
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 03 June 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.884188
This article is part of the Research Topic Immunological Landscape and Management of Kidney Diseases View all 7 articles
Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis is a destructive small vessel vasculitis affecting multiple organs. Renal involvement often leads to end-stage renal disease and increases mortality. Prompt diagnosis and initiation of adequate immunosuppressive therapy are critical for the best patient and kidney outcomes. However, considerable heterogeneity in symptoms and severity across the patients frequently hinder the diagnosis and management. The objective of this review is to emphasize the heterogeneity of the ANCA-associated vasculitis, facilitate the recognition and give guidance to the therapeutical possibilities. We present epidemiologic and risk factors, pathogenesis, and provide comprehensive clinical features of the disease. This article also focuses on the currently available therapeutic options and emerging cellular and molecular targets for the management of systemic and especially renal disease. We conducted extensive literature research published on PubMed and Google Scholar. We systematically reviewed, analyzed, and assembled databases, covering a broad spectrum of aspects of the disease. We compared and summarized the recommendations of two recent guidelines on ANCA-associated vasculitis. The incidence of ANCA-associated vasculitis, hence glomerulonephritis shows a steady increase. Familiarity with the presenting symptoms and laboratory abnormalities are necessary for rapid diagnosis. Early initiation of treatment is the key aspect for favorable patient and renal outcomes. A better understanding of the pathogenesis constantly leads to more targeted and therefore more efficient and less toxic treatment.
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is an inflammatory disorder of the small arteries, resulting in vascular destruction and tissue necrosis affecting various organs (1). The major clinicopathological categories of AAV are microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA). According to serology, anti-myeloperoxidase (aMPO) and anti-proteinase 3 (aPR3) positive diseases are distinguished.
Glomerulonephritis is a frequent manifestation of AAV, presenting in almost all cases of MPA and frequently in GPA (2, 3). Renal involvement is of particular importance since it affects renal and overall patient morbidity and mortality (4). Despite advances in treatment, renal involvement in AAV still bears high mortality (3, 4). Therefore, early recognition and treatment can decrease the disease burden on patients and healthcare costs.
The worldwide reported prevalence of AAV ranges from 4.6 to 21.8 cases per one million person-years (5–7). Most studies agree that its incidence increases over time (5, 8, 9). However, the rise might be explained by better recognition and more widespread availability of the ANCA test (10, 11).
There is an obvious geographical difference between the distributions of MPA and GPA; MPA is more frequent in Southern Europe and Asia, while GPA is more prevalent in Northern Europe and Australia (5, 9, 12). The geographical distribution of the serotypes is partially explained by the ambient ultraviolet radiation levels and latitude but also proposes a heterogeneity in the genetic background (13). The average onset of AAV is 65 years and prevalence peaks in the 70–75-year age group (7, 12, 14), but it is not limited to adults (15, 16). Patients with renal involvement are 10 years older on average compared to those without it (4). Though AAV displays a slight overall male predominance (8, 9, 17–19), renal involvement is more often present in females (4, 14, 20), which may be explained by the female predominance of MPO-AAV and its higher incidence with glomerulonephritis. PR3-AAV, on the other hand, is associated with male gender, a younger age, and a higher glomerular filtration rate at the diagnosis (13).
Meta-analysis and genome-wide association studies (GWAS) confirm different genetic background for MPO- and PR3-AAV (21, 22).
According to the current hypothesis, AAV occurs in genetically predisposed patients who were exposed to certain environmental factors. GWAS identified several human leukocyte antigen (HLA) and non-HLA regions associated with AAV. GPA is linked to HLA-DP1, α1-antitrypsin (SERPINA) and proteinase 3, MPA to HLA-DQ, and EGPA to HLA-DRB4. Single-nucleotide polymorphism in protein tyrosine phosphatase (PTPN22), cytotoxic T lymphocyte-associated antigen-4 (CTLA4), Toll-like receptor 9, Fc gamma receptors, interleukin-10 and 2 were also associated with higher occurrence of AAV (23, 24). Environmental risk factors include certain medications, silica dust, organic solvents, and most commonly, infections (25). Recently COVID-19 (coronavirus disease) vaccinations were also reported as triggering factors in some case reports (26–30).
The key concept in the pathogenesis is the priming of the neutrophil granulocytes. During priming, neutrophil responsiveness is amplified by an activating stimulus by prior exposure to an agent. Examples of priming agents include tumor necrosis alpha (TNFα), interleukin (IL)-1,−6, interferon-gamma (IFNγ), lipopolysaccharides, substance P, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (31, 32). Additionally, complement factor 5a (C5a) from the alternative complement pathway can also prime neutrophils by binding its receptors (32, 33). As a consequence of priming, antimicrobial activity increases, and MPO or PR3 antigens are expressed on the cell membrane. Cross-binding of the antigens and the surface Fcγ receptors by ANCA causes uncontrolled activation, resulting in excessive formation of cytokines, reactive oxygen species (ROS), lytic enzymes, and neutrophil extracellular traps (NETs) (31, 33). NETs are mesh-like structures of nuclear and mitochondrial decondensed chromatin, histones, cytoplasmic, and granule proteins (34). By exposing intracellular molecules, granular enzymes (e.g., PR3 and MPO), and ROS, NETs play a role in both vascular damages by a direct cytotoxic effect on endothelial cells and the generation of MPO-, PR3-, and atypical ANCA antibodies (34, 35).
MPO rests in the azurophil granules, bound to neutrophil elastase (NE), cathepsin G, lactoferrin, PR3, and lysozyme, forming azurosomes. MPO aids the release of neutrophil elastase into the cytoplasm by oxidizing it. NE binds to F-actin and degrades the cytoskeleton, making its way to the nucleus. Cytoskeletal degradation inhibits concomitant phagocytosis, committing cells to NETosis. NE also cooperates with gasdermin D in the destruction of the nuclear envelope by forming pores and allowing water influx. Entering the nucleus, NE and MPO decondense chromatin by degrading histones (36). This process is also promoted by the peptidyl arginine deiminase type 4 (PADI4) enzyme, which catalyzes histone arginine conversion to citrulline, reducing positive charge and loosening the compact chromatin structure (34, 35). Another detrimental effect of NETs is thrombocyte aggregation and enhancing coagulation (37). NETs serve as a scaffold in which platelets and red blood cells can accumulate, while H3 and H4 histones induce platelet aggregation. Furthermore, extranuclear DNA and histones serve as danger-associated molecular patterns (DAMPs) and induce apoptosis, necrosis, necroptosis, and pyroptosis leading to further inflammation (34). NET formation normally is counterbalanced by the activity of the endonuclease enzymes, e.g., Deoxyribonuclease-1 (DNASE1). Excessive formation and/or impaired clearance have a pathogenic role in autoimmune diseases, e.g., AAV, and systemic lupus erythematosus (38).
NET antigens are presented to naive T cells by dendritic cells that differentiate into CD4+ T helper (Th) cells. CD4+ T cells promote the differentiation of B-cells into ANCA-producing plasma cells. Cell-free DNA stimulates B-cells via Toll-like receptor 9 (TLR9), enhancing antibody production (33). Activated neutrophils release B cell-activating factor (BAFF) or B lymphocyte stimulator (BLyS) which is involved in enhancing B cell receptor signaling by upregulating the expression of the co-receptors CD21 and CD19, and prolonging B-cell survival by decreasing pro-apoptotic proteins (39).
Necrotizing granulomas are structures which can serve as a niche to induce differentiation and maturation of T cells. Macrophage-T cell interaction is the sine qua non of granuloma formation. In the early phase of granuloma formation, neutrophil invasion and the release of lytic enzymes result in the formation of a necrotic core lesion. Over time, macrophages are recruited, primarily to phagocytose the apoptotic debris. This process is called efferocytosis. A defect in efferocytosis is postulated to be a key step in the formation and in the inability of resolution of the granulomas. Anti-PR3 antibodies halt the clearance of PR3-expressing or ANCA opsonized cells and increase pro-inflammatory cytokine and chemokine release, attracting further neutrophils and T-cells (40). IL-23 tends to polarize T cells toward the TH17 phenotype (33). This subset of T cells releases a great quantity of IL-17,−21,−22, and TNFα, which leads to a vicious circle by stimulating macrophages and priming neutrophils. IL-17 is also implicated to have a major role in granuloma formation by activating endothelial and epithelial cells, causing macrophage and dendritic cell fusion (41).
To conclude, neutrophils play a key part in tissue damage in AAV, but B- and T-lymphocytes have also an important role to maintain the pathognomonic state, providing a wide range of targets for therapeutic intervention.
Renal involvement in AAV is heterogeneous. The most aggressive renal manifestation of AAV is the rapidly progressive glomerulonephritis syndrome (RPGN), with a marked decrease in glomerular filtration rate (GFR) over some weeks or months, sometimes accompanied by oliguria (2). AAV, however, frequently appears as persistent microscopic and dysmorphic hematuria, sub-nephrotic proteinuria, and systemic hypertension, with or without azotemia (2, 42). Such clinical pictures mandate the serological investigation of ANCAs, and according to the latest guidelines, a solid ANCA-positivity with relevant clinical symptoms may be sufficient to start the remission induction treatment without renal biopsy (43). However, the biopsy is highly recommended and provides the “gold-standard” diagnostic results, especially in the case of an ambiguous serology or unusual clinical presentation.
Considering the systemic nature of the disease, the involvement of various organs may complete the clinical picture, and the exclusion of alternative diagnoses, especially infections, is a crucial aspect of the evaluation. Extra-renal manifestations are also particularly important clues for the prompt diagnosis of ANCA-associated glomerulonephritis when rapid renal involvement is not present (Table 1) (2, 42, 44–74). Fever, malaise, anorexia, weight loss, arthralgia, and myalgia are common nonspecific manifestations of autoimmune diseases that should always raise the possibility of systemic vasculitis. Most of the key symptoms such as chronic nasal discharge, nasal polyps, sinusitis, recurrent otitis, persistent cough, skin lesions, etc. (Figure 1) may be mild and the patients may not attribute importance to them, resulting in diagnostic delay. Misdiagnosis may also be due to the presence of rare AAV-associated symptoms. For example, gastrointestinal manifestation is thought to be a rare complication of AAV, however, several case reports support the opposite. Even with histological evaluation, the diagnosis can be difficult, as the granulomatous lesions of GPA may make the involvement almost indistinguishable from inflammatory bowel disease (57, 69).
In summary, considerable heterogeneity in symptoms and severity across the patients hinder the diagnosis and may delay proper treatment of the disease. The diagnosis of AAV starts with thinking of it.
Since symptoms of vasculitis can be excessively overlapping, the Diagnostic and Classification Criteria in Vasculitis (DCVAS) study developed classification criteria to differentiate them. Results were published in 2022. These revised classification criteria were needed to increase sensitivity compared to the 1990 American College of Rheumatology (ACR) criteria. Since 1990, ANCA testing became widely available, as well as diagnostic imaging tools. On top of it, the recent classification included patients with MPA, which was not the case with the previous one. Another advantage is the shifting toward weighted criteria which enables a more discriminative classification (75–77).
The diagnostic hallmarks of AAV are the anti-neutrophil antibodies. Their diagnostics are based on indirect immunofluorescence (IIF) of ethanol-fixed neutrophils or enzyme-linked immunosorbent assay (ELISA). IIF is more sensitive, while ELISA is more specific. Their use varies around the world and between laboratories. Based on IIF, there are two types of fluorescence patterns with ANCA positive sera: cytoplasmic (c-ANCA), and perinuclear (p-ANCA). The mixture of these antibodies is defined as atypical ANCA. Antigens are located in the granules. Cytoplasmic ANCA pattern can be detected throughout the cytoplasm, and it is most commonly directed against proteinase 3 (PR3). The perinuclear pattern is artifactual and results from alcohol fixation. After fixation, positively charged granular constitutes, especially myeloperoxidase (MPO), rearrange to the negatively charged nucleus membrane and can be stained with anti-myeloperoxidase antibodies (44, 78).
ANCA antibodies are positive in 80–95% of ANCA-associated vasculitis patients. PR3- is strongly linked to GPA, while MPO-ANCA is to MPA and EGPA. Renal limited disease is usually MPO-ANCA positive as well (79). Nonetheless, 20–30% of the patients with clinical AAV have atypical ANCA, and 10–20% of them are ANCA negative, posing a diagnostic challenge and potential treatment delay (80–82). On the other hand, naturally occurring ANCA antibodies can also be detected in healthy individuals, accounting for false-positive cases.
Atypical ANCA antibodies can be detected in drug-induced vasculitis, inflammatory bowel diseases, rheumatoid arthritis, and systemic lupus erythematosus (83). Perinuclear-ANCA positivity is detected in inflammatory bowel diseases, especially in ulcerative colitis and in autoimmune hepatobiliary diseases (84). ANCA positivity may be noticed in bacterial endocarditis, chronic infections, malignancy, myeloma multiplex, cryoglobulinemia, idiopathic pulmonary fibrosis, and cystic fibrosis (81, 85–89). IgA (immunoglobulin A) nephropathy cases with ANCA positivity were also reported (35, 90). Anti-GBM (glomerular basal membrane) disease may also present with ANCA positivity, and in 5% of the cases, ANCA antibodies precede the development of anti-GBM antibodies by months (91–93). On the other hand, ANCA positivity (usually MPO-ANCA) in anti-GBM patients occurs in 35% of patients. Anti-GBM positive AAV patients' disease resembles more that of anti-GBM disease, and they have a higher risk of end-stage renal disease (ESRD) (19, 94).
