
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 16 June 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.883121
This article is part of the Research Topic Stevens Johnson Syndrome: Past, Present, and Future Directions View all 11 articles
Epidermal necrolysis, the unifying term for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), is a severe cutaneous drug reaction associated with high morbidity and mortality. Given the rarity of this disease, large-scale prospective research studies are limited. Significant institutional and geographical variations in treatment practices highlight the need for standardization of clinical assessment scores and prioritization of research outcome measures in epidermal necrolysis. At the present, clinical assessment is typically simplified to total body surface area (BSA) involvement, with little focus on morphology. Validated clinical scoring systems are used as mortality prognostication tools, with SCORTEN being the best-validated tool thus far, although the ABCD-10 has also been recently introduced. These tools are imperfect in that they tend to either overestimate or underestimate mortality in certain populations and are not designed to monitor disease progression. Although mortality is often used as a primary endpoint for epidermal necrolysis studies, this outcome fails to capture more nuanced changes in skin disease such as arrest of disease progression while also lacking a validated skin-directed inclusion criterion to stratify patients based on the severity of skin disease at study entry. In addition to mortality, many studies also use BSA stabilization or time to re-epithelialization as endpoints, although these are not clearly defined morphologically, and inter- and intra-rater reliability are unclear. More specific, validated cutaneous assessment scores are necessary in order advance therapeutic options for epidermal necrolysis. In this review, we summarize the strengths and weaknesses of current clinical assessment practices in epidermal necrolysis and highlight the need for standardized research tools to monitor cutaneous involvement throughout the hospitalization.
Epidermal necrolysis, the unifying term for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), is a severe cutaneous drug reaction associated with high morbidity and mortality (1–3). It is considered to be the most life-threatening dermatologic disease with a mortality incidence of 15% overall, and up to 50% in the elderly (4, 5). Increasing recognition is also being given to the long-term multisystem sequelae of epidermal necrosis present in the majority of survivors, including permanent mucosal damage, cutaneous dyspigmentation and scarring, and resultant mental illness (5). Despite its severity, epidermal necrosis has no FDA-approved therapeutics in use. Treatment, including no treatment, varies significantly by physician specialty, institutional geography, and institutional experiences. In this review, we summarize the strengths and weaknesses of current clinical assessment practices epidermal necrolysis and highlight the need for standardized research tools to monitor cutaneous involvement throughout hospitalization. More specific, validated cutaneous assessment scores are necessary to appropriately risk-stratify patients on study entry, assess skin disease change in response to treatment, and ultimately advance therapeutic options for epidermal necrolysis.
The severity-of-illness score for TEN (SCORTEN) is a mortality prognostication tool for epidermal necrolysis (1). It was developed in 2000 by a team in France, using 165 patients to identify significant variables via a logistic regression model and 75 patients to internally validate the results (1). From this model, the researchers identified seven equally weighted parameters that are risk factors for death: age >40 years, malignancy, heart rate >120 beats per minute, initial percentage of epidermal detachment >10%, serum urea >10 mmol/L, serum glucose >14 mmol/L, and bicarbonate <20 mmol/L (score range: 0–7, Table 1). Collectively, these comprise the SCORTEN, which can predict risk of mortality ranging from 3.2 to 90.0%. Originally, this score was meant to be calculated once within 24 h of admission. Despite this initial intent, authors from this group later published an analysis that demonstrated SCORTEN performance on the first 5 days of hospitalization remained high (and performed even better on day 3), and thus recommended SCORTEN calculation on both days 1 and 3 (6).
In the two decades following its conception, SCORTEN has been widely used and validated in patient populations around the world. In an effort to summarize its use over the past two decades, a group of researchers performed a meta-analysis to better understand the accuracy of SCORTEN in predicting mortality (7). Overall, 64 studies were included. SCORTEN was found to be an overall good predictor of mortality but tends to underestimate mortality for values <3 and overestimate for values >3. Certain factors were associated with reduced predictive accuracy, such as mean age of patients and ending year of the study. SCORTEN tended to underestimate mortality in older cohorts of patients and overestimate mortality in more recent studies. BSA involvement may influence SCORTEN predictiveness, although the results are more varied. One study found that SCORTEN underestimated mortality for a cohort of patients with TEN (BSA > 30%) (8), but another study found SCORTEN retained good predictive ability in burn center patients (9).
