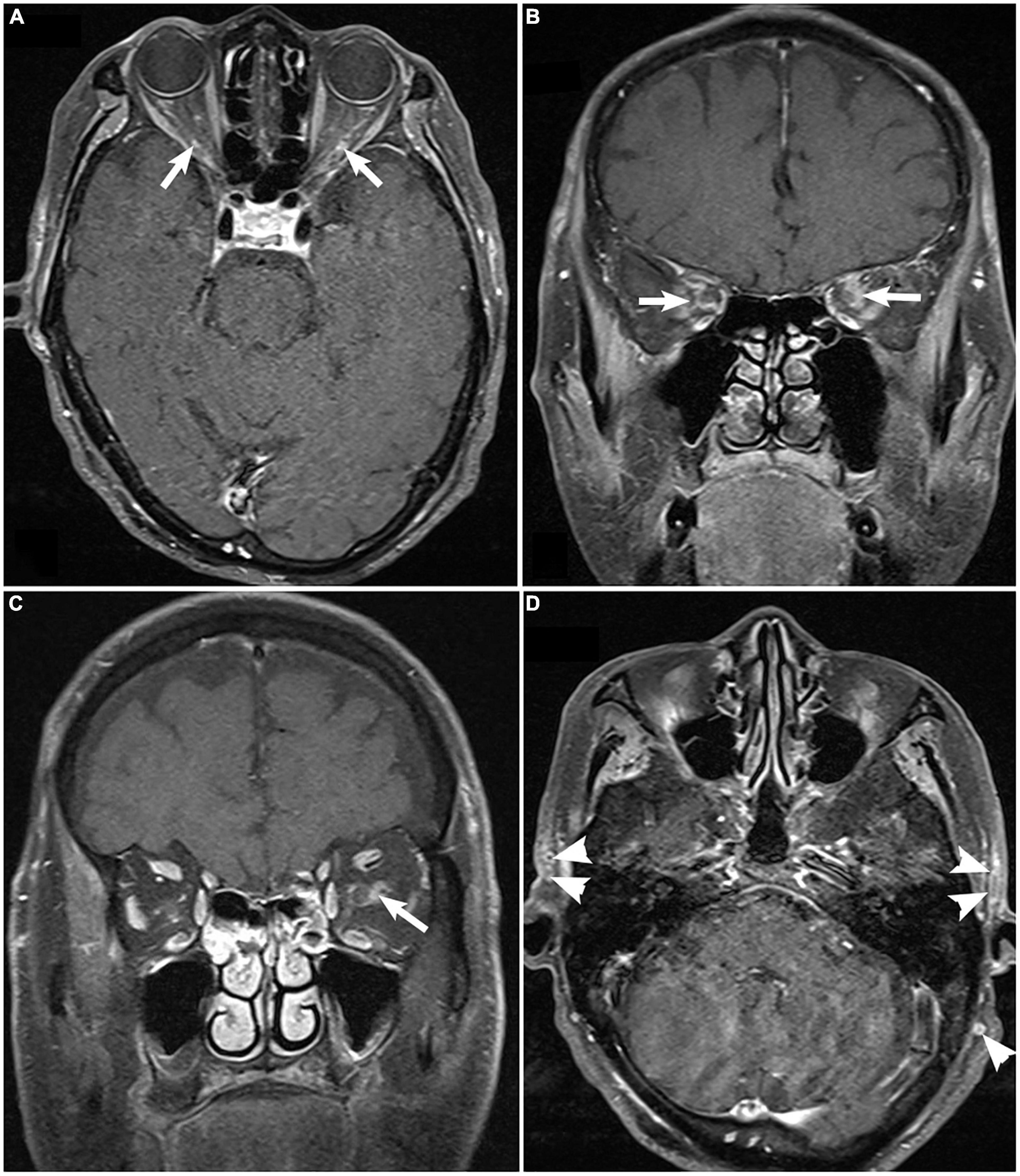
Figure 4. Fat-suppressed contrast-enhanced T1-weighted magnetic resonance imaging of the orbit demonstrating enhancement of bilateral optic nerve sheaths [arrow; (A), axial; (B), coronal], unilateral enhancement of optic nerve sheaths [(C), arrow], and enhancement and thickening superficial temporal artery [(D), arrowhead].