In terms of complement levels, there is a controversy in the literature. Some authors declare that complement levels stay within the normal range, while others state that complement levels rise significantly in AAV, as a consequence of fulminant immune system activation (32, 95). Moreover, low levels of complement are also reported. The decreased level of C3 is associated with worse renal outcome which emphasizes the activation and the possible pathognomic role of the alternative pathway (96, 97).
Thrombocytosis, normochromic normocytic anemia, and leukocytosis are common findings in complete blood count. EGPA manifests with eosinophilia, which is commonly >10% of the total leukocyte count and is frequently accompanied by elevated serum IgE levels. Although C-reactive protein (CRP) and erythrocyte sedimentation rate elevate, these markers have low specificity (98).
Renal manifestation is evaluated by serum creatinine, blood urea nitrogen, and urine analysis. Proteinuria, hematuria, and urine sediment finding with dysmorphic erythrocytes and red blood cell casts aid the prompt diagnosis of the glomerular involvement.
Since pathological features in the specimen do not alter the choice and the duration of the therapy currently, the need for renal biopsy in all AAV patients is controversial. The EULAR/ERA-EDTA (European League Against Rheumatism/European Renal Association) recommendations in 2016 supported the need for a renal biopsy to assist the diagnosis and prognosis (Level of evidence 3; grade of recommendation C) (99). In selected cases, where the clinical presentation is compatible with ANCA-associated vasculitis and ANCA positivity is solid, and there is a low probability of secondary vasculitis and false-positive ANCA, treatment can be commenced without renal biopsy (81). Out of consideration of prognostic factors, however, sampling can be performed (100). In case of an ambiguous serology result (negativity or positivity in low titer, atypical antibodies) or unusual clinical presentation, the biopsy is highly recommended. In these cases, the principal role of the biopsy is to provide diagnostic information. The diagnostic yield of a kidney biopsy is especially high in GPA (91.5%) (101). According to the newest KDIGO (Kidney Disease Improving Global Outcome) 2021 guideline on glomerular diseases, renal biopsy remains the gold standard in the diagnostic process, therefore, it should always be considered in patients with ANCA-associated glomerulonephritis. On the other hand, when the clinical presentation is unquestionable, waiting for a biopsy should not delay the start of the proper treatment and biopsy can be performed later, “when feasible.” This phrase, however, may leave an alternative to skip biopsy in some patients, especially in the elderly, and in those, who are in poor general condition with obvious clinical signs for AAV and with good response to the remission induction therapy (It is important to note, that the statements regarding the need of a kidney biopsy were a practice point, not a recommendation, because of the lack of conclusive evidence.) (43).
The histopathological feature of ANCA-associated glomerulonephritis is pauci-immune small-vessel vasculitis. The term “pauci” refers to the scarcity of immunoglobulin and complement deposition detected by indirect immunofluorescence. In the earliest phase, light microscopy reveals neutrophil invasion occurring in the vessel walls, and it is gradually overtaken by mononuclear leukocytes. Leukocyte fragmentation (leukocytoclasia) can be observed.
Segmental fibrinoid necrosis, with or without cellular and fibrocellular extracapillary crescents are common findings in early vascular lesions (Figure 2). Although severe acute lesions may appear as large concentric crescents or widespread sclerosis it is usually a hallmark of chronic inflammation (102). Apart from glomerulonephritis, AAV patients might have renal arteritis. On rare occasions, medullary angiitis manifests as medullary interstitial disease and papillary necrosis in severe cases (103). There are main histopathologic types (Berden classification) that provide information about the prognosis: sclerotic (>50% globally sclerotic glomeruli), focal class (>50% normal glomeruli), crescentic class (>50% of glomeruli with cellular crescents), or mixed class in which no dominant feature can be detected. Renal survival (time to end-stage renal failure) at 5 years from the time of the diagnosis was 93% in patients with focal, 76% with crescentic, 61% with mixed, and 50% with sclerotic categories at the time of the diagnosis (100).
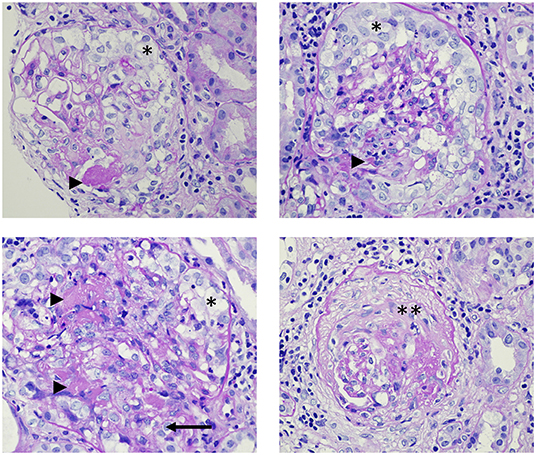
Figure 2. Histopathology images from the renal biopsy of a patient with ANCA-associated glomerulonephritis. The patient has renal limited vasculitis with anti-myeloperoxidase positivity, causing rapidly progressive glomerulonephritis syndrome. Arrowheads: fibrin; *: cellular crescents; **: sclerotic crescent; arrow: rupture of the Bowman's capsule. Light microscopy, Periodic Acid-Schiff (PAS) staining, magnification: 600x.
GPA and EGPA patients rarely have necrotizing granulomatous lesions in the kidney biopsy specimens. Concomitant eosinophilic infiltration may be detected in GPA and it is prominent in EGPA (104).
Repeat biopsies to monitor kidney function and the success of therapy are not performed routinely, however, they may have a role in assessing disease activity. Clinical biomarkers, such as hematuria and serum creatinine do not reflect disease activity specifically and sensitively enough, thus they can be mistaken for relapses. Approximately half of the patients who underwent rebiopsy had alteration in the Berden classification, up-or downstepping in the classification hierarchy (105, 106). This finding suggests that interval biopsy could aid clinicians to develop more appropriate and personalized treatment and predict kidney outcomes more precisely than the initial biopsy.
As discussed above, cutaneous manifestations can appear in a wide variability. Thus, diagnosis can be challenging, in which skin biopsy could aid the work. Lung biopsy is rare and only conducted in the absence of systemic manifestations or when serology cannot prove the diagnosis. It is to rule out alternative diagnoses since granulomas do not have a specific sign on imaging examinations, they can mimic tumors and infections as well (72).
The chronic relapsing-remitting nature of AAV necessitates tight follow-up of disease activity to provide appropriate management. The Birmingham Vasculitis Activity Score V3.0 (BVAS) is an approved, easily available, and effective tool to evaluate disease severity in everyday clinical work. The scoring system scrutinizes 9 organ involvement, and 66 attributes altogether and assesses the symptoms according to their persistent or intermittent nature. Clinical features are weighted according to their clinical relevance (107, 108). The score has a prognostic value in the short and medium-term mortality and also suggests responsiveness to therapy (108). Other scores, such as Vasculitis Damage Index (109), Disease Extent Index (110), Five Factor Score for EGPA (111) are also validated and reliable (112).
One of the main aims of this review is to summarize the recent recommendations for the management of ANCA-associated glomerulonephritis in light of two recent guidelines: KDIGO and the American College of Rheumatology/Vasculitis Foundation (ACR) guideline from 2021 (43, 113). As glomerulonephritis is more prominent in GPA/MPA, EGPA treatment is not discussed in this section.
The treatment of AAV varies according to organ involvement and disease severity. ANCA-associated glomerulonephritis is considered an organ- or life-threatening disease, therefore, demands induction and maintenance immunosuppression (Figure 3).
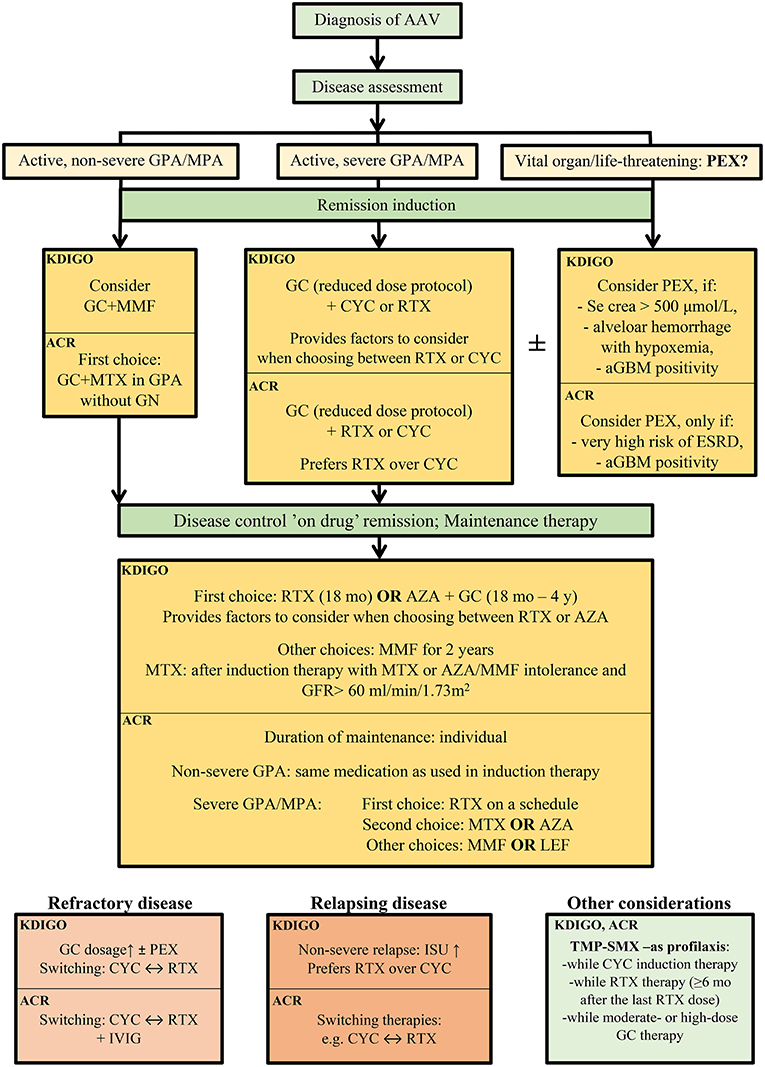
Figure 3. Therapeutic recommendation of the remission induction therapy in ANCA-associated glomerulonephritis, according the current guidelines, KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases (43) and American College of Rheumatology 2021 (113). AAV, ANCA-associated vasculitis; KDIGO, Kidney Disease Improving Global Outcome; ACR, American College of Rheumatology; aGBM, anti-glomerular basal membrane antibody; AZA, azathioprine; crea, creatinine; CYC; cyclophosphamide; ESRD; end-stage renal disease; GC, glucocorticoids; GFR, glomerular filtration rate; GN, glomerulonephritis; GPA, granulomatosis with polyangiitis; ISU, immunosuppressive therapy; IVIG, intravenous immunoglobulin; LEF, leflunomide; MMF, mycophenolate mofetil; mo, months; MPA, microscopic polyangiitis; MTX; methotrexate; PEX; plasma exchange; RTX, rituximab; TMP-SMX, trimethoprim/sulfamethoxazole; y, years.
The standard induction immunosuppression therapy is glucocorticoids combined with cyclophosphamide (CYC) and/or rituximab (RTX) (Figure 3, Table 2).
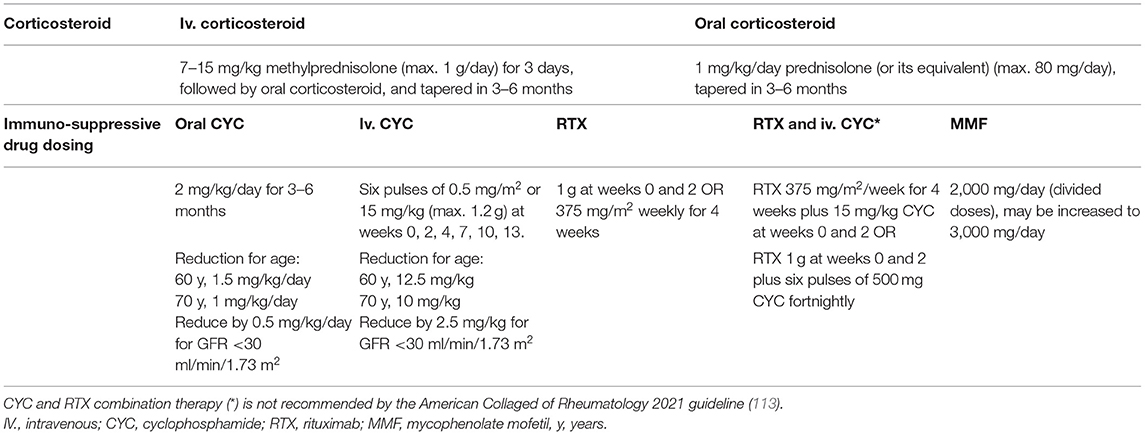
Table 2. Remission induction therapy in ANCA-associated glomerulonephritis according to the KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases (43).