Perhaps the most common criticism of SCORTEN is that it simplifies continuous and dynamic biologic measurements into dichotomous variables, thereby losing a significant amount of information in the process, particularly in the skin assessment which does not regard morphology or locations. Additionally, SCORTEN was originally meant to be used at a single timepoint rather than as a daily monitoring tool. Interestingly some studies have found that either delayed or sequential use of SCORTEN provides improved prognostication (6, 10). Another common concern is that defining BSA remains somewhat subjective, and may vary from one provider to another depending on how BSA involvement is estimated and whether the provider measures only desquamated skin vs. skin with bullae.
In response to this, a group of researchers designed a refined model from 369 patients in the RegiSCAR study that they termed the auxiliary score which scores both age and BSA differently (11). The auxiliary score divides age into three groups (31–55, 56–75, and ≥75 years). The score additionally uses a higher cutoff to differentiate between BSA involvement at >30%. Some studies have found that models that differentiate between BSA >30%, as in TEN, may have better prognostic ability (8, 10, 11). However, authors of the auxiliary score concluded that SCORTEN should remain the model of choice in the clinical setting, whereas the auxiliary score may be useful in retrospective research with missing biochemical data.
The role of other biochemical markers in predicting mortality risk has also been investigated. A group recently found that the ratio of red cell distribution width to hemoglobin (RDW/Hb) is predictive of mortality (12). They incorporated this value into the SCORTEN and named this new model the Re-SCORTEN. Overall, they found improved mortality prognostication with this revised model as compared to SCORTEN alone, but this scoring model has not yet been validated in other populations.
Despite these critiques, SCORTEN has remained the gold standard for not only predicting patient mortality, but is also frequently used in study outcomes to compare therapy efficacy by survival to expected mortality, as well as compare quality of care between institutions (13, 14).
Another recently devised mortality prognostication tool for epidermal necrolysis is ABCD-10. The ABCD-10 is calculated using the following metrics: age over 50 years (one point), bicarbonate level <20 mmol/L (one point), cancer present and active (two points), dialysis prior to admission (3 points), and epidermal detachment ≥10% body surface area on admission (one point) (Table 1) (13). Despite its recency in development, ABCD-10 offers many strengths when assessing patients with epidermal necrolysis. In comparison to SCORTEN, ABCD-10 takes includes patients with end stage renal disease (using prior dialysis as a proxy) and more heavily weighs cancer diagnosis (Figure 1). Authors of ABCD-10 discovered that undergoing dialysis prior to admission was associated with a more than 15-fold increased risk of death in comparison to those not undergoing dialysis (13). In additional studies since its inception, ABCD-10 has been validated in external cohorts as having good discriminatory capability similar to that of SCORTEN (15). With continuing advances in supportive care and intensive treatments, as well as varying treatment protocols across institutions, ABCD-10 is a great step toward improving prognostic information of epidermal necrolysis patients.
