
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 04 May 2022
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.882341
 Yuan-Hui Liu1†
Yuan-Hui Liu1† Zhi-Yuan Cao2†
Zhi-Yuan Cao2† Yi-Ning Dai1†
Yi-Ning Dai1† Li-Huan Zeng1
Li-Huan Zeng1 Ye-Shen Zhang1
Ye-Shen Zhang1 Hua-Lin Fan1
Hua-Lin Fan1 Chong-Yang Duan2
Chong-Yang Duan2 Ning Tan1*
Ning Tan1* Peng-Cheng He1*
Peng-Cheng He1*Background: Infections are not common but important in patients with acute myocardial infarction, and are associated with worse outcomes. Infection was proved to be associated with the use of proton pump inhibitor (PPI) in several cohorts. It remains unclear whether PPI usage affects infection in patients with acute myocardial infarction.
Methods: We consecutively enrolled patients with ST-elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI) from January 2010 to June 2018. All patients were divided into the PPI group and non-PPI group according to whether the PPI was used. The primary endpoint was the development of infection during hospitalization.
Results: A total of 3027 patients were finally enrolled, with a mean age of 62.2 ± 12.6 years. 310 (10.2%) patients were developed infection during hospitalization. Baseline characteristics were similar between the PPI and non-PPI groups (n = 584 for each group) after propensity score analysis. PPI usage was significantly associated with infection based on the propensity score matching analysis (adjusted OR = 1.62, 95% CI = 1.02-2.57, P = 0.041). Comparing to patients with non-PPI usage, PPI administration was positively associated with higher risk of in-hospital all-cause mortality (adjusted OR = 3.25, 95% CI = 1.06-9.97, P = 0.039) and in-hospital major adverse clinical events (adjusted OR = 3.71, 95% CI = 1.61-8.56, P = 0.002). Subgroup analysis demonstrated that the impact of PPI on infection was not significantly different among patients with or without diabetes and patients with age ≥65 years or age <65 years.
Conclusion: PPI usage was related to a higher incidence of infection during hospitalization, in-hospital all-cause mortality, and in-hospital major adverse clinical events (MACE) in STEMI patients.
Infections during hospitalization can prolong the length of intensive care unit stay and increase short-term mortality as high as tenfold for patients with acute myocardial infarction (AMI) (1–3). Standardized antibiotic therapy can be used to control an infection, but it is unlikely to reverse the worsened condition the infection typically causes. Therefore, attention should be paid to the strategies for reducing infection risk.
Proton pump inhibitors (PPIs) are routinely used to prevent gastrointestinal bleeding (4–6). The suggestions from international guidelines are diverse regarding the use of PPI treatment in post-AMI patients who receive dual antiplatelet therapy (DAPT), and the possible benefits and concerns should be carefully weighed (7–9). A large-scale study of 46301 AMI patients with DAPT found that the clinical benefits of PPI treatment were limited, even when used in accordance with the European Cardiology Society (ESC) guidelines risk stratification strategy (10) suggesting caution is warranted in prescribing PPIs. In addition, a major concern is that PPI usage may be associated with a high risk of pulmonary infection in patients with stroke (11) or critical illness (5). However, such effect was not proven in patients with cardiovascular disease. Furthermore, it remains unknown whether PPI use is associated with infection in AMI patients with a greater risk of infection. We aimed to explore the relationship between PPI use and infection in patients with ST-elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI).
Patients with a primary diagnosis of STEMI and admitted for undergoing PCI in Guangdong Provincial People’s Hospital from January 2010 to June 2018 were enrolled. The current updated 2017 ESC guideline was used for STEMI diagnosis (12). The exclusion criteria were on hemodialysis at admission, undergoing cardiac surgery, chronic inflammatory disease, readmission to hospital, using histamine 2-receptor antagonists or omeprazole (not recommended in patients who received clopidogrel), starting PPI treatment after infection and without PCI. The study flow is shown in Figure 1. This study was conducted based on the Code of Ethics of the 1964 Declaration of Helsinki and its later amendments. The study protocol was approved by the Guangdong Provincial People’s Hospital Ethics Committee (No.GDREC2016378H), and the written informed consents were acquired.