Glucocorticoid (GC) treatment may include high dose (1–3 g) of intravenous (IV) methylprednisolone in patients presenting with RPGN, and daily oral glucocorticoids tapered over 5 months in all patients with organ-threatening disease. IV methylprednisolone is routinely administered in severe diseases; however, it has never been tested in randomized controlled trials. The recent ACR guideline recommends - as an ungraded position statement - either IV pulse or high-dose oral glucocorticoid treatment as a part of the initial therapy of active, severe GPA/MPA (113). As prolonged administration and high dose oral corticosteroids are associated with high risk of adverse events, recent recommendations favor therapies with accelerated taper, thus lower glucocorticoid requirements. According to the results of the Plasma Exchange and Glucocorticoids for Treatment of Anti-Neutrophil Cytoplasm Antibody (ANCA)-Associated Vasculitis (PEXIVAS) trial, rapid tapering of glucocorticoids may eliminate some side effects without altering the efficacy of the therapy (114). Based on the protocol and results of the PEXIVAS study, both KDIGO and ACR 2021 guidelines recommend the reduced-dose glucocorticoid regimen over the standard dose in patients with active, severe GPA/MPA (Level of evidence: very low to moderate) (43, 113). The use of rituximab in combination with intravenous or oral cyclophosphamide permits lower glucocorticoid exposure as well (115). The oral complement C5a receptor inhibitor, avacopan as a potential alternative to glucocorticoids, may also reduce glucocorticoid-related toxicity and improve patent quality of life (116).
Cyclophosphamide has been used for induction immunosuppression of AAV for almost 50 years now (117). The European Vasculitis Study Group compared daily oral CYC with intravenous pulse CYC regimens (CYCLOPS trial) and found that both routes of admission induced remission. As a result of the decreased cumulative dose, pulse CYC was associated with fewer cases of leukopenia and infection, but also demonstrated a tendency toward a higher risk of disease relapse (118). Older adults and patients with impaired renal function require dose reductions (Table 2) (43).
The Rituximab for ANCA-Associated Vasculitis (RAVE) and the Rituximab versus Cyclophosphamide in ANCA-associated Renal Vasculitis (RITUXVAS) trials compared RTX- to CYC-based induction treatment. Remission rates, relapse rates, or adverse events (119, 120) were similar for the two drugs. RTX is also highly efficient in relapsing disease to re-induce remission. (121). RTX provided a higher initial remission rate in aPR3-ANCA positive patients (122). Phenotypic characterization of B cells is suggested to aid the selection of therapy. AAV patients with a higher level of class-switched memory B cells or IgD−CD27− B cells, rituximab is likely to be more effective than cyclophosphamide, enabling earlier glucocorticoid tapering and better survival rates (123). In severe kidney disease (serum creatinine > 4 mg/dL or 354 μmol/L), however, no randomized clinical trials were conducted with RTX alone, only in combination with CYC (124). The potential superiority of the combination therapy is currently under investigation in the ongoing ENDURRANCE-1 randomized-controlled trial (NCT03942887). The main difference between the recent ACR and KDIGO guidelines from 2021 is related to the CYC vs. RTX use in the remission induction therapy. ACR recommends RTX over CYC for patients with active, severe GPA/MPA (113), while the KDIGO recommendation does not declare superiority but provides factors to consider while choosing from the protocols. RTX is preferred in children, adolescents, and reproductive-aged patients, in frail older adults, in relapsing or PR3-ANCA disease, or when glucocorticoid-sparing is especially important. CYC remained the first choice in severe kidney disease (serum creatinine > 4 mg/dL or 354 μmol/L), because of the lack of evidence from randomized controlled trials (43). Based on the protocol of the RITUXIVAS study, KDIGO provides a combination regimen of RTX and CYC (practice point, not a recommendation), while ACR does not suggest combined therapy.
A randomized controlled trial compared mycophenolate mofetil (MMF) to pulse cyclophosphamide and found mycophenolate non-inferior for remission induction in patients with estimated GFR >15 ml/min/1.73 m2. However, relapse rates were higher, mostly in PR3-ANCA positive patients (125). This suggests that MMF could be utilized as first-line induction therapy in MPO-ANCA positive individuals with moderate renal damage (126). In fact, the latest KDIGO recommendation also included MMF as induction in the therapeutic palette (Table 2) (43). According to the ACR guideline, MMF may be a secondary choice in active, non-severe GPA, or tertiary choice in the maintenance therapy of active severe GPA/MPA (113).
Methotrexate (MTX) is the first choice combined with GC in active, non-severe GPA according to the ACR guideline (113). MTX is recommended over CYC or RTX in this patient group, based on its less toxicity and greater clinical experience. The definition of non-severe disease is vasculitis without organ- or life-threatening manifestations (e.g., rhinosinusitis, mild systemic symptoms, mild arthritis, etc.), so it is not to be considered in active glomerulonephritis. The KDIGO group focused on AAV with glomerulonephritis and recommends MTX only as maintenance therapy if azathioprine (AZA) or MMF are not tolerated and eGFR >60 ml/min/1.73 m2.
In refractory diseases, glucocorticoid dosage may be increased with the addition of CYC (if previously RTX was used) or with the addition of RTX (if previously CYC was used). Plasmapheresis may also be part of the treatment in poor responders (43), according to the KDIGO guideline. ACR recommends switching between CYC or RTX therapies in active, severe refractory GPA/MPA over combining the two therapies. Intravenous immunoglobulin (IVIG) may be also added to refractory GPA/MPA (ACR recommendation) (113). Secondary causes of AAV should always be excluded in the case of refractory disease (drug- or malignancy-induced AAV, infections). The importance of the kidney biopsy is clear in refractory diseases to rule out other underlying kidney diseases and evaluate disease activity or chronic, irreversible tissue damage.
Since anti-neutrophil-associated antibodies are pathogenic in AAV, their rapid removal by plasmapheresis may control disease activity and prevent organ damage. Plasma exchange also removes cytokines and inflammatory mediators that enhance the harmful effects of the autoantibodies (127). The addition of plasmapheresis to standard therapy, however, is a controversial issue. The Plasma Exchange for Renal Vasculitis (MEPEX) trial demonstrated that plasmapheresis improved renal outcome in patients with severe kidney disease (128). In the PEXIVAS trial, however, plasmapheresis conferred survival benefits neither in patients with eGFR <50 ml/min/1,73m2 nor in patients with lung hemorrhage (114), and it only reduces the risk of ESRD within 12 months. On the other hand, it increases the risk of serious infections, particularly in the first year of treatment (129). The lack of kidney biopsy during the enrollment in this trial, however, limits the generalizability of the results. According to the American Society of Apheresis (ASFA), plasmapheresis is recommended in cases of biopsy-proven RPGN, signs of active inflammation in the glomeruli such as fibrinoid necrosis or crescents with a small degree of fibrosis, and a fulminant clinical course (130). This recommendation overlaps with the latest KDIGO guideline (43), where plasmapheresis is recommended only in the case of severe RPGN (serum creatinine > 500 μmol/L or 5.65 mg/dL), if alveolar hemorrhage with hypoxemia, or anti-GBM positivity is present (Practice point, not a recommendation). ACR guideline is also against the routine addition of plasma exchange in active severe GPA/MPA with glomerulonephritis (level of evidence is low to high), and plasmapheresis may be considered only in patients with a high risk for ESRD or with anti-GBM positivity (113) (Figure 3).
After the induction therapy, a lower level of immune suppression is required to maintain the achieved remission and prevent relapse. The most common maintenance therapy comprises a reduced dose of glucocorticoids with azathioprine (AZA) or RTX (without GC) (Table 3).
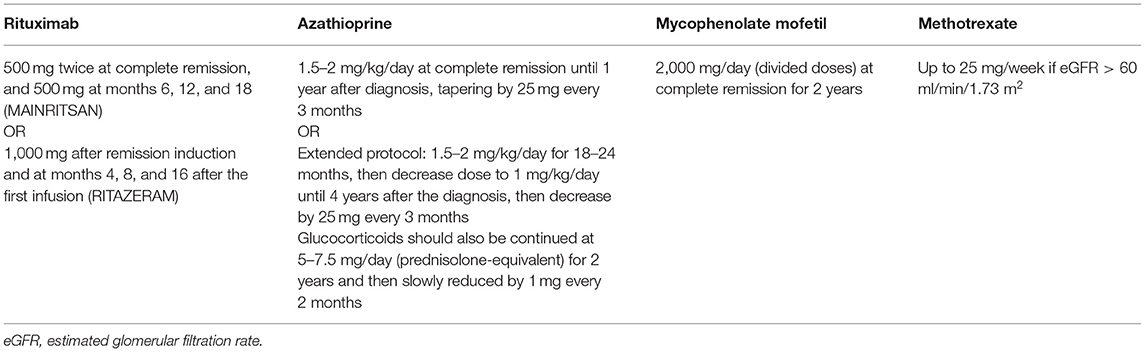
Table 3. Immunosuppressive maintenance therapy in ANCA-associated glomerulonephritis according to the KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases (43).
AZA proved to be a safe and efficient alternative to oral CYC for maintenance therapy in the Cyclophosphamide vs. Azathioprine for Early Remission Phase of Vasculitis (CYCAZAREM) trial. Severe adverse events and relapse rates did not differ between the groups, however, AZA treatment resulted in fewer cases of leukopenia (131).
According to the Maintenance of Remission using Rituximab in Systemic ANCA-associated Vasculitis (MAINRITSAN) trial, RTX-treated patients experience fewer major relapses than AZA-treated peers, and RTX was superior to AZA in PR3-ANCA positive patients (132). There are more types of protocols with different doses of RTX in the clinical trials and comparative trial has not been conducted yet (500 mg IV every 6 months – MAINRITSAN scheme, approved by FDA (U.S. Food and Drug Administration) vs 1,000 mg IV every 4 months – RITAZAREM scheme), so the optimal protocol is yet to be determined (132, 133). However, the efficient but lower dose of RTX might be a better choice to reduce side effects. The efficiency of RTX vs. AZA treatment in relapsing disease is under investigation in the Rituximab vs. Azathioprine as Therapy for Maintenance of Remission for Anti-neutrophil Cytoplasm Antibody-associated Vasculitis (RITAZAREM, NCT01697267) trial (133).
As compared to AZA, maintenance therapy with MMF resulted in the same level of adverse effects, proteinuria, glomerular filtration rate, and disease activity score, but a higher relapse rate (IMPROVE) (134). MTX, however, may serve as an alternative for AZA, at least in patients with preserved renal function (eGFR > 60 ml/min/1,73 m2). According to the WEGENT trial, MTX- and AZA-based maintenance therapies are equally effective in preventing relapse and provide similar long-term outcomes (135).
While high doses of glucocorticoids and associated toxicity is a relevant concern in induction therapy, it must be noted that the prolonged administration of low-dose glucocorticoids seems to contribute to efficient maintenance therapy and also prevents relapses (136). According to KDIGO and ACR recommendations, AZA treatment has to be combined with low-dose glucocorticoids, whereas RTX treatment is not. In the MAINRITSAN trial, however, RTX was applied together with low-dose prednisone treatment for at least 18 months, and the prolonged glucocorticoid treatment could have contributed to the unexpectedly low major relapse rate (132).
The recommendations regarding the first choice of maintenance therapy are slightly different according to the most recent guidelines. KDIGO recommends either RTX or AZA with low-dose GC for maintenance (level and quality of evidence 1C) while providing factors to consider choosing the best therapy for individual patients. RTX is preferred in relapsing disease, in PR3-ANCA disease, frail or older adults, when GC-sparing is important, or in AZA-allergy. AZA is preferred with hepatitis B exposure, low baseline immunoglobulin G (IgG < 300 mg/dl) level, or when RTX is not available (43). MMF provides an alternative to RTX or AZA, while MTX can be used if kidney function is preserved, according to the KDIGO group. On the other hand, ACR recommends RTX on a schedule-based protocol in the maintenance therapy of active, severe GPA/MPA (level of evidence: very low to moderate) over other therapies and suggests IVIG administration if IgG level is low during RTX treatment and recurrent infections are present (113). MTX, AZA, MMF, and leflunomide could be secondary or tertiary choice during maintenance. In active, non-severe GPA, the first choice of maintenance is the same medication as the remission was reached with and therapy switching is needed only in relapsing disease (113) (Figure 3).
The optimal duration of maintenance treatment is yet to be determined. Too early withdrawal of therapy increases the risk of relapse, while prolonged immunosuppression is associated with greater toxicity. The current KDIGO guideline suggests AZA to be administered for at least 18 months and therapy may extend to 4 years (43). RTX maintenance was studied up to 18 months. While individually tailored rituximab regimens – administration upon B-cell return or serologic relapse – are as effective as fixed-schedule ones in preventing relapse, based on clinical experience and the limited power of the related trial, ACR recommends scheduled RTX re-dosing during remission maintenance (Level of evidence very low to low) (113, 137).
Although induction protocols are commonly effective in remission induction, maintenance regimens vary in efficacy. Depending on the treatment regimens, relapse rates range from 30 to 50% at 5 years (138).
The rise in the ANCA titer is suggested as an early sign of the impending relapse. Doubling of ANCA-level in the previous 3 months provides an 11-times higher chance for relapse in the next 1.5 years (139). Nevertheless, it is still debatable whether the ANCA persistence or the rise could predict the disease activity and should lead to an abrupt change of therapy (140, 141). In many cases, ANCA persistence and even elevation in ANCA-titers are not associated with worsening clinical features, though ANCA persistence is often linked to partial remission or subclinical appearance, and a “second hit” is required to trigger a manifest vasculitis. In addition, ANCA-titers have a modest correlation with disease activity (140).
The reason behind this observation is not entirely clear, but it might trace back to the pathogenicity of ANCA: epitope specificity and sialylation ratio which are thought to regulate ANCA reactivity (142, 143).