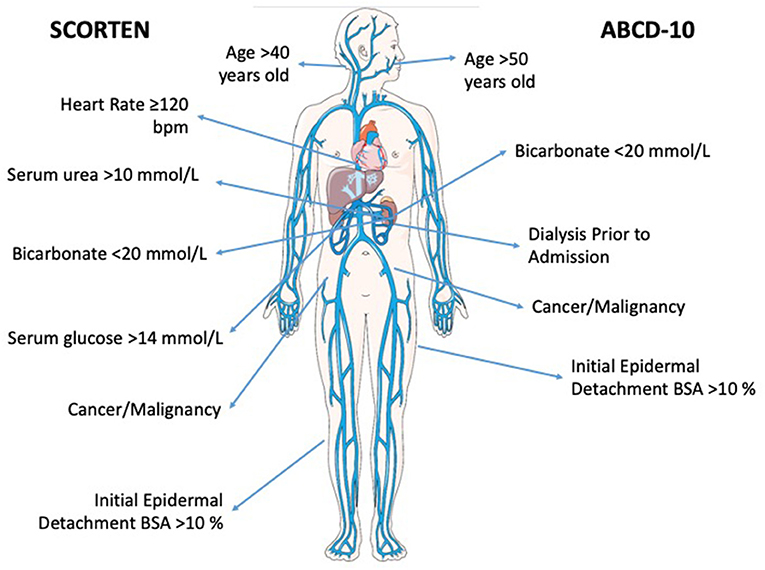
Figure 1. Bioicon representation of the prognostic factors associated with both SCORTEN and ABCD-10 scoring systems. Venous-circulation-body icon by Servier https://smart.servier.com/ is licensed under CC-BY 3.0 Unported https://creativecommons.org/licenses/by/3.0/.
While ABCD-10 has good discriminatory ability, multiple studies have showed that it underperforms in comparison to SCORTEN (3, 7, 15, 16). Specifically, one retrospective cohort study in Singapore found that in both patients treated with supportive care or immunomodulatory therapy, ABCD-10 underestimated mortality at lower score ranges and overestimated mortality at higher score ranges (15). Authors of another large retrospective study in the United States postulated that ABCD-10 underperformed SCORTEN due to the lower rates of dialysis and cancer in their population (3). Furthermore, some researchers have suggested that SCORTEN already adequately captures kidney disease as a co-morbidity by included serum urea and bicarbonate levels, given evidence of multicollinearity between dialysis and serum bicarbonate levels (15).
Further studies are needed to better understand the applicability of ABCD-10. Still, it is limited in its usefulness in epidermal necrolysis assessment, as it cannot be used to monitor cutaneous involvement throughout hospitalization and responsiveness to treatment.
While SCORTEN and ABCD-10 are commonly used mortality prognostication tools for epidermal necrolysis, to determine therapeutic efficacy, other clinical endpoints are needed to monitor disease response to interventions. Formal endpoints in clinical trials for patients with epidermal necrolysis have not been standardized. A query of the ClinicalTrials.Gov database for trials evaluating interventions for patients with epidermal necrolysis demonstrated high variability in primary and secondary outcomes (Table 2). Overall, outcomes among clinical trials and retrospective studies are generally grouped into three categories: (1) the standardized mortality ratio, (2) clinical outcomes, and (3) cutaneous response to treatment.
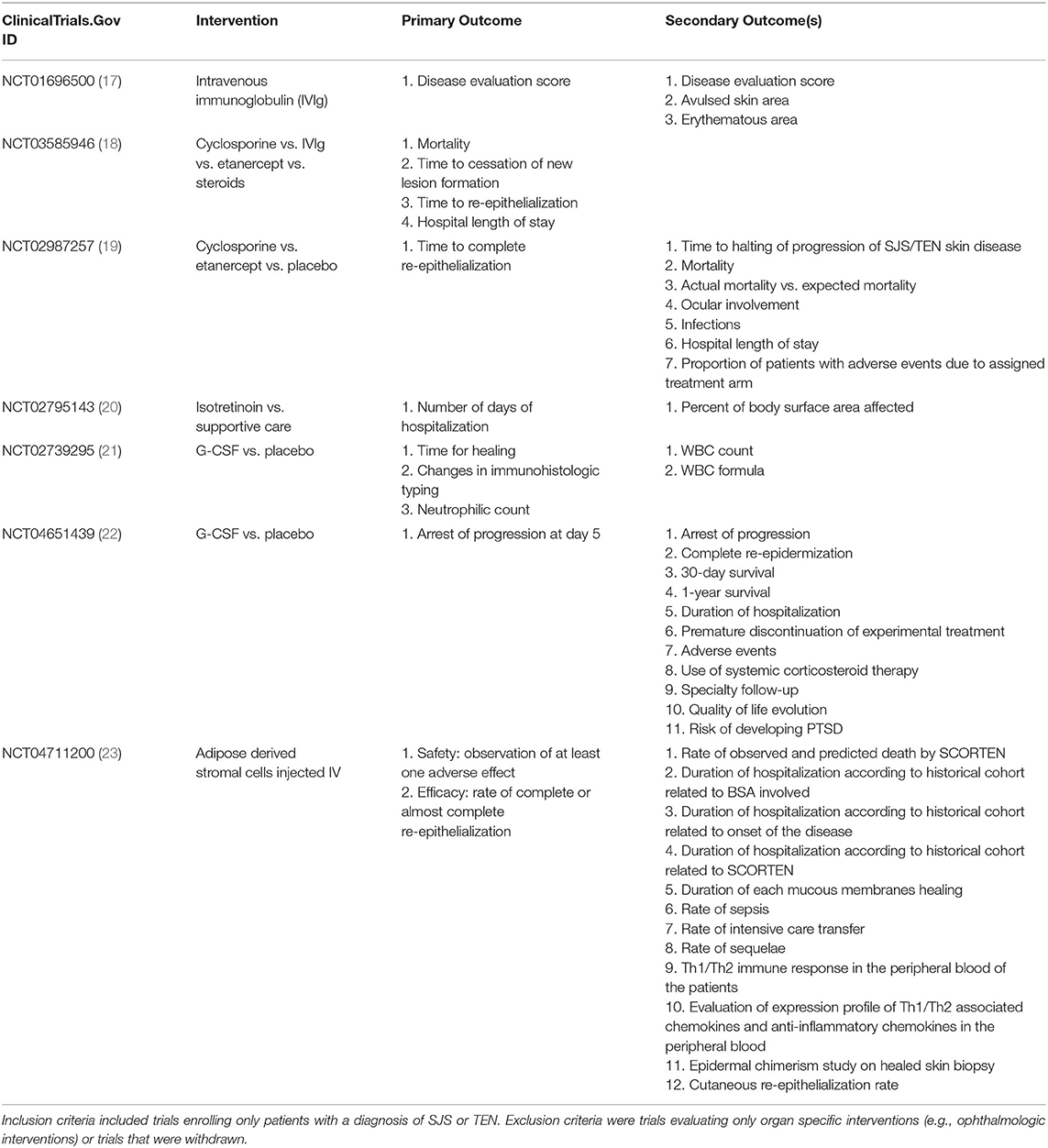