Temporal Artery Biopsy
Among the eight patients who underwent temporal artery biopsy, seven (87.5%) demonstrated positive results in terms of classic GCA pathological changes, such as arterial wall inflammation with mononuclear cell infiltration, multinucleated giant cells, and luminal stenosis or occlusion (Figure 5).
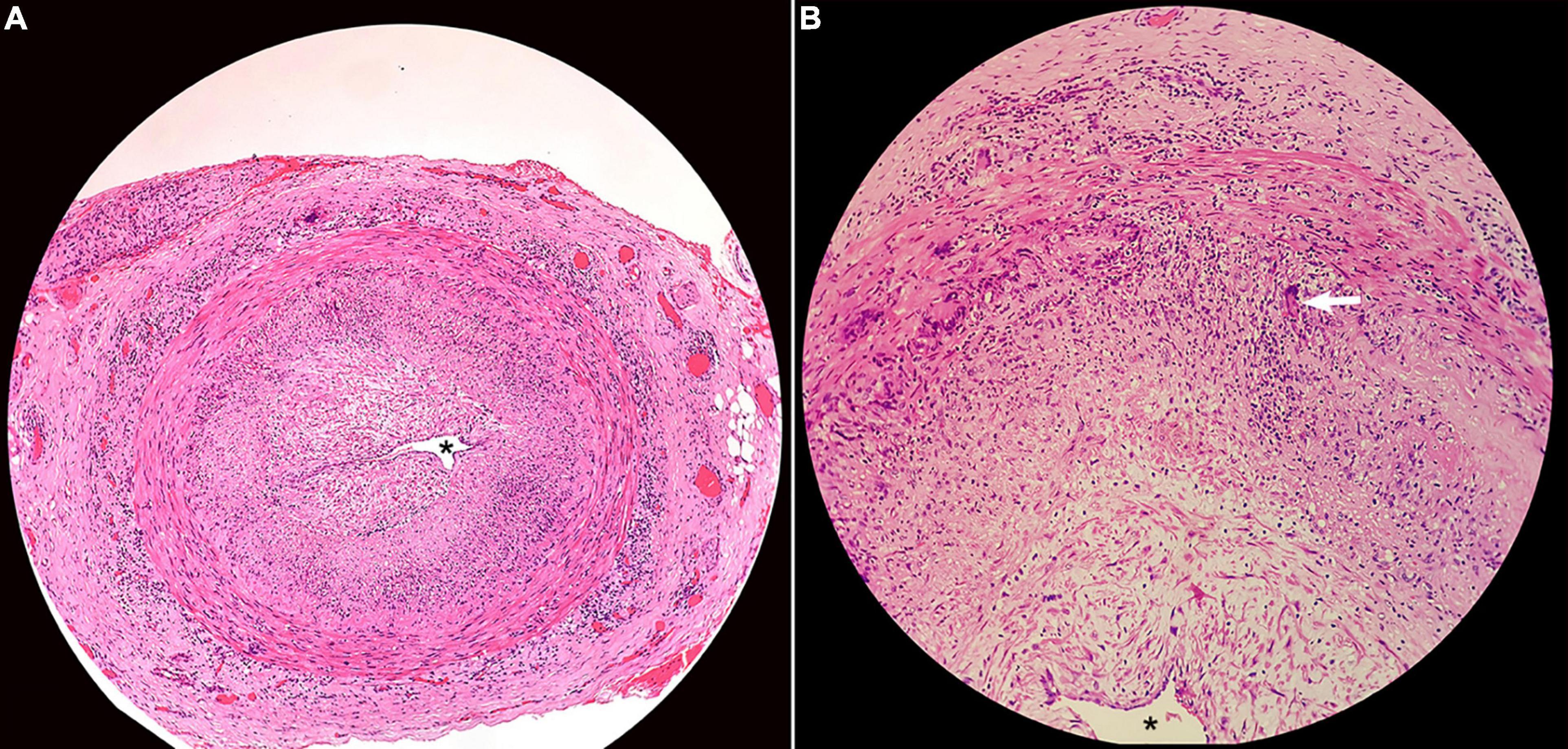
Figure 5. Histopathology of superficial temporal artery biopsy showing (A) transmural inflammation of the artery wall, intimal thickening, and near obliteration of the artery lumen (asterisk; H&E × 40); (B) multinuclear giant cell (arrow; H&E × 200).