Identifying clinical features and/or biomarkers to predict an imminent relapse reliably would be essential in the chronic care and follow-up in AAV patients and it remains a key research priority. Anti-PR3 positivity, granulomatous disease, lower serum creatinine at presentation, nasal Staphylococcus aureus colonization, ear-nose-throat involvement, and prior relapses convey a higher relapse risk (144). Though BVAS (v3) is usually used in clinical trials to measure symptoms objectively, it can be an appropriate tool in everyday practice to evaluate the symptoms of AAV. The relapse of the disease can affect a previously not involved organ system (145), so a proper, structured checklist of the possible symptoms (persistent and new-onset) aids the physicians' work. Online BVAS (v3) calculators are available and make it easy to register the patients' disease activity score in their documentation during the follow-up period (146). Both recent guidelines agree on immunosuppressive therapy dosing should not be based on ANCA titers only but the patient's clinical symptoms parallel with diagnostic findings should be considered in treatment decisions (43, 113).
Patients with severe relapsing disease should be treated by reintroducing induction therapy (43, 113). According to the current guidelines, RTX is preferred to be administered during the relapse, as higher remission rates were found with RTX compared to CYC in relapsing disease in a post hoc analysis of the RAVE trial, especially in PR3-ANCA positive patients (122). For patients with severe relapse while receiving RTX for remission maintenance, switching therapy to CYC is a better choice than receiving RTX for remission re-induction, especially if the patient recently received RTX (113). If CYC has been chosen to induce remission in relapsing disease, it is very important to calculate the cumulative dosage, as the risk of malignancies increases significantly above the total of 36 g of CYC administered (147). In mild relapses, CYC should be avoided (43).
High-dose (IVIG) has immunomodulatory effects via neutralizing complements, inhibiting the maturation and function of dendritic cells, inflammatory cytokines, enhancing the expansion of regulating T-cells, and decreasing Th1 and Th17 cells. It also helps to neutralize autoantibodies (148). IVIG is proved to be a beneficial adjunctive therapy not only in refractory but also in relapsing AAV diseases by decreasing disease activity and having an acceptable safety profile (149). IVIG can be used as a salvage therapy in active GPA/MPA in special patient groups when other therapies are contraindicated (e.g., during pregnancy) (113).
To conclude, recognizing patients with a high risk of renal relapse would benefit clinical care and therapy optimization. Clinical relapse occurs mainly at the initiation of the maintenance therapies or during their tapering (140) which suggests that the timing to treat relapse and precise therapy regimes still need to be elucidated.
As discussed above, histologic findings - according to the Berden classification - correlate well with the prognosis. Focal pattern is associated with the best, while sclerotic histology is related to the poorest outcomes. Ambivalently, the ESRD-free survival rate is surprisingly high in the sclerotic group: it remains 50% until 5 years and only lowers at around 7 years, while in other categories, it worsens gradually (100). The sclerotic group of patients cannot be considered homogenous. Patients with an eGFR of more than 15 ml/min/1.73 m2 at baseline have a substantially higher long-term ESRD-free survival rate, and their renal survival rate is approximately the same as in patients with mixed or crescentic pattern. Sclerotic histology with eGFR <15 ml/min/1.73 m2 however, is proposed to have a poor prognosis (150). Glomerular crescents are correlated with a better outcome than sclerosis, suggesting that active inflammation may be suppressed or even treated with immunosuppressive therapy, while chronic lesions are most likely to be irreversible. Sclerosis and its extent is associated with advanced chronicity: the longer the time from the diagnosis, the worse the prognosis (151, 152). ANCA Renal Risk Score (ARRS) predicts renal outcome according to the percentage of normal glomeruli, tubular atrophy, interstitial fibrosis, and renal function at the time of the diagnosis (153). Chronicity Score by Mayo Clinic/Renal Pathology Society (MCCS) includes glomerulosclerosis, interstitial fibrosis, tubular atrophy, and arteriosclerosis. Higher MCCS grades are associated with decreased renal function and poor outcome (154), as well as treatment resistance (155).
As regards clinical parameters, older age, lower GFR, and MPO-ANCA positivity are associated with poorer renal outcome (151). Treatment resistance was also anticipated by anti-MPO positivity, female sex, and black race (156). Patients who underwent B cell depletion and have a low or decreasing number of CD5+ B cells after repopulation are more likely to have a shorter relapse time. Pulmonary hemorrhage correlated with overall poor outcome as well (157, 158).
Cardiovascular diseases are the most common cause of mortality in AAV after the first year (67). Autoimmune abnormalities are known to play a role in atherogenesis and the acceleration of atherosclerosis (68). As a result, hypertension, the risk of ischemic heart disease, therefore cardiomyopathy, myocardial infarction, transient ischemic attack, and stroke increase in AAV patients (67, 159). Venous thrombotic events are also frequently seen in association with AAV (66, 160). Serosal involvement is rare compared to other autoimmune diseases and occurs mainly in EGPA (70). Besides the damage to the small vessels and chronic systemic inflammatory state, prolonged glucocorticoid treatment can enhance cardiovascular morbidity in AAV patients. It is, therefore, prudent to evaluate the patients' traditional cardiovascular risk factors (smoking habits, dyslipidemia, hypertension, diabetes mellitus, obesity, physical inactivity), and address them during their chronic care.
AAV is associated with an increased risk of cancer development, and malignancies particularly affect the urinary tract and the skin. Hematologic malignancies are also more frequent (161). Chronic inflammation is known to be a risk factor in oncogenesis. By creating excessive oxidative stress, enhancing cell proliferation, angiogenesis, and cell invasion, inflammation favors tumor-microenvironment (162). Long-standing immune activation leads to excessive immune cell proliferation and a greater risk of acquired genetic aberrations, and resistance to apoptosis, therefore to a rise of a malignant clone (163).
Immune surveillance is the process, in which the immune system recognizes precancerous or cancerous cells and eliminates them. Autoimmune diseases mean immune dysregulation, hence impaired removal of the altered cells. Surveillance is damaged by immunosuppressive therapies as well (164), and in addition, many of them have a direct cytotoxic and carcinogenic effect (161, 165).
CYC is particularly associated with oncogenic features. Its metabolites, acrolein, and chloro-acetaldehyde, cause DNA breakage. They are excreted in the urine and get in direct contact with the urothelium. The accumulation of toxic metabolites in the bladder can cause bladder cancer (161), the incidence of which may be decreased by hyperhydration and by the use of sodium 2-mercaptoethanesulfonate (MESNA) (161). As mentioned above, high cumulative CYC dosage increases the risk of malignancies (147).
Of note, rituximab does not increase the risk of malignancy compared to the general population (166).
Vasculitis and infections are tightly linked. Bacterial infections can initiate ANCA production and infections are common complications of immunosuppressive therapy. They are reported in 20–76% of the cases of which three-quarter takes place during induction therapy (167, 168). In fact, infections remain the major cause of death (159, 167, 168). Renal involvement raises the risk of serious infections, and it is linked to worse outcome as well as older age, higher BVAS score, diabetes, and smoking (168). Pneumonia is the most common form of infection, followed by sepsis, urinary tract, and skin infections (168). Bacterial infections owe up to 80% of the infections, and Staphylococcus aureus, Acinetobacter baumanni, Escherichia coli, Klebsiella species, Enterococci, and Pseudomonas aeruginosa are frequent underlying pathogens. Viral infections are usually caused by Cytomegalovirus, Varicella-Zoster, Influenzae, and Herpes simplex virus, and they occur in 10–15% of the cases. Fungal and parasitic infections contribute to about 10% of the infections, of which Pneumocystis jiroveci, Aspergillus fumigatus, and Candida species, are the most common pathogens (168, 169). For the prevention of opportunistic infections, low-dose sulfamethoxazole/trimethoprim or pentamidine is recommended for the course of the cyclophosphamide treatment, and at least 6 months after rituximab induction therapy (43, 113). Prophylactic antiviral and antifungal agents could be considered in severely immunocompromised patients (170).
Hepatitis B and C reactivation can occur as a consequence of immunosuppressive therapy; therefore, screening is advised before treatment. Preemptive antiviral prophylaxis (lamivudine, entecavir, or tenofovir) should be prescribed if the patient has a history of hepatitis B or C infection (171). To avoid the flare-up of latent tuberculosis, QuantiFERON-TB Gold screening is also rational prior to the initiation of the immunosuppressive treatment, however, there is a high rate of indeterminate results in patients with systemic vasculitis (172).
A severe but very rare infectious complication of rituximab treatment is progressive multifocal leukoencephalopathy (PML), caused by the JC polyoma virus. JC virus infects commonly and persistently the urinary tract of adults. The PML-associated neurotropic JC virus strain contains distinctive mutations in the surface loops of the major capsid protein, leading to escape from antibody-mediated neutralization. PML used to be the rare complication of HIV (human immunodeficiency virus) infected patients, but it has become more frequently related to immunosuppressive treatments with monoclonal antibodies. The cumulative reporting rates of confirmed PML in HIV-negative patients were at about two per 100 000 patients in the period 1998 to 2007, then increased to 7 to 8 per 100 000, which included patients with hematologic and oncologic diseases. The higher number of registered cases is partly due to the better awareness of the disease (173).
Live attenuated vaccines are contraindicated during immunosuppressive therapies. Inactivated vaccines are recommended at least 2 weeks prior to the initiation of the treatment. In case of rituximab, vaccines should be given at least 5 months after the last dose and at least 4 weeks before the administration (174).
Despite the evolution of immunosuppressive therapy in AAV, 20–25% of the patients develop ESRD and need renal replacement therapy (126). More AAV patients receive hemodialysis than peritoneal dialysis, and the relapse rate of AVV in dialyzed patients is low (0.08 episodes per person-year) (175). It is still controversial whether to continue immunosuppressive maintenance therapy in patients on dialysis. Continuing the therapy raises the risk of infectious diseases (175), while the withdrawal may expose the patients to extra-renal manifestations. In MPO-ANCA diseases without extrarenal manifestations, the withdrawal of the maintenance treatment after 6 months seems to be a safe strategy (126). The recent KDIGO guideline suggests discontinuing immunosuppressive therapy after 3 months in patients on dialysis and without any extrarenal manifestation (Practice point) (43). However, a randomized controlled trial needs to be conducted to address this question, such as the ongoing MASTER ANCA trial (NCT03323476).
Renal transplantation provides better quality of life for patients with ESRD. Patients and transplant kidney survival is similar to nondiabetic patients (176). The relapse rate is low (it was 0.02–0.028 episodes per patient-year in a multicenter retrospective cohort) and was not affected by the ANCA subtype or the duration of remission before transplantation (177, 178). The current KDIGO guideline suggests withholding transplantation until the patient is in complete clinical remission for ≥6 months. Persistence of elevated but stable ANCA titers should not delay transplantation (43). Relapse should be confirmed by histopathology, and the same treatment protocol is recommended for the relapse as discussed above (43).
Bortezomib is a proteasome inhibitor, which is implicated to inhibit NF-kB, halting cell proliferation, and committing B-cells to apoptosis (179). Bortezomib proved to be efficient in a mouse model of MPO-ANCA glomerulonephritis (180). A case study reported complete remission in a refractory disease (181). However, clinical trials have not been performed because of its frequent adverse events (182, 183).
Adding hydroxychloroquine to standard therapy proved to be effective in reducing relapses, and steroid doses. It helped to improve symptoms and it was well tolerated as well (184). An ongoing randomized-controlled study (HAVEN) is assessing its efficacy (NCT04316494).
Belimumab, an anti-BAFF monoclonal antibody may inhibit the AAV-associated overexpression of BAFF (185) and promote B-cell apoptosis. Thus, the formation of mature, autoreactive B-cells is impeded and class switching and Th1 responses are also altered favorably. Belimumab was studied as a potential maintenance agent, but it did not reduce the risk of relapse (186).
An ongoing study (COMBIVAS) evaluates the efficacy of combined rituximab and belimumab (NCT03967925), and another one (BREVAS) belimumab combined with azathioprine (186).
Abatacept is a fusion protein of the Fc region of human IgG1 and the extracellular domain of the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4/CD152). CTLA-4 is a constitutively expressed receptor on regulatory T-cells, and it is homologous to the co-stimulatory T-cell protein, CD28. By binding to CD80 and CD86 on antigen-presenting cells with a higher affinity than CD28, abatacept prevents T-cell activation (187). A study proved abatacept effective and well-tolerated in relapsing GPA patients (188), and the results of a double-blind randomized controlled trial of abatacept as first-line treatment for AAV (ABAVAS) are expected to be published soon (NCT00482066). It is to note that these studies excluded patients with severe disease, including glomerulonephritis.
A case report of two patients demonstrated remission with the use of C5 inhibitor, eculizumab, but it has never been studied more thoroughly (189).
A randomized study, Alemtuzumab for ANCA Associated Refractory Vasculitis (ALEVIATE), studied alemtuzumab, a monoclonal antibody against the CD52 on T-cells, targeting them to destruction (190). Although the majority of patients reached remission at 6 months, adverse effects were more common than in standard therapies. Alemtuzumab also triggered vasculitis in several case studies (191, 192).
Tocilizumab, a humanized interleukin-6 receptor antibody, could be a potential therapeutic drug in AAV. The efficacy of tocilizumab has already been proved for large-vessel vasculitis, and some studies also detected clinical remission in AAV (193). Large-scale clinical trials, like SATELITE (NCT04871191), are needed to determine the long-term renal and patient outcomes (33).
Promising results have recently been published from the European IXCHANGE study of an anti-C5a antibody, vilobelimab. Vilobelimab proved to be efficient in maintaining therapy in terms of remission with good safety and tolerability profile. Vilobelimab use is suspected to be able to lower glucocorticoid dosage and its toxicity (NCT03712345).