Table 2. Endpoints in trials registered at ClinicalTrials.Gov for epidermal necrolysis interventions.
One of the most common primary endpoints utilized in epidermal necrolysis studies is the standardized mortality ratio (SMR), defined as the ratio of observed deaths in comparison to deaths predicted by SCORTEN (13, 24–28). For example, a retrospective cohort analysis on 377 patients across multiple institutions in the United States stratified SMR by therapeutic approach, and demonstrated that combination of intravenous immunoglobulin and steroid use led to the lowest SMR of 0.52 [95% confidence interval (CI) 0.21–0.79] (27). However, the SMR for all patients in this cohort was 0.70 (95% CI 0.58–0.79), suggesting that SCORTEN as a whole overestimated mortality risk in this patient cohort. This has been reflected in other studies that use the SMR (29).
Many studies commonly employ basic clinical outcomes, such as length of stay, development of sepsis, and mortality. In a systematic review of the efficacy of intravenous immunoglobulin in the treatment of epidermal necrolysis, clinical endpoints were defined as mortality rates, length of hospital stay, time to disease cessation, and time to skin healing (30). A recent European multicenter study sought to assess overall treatment approaches including supportive care only as the reference group and the treatment groups were systemic glucocorticoids, cyclosporine, intravenous immunoglobulin, and antitumor necrosis factor agents (2). This study classified outcomes as risk of infection, body surface area detachment in the acute phase, and an overall 6-week mortality rate between treatment groups (2). Furthermore, participants were also evaluated for long-term outcomes defined as the development of severe acute complications which included septicemia, acute kidney injury, pulmonary infection, or respiratory distress requiring mechanical ventilation (2). While some of these outcomes are standard clinical outcomes including complicating infections, others are more specific to the disease and lack the validation to confirm their utility such as time to disease cessation, skin healing, and body surface area detachment in the acute phase.