Treatment and Prognosis
All patients underwent cortical steroid treatment after GCA diagnosis. Intravenous methylprednisolone 500 mg/d to 1 g/d were administered to 11 patients and low dose methylprednisolone 120 mg/d or oral prednisone 60 mg/d was prescribed for patients with intolerance due to contraindications and old age. Immunosuppressive agents such as methotrexate were added while oral prednisone was tapered to a low dosage around 5 to 15 mg/d. Tocilizumab was prescribed and well tolerated in two patients. Final visual functions were very poor in most patients, except in one patient who initially presented with only a headache.
Discussion
The low incidence of GCA among Asians is considered to be associated with the different type of human leukocyte antigen observed in this population (11, 12). In a comparative study by Pereira et al., only one Asian case was reported during an 8-year period (1), whereas in our territory eye center division of neuro-ophthalmology in Shanghai, 15 consecutive cases have been diagnosed within the last 5 years. To date, this is the only case series report on GCA presenting with ocular manifestations in China. It is also a notably large case series report on Asian populations. Our data suggested that ophthalmologists need to remain vigilant about GCA being potentially underdiagnosed among elderly Asian patients with ischemic conditions such as ophthalmic ischemia, central retinal artery occlusion (CRAO)/AION, and AION combined with CLAO; in particular, the latter was highly indicative of a PCA occlusion. There were no cases of branch retinal artery occlusion in this series since they lack the internal elastic lamina (13, 14).
Female predominance has been reported in the literature among both Caucasian and Asian ethnicities (1, 7, 15). The male/female ratio in our case series was 2:1, which was consistent with the ratio found in a small case series with Thai patients (2); both of these case series were composed mainly of patients with AION.
Systemic symptoms such as headache, TMVL, weight loss, and scalp tenderness were common complaints in this study of which headache could be the only presenting symptom. Although TMVL was also very common, it was reported only when the patients were specifically asked. Diplopia and visual hallucinations were not reported by any patient. Unlike Caucasians, Asian patients with GCA usually present with non-specific symptoms such as headache, weight loss, malaise, fatigue, and fever, rather than jaw claudication or double vision. This results in delayed diagnosis by physicians or rheumatologists.
Due to a lack of specific blood biomarkers for the diagnosis of GCA, routine complete blood count, ESR, and CRP are very useful ancillary tests that can quickly distinguish patients with arteritic AION from those with non-arteritic AION. Elevated ESR and CRP levels, combined with anemia, are highly indicative of the necessity for further work-up.
According to the literature, the incidence of visual symptoms in GCA can be as high as 70%, and 20% of patients may experience permanent visual loss (15, 16). In our case series, he visual acuity most tested in the affected eye was light perception, and the outcome was also very poo. The fundus appearance revealed classic chalky-white swelling in the acute stage, and retinal cotton-wool spots might have been an early sign of arterial ischemia. Peripapillary atrophy is another feature in patients with chronic ischemia, which can be observed in the acute stage. The simultaneous presence of AION and CRAO indicates PCA involvement and is highly indicative of GCA causing arteritic AION rather than non-arteritic AION. In addition, AION combined with CRAO/branch retinal artery occlusion, which indicates OA involvement, was not observed in our series.
Due to the considerably low temporal artery biopsy rate in China, GCA is rarely reported by ophthalmologists in this country; therefore, GCA remains underdiagnosed among the AION population, especially in the elderly. Temporal artery ultrasonography, which is non-invasive and cost-effective, is recommended by the European League Against Rheumatism as the first-line study for evaluating large-vessel vasculitis and has been widely utilized in our clinic recently for evaluating high-risk GCA patients (17–19). CDUS can detect the blood flow in ocular arteries, including the OA, CRA, and PCA, and the high-frequency linear probe can detect the superficial cutaneous temporal artery. The hypoechoic halo sign is indicative of inflammatory thickening of the intima with stenosis/occlusion of the lumen; these phenomena are consistent with pathological changes in GCA. According to our data, the halo sign is a highly specific indicator for GCA diagnosis and can also guide the side for biopsy. However, a limitation associated with using ultrasound for detection of temporal artery vasculitis is operator dependence.