The pivotal role of peptidylarginin-deiminase 4 (PADI) in NET formation has been demonstrated. Correspondingly, PADI inhibitors reduce histone citrullination and NET release both in vivo and in vitro (194). Selective PADI-4 inhibitor, GSK484 was successful in anti-MPO glomerulonephritis by reducing the inflammatory response and the subsequent segmental necrosis (195). Hence, PADI inhibitors could potentially contribute to the therapeutic armamentarium of NETosis-associated diseases.
Leflunomide is a dihydro-orotate-dehydrogenase inhibitor which is responsible for pyrimidine synthesis. By halting the synthetic pathway, it arrests the actively proliferating T-, and B-lymphocytes and proved efficient in a small number of patients (196). Currently, a study is comparing the efficacy of azathioprine and leflunomide as a maintenance regime (NCT04737343).
Figure 4 summarizes the therapeutic evolution of ANCA-associated glomerulonephritis, with the milestones of the currently used therapies (Figure 4A), and previous trials with medications with positive outcome but not used in practice with ongoing trials (Figure 4B) (181, 184, 186, 189, 193, 197–199) (NCT03712345, NCT04316494, NCT00482066, NCT04737343, NCT00482066, NCT04871191, NCT04973033, NCT03942887, NCT02749292, NCT03967925).
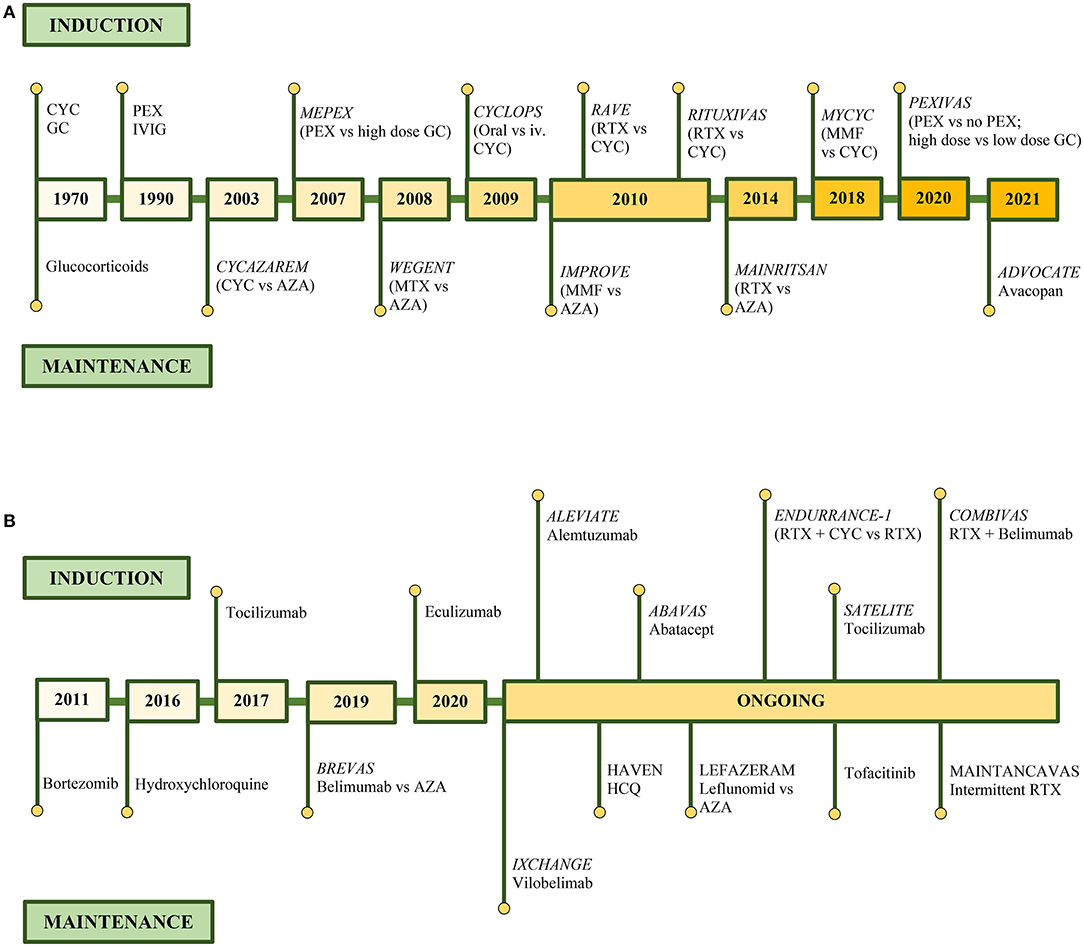
Figure 4. Therapeutic evolution of ANCA-associated glomerulonephritis. Clinical trials in remission induction and maintenance therapy of currently used medications (A), and medications with positive outcome but currently not presented in guidelines and on-going clinical trials (B).
The initially nonspecific, then heterogenic symptoms can mislead the diagnostic approach and cause substantial delay in the adequate treatment of ANCA-associated glomerulonephritis. In the absence of distinct symptoms, renal-limited and not rapidly progressive forms are also challenging to identify. The clinical features and laboratory parameters of ANCA-associated vasculitis can mimic infection, malignancy, and other autoimmune diseases. ANCA-negative and atypical ANCA cases can complicate the road to the diagnosis. The first but necessary step of the diagnostic approach is to think of it. Although the treatment options are continuing to improve, the prompt diagnosis and treatment initiation of ANCA-associated glomerulonephritis have remained crucial factors in patient and renal outcome.
AM, PS, and NL participated in reviewing the literature and writing the manuscript. All authors agree to be accountable for the content of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The images in Figure 2 were provided by Dr. Magdolna Kardos, MD, nephropathologist, Institute of Pathology, Forensic and Insurance Medicine, Semmelweis University, Budapest, Hungary. We would like to thank Mbuotidem Jeremiah Thomas, MD, Ph.D. for the overall English proofreading.
1. Tan JA, Dehghan N, Chen W, Xie H, Esdaile JM, Avina-Zubieta JA. Mortality in ANCA-associated vasculitis: ameta-analysis of observational studies. Ann Rheum Dis. (2017) 76:1566–74. doi: 10.1136/annrheumdis-2016-210942
2. Sinico RA, Di Toma L, Radice A. Renal involvement in anti-neutrophil cytoplasmic autoantibody associated vasculitis. Autoimmun Rev. (2013) 12:477–82. doi: 10.1016/j.autrev.2012.08.006
3. Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. (2003) 41:776–84. doi: 10.1016/S0272-6386(03)00025-8
4. Berti A, Cornec-Le Gall E, Cornec D, Casal Moura M, Matteson EL, Crowson CS, et al. Incidence, prevalence, mortality and chronic renal damage of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in a 20-year population-based cohort. Nephrol Dial Transplant. (2019) 34:1508–17. doi: 10.1093/ndt/gfy250
5. Mohammad AJ. An update on the epidemiology of ANCA-associated vasculitis. Rheumatology (Oxford). (2020) 59(Suppl 3):iii42-iii50. doi: 10.1093/rheumatology/keaa089
6. Quartuccio L, Treppo E, Valent F, De Vita S. Healthcare and economic burden of ANCA-associated vasculitis in Italy: an integrated analysis from clinical and administrative databases. Intern Emerg Med. (2021) 16:581–9. doi: 10.1007/s11739-020-02431-y
7. Ntatsaki E, Watts RA, Scott DG. Epidemiology of ANCA-associated vasculitis. Rheum Dis Clin North Am. (2010) 36:447–61. doi: 10.1016/j.rdc.2010.04.002
8. Lane SE, Watts R, Scott DG. Epidemiology of systemic vasculitis. Curr Rheumatol Rep. (2005) 7:270–5. doi: 10.1007/s11926-005-0036-5
9. Gibson A, Stamp LK, Chapman PT, O'Donnell JL. The epidemiology of Wegener's granulomatosis and microscopic polyangiitis in a Southern Hemisphere region. Rheumatology (Oxford). (2006) 45:624–8. doi: 10.1093/rheumatology/kei259
10. Cornec D, Cornec-Le Gall E, Specks U. Clinical trials in antineutrophil cytoplasmic antibody-associated vasculitis: what we have learnt so far, and what we still have to learn. Nephrol Dial Transplant. (2017) 32(suppl_1):i37-i47. doi: 10.1093/ndt/gfw384
11. Chen M, Kallenberg CG. The environment, geoepidemiology and ANCA-associated vasculitides. Autoimmun Rev. (2010) 9:A293–8. doi: 10.1016/j.autrev.2009.10.008
12. Berti A, Cornec D, Crowson CS, Specks U, Matteson EL. The epidemiology of antineutrophil cytoplasmic autoantibody-associated vasculitis in Olmsted County, Minnesota: a twenty-year US population-based study. Arthritis Rheumatol. (2017) 69:2338–50. doi: 10.1002/art.40313
13. Weiner M, Bjørneklett R, Hrušková Z, Mackinnon B, Poulton CJ, Sindelar L, et al. Proteinase-3 and myeloperoxidase serotype in relation to demographic factors and geographic distribution in anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Nephrol Dial Transplant. (2019) 34:301–8. doi: 10.1093/ndt/gfy106
14. Fujimoto S, Uezono S, Hisanaga S, Fukudome K, Kobayashi S, Suzuki K, et al. Incidence of ANCA-associated primary renal vasculitis in the Miyazaki Prefecture: the first population-based, retrospective, epidemiologic survey in Japan. Clin J Am Soc Nephrol. (2006) 1:1016–22. doi: 10.2215/CJN.01461005
15. Grisaru S, Yuen GW, Miettunen PM, Hamiwka LA. Incidence of Wegener's granulomatosis in children. J Rheumatol. (2010) 37:440–2. doi: 10.3899/jrheum.090688
16. Celakil ME, Yucel BB, Ozod UK, Bek K. Anca-associated crescentic glomerulonephritis in a child with isolated renal involvement. J Bras Nefrol. (2019) 41:293–5. doi: 10.1590/2175-8239-jbn-2018-0062
17. Watts RA, Gonzalez-Gay MA, Lane SE, Garcia-Porrua C, Bentham G, Scott DG. Geoepidemiology of systemic vasculitis: comparison of the incidence in two regions of Europe. Ann Rheum Dis. (2001) 60:170–2. doi: 10.1136/ard.60.2.170
18. Gonzalez-Gay MA, Garcia-Porrua C, Guerrero J, Rodriguez-Ledo P, Llorca J. The epidemiology of the primary systemic vasculitides in northwest Spain: implications of the Chapel Hill Consensus Conference definitions. Arthritis Rheum. (2003) 49:388–93. doi: 10.1002/art.11115
19. Jennette JC, Nachman PH. ANCA Glomerulonephritis and Vasculitis. Clin J Am Soc Nephrol. (2017) 12:1680–91. doi: 10.2215/CJN.02500317
20. Molnar A, Thomas MJ, Fintha A, Kardos M, Dobi D, Tisler A, et al. Kidney biopsy-based epidemiologic analysis shows growing biopsy rate among the elderly. Sci Rep. (2021) 11:24479. doi: 10.1038/s41598-021-04274-9
21. Bonatti F, Reina M, Neri TM, Martorana D. Genetic susceptibility to ANCA-associated vasculitis: state of the art. Front Immunol. (2014) 5:577. doi: 10.3389/fimmu.2014.00577
22. Lee KS, Kronbichler A, Pereira Vasconcelos DF, Pereira da Silva FR, Ko Y, Oh YS, et al. Genetic Variants in antineutrophil cytoplasmic antibody-associated vasculitis: a bayesian approach and systematic review. J Clin Med. (2019) 8:266. doi: 10.3390/jcm8020266
23. Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DRW, et al. Genetically Distinct Subsets within ANCA-Associated Vasculitis. N Engl J Med. (2012) 367:214–23. doi: 10.1056/NEJMoa1108735
24. Li W, Huang H, Cai M, Yuan T, Sheng Y. Antineutrophil cytoplasmic antibody-associated vasculitis update: genetic pathogenesis. Front Immunol. (2021) 12:624848. doi: 10.3389/fimmu.2021.624848
25. Scott J, Hartnett J, Mockler D, Little MA. Environmental risk factors associated with ANCA associated vasculitis: a systematic mapping review. Autoimmun Rev. (2020) 19:102660. doi: 10.1016/j.autrev.2020.102660
26. Shakoor MT, Birkenbach MP, Lynch M. ANCA-Associated Vasculitis Following Pfizer-BioNTech COVID-19 Vaccine. Am J Kidney Dis. (2021) 78:611–3. doi: 10.1053/j.ajkd.2021.06.016
27. Sekar A, Campbell R, Tabbara J, Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. (2021) 100:473–4. doi: 10.1016/j.kint.2021.05.017
28. Feghali EJ, Zafar M, Abid S, Santoriello D, Mehta S. De-novo Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Following the mRNA-1273 (Moderna) Vaccine for COVID-19. Cureus. (2021) 13:e19616. doi: 10.7759/cureus.19616
29. Hakroush S, Tampe B. Case Report: ANCA-Associated Vasculitis Presenting With Rhabdomyolysis and Pauci-Immune Crescentic Glomerulonephritis After Pfizer-BioNTech COVID-19 mRNA Vaccination. Front Immunol. (2021) 12:762006. doi: 10.3389/fimmu.2021.762006
30. Chen CC, Chen HY, Lu CC, Lin SH. Case report: anti-neutrophil cytoplasmic antibody-associated vasculitis with acute renal failure and pulmonary hemorrhage may occur after COVID-19 vaccination. Front Med (Lausanne). (2021) 8:765447. doi: 10.3389/fmed.2021.765447
31. Shah R, Thomas R, Mehta DS. Neutrophil priming: Implications in periodontal disease. J Indian Soc Periodontol. (2017) 21:180–5. doi: 10.4103/jisp.jisp_385_15
32. Kronbichler A, Lee KH, Denicolo S, Choi D, Lee H, Ahn D, et al. Immunopathogenesis of ANCA-Associated Vasculitis. Int J Mol Sci. (2020) 21:7319. doi: 10.3390/ijms21197319
33. Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol. (2019) 15:91–101. doi: 10.1038/s41584-018-0145-y
34. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. (2018) 18:134–47. doi: 10.1038/nri.2017.105
35. Yang H, Biermann MH, Brauner JM, Liu Y, Zhao Y, Herrmann M. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front Immunol. (2016) 7:302. doi: 10.3389/fimmu.2016.00302
36. Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V, A. myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. (2014) 8:883–96. doi: 10.1016/j.celrep.2014.06.044
37. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. (2010) 107:15880–5. doi: 10.1073/pnas.1005743107
38. van Dam LS, Rabelink TJ, van Kooten C, Teng YKO. Clinical Implications of Excessive neutrophil extracellular trap formation in renal autoimmune diseases. Kidney Int Rep. (2019) 4:196–211. doi: 10.1016/j.ekir.2018.11.005
39. Ramanujam M, Davidson A. The current status of targeting BAFF/BLyS for autoimmune diseases. Arthritis Res Ther. (2004) 6:197. doi: 10.1186/ar1222
40. Vegting Y, Vogt L, Anders HJ, de Winther MPJ, Bemelman FJ, Hilhorst ML. Monocytes and macrophages in ANCA-associated vasculitis. Autoimmun Rev. (2021) 20:102911. doi: 10.1016/j.autrev.2021.102911
41. Abdulahad WH, Lamprecht P, Kallenberg CG. T-helper cells as new players in ANCA-associated vasculitides. Arthritis Res Ther. (2011) 13:236. doi: 10.1186/ar3362
42. Geetha D, Seo P, Ellis C, Kuperman M, Levine SM. Persistent or new onset microscopic hematuria in patients with small vessel vasculitis in remission: findings on renal biopsy. J Rheumatol. (2012) 39:1413–7. doi: 10.3899/jrheum.111608
43. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. (2021) 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
44. Falk RJ, Hogan S, Carey TS, Jennette JC. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The glomerular disease collaborative network. Ann Intern Med. (1990) 113:656–63. doi: 10.7326/0003-4819-113-9-656
45. Chen KR. Skin involvement in ANCA-associated vasculitis. Clin Exp Nephrol. (2013) 17:676–82. doi: 10.1007/s10157-012-0736-x
46. Kawakami T, Soma Y, Kawasaki K, Kawase A, Mizoguchi M. Initial cutaneous manifestations consistent with mononeuropathy multiplex in Churg-Strauss syndrome. Arch Dermatol. (2005) 141:873–8. doi: 10.1001/archderm.141.7.873
47. Marzano AV, Raimondo MG, Berti E, Meroni PL, Ingegnoli F. Cutaneous Manifestations of ANCA-Associated Small Vessels Vasculitis. Clin Rev Allergy Immunol. (2017) 53:428–38. doi: 10.1007/s12016-017-8616-5
48. Almouhawis HA, Leao JC, Fedele S, Porter SR. Wegener's granulomatosis: a review of clinical features and an update in diagnosis and treatment. J Oral Pathol Med. (2013) 42:507–16. doi: 10.1111/jop.12030
49. Seo P, Stone JH. The antineutrophil cytoplasmic antibody-associated vasculitides. Am J Med. (2004) 117:39–50. doi: 10.1016/j.amjmed.2004.02.030
50. Thickett DR, Richter AG, Nathani N, Perkins GD, Harper L. Pulmonary manifestations of anti-neutrophil cytoplasmic antibody (ANCA)-positive vasculitis. Rheumatology (Oxford). (2006) 45:261–8. doi: 10.1093/rheumatology/kei217
51. Vanoli J, Riva M, Vergnano B, D'Andrea G, L'Imperio V, Pozzi MR, et al. Granulomatosis with polyangiitis presenting with diffuse alveolar hemorrhage requiring extracorporeal membrane oxygenation with rapid multiorgan relapse: a case report. Medicine (Baltimore). (2017) 96:e6024. doi: 10.1097/MD.0000000000006024
52. Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine (Baltimore). (1999) 78:26–37. doi: 10.1097/00005792-199901000-00003
53. Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. (2013) 65:270–81. doi: 10.1002/art.37721
54. Bacciu A, Buzio C, Giordano D, Pasanisi E, Vincenti V, Mercante G, et al. Nasal polyposis in Churg-Strauss syndrome. Laryngoscope. (2008) 118:325–9. doi: 10.1097/MLG.0b013e318159889d
55. Bacciu A, Bacciu S, Mercante G, Ingegnoli F, Grasselli C, Vaglio A, et al. Ear, nose and throat manifestations of Churg-Strauss syndrome. Acta Otolaryngol. (2006) 126:503–9. doi: 10.1080/00016480500437435
56. Tzelepis GE, Kokosi M, Tzioufas A, Toya SP, Boki KA, Zormpala A, et al. Prevalence and outcome of pulmonary fibrosis in microscopic polyangiitis. Eur Respir J. (2010) 36:116–21. doi: 10.1183/09031936.00110109
57. Deniz K, Ozşeker HS, Balas S, Akpýnar E, Sökmensüer C. Intestinal involvement in Wegener's granulomatosis. J Gastrointestin Liver Dis. (2007) 16:329–31.
58. Kawasaki K, Eizuka M, Murata O, Ishida K, Nakamura S, Sugai T, et al. Eosinophilic granulomatosis with polyangiitis involving the small intestine: radiographic and endoscopic findings. Endoscopy. (2015) 47 (Suppl 1) UCTN:E492-4. doi: 10.1055/s-0034-1393140
59. Franco DL, Ruff K, Mertz L, Lam-Himlin DM, Heigh R. Eosinophilic granulomatosis with polyangiitis and diffuse gastrointestinal involvement. Case Rep Gastroenterol. (2014) 8:329–36. doi: 10.1159/000369129
60. Rowaiye OO, Kusztal M, Klinger M. The kidneys and ANCA-associated vasculitis: from pathogenesis to diagnosis. Clin Kidney J. (2015) 8:343–50. doi: 10.1093/ckj/sfv020
61. Zheng Y, Zhang Y, Cai M, Lai N, Chen Z, Ding M. Central nervous system involvement in ANCA-associated vasculitis: what neurologists need to know. Front Neurol. (2018) 9:1166. doi: 10.3389/fneur.2018.01166
62. Holle JU, Gross WL. Neurological involvement in Wegener's granulomatosis. Curr Opin Rheumatol. (2011) 23:7–11. doi: 10.1097/BOR.0b013e32834115f9
63. Esposito D, Trimpou P, Giugliano D, Dehlin M, Ragnarsson O. Pituitary dysfunction in granulomatosis with polyangiitis. Pituitary. (2017) 20:594–601. doi: 10.1007/s11102-017-0811-0
64. Kubal AA, Perez VL. Ocular manifestations of ANCA-associated vasculitis. Rheum Dis Clin North Am. (2010) 36:573–86. doi: 10.1016/j.rdc.2010.05.005
65. Sorin SM, Răzvan-Marian M, Daniela MM, Dan-Alexandru T. Therapy of ocular complications in ANCA+ associated vasculitis. Rom J Ophthalmol. (2021) 65:10–4. doi: 10.22336/rjo.2021.3
66. Merkel PA, Lo GH, Holbrook JT, Tibbs AK, Allen NB, Davis JC Jr, et al. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener's Clinical Occurrence of Thrombosis (WeCLOT) Study. Ann Intern Med. (2005) 142:620–6. doi: 10.7326/0003-4819-142-8-200505030-00011
67. Kronbichler A, Leierer J, Gauckler P, Shin JI. Comorbidities in ANCA-associated vasculitis. Rheumatology (Oxford). (2020) 59(Suppl 3):iii79-iii83. doi: 10.1093/rheumatology/kez617
68. Cohen Tervaert JW. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract Res Clin Rheumatol. (2013) 27:33–44. doi: 10.1016/j.berh.2012.12.004
69. Ledó N, Petho ÁG. Gastrointestinal symptoms as first remarkable signs of ANCA-associated granulomatosis with polyangiitis: a case report and reviews. BMC Gastroenterol. (2021) 21:158. doi: 10.1186/s12876-021-01730-8
70. Thompson GE, Bourne MH Jr, Casal Moura M, Baqir M, Cartin-Ceba R, Makol A, et al. Pleuritis and pericarditis in antineutrophil cytoplasmic autoantibody-associated vasculitis. Chest. (2021) 160:572–81. doi: 10.1016/j.chest.2021.02.049
71. Padoan R, Campaniello D, Felicetti M, Cazzador D, Schiavon F. Ear, nose, and throat in ANCA-associated vasculitis: a comprehensive review. Vessel Plus. (2021) 5:41. doi: 10.20517/2574-1209.2021.41
72. Sacoto G, Boukhlal S, Specks U, Flores-Suárez LF, Cornec D. Lung involvement in ANCA-associated vasculitis. La Presse Médicale. (2020) 49:104039. doi: 10.1016/j.lpm.2020.104039
73. Hatemi I, Hatemi G, Çelik AF. Systemic vasculitis and the gut. Curr Opin Rheumatol. (2017) 29:33–8. doi: 10.1097/BOR.0000000000000344
74. Liu S, Guo L, Zhang Z, Li M, Zeng X, Wang L, et al. Cardiac manifestations of eosinophilic granulomatosis with polyangiitis from a single-center cohort in China: clinical features and associated factors. Ther Adv Chronic Dis. (2021) 12:2040622320987051. doi: 10.1177/2040622320987051
75. Suppiah R, Robson JC, Grayson PC, Ponte C, Craven A, Khalid S, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Microscopic Polyangiitis. Arthritis Rheumatol. (2022) 74:400–6. doi: 10.1002/art.41983
76. Robson JC, Grayson PC, Ponte C, Suppiah R, Craven A, Judge A, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann Rheum Dis. (2022) 81:315–20. doi: 10.1136/annrheumdis-2021-221795
77. Grayson PC, Ponte C, Suppiah R, Robson JC, Craven A, Judge A, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann Rheum Dis. (2022) 81:309–14. doi: 10.1136/annrheumdis-2021-221794
78. Panda R, Krieger T, Hopf L, Renne T, Haag F, Rober N, et al. Neutrophil extracellular traps contain selected antigens of anti-neutrophil cytoplasmic antibodies. Front Immunol. (2017) 8:439. doi: 10.3389/fimmu.2017.00439
79. Khan AJJ, Khan NAJ. Renal limited ANCA-positive vasculitis: a rare manifestation of a rare disease. J Investig Med High Impact Case Rep. (2020) 8:2324709620974874. doi: 10.1177/2324709620974874
80. Mollaeian A, Chan N, Aloor R, Iding JS, Arend LJ, Saeidabadi SHF, et al. ANCA-negative microscopic polyangiitis with diffuse alveolar hemorrhage masquerading as congestive heart failure. Autoimmunity Highlights. (2021) 12:1. doi: 10.1186/s13317-020-00143-z
81. Jayne D. Vasculitis-when can biopsy be avoided? Nephrol Dial Transplant. (2017) 32:1454–6. doi: 10.1093/ndt/gfx248
82. Eisenberger U, Fakhouri F, Vanhille P, Beaufils H, Mahr A, Guillevin L, et al. ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant. (2005) 20:1392–9. doi: 10.1093/ndt/gfh830
83. Terjung B, Spengler U, Sauerbruch T, Worman HJ. “Atypical p-ANCA” in IBD and hepatobiliary disorders react with a 50-kilodalton nuclear envelope protein of neutrophils and myeloid cell lines. Gastroenterology. (2000) 119:310–22. doi: 10.1053/gast.2000.9366
84. Roozendaal C, Kallenberg CG. Are anti-neutrophil cytoplasmic antibodies (ANCA) clinically useful in inflammatory bowel disease (IBD)? Clin Exp Immunol. (1999) 116:206–13. doi: 10.1046/j.1365-2249.1999.00905.x
85. Ghosh GC, Sharma B, Katageri B, Bhardwaj M, ANCA positivity in a patient with infective endocarditis-associated glomerulonephritis: a diagnostic dilemma. Yale J Biol Med. (2014) 87:373–7.
86. Suarez-Cuartin G, Molina-Molina M. Clinical implications of ANCA positivity in idiopathic pulmonary fibrosis patients. Breathe. (2020) 16:190321. doi: 10.1183/20734735.0321-2019
87. Sediva A, Bartunkova J, Kolarova I, Hrusak O, Vavrova V, Macek M Jr, et al. Antineutrophil cytoplasmic autoantibodies (ANCA) in children with cystic fibrosis. J Autoimmun. (1998) 11:185–90. doi: 10.1006/jaut.1997.0186
88. Liu H, Xiong J, Zhang J, Zhang Y, Nie L, Wang Y, et al. Possible intrinsic association of anti-neutrophil cytoplasmic antibody-associated vasculitis coexisting with multiple myeloma. Oncol Lett. (2016) 12:2084–6. doi: 10.3892/ol.2016.4855
89. Roper T, Elias R, Jayawardene S. A case of myeloma kidney with perinuclear anti-neutrophil cytoplasmic antibody and anti-myeloperoxidase positivity: the importance of determining the true cause of renal impairment. Case Rep Nephrol Dial. (2020) 10:79–85. doi: 10.1159/000509099
90. Chebotareva N, Kamyshova E, Bulanov N, Lysenko L, Moiseev S. Antineutrophil cytoplasmic autoantibody (ANCA) positive immunoglobulin A (IgA) nephropathy: Case reports and review of literature. Egypt Rheumatol. (2020) 42:251–4. doi: 10.1016/j.ejr.2020.06.002
91. Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. (2004) 66:1535–40. doi: 10.1111/j.1523-1755.2004.00917.x
92. McAdoo SP, Medjeral-Thomas N, Gopaluni S, Tanna A, Mansfield N, Galliford J, et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant. (2018) 33:899. doi: 10.1093/ndt/gfy075
93. Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. (2003) 63:1164–77. doi: 10.1046/j.1523-1755.2003.00843.x
94. J DEZ, Taylor D, Thein H, Yehia M. Incidence and features of dual anti-GBM-positive and ANCA-positive patients. Nephrology (Carlton). (2011) 16:725–9. doi: 10.1111/j.1440-1797.2011.01484.x
95. Moiseev S, Lee JM, Zykova A, Bulanov N, Novikov P, Gitel E, et al. The alternative complement pathway in ANCA-associated vasculitis: further evidence and a meta-analysis. Clin Exp Immunol. (2020) 202:394–402. doi: 10.1111/cei.13498
96. Crnogorac M, Horvatic I, Kacinari P, Ljubanovic DG, Galesic K. Serum C3 complement levels in ANCA associated vasculitis at diagnosis is a predictor of patient and renal outcome. J Nephrol. (2018) 31:257–62. doi: 10.1007/s40620-017-0445-3
97. Manenti L, Vaglio A, Gnappi E, Maggiore U, Allegri L, Allinovi M, et al. Association of serum C3 concentration and histologic signs of thrombotic microangiopathy with outcomes among patients with ANCA-associated renal vasculitis. Clin J Am Soc Nephrol. (2015) 10:2143–51. doi: 10.2215/CJN.00120115
98. Kawamura T, Usui J, Kaneko S, Tsunoda R, Imai E, Kai H, et al. Anaemia is an essential complication of ANCA-associated renal vasculitis: a single center cohort study. BMC Nephrol. (2017) 18:337. doi: 10.1186/s12882-017-0754-8
99. Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. (2016) 75:1583–94. doi: 10.1136/annrheumdis-2016-209133
100. Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. (2010) 21:1628–36. doi: 10.1681/ASN.2010050477
101. Aasarød K, Bostad L, Hammerstrøm J, Jørstad S, Iversen BM. Renal histopathology and clinical course in 94 patients with Wegener's granulomatosis. Nephrol Dial Transplantat. (2001) 16:953–60. doi: 10.1093/ndt/16.5.953
102. Vizjak A, Rott T, Koselj-Kajtna M, Rozman B, Kaplan-Pavlovcic S, Ferluga D. Histologic and immunohistologic study and clinical presentation of ANCA-associated glomerulonephritis with correlation to ANCA antigen specificity. Am J Kidney Dis. (2003) 41:539–49. doi: 10.1053/ajkd.2003.50142
103. Klein J, Rodriguez W, Kuperman M, Szerlip H. Medullary angiitis and pauci-immune crescentic glomerulonephritis. Proc (Bayl Univ Med Cent). (2017) 30:351–2. doi: 10.1080/08998280.2017.11929645
104. Doreille A, Buob D, Bay P, Julien M, Riviere F, Rafat C. Renal Involvement in Eosinophilic Granulomatosis With Polyangiitis. Kidney Int Rep. (2021) 6:2718–21. doi: 10.1016/j.ekir.2021.07.002
105. Hruskova Z, Honsova E, Berden AE, Rychlik I, Lanska V, Zabka J, et al. Repeat protocol renal biopsy in ANCA-associated renal vasculitis. Nephrol Dial Transplantat. (2014) 29:1728–32. doi: 10.1093/ndt/gfu042
106. Chapman GB, Farrah TE, Chapman FA, Pugh D, Bellamy COC, Lahiri R, et al. Utility of interval kidney biopsy in ANCA-associated vasculitis. Rheumatology. (2021) 61:1966–74. doi: 10.1093/rheumatology/keab695
107. Yumura W, Kobayashi S, Suka M, Hayashi T, Ito S, Nagafuchi H, et al. Assessment of the Birmingham vasculitis activity score in patients with MPO-ANCA-associated vasculitis: sub-analysis from a study by the Japanese Study Group for MPO-ANCA-associated vasculitis. Mod Rheumatol. (2014) 24:304–9. doi: 10.3109/14397595.2013.854075
108. Luqmani RA. Disease assessment in systemic vasculitis. Nephrol Dial Transplant. (2015) 30 Suppl 1:i76–82. doi: 10.1093/ndt/gfv002
109. Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. (1997) 40:371–80. doi: 10.1002/art.1780400222
110. de Groot K, Gross WL, Herlyn K, Reinhold-Keller E. Development and validation of a disease extent index for Wegener's granulomatosis. Clin Nephrol. (2001) 55:31–8.
111. Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore). (1996) 75:17–28. doi: 10.1097/00005792-199601000-00003
112. Merkel PA, Cuthbertson DD, Hellmich B, Hoffman GS, Jayne DR, Kallenberg CG, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis. (2009) 68:103–6. doi: 10.1136/ard.2008.097758
113. Chung SA, Langford CA, Maz M, Abril A, Gorelik M, Guyatt G, et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody–Associated Vasculitis. Arthritis Rheumatol. (2021) 73:1366–83. doi: 10.1002/art.41773
114. Walsh M, Merkel PA, Peh C-A, Szpirt WM, Puéchal X, Fujimoto S, et al. Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis. N Engl J Med. (2020) 382:622–31. doi: 10.1056/NEJMoa1803537
115. Cortazar FB, Muhsin SA, Pendergraft WF 3rd, Wallace ZS, Dunbar C, Laliberte K, et al. Combination Therapy With Rituximab and Cyclophosphamide for Remission Induction in ANCA Vasculitis. Kidney Int Rep. (2018) 3:394–402. doi: 10.1016/j.ekir.2017.11.004
116. Jayne DRW, Merkel PA, Schall TJ, Bekker P. Avacopan for the Treatment of ANCA-Associated Vasculitis. N Engl J Med. (2021) 384:599–609. doi: 10.1056/NEJMoa2023386
117. Fauci AS, Wolff SM. Wegener's granulomatosis: studies in eighteen patients and a review of the literature. Medicine. (1973) 52:535–61. doi: 10.1097/00005792-197311000-00002
118. de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. (2009) 150:670–80. doi: 10.7326/0003-4819-150-10-200905190-00004
119. Geetha D, Specks U, Stone JH, Merkel PA, Seo P, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis with renal involvement. J Am Soc Nephrol. (2015) 26:976–85. doi: 10.1681/ASN.2014010046
120. Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. (2010) 363:221–32. doi: 10.1056/NEJMoa0909905
121. Smith RM, Jones RB, Specks U, Bond S, Nodale M, Aljayyousi R, et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis. (2020) 79:1243–9. doi: 10.1136/annrheumdis-2019-216863
122. Unizony S, Villarreal M, Miloslavsky EM, Lu N, Merkel PA, Spiera R, et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Ann Rheum Dis. (2016) 75:1166–9. doi: 10.1136/annrheumdis-2015-208073
123. Miyazaki Y, Nakayamada S, Kubo S, Ishikawa Y, Yoshikawa M, Sakata K, et al. Favorable efficacy of rituximab in ANCA-associated vasculitis patients with excessive B cell differentiation. Arthritis Res Ther. (2020) 22:141. doi: 10.1186/s13075-020-02215-x
124. Jones RB, Cohen Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus Cyclophosphamide in ANCA-Associated Renal Vasculitis. N Engl J Med. (2010) 363:211–20. doi: 10.1056/NEJMoa0909169
125. Jones RB, Hiemstra TF, Ballarin J, Blockmans DE, Brogan P, Bruchfeld A, et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann Rheum Dis. (2019) 78:399–405. doi: 10.1136/annrheumdis-2018-214245
126. Geetha D, Jefferson JA. ANCA-Associated Vasculitis: Core Curriculum 2020. Am J Kidney Dis. (2020) 75:124–37. doi: 10.1053/j.ajkd.2019.04.031
127. Walsh M, Catapano F, Szpirt W, Thorlund K, Bruchfeld A, Guillevin L, et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta-analysis. Am J Kidney Dis. (2011) 57:566–74. doi: 10.1053/j.ajkd.2010.10.049
128. Jayne DRW, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized Trial of Plasma Exchange or High-Dosage Methylprednisolone as Adjunctive Therapy for Severe Renal Vasculitis. J Am Soc Nephrol. (2007) 18:2180–8. doi: 10.1681/ASN.2007010090
129. Walsh M, Collister D, Zeng L, Merkel PA, Pusey CD, Guyatt G, et al. The effects of plasma exchange in patients with ANCA-associated vasculitis: an updated systematic review and meta-analysis. BMJ. (2022) 376:e064604. doi: 10.1136/bmj-2021-064604
130. Balogun RA, Sanchez AP, Klingel R, Witt V, Aqui N, Meyer E, et al. Update to the ASFA guidelines on the use of therapeutic apheresis in ANCA-associated vasculitis. J Clin Apher. (2020) 35:493–9. doi: 10.1002/jca.21820
131. Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JWC, Dadoniené J, et al. A Randomized Trial of Maintenance Therapy for Vasculitis Associated with Antineutrophil Cytoplasmic Autoantibodies. N Engl J Med. (2003) 349:36–44. doi: 10.1056/NEJMoa020286
132. Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. (2014) 371:1771–80. doi: 10.1056/NEJMoa1404231
133. Gopaluni S, Smith RM, Lewin M, McAlear CA, Mynard K, Jones RB, et al. Rituximab versus azathioprine as therapy for maintenance of remission for anti-neutrophil cytoplasm antibody-associated vasculitis (RITAZAREM): study protocol for a randomized controlled trial. Trials. (2017) 18:112. doi: 10.1186/s13063-017-1857-z
134. Hiemstra TF, Walsh M, Mahr A, Savage CO, de Groot K, Harper L, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. (2010) 304:2381–8. doi: 10.1001/jama.2010.1658
135. Pagnoux C, Mahr A, Hamidou MA, Boffa JJ, Ruivard M, Ducroix JP, et al. Azathioprine or Methotrexate Maintenance for ANCA-Associated Vasculitis. N Engl J Med. (2008) 359:2790–803. doi: 10.1056/NEJMoa0802311
136. Walsh M, Merkel PA, Mahr A, Jayne D. Effects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis. Arthritis Care Res (Hoboken). (2010) 62:1166–73. doi: 10.1002/acr.20176
137. Correction: Correction: Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre randomised controlled phase III trial (MAINRITSAN2). Ann Rheum Dis. (2019) 78:e101-e. doi: 10.1136/annrheumdis-2017-212878corr1
138. King C, Druce KL, Nightingale P, Kay E, Basu N, Salama AD, et al. Predicting relapse in anti-neutrophil cytoplasmic antibody-associated vasculitis: A Systematic review and meta-analysis. Rheumatol Adv Pract. (2021) 5:1–9. doi: 10.1093/rap/rkab018
139. Salama AD. Relapse in Anti-Neutrophil Cytoplasm Antibody (ANCA)-Associated Vasculitis. Kidney Int Rep. (2020) 5:7–12. doi: 10.1016/j.ekir.2019.10.005
140. Cohen Tervaert JW, Damoiseaux J. Antineutrophil Cytoplasmic Autoantibodies: How Are They Detected and What Is Their Use for Diagnosis, Classification and Follow-up? Clin Rev Allergy Immunol. (2012) 43:211–9. doi: 10.1007/s12016-012-8320-4
141. Kemna MJ, Damoiseaux J, Austen J, Winkens B, Peters J, van Paassen P, et al. ANCA as a Predictor of Relapse: Useful in Patients with Renal Involvement But Not in Patients with Nonrenal Disease. J Am Soc Nephrol. (2015) 26:537–42. doi: 10.1681/ASN.2013111233
142. Espy C, Morelle W, Kavian N, Grange P, Goulvestre C, Viallon V, et al. Sialylation levels of anti–proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener's). Arthritis Rheumatism. (2011) 63:2105–15. doi: 10.1002/art.30362
143. Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest. (2013) 123:1773–83. doi: 10.1172/JCI65292
144. McClure M, Jones RB. Treatment of Relapses in ANCA-Associated Vasculitis. Clin J Am Soc Nephrol. (2019) 14:967–9. doi: 10.2215/CJN.06250519
145. Reinhold-Keller E, Beuge N, Latza U, de Groot K, Rudert H, Nölle B, et al. An interdisciplinary approach to the care of patients with Wegener's granulomatosis: long-term outcome in 155 patients. Arthritis Rheum. (2000) 43:1021–32. doi: 10.1002/1529-0131(200005)43:5<1021::AID-ANR10>3.0.CO;2-J
146. Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis. (2009) 68:1827–32. doi: 10.1136/ard.2008.101279
147. Faurschou M, Sorensen IJ, Mellemkjaer L, Loft AG, Thomsen BS, Tvede N, et al. Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol. (2008) 35:100–5.
148. Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. (2012) 367:2015–25. doi: 10.1056/NEJMra1009433
149. Crickx E, Machelart I, Lazaro E, Kahn JE, Cohen-Aubart F, Martin T, et al. Intravenous Immunoglobulin as an Immunomodulating Agent in Antineutrophil Cytoplasmic Antibody-Associated Vasculitides: A French Nationwide Study of Ninety-Two Patients. Arthritis Rheumatol. (2016) 68:702–12. doi: 10.1002/art.39472
150. Bjørneklett R, Solbakken V, Bostad L, Fismen AS. Prognostic factors in anti-neutrophil cytoplasmic antibody-associated glomerulonephritis with severe glomerular sclerosis: a national registry-based cohort study. Patholog Res Int. (2018) 2018:5653612. doi: 10.1155/2018/5653612
151. Salmela A, Törnroth T, Poussa T, Ekstrand A. Prognostic factors for survival and relapse in ANCA-Associated vasculitis with renal involvement: a Clinical Long-Term Follow-up Study. Int J Nephrol. (2018) 2018:6369814. doi: 10.1155/2018/6369814
152. Lal DP, O'Donoghue DJ, Haeney M. Effect of diagnostic delay on disease severity and outcome in glomerulonephritis caused by anti-neutrophil cytoplasmic antibodies. J Clin Pathol. (1996) 49:942–4. doi: 10.1136/jcp.49.11.942
153. Brix SR, Noriega M, Tennstedt P, Vettorazzi E, Busch M, Nitschke M, et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. (2018) 94:1177–88. doi: 10.1016/j.kint.2018.07.020
154. Moura MC, Fervenza FC, Specks U, Sethi S. Kidney biopsy chronicity grading in antineutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant. (2021) 16:329–31. doi: 10.1093/ndt/gfab250
155. Hakroush S, Tampe D, Korsten P, Ströbel P, Tampe B. Systematic scoring of tubular injury patterns reveals interplay between distinct tubular and glomerular lesions in ANCA-associated glomerulonephritis. J Clin Med. (2021) 10:2682. doi: 10.3390/jcm10122682
156. Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. (2005) 143:621–31. doi: 10.7326/0003-4819-143-9-200511010-00005
157. Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. (1996) 7:23–32. doi: 10.1681/ASN.V7123
158. Hruskova Z, Casian AL, Konopasek P, Svobodova B, Frausova D, Lanska V, et al. Long-term outcome of severe alveolar haemorrhage in ANCA-associated vasculitis: a retrospective cohort study. Scand J Rheumatol. (2013) 42:211–4. doi: 10.3109/03009742.2012.754939
159. Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Höglund P, et al. Damage in the anca-associated vasculitides: long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis. (2015) 74:177–84. doi: 10.1136/annrheumdis-2013-203927
160. Liapi M, Jayne D, Merkel PA, Segelmark M, Mohammad AJ. Venous thromboembolism in ANCA-associated vasculitis: a population-based cohort study. Rheumatology (Oxford). (2021) 60:4616–23. doi: 10.1093/rheumatology/keab057
161. Mahr A, Heijl C, Le Guenno G, Faurschou M. ANCA-associated vasculitis and malignancy: Current evidence for cause and consequence relationships. Best Pract Res Clin Rheumatol. (2013) 27:45–56. doi: 10.1016/j.berh.2012.12.003
162. Chow MT, Moller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol. (2012) 22:23–32. doi: 10.1016/j.semcancer.2011.12.004
163. Pereira MI, Medeiros JA. Role of Helicobacter pylori in gastric mucosa-associated lymphoid tissue lymphomas. World J Gastroenterol. (2014) 20:684–98. doi: 10.3748/wjg.v20.i3.684
164. Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. (2007) 117:1137–46. doi: 10.1172/JCI31405
165. Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. (2009) 6:638–47. doi: 10.1038/nrclinonc.2009.146
166. van Daalen EE, Rizzo R, Kronbichler A, Wolterbeek R, Bruijn JA, Jayne DR, et al. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis. (2017) 76:1064–9. doi: 10.1136/annrheumdis-2016-209925
167. Haris Á, Polner K, Arányi J, Braunitzer H, Kaszás I. Incidence and clinical predictors of infections in patients treated with severe systemic ANCA-associated vasculitis. Physiol Int. (2021) 108:66–79. doi: 10.1556/2060.2021.00006
168. Yang L, Xie H, Liu Z, Chen Y, Wang J, Zhang H, et al. Risk factors for infectious complications of ANCA-associated vasculitis: a cohort study. BMC Nephrol. (2018) 19:138. doi: 10.1186/s12882-018-0933-2
169. Jourdain P, Brilland B, Medhioub O, Caron J, Samoreau C, Djema A, et al. Incidence and Temporal Trend in Risk Factors of Severe Infections in ANCA-Glomerulonephritis Patients. Kidney Int Rep. (2021) 6:1161–5. doi: 10.1016/j.ekir.2020.12.037
170. Kronbichler A, Jayne DRW, Mayer G. Frequency, risk factors and prophylaxis of infection in ANCA-associated vasculitis. Eur J Clin Invest. (2015) 45:346–68. doi: 10.1111/eci.12410
171. Malpica L, van Duin D, Moll S. Preventing infectious complications when treating non-malignant immune-mediated hematologic disorders. Am J Hematol. (2019) 94:1396–412. doi: 10.1002/ajh.25642
172. Rousset S, Treiner E, Moulis G, Pugnet G, Astudillo L, Paricaud K, et al. High rate of indeterminate results of the QuantiFERON-TB Gold in-tube test, third generation, in patients with systemic vasculitis. Rheumatology. (2019) 59:1006–10. doi: 10.1093/rheumatology/kez390
173. Focosi D, Tuccori M, Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: What do we know after 20 years of rituximab. Rev Med Virol. (2019) 29:e2077. doi: 10.1002/rmv.2077
174. Papp KA, Haraoui B, Kumar D, Marshall JK, Bissonnette R, Bitton A, et al. Vaccination guidelines for patients with immune-mediated disorders taking immunosuppressive therapies: executive summary. J Rheumatol. (2019) 46:751–4. doi: 10.3899/jrheum.180784
175. Lionaki S, Hogan SL, Jennette CE, Hu Y, Hamra JB, Jennette JC, et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. (2009) 76:644–51. doi: 10.1038/ki.2009.218
176. Hruskova Z, Stel VS, Jayne D, Aasarød K, De Meester J, Ekstrand A, et al. Characteristics and Outcomes of Granulomatosis With Polyangiitis (Wegener) and Microscopic Polyangiitis Requiring Renal Replacement Therapy: Results From the European Renal Association-European Dialysis and Transplant Association Registry. Am J Kidney Dis. (2015) 66:613–20. doi: 10.1053/j.ajkd.2015.03.025
177. Geetha D, Eirin A, True K, Valentina Irazabal M, Specks U, Seo P, et al. Renal transplantation in antineutrophil cytoplasmic antibody-associated vasculitis: a multicenter experience. Transplantation. (2011) 91:1370–5. doi: 10.1097/TP.0b013e31821ab9aa
178. Göçeroglu A, Rahmattulla C, Berden AE, Reinders ME, Wolterbeek R, Steenbergen EJ, et al. The Dutch Transplantation in Vasculitis (DUTRAVAS) Study: Outcome of Renal Transplantation in Antineutrophil Cytoplasmic Antibody-associated Glomerulonephritis. Transplantation. (2016) 100:916–24. doi: 10.1097/TP.0000000000000910
179. Maruyama H, Hirayama K, Yamashita M, Ohgi K, Tsujimoto R, Takayasu M, et al. Serum 20S proteasome levels are associated with disease activity in MPO-ANCA-associated microscopic polyangiitis. BMC Rheumatol. (2020) 4:36. doi: 10.1186/s41927-020-00137-4
180. Bontscho J, Schreiber A, Manz RA, Schneider W, Luft FC, Kettritz R. Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis. J Am Soc Nephrol. (2011) 22:336–48. doi: 10.1681/ASN.2010010034
181. Novikov P, Moiseev S, Bulanov N, Shchegoleva E. Bortezomib in refractory ANCA-associated vasculitis: a new option? Ann Rheum Dis. (2016) 75:e9. doi: 10.1136/annrheumdis-2015-207947
182. Alexander T, Sarfert R, Klotsche J, Kuhl AA, Rubbert-Roth A, Lorenz HM, et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis. (2015) 74:1474–8. doi: 10.1136/annrheumdis-2014-206016
183. Almaani S, Fussner LA, Brodsky S, Meara AS, Jayne D. ANCA-associated vasculitis: an update. J Clin Med. (2021) 10:1446. doi: 10.3390/jcm10071446
184. Casian A, Sangle S, D'Cruz D. AB0531 The role of hydroxychloroquine in ANCA positive and negative vasculitis. Ann Rheum Dis. (2016) 75:1086–7. doi: 10.1136/annrheumdis-2016-eular.5464
185. Lenert A, Lenert P. Current and emerging treatment options for ANCA-associated vasculitis: potential role of belimumab and other BAFF/APRIL targeting agents. Drug Des Devel Ther. (2015) 9:333–47. doi: 10.2147/DDDT.S67264
186. Jayne D, Blockmans D, Luqmani R, Moiseev S, Ji B, Green Y, et al. Efficacy and Safety of Belimumab and Azathioprine for Maintenance of Remission in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Randomized Controlled Study. Arthritis Rheumatol. (2019) 71:952–63. doi: 10.1002/art.40802
187. Korhonen R, Moilanen E. Abatacept, a Novel CD80/86–CD28 T Cell Co-stimulation Modulator, in the Treatment of Rheumatoid Arthritis. Basic Clin Pharmacol Toxicol. (2009) 104:276–84. doi: 10.1111/j.1742-7843.2009.00375.x
188. Langford CA, Monach PA, Specks U, Seo P, Cuthbertson D, McAlear CA, et al. An open-label trial of abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener's). Ann Rheum Dis. (2014) 73:1376–9. doi: 10.1136/annrheumdis-2013-204164
189. Huizenga N, Zonozi R, Rosenthal J, Laliberte K, Niles JL, Cortazar FB. Treatment of Aggressive Antineutrophil Cytoplasmic Antibody-Associated Vasculitis With Eculizumab. Kidney Int Rep. (2020) 5:542–5. doi: 10.1016/j.ekir.2019.11.021
190. Prendecki M, McAdoo SP. New Therapeutic Targets in Antineutrophil Cytoplasm Antibody–Associated Vasculitis. Arthritis Rheumatol. (2021) 73:361–70. doi: 10.1002/art.41407
191. Azevedo CJ, Zamvil SS. New cases of vasculitis after alemtuzumab. Mult Scler. (2020) 26:1606–8. doi: 10.1177/1352458520908040
192. Horisberger A, Pantazou V, Cuendet G, Ribi C, Dunet V, Théaudin M. ANCA-associated life-threatening systemic vasculitis after alemtuzumab treatment for multiple sclerosis. Mult Scler. (2020) 26:1599–602. doi: 10.1177/1352458519895449
193. Sakai R, Kondo T, Kurasawa T, Nishi E, Okuyama A, Chino K, et al. Current clinical evidence of tocilizumab for the treatment of ANCA-associated vasculitis: a prospective case series for microscopic polyangiitis in a combination with corticosteroids and literature review. Clin Rheumatol. (2017) 36:2383–92. doi: 10.1007/s10067-017-3752-0
194. Kusunoki Y, Nakazawa D, Shida H, Hattanda F, Miyoshi A, Masuda S, et al. Peptidylarginine Deiminase Inhibitor Suppresses Neutrophil Extracellular Trap Formation and MPO-ANCA Production. Front Immunol. (2016) 7:227. doi: 10.3389/fimmu.2016.00227
195. O'Sullivan KM, Gan PY, Kitching AR, Holdsworth S. SAT-012 Peptidyl Arginase Deiminase 4 inhibition attenuates inflammation in murine experimental myeloperoxidase cytoplasmic antibody associated glomerulonephritis. Kidney Int Rep. (2019) 4:S6. doi: 10.1016/j.ekir.2019.05.033
196. Mustapha N, Barra L, Carette S, Cuthbertson D, Khalidi NA, Koening CL, et al. Efficacy of leflunomide in the treatment of vasculitis. Clin Exp Rheumatol. (2021) 39 (Suppl 129):114–8. doi: 10.55563/clinexprheumatol/ve38dj
197. Gopaluni S, Goymer D, McClure M, Cahill H, Broadhurst E, Smith R, et al. 302. Alemtuzumab for relapsing and refractory primary systemic vasculitis - a trial of efficacy and safety (aleviate): a randomized open-label phase II clinical trial. Rheumatology. (2019) 58:135–6. doi: 10.1093/rheumatology/kez063.026
198. Guntur VP, Manka LA, Denson JL, Dunn RM, Dollin YT, Gill M, et al. Benralizumab as a Steroid-Sparing Treatment Option in Eosinophilic Granulomatosis with Polyangiitis. J Allergy Clin Immunol Pract. (2021) 9:1186–93.e1. doi: 10.1016/j.jaip.2020.09.054
Keywords: ANCA-associated vasculitis, glomerulonephritis, microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), autoimmune kidney disease
Citation: Molnár A, Studinger P and Ledó N (2022) Diagnostic and Therapeutic Approach in ANCA-Associated Glomerulonephritis: A Review on Management Strategies. Front. Med. 9:884188. doi: 10.3389/fmed.2022.884188
Received: 25 February 2022; Accepted: 18 May 2022;
Published: 03 June 2022.
Edited by:
Guangyan Cai, Chinese PLA General Hospital, ChinaCopyright © 2022 Molnár, Studinger and Ledó. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nóra Ledó, bGVkby5ub3JhQG1lZC5zZW1tZWx3ZWlzLXVuaXYuaHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.