Disease severity is also utilized as an outcome measure, with severity measurements varying between studies. In a study assessing burn unit transfers, disease severity was classified as total body surface area as well as the Acute Physiology and Chronic Health Evaluation (APACHE) score (31). Conversely, other trials utilized their own severity illness scores by developing rating scales which combined lesion characteristics and patient general conditions (32). While these assessments are commonly used for burn and ICU patients, they are of uncertain utility as a primary outcome measure for an intervention to be beneficial.
In addition to mortality and systemic disease severity as primary endpoints, cutaneous signs are an important outcome measure. The most frequently used cutaneous outcomes include time to skin re-epithelialization and body surface area stabilization from the acute phase. However, there are no standardized morphological assessments for cutaneous resolution of the acute phase and therefore, these outcomes are subject to provider bias and unclear validity. Furthermore, these cutaneous endpoints are not sensitive to special site areas such as the mucous membranes. As alluded to previously, subjectivity also arises in grading of BSA involvement. Some studies utilized a cutaneous measure of total BSA of detached and detachable skin (25, 30) that did not include strictly purpuric lesions, while another study defined cutaneous endpoints as the onset of spontaneous resolution of the acute phase (33). Clearly, more discrete skin scoring assessments and instruments are necessary to be validated for the success of future clinical studies in this disease. Further, improved cutaneous scoring assessments are critical not only as an outcome measure, but as an entry criterion for research studies to ensure balanced randomization across institutions.
The lack of standardized endpoint measures in epidermal necrolysis is a significant barrier in the development of regulatory approved therapies. At the current time, there exists a panoply of drugs, wound care, and supportive care regimens that lack strong evidence for efficacy for treating this disease. Efforts to improve treatment options and reduce mortality require standardized clinical outcomes that are more finely tuned to risk-stratifying patients at entry, then detecting treatment response. Recently some there have been some attempts at standardization of quantitative endpoints via a survey that identified minimally clinical important differences (MCID), defined as the smallest change in a treatment outcome that a patient or clinician would identify as important and indicate a change in management (34).
Further work is required on standardizing outcome measures and validating skin assessments. We recommend the development of a consensus morphological assessment of cutaneous morphologies and locations of involvement, from which cutaneous endpoints can be reliably measured. Without these standardizations, therapeutic treatments and interventions will remain limited with a bias toward lack of intervention efficacy.
AD, SH, MW, and BK contributed to the writing of the manuscript. AD and BK prepared the final manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. (2000) 115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x
2. Kridin K, Brüggen M-C, Chua S-L, Bygum A, Walsh S, Nägeli MC, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. (2021) 157:1182–90. doi: 10.1001/jamadermatol.2021.3154
3. Duplisea MJ, Roberson ML, Chrisco L, Strassle PD, Williams FN, Ziemer CM. Performance of ABCD-10 and SCORTEN mortality prediction models in a cohort of patients with Stevens-Johnson syndrome/toxic epidermal necrolysis. J Am Acad Dermatol. (2021) 85:873–7. doi: 10.1016/j.jaad.2021.04.082
4. Phillips EJ, Bouchard CS, Divito SJ. Stevens-johnson syndrome and toxic epidermal necrolysis-coordinating research priorities to move the field forward. JAMA Dermatol. (2022). doi: 10.1001/jamadermatol.2022.0484. [Epub ahead of print].