MRI is another non-invasive technique for evaluating arteries and determining a diagnosis (17, 20). In addition to the cranial arteries, enhancement of the optic nerve sheath in orbit MRI has been reported as one characteristic of GCA, which was observed in our case series as well (20, 21). Although perineural enhancement is a non-specific phenomenon which can also be observed in infectious and other autoimmune optic neuropathies such as sarcoidosis, MRI on a patient with potential GCA is still crucial to exclude malignant conditions.
The limitations of the study include the short follow-up of patients which limited our ability to obtain long-term effects of immune therapies like Tocilizumab. Furthermore, future research should focus on identifying specific biomarkers of GCA pathogenesis in the Chinese population.
In summary, GCA may be underdiagnosed in elderly Chinese patients with ocular ischemic conditions. The profound visual loss associated with AION/CRAO in elderly patients is highly indicative of GCA in cases of elevated ESR or CRP levels. The hypoechoic halo sign found on ultrasound of the temporal artery is a very sensitive and specific indicator for diagnosis and should be utilized to guide biopsy.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Review Board of the Eye Ear Nose and Throat Hospital of the Fudan University Shanghai. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QC and GT conceived the study. DG and YB performed the biopsy and pathology. WC and JZ participated in the diagnosis. CF and PS collected and interpreted data. QC and CF drafted the manuscript. GT revised the manuscript. XS sponsored by funding. All authors have approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2020YFA0112700).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
AION, anterior ischemic optic neuropathy; CDUS, color duplex ultrasonography; CLAO, cilioretinal artery occlusion; CRA, central retinal artery; CRAO, central retinal artery occlusion; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GCA, Giant cell arteritis; OA, ophthalmic artery; OCT, optical coherence tomography; PCA, posterior ciliary artery; TMVL, transient monocular visual loss.
References
1. Pereira LS, Yoon MK, Hwang TN, Hong JE, Ray K, Porco T, et al. Giant cell arteritis in Asians: a comparative study. Br J Ophthalmol. (2011) 95:214–6. doi: 10.1136/bjo.2009.177220
2. Attaseth T, Vanikieti K, Poonyathalang A, Preechawat P, Jindahra P, Wattanatranon D. Anterior ischemic optic neuropathy due to biopsy-proven giant cell arteritis in Thai patients. Clin Ophthalmol. (2015) 9:1071–5. doi: 10.2147/OPTH.S82898
3. Cha DM, Lee T, Choe G, Yang HK, Hwang JM. Silent giant cell arteritis in an elderly Korean woman. Korean J Ophthalmol. (2013) 27:224–7. doi: 10.3341/kjo.2013.27.3.224
4. Liou LM, Khor GT, Lan SH, Lai CL. Giant cell arteritis with multiple cranial nerve palsy and reversible proptosis: a case report. Headache. (2007) 47:1451–3. doi: 10.1111/j.1526-4610.2007.00952.x
5. Wang X, Hu Z, Lu W, Tang X, Zeng L, Zhang J, et al. Giant cell arteritis: a rare disease in Asians. J Clin Rheumatol. (2009) 15:48. doi: 10.1097/RHU.0b013e31819632e1
6. Tian G, Chen W, Chen Q, Wang M, Zhao G, Li Z, et al. Giant cell arteritis presenting as bilateral anterior ischemic optic neuropathy: a biopsy-proven case report in Chinese patient. BMC Ophthalmol. (2018) 18:282. doi: 10.1186/s12886-018-0953-5
7. Kobayashi S, Yano T, Matsumoto Y, Numano F, Nakajima N, Yasuda K, et al. Clinical and epidemiologic analysis of giant cell (temporal) arteritis from a nationwide survey in 1998 in Japan: the first government-supported nationwide survey. Arthritis Rheum. (2003) 49:594–8. doi: 10.1002/art.11195
8. Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. (1990) 33:1122–8. doi: 10.1002/art.1780330810
9. Zhang Y, Wang D, Chu X, Zhang W, Zeng X. Differences in clinical manifestations and prognosis of Chinese giant cell arteritis patients with or without polymyalgia rheumatica. Ir J Med Sci. (2019) 188:713–20. doi: 10.1007/s11845-018-1903-1