Baseline blood samples were collected within 24 h after admission. Laboratory examination evaluated troponin I/T, blood lipids, electrolytes, serum creatinine, and other conventional parameters. A Chest X-ray was routinely performed at admission and the computed tomography scan was conducted at the physician’s discretion. The infection indicators, including culture (blood, sputum, urine, or wound), were measured when necessary. An ultrasonic cardiogram was performed during hospitalization. Coronary angiogram and PCI were performed by the cardiologists in accordance with standard interventional techniques (13).
Whether patients were administrated with PPI or not was decided at the discretion of cardiologists based on patients’ condition, and the PPIs were given by oral. Patients were separated into two groups (PPI group: received PPI within one week before admission or starting at admission until discharge; non-PPI group: did not receive PPI within one week before admission and during hospitalization). The dose and type of PPI and other medication, including antiplatelet agents, statins, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and beta-blockers, were prescribed at the discretion of a cardiologist.
The primary endpoint was infection during hospitalization, defined as infection requiring antibiotics (reflecting the clinical influence of infection compatible with the necessity for additional treatment) (14). Whether the patients needed an antibiotic was decided by the physicians, based on the sign, symptoms, and laboratory examination (such as white blood cells, C-reactive protein, and procalcitonin et al.) suggesting infection. The investigator identified infection events based on the medical record of antibiotic use and infectious features. The definition of infection was also determined in medical records at discharge with infection ICD-10 codes. Infections were classified into pulmonary infections, urinary infections, or other infections (such as abdominal sepsis, primary bacteremia, and unidentified primary infection site). Infections diagnosed within the first 72 h of hospital admission were considered as community-acquired infections, and infections diagnosed after 72 h were considered as hospital-acquired infections (15). Secondary endpoints were considered to be in-hospital all-cause mortality and in-hospital major adverse clinical events (MACE) including in-hospital all-cause mortality, stroke, recurrence of myocardial infarction, or repeated revascularization.
Continuous data are shown as mean ± SD and categorical data are expressed as percentages. The Student’s t test or analysis of variance was chosen to compare between groups when the data were normally distributed. The Wilcoxon rank-sum test was selected for comparative analysis. A multivariable logistic regression model was performed to identify the risk factors of infection, in-hospital all-cause mortality, and in-hospital MACE. To evaluate the impact of PPI on clinical outcomes, two different methods were used to adjust or balance baseline confounding factors: (1) multivariable analysis and (2) propensity score analysis. Potential confounders that were significant in the univariate analysis or that were clinically important were included in the multivariable logistic models. The following variables were ultimately included for infection: PPI use, age, sex, diabetes, anemia, peptic ulcers, hypertension, smoking, chronic obstructive pulmonary disease, prior myocardial infarction, prior stroke, white blood cell count, serum albumin levels, estimated glomerular filtration rate, DAPT, and femoral arterial access during PCI. The odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated.
Three propensity score methods were conducted to test the robustness of the results: matching on the propensity score (caliper matching method of 1:1 without replacement was used with caliper set as 0.25 standard deviations of the propensity scores), covariate adjustment using the propensity score, and stratification on the quintiles of the propensity score. The propensity score model development was done using the following steps sequentially. First, the factors were all included in the logistic regression model to predict the usage of PPI. Second, for each continuous variable, the model in step one was compared with a model that incorporated the restricted cubic spline function with three knots for all continuous variables. Non-significant cubic splines were excluded. Third, we examined potential interactions between predictor variables by stepwise logistic regression (entry significant level = 0.2 and stay significant level = 0.05). Due to a large number of potential interactions, only two-way interaction was considered. Interactions that were found to be significant were then retained for subsequent steps. Fourth, we then developed a full model including all main effects, cubic-spline representations for continuous variables, and interactions that were identified in the second and third steps. This final model was then used to estimate the propensity scores. For propensity score matching analysis, patients were matched in a 1:1 (PPI group vs. non-PPI group) ratio, and a standardized difference of less than 10% was used to indicate a negligible difference in the covariates between the groups. All factors listed in Table 1 except serum creatinine [instead of eGFR (estimated glomerular filtration rate)], anemia (instead of hemoglobin), left ventricular ejection fraction, period of antibiotics, and length of hospital stay, and HbA1c were considered in the propensity score model development. For propensity score adjustment analysis, logistic regression by adjustment of only the propensity score was used to test the association of PPIs with the risk of in-hospital clinical outcomes. In the stratification analysis, patients were grouped into quintiles based on the propensity score. We also performed the analysis based on the subgroup of age ≥ 65 years, diabetes, hypertension, smoking, and eGFR < 60 mL/min/1.73 m2 to evaluate the role of PPI on infection. A P value less than 0.05 was considered statistically significant. The analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
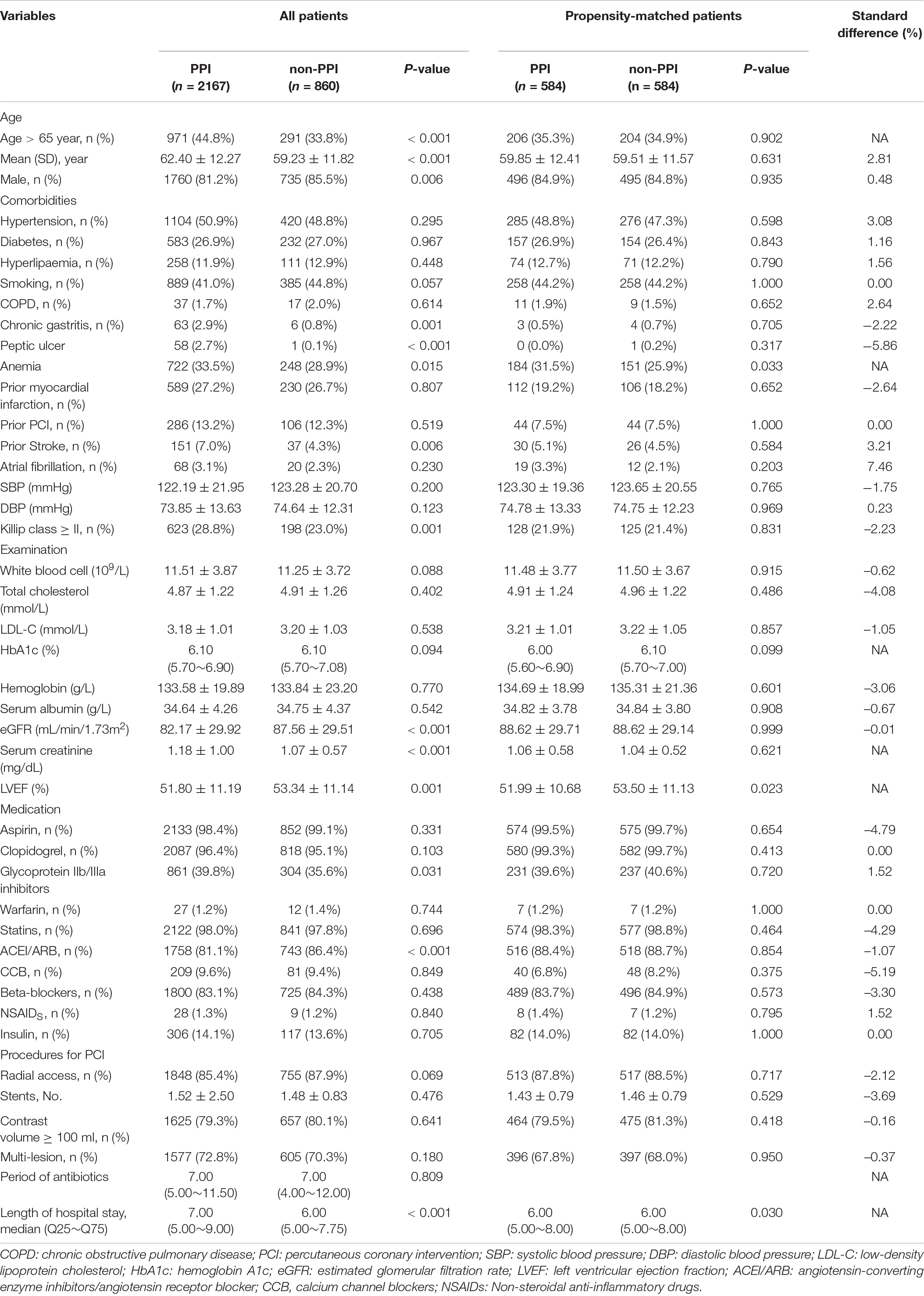
Table 1. Differences in baseline characteristics between patients with and without proton pump inhibitor treatment.
For sample size analysis, we firstly applied the rule of thumb. Events per variable should be 10 or greater. In our database, 310 patients developed an infection. It means we can adjust about 30 risk factors, and we thought the sample size was relatively enough. Furthermore, the sample size was also evaluated based on the logistic regression. A sample size of 3018 observations (of which 70% are in the non-PPI group and 30% are in the PPI group) achieved 80% power at a 0.05 significance level to detect an odds ratio of 1.5 with the R-Squared of the PPI group with other covariables set to be 0.30.
A total of 3,027 patients (17.6% female) were finally enrolled, and the mean age was 62.2 ± 12.6 years. The participants were separated into a PPI group (n = 2167) and a non-PPI group (n = 860). Patients treated with PPI are more likely to be older (44.8 vs. 33.8%, P < 0.001), be female (18.8 vs. 14.5%, P = 0.006), have a history of anemia (33.5 vs. 28.9%, P = 0.015), stroke (7.0 vs. 4.3%, P = 0.006), chronic gastritis (2.9 vs. 0.8%, P = 0.001), and peptic ulcer (2.7 vs. 0.1%, P < 0.001) than those without PPI usage. The percentage of hypertension, diabetes, hyperlipaemia, smoking, chronic obstructive pulmonary disease, prior myocardial infarction, and atrial fibrillation were similar between the two groups. Patients treated with PPI have lower level of left ventricular ejection fraction (51.80 ± 11.19 versus 53.34 ± 11.14, P = 0.001), eGFR (82.17 ± 29.92 mL/min/1.73 m2 versus 87.56 ± 29.51 mL/min/1.73 m2, P < 0.001), and higher level of serum creatinine (1.18 ± 1.00 mg/dL versus 1.07 ± 0.57 mg/dL, P < 0.001). The levels of white blood cells, total cholesterol, hemoglobin, and serum albumin were not significantly different between the two groups. Besides, patients treated with PPI had a higher rate of Killip class ≥ II and more use of glycoprotein IIb/IIIa inhibitors, but less use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers than those without PPI treatment. In-hospital other medications administrated, and PCI procedures were similar between the groups. In addition, the PPI group had a longer duration of hospital stay (7.00 days versus 6.00 days, P < 0.001) (Table 1).
A total of 310 (10.2%) patients developed an infection during hospitalization. The most common infection was pulmonary infection (70.0%), followed by urinary tract infection (15.5%). Patients with PPI treatment had significantly higher rates of infection than those without PPI treatment (11.5% vs. 7.0%, P < 0.001), including hospital-acquired infections (32.8% vs. 21.7%). However, the infection type was similar between the patients with and without PPI treatment (P = 0.390). The percentage of ventilator use was higher in patients with PPI treatment than in those without PPI treatment (4.8% versus 2.4%, P = 0.003). In addition, patients in the PPI group had a higher incidence of in-hospital all-cause mortality (3.9% vs. 1.5%, P < 0.001) and in-hospital MACE (5.8% vs. 2.1%, P < 0.001), compared with the patients in the non-PPI group.
Proton pump inhibitor use was significantly associated with the development of infection (adjusted OR = 1.76, 95% CI = 1.21–2.57, P = 0.003) and pulmonary infection, after adjusting for the confounding variables using multivariable logistic regression analysis (Table 2). Similar results were found for the incidence of in-hospital all-cause mortality and in-hospital MACE (Table 2). In addition, comparing to patients without PPI usage, patients with PPI usage were associated with a relatively higher risk of in-hospital all-cause mortality (OR = 1.89, 95% CI = 0.92–3.87, P = 0.082), and higher risk of in-hospital MACE (OR = 2.10, 95% CI = 1.18–3.73, P = 0.011) when further adjusting infection (Table 3).
Patients treated with and without PPI were then matched 1:1 to create a final cohort of 584 patients per group. The differences in baseline characteristics between the groups were eliminated by matching with all variables of a standardized difference of <10% (Table 1). After matching, the percentage of ventilator use was similar between the two groups (1.2 vs. 2.4%, P = 0.123). However, the rate of infection in the PPI group was still higher than that of the non-PPI group (9.1 vs. 6.0%, P = 0.046) (Figure 2). Similar results were found about the risk of in-hospital all-cause mortality (2.4 vs. 0.9%, P = 0.037) and in-hospital MACE (4.6 vs. 1.4%, P = 0.001).
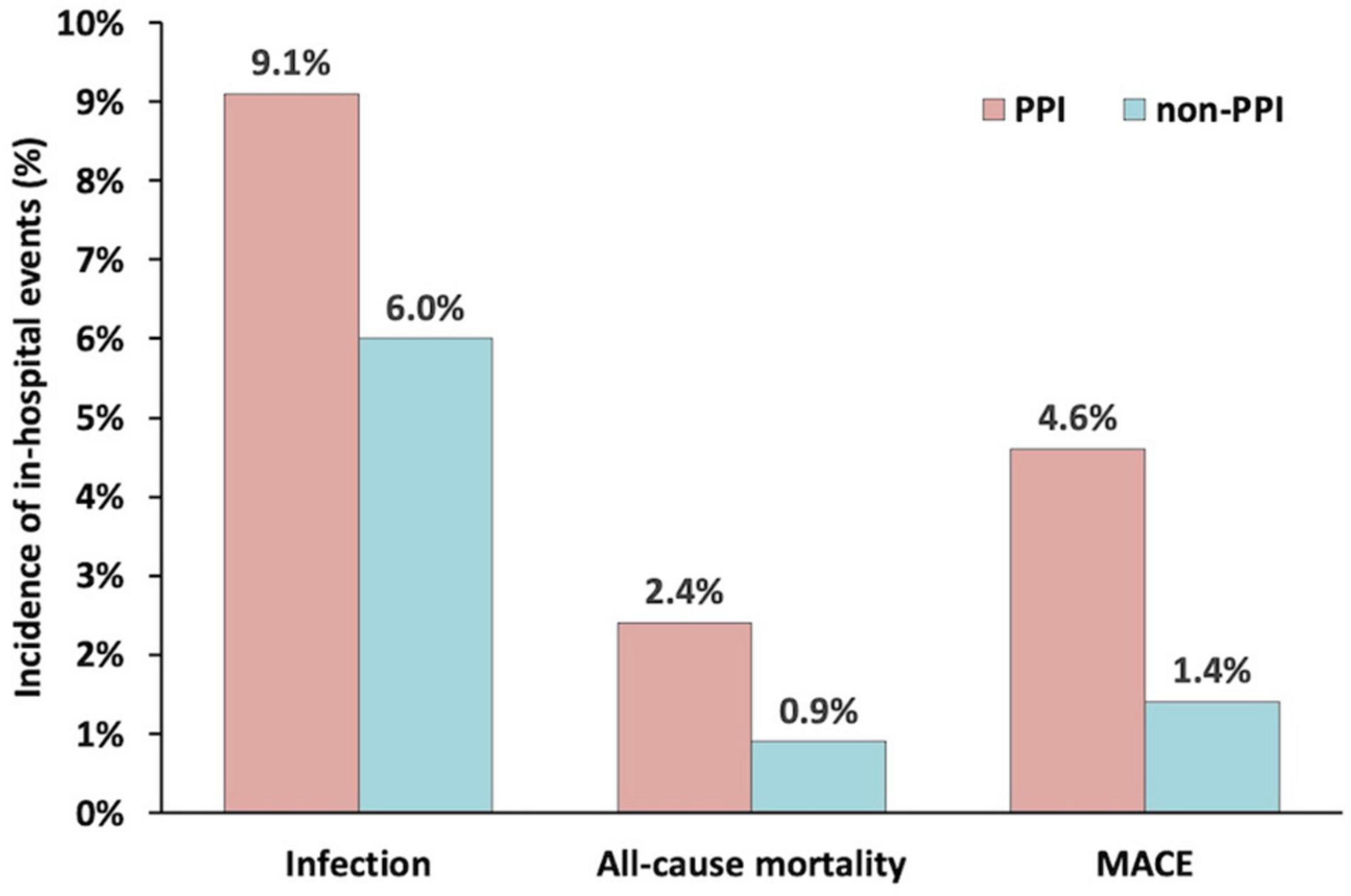
Figure 2. The incidence of infection, all-cause mortality, and major adverse clinical events during hospitalization in the propensity score matched groups.
PPI usage was significantly related to infection based on the propensity score matching analysis (adjusted OR = 1.62, 95% CI = 1.02–2.57, P = 0.041), propensity score adjustment analysis (adjusted OR = 1.52, 95% CI = 1.06–2.18, P = 0.023), and stratification analysis (adjusted OR = 1.53, 95% CI = 1.06–2.20, P = 0.022). Both propensity score analyses demonstrated that PPI administration was positively associated with in-hospital all-cause mortality and in-hospital MACE (Table 4).
The impact of PPI on infection was not significantly different among patients with or without diabetes and those patients with age ≥ 65 years or age < 65 years. A significant interaction of the effect of PPI on the infection has been found between patients with or without smoking, hypertension, and with eGFR > 60 mL/min/1.73 m2 or < 60 mL/min/1.73m2. Patients with a history of hypertension or with eGFR < 60 mL/min/1.73 m2 had a higher risk of infections than those without a history of hypertension or with eGFR > 60 mL/min/1.73 m2, when they were treated with PPI. However, patients treated with PPI and without smoking had a higher risk of infections than those with smoking (Figure 3).
The present study demonstrated that PPI treatment was related to a high risk of infection during hospitalization and other in-hospital clinical outcomes.
Growing evidence from large-scale epidemiology studies has revealed that PPI use was associated with elevated risks of both community-acquired and hospital-acquired pneumonia (16–18). Studies exploring PPI usage and pulmonary infection were based on critical illness cohorts. A meta-analysis of 72 randomized controlled trials suggested that PPI administration might increase pneumonia in clinical illness, which was supported by clinical practice guidelines in 2020 (19, 20). However, another meta-analysis indicated that prophylactic PPI administration might have no significant impact on pneumonia, the discrepancies may be due to methodologic differences or insufficient data (5). Compared to patients with AMI, critical illness patients also had high rates of infection, but were more complex with fixed diseases; infection development in the critical illness cohort might be related to the ventilators and prolonged intensive care unit stays (21, 22). Variation in the factors involved in the development of infection made these two cohorts independent of each other. Thus, the evidence from a critical illness cohort might be not suitable extrapolation to an AMI cohort.
PPI treatment has been proven to be associated with increased odds of hospital-acquired pneumonia in patients with acute stroke (11). However, it is unclear whether these results can be extrapolated to patients with AMI. In a large multicenter randomized study in patients with stable coronary or peripheral arterial disease who were given rivaroxaban or aspirin, long-term PPI usage was not associated with increased pneumonia (6). The incidence of pneumonia was only 2.8% in these patients compared to ours, likely due to the inclusion of low-risk patients, such as those with stable coronary disease. An observational study including 332 acute coronary syndrome patients found that the incidence of hospital-acquired pneumonia was not significantly different between patients who received PPI treatment and those who did not (23). First of all, the cohort sizes were small; and they included patients with high risk for gastrointestinal hemorrhage and mortality, the 30-day mortality reached 10%, which further limited the assessment of PPI for pneumonia. Second, only hospital-acquired pneumonia was evaluated that largely underestimating the pneumonia diagnosis within 