5. Chang W-C, Abe R, Anderson P, Anderson W, Ardern-Jones MR, Beachkofsky TM, et al. SJS/TEN 2019: from science to translation. J Dermatol Sci. (2020) 98:2–12. doi: 10.1016/j.jdermsci.2020.02.003
6. Guégan S, Bastuji-Garin S, Poszepczynska-Guigné E, Roujeau J-C, Revuz J. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. (2006) 126:272–6. doi: 10.1038/sj.jid.5700068
7. Torres-Navarro I, Briz-Redón Á, Botella-Estrada R. Accuracy of SCORTEN to predict the prognosis of Stevens-Johnson syndrome/toxic epidermal necrolysis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. (2020) 34:2066–77. doi: 10.1111/jdv.16137
8. Hu C-H, Chang N-J, Liu EK-W, Chuang S-S, Chung W-H, Yang J-Y. SCORTEN and impaired renal function related to mortality of toxic epidermal necrolysis syndrome patients in the Asian population. J Eur Acad Dermatol Venereol. (2013) 27:628–33. doi: 10.1111/j.1468-3083.2012.04502.x
9. Cartotto R, Mayich M, Nickerson D, Gomez M. SCORTEN accurately predicts mortality among toxic epidermal necrolysis patients treated in a burn center. J Burn Care Res. (2008) 29:141–6. doi: 10.1097/BCR.0b013e31815f3865
10. Bansal S, Garg VK, Sardana K, Sarkar R. A clinicotherapeutic analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis with an emphasis on the predictive value and accuracy of SCORe of Toxic Epidermal Necrolysis. Int J Dermatol. (2015) 54:e18–26. doi: 10.1111/ijd.12466
11. Sekula P, Liss Y, Davidovici B, Dunant A, Roujeau J-C, Kardaun S, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. (2011) 32:237–45. doi: 10.1097/BCR.0b013e31820aafbc
12. Koh HK, Fook-Chong SMC, Lee HY. Improvement of mortality prognostication in patients with epidermal necrolysis: the role of novel inflammatory markers and proposed revision of SCORTEN (Re-SCORTEN). JAMA Dermatol. (2021) 158:160–66. doi: 10.1001/jamadermatol.2021.5119
13. Noe MH, Rosenbach M, Hubbard RA, Mostaghimi A, Cardones AR, Chen JK, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. (2019) 155:448–54. doi: 10.1001/jamadermatol.2019.0998
14. Nizamoglu M, Ward JA, Frew Q, Gerrish H, Martin N, Shaw A, et al. Improving mortality outcomes of Stevens Johnson syndrome/toxic epidermal necrolysis: a regional burns centre experience. Burns J Int Soc Burn Inj. (2018) 44:603–11. doi: 10.1016/j.burns.2017.09.015
15. Koh HK, Fook-Chong S, Lee HY. Assessment and comparison of performance of ABCD-10 and SCORTEN in prognostication of epidermal necrolysis. JAMA Dermatol. (2020) 156:1294–9. doi: 10.1001/jamadermatol.2020.3654
16. Suo H, Jiang B, Sun X, Dong J, Alamgir M, Guan X, et al. Comparing the accuracy of ABCD-10 and SCORTEN in predicting the in-hospital mortality of Stevens-Johnson syndrome/toxic epidermal necrolysis: a multi-institutional study from central China. Dermatol Basel Switz. (2021) 1–9. doi: 10.1159/000520494. [Epub ahead of print].
17. NPB-01(Intravenous Immunoglobulin) Therapy for Patients With Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Unresponsive to Corticosteroids. Identifier NCT01696500 (2012–2014). Available online at: https://clinicaltrials.gov/ct2/show/NCT01696500 (accessed May 2, 2022.)
18. Kroshinsky D. A Prospective Multicenter Cohort Study Assessing Outcomes in Stevens Johnsons Syndrome and Toxic Epidermal Necrolysis. Identifier NCT03585946 Available online at: https://clinicaltrials.gov/ct2/show/NCT03585946 (accessed May 2, 2022.)
19. Phillips EJ. NATIENS: A Phase III Randomized Double-Blinded Placebo Controlled Study to Determine the Optimal Management and Mechanisms of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Identifier NCT02987257. Available online at: https://clinicaltrials.gov/ct2/show/NCT02987257 (accessed May 2, 2022.)
20. Saavedra AP. Evaluating the Effect of Isotretinoin in Regulatory T-Cell Function in Adverse Cutaneous Drug Eruptions (ACDEs): A Pilot Study. Identifier NCT02795143. Available online at: https://clinicaltrials.gov/ct2/show/NCT02795143 (accessed May 2, 2022.)
21. Rousseau A. Evaluation of G-CSF as a Treatment of Toxic Epidermal Necrolysis. Identifier NCT02739295. Available online at: https://clinicaltrials.gov/ct2/show/NCT02739295 (accessed May 2, 2022.)
22. Ben Said B. Evaluating the Therapeutic Efficacy of Filgrastim in Severe Bullous Drug Eruptions (Lyell and Stevens-Johnson Syndromes). Identifier NCT04651439. Available online at: https://clinicaltrials.gov/ct2/show/NCT04651439 (accessed May 2, 2022.)
23. Oro S. Mesenchymal Stromal Cells Treatment in Lyell Syndrome: A Pilot Phase 1-2 Open Trial. Identifier NCT04711200. Available online at: https://clinicaltrials.gov/ct2/show/NCT04711200 (accessed May 2, 2022.)
24. Wang C-W, Yang L-Y, Chen C-B, Ho H-C, Hung S-I, Yang C-H, et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. (2018) 128:985–96. doi: 10.1172/JCI93349
25. Bachot N, Revuz J, Roujeau J-C. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol. (2003) 139:33–6. doi: 10.1001/archderm.139.1.33
26. Pushker N, Gorimanipalli B, Sharma N, Kashyap S, Bajaj MS. Mucous membrane grafting (fibrin glue vs. suture) for lid margin pathologies in Stevens-Johnson syndrome: randomized comparative study. Eye. (2021) 35:1985–92. doi: 10.1038/s41433-020-01203-4
27. Micheletti RG, Chiesa-Fuxench Z, Noe MH, Stephen S, Aleshin M, Agarwal A, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. (2018) 138:2315–21. doi: 10.1016/j.jid.2018.04.027
28. Tsai T-Y, Huang I-H, Chao Y-C, Li H, Hsieh T-S, Wang H-H, et al. Treating toxic epidermal necrolysis with systemic immunomodulating therapies: a systematic review and network meta-analysis. J Am Acad Dermatol. (2021) 84:390–7. doi: 10.1016/j.jaad.2020.08.122
29. Imahara SD, Holmes JH, Heimbach DM, Engrav LE, Honari S, Klein MB, et al. SCORTEN overestimates mortality in the setting of a standardized treatment protocol. J Burn Care Res. (2006) 27:270–5. doi: 10.1097/01.BCR.0000216532.71360.9B
30. Huang Y-C, Li Y-C, Chen T-J. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol. (2012) 167:424–32. doi: 10.1111/j.1365-2133.2012.10965.x
31. Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. (2010) 5:39. doi: 10.1186/1750-1172-5-39
32. Aihara M, Kano Y, Fujita H, Kambara T, Matsukura S, Katayama I, et al. Efficacy of additional i.v. immunoglobulin to steroid therapy in Stevens-Johnson syndrome and toxic epidermal necrolysis. J Dermatol. (2015) 42:768–77. doi: 10.1111/1346-8138.12925
33. Kakourou T, Klontza D, Soteropoulou F, Kattamis C. Corticosteroid treatment of erythema multiforme major (Stevens-Johnson syndrome) in children. Eur J Pediatr. (1997) 156:90–3. doi: 10.1007/s004310050561
Keywords: SJS/TEN, scoring assessment, drug reaction, epidermal necrolysis, dermatology
Citation: Dobry AS, Himed S, Waters M and Kaffenberger BH (2022) Scoring Assessments in Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Front. Med. 9:883121. doi: 10.3389/fmed.2022.883121
Received: 24 February 2022; Accepted: 25 May 2022;
Published: 16 June 2022.
Edited by:
Elizabeth Phillips, Vanderbilt University, United StatesReviewed by:
Paola Savoia, Università degli Studi del Piemonte Orientale, ItalyCopyright © 2022 Dobry, Himed, Waters and Kaffenberger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allison S. Dobry, YWxsaXNvbi5kb2JyeUB1Y3NmLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.