10. Liu T, Volpe JN, Galetta LS. Neuro-Ophthalmology: Diagnosis and Management. 2nd ed. Amsterdam: Elsevier Inc (2001).
11. Hunder GG, Lie JT, Goronzy JJ, Weyand CM. Pathogenesis of giant cell arteritis. Arthritis Rheum. (1993) 36:757–61. doi: 10.1002/art.1780360604
12. Hashimoto H. Takayasu’s arteritis and giant cell (temporal or cranial) arteritis. Intern Med. (2000) 39:4–5. doi: 10.2169/internalmedicine.39.4
13. Vodopivec I, Rizzo JF III. Ophthalmic manifestations of giant cell arteritis. Rheumatology. (2018) 57:ii63–72. doi: 10.1093/rheumatology/kex428
14. Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. (2011) 30:359–94. doi: 10.1016/j.preteyeres.2011.05.001
15. Chen JJ, Leavitt JA, Fang C, Crowson CS, Matteson EL, Warrington KJ. Evaluating the incidence of arteritic ischemic optic neuropathy and other causes of vision loss from giant cell arteritis. Ophthalmology. (2016) 123:1999–2003. doi: 10.1016/j.ophtha.2016.05.008
16. Glutz von Blotzheim S, Borruat FX. Neuro-ophthalmic complications of biopsy-proven giant cell arteritis. Eur J Ophthalmol. (1997) 7:375–82. doi: 10.1177/112067219700700412
17. Ninan JV, Lester S, Hill CL. Diagnosis and management of giant cell arteritis: an Asia-Pacific perspective. Int J Rheum Dis. (2019) 22:28–40. doi: 10.1111/1756-185X.13297
18. Serling-Boyd N, Stone JH. Recent advances in the diagnosis and management of giant cell arteritis. Curr Opin Rheumatol. (2020) 32:201–7. doi: 10.1097/BOR.0000000000000700
19. Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. (2018) 77:636–43. doi: 10.1136/annrheumdis-2017-212649
20. Klink T, Geiger J, Both M, Ness T, Heinzelmann S, Reinhard M, et al. Giant cell arteritis: diagnostic accuracy of MR imaging of superficial cranial arteries in initial diagnosis-results from a multicenter trial. Radiology. (2014) 273:844–52. doi: 10.1148/radiol.14140056
Keywords: giant cell arteritis (GCA), anterior ischemic optic neuropathy (AION), arteritic anterior ischemic optic neuropathy, color duplex ultrasonography, superficial temporal artery biopsy
Citation: Chen Q, Chen W, Feng C, Gong D, Zhang J, Bi Y, Sun P, Sun X and Tian G (2022) Giant Cell Arteritis Presenting With Ocular Symptoms: Clinical Characteristics and Multimodal Imaging in a Chinese Case Series. Front. Med. 9:885463. doi: 10.3389/fmed.2022.885463
Received: 28 February 2022; Accepted: 25 May 2022;
Published: 20 June 2022.
Edited by:
Anna Maria Roszkowska, University of Messina, ItalyReviewed by:
Winfried Amoaku, University of Nottingham, United KingdomBayan Al Othman, University of Rochester, United States
Copyright © 2022 Chen, Chen, Feng, Gong, Zhang, Bi, Sun, Sun and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohong Tian, dmFsZW50aWFuOTlAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Qian Chen
Qian Chen Weimin Chen
Weimin Chen Chaoyi Feng
Chaoyi Feng Deshan Gong3
Deshan Gong3 Jiong Zhang
Jiong Zhang Yingwen Bi
Yingwen Bi Ping Sun
Ping Sun Xinghuai Sun
Xinghuai Sun Guohong Tian
Guohong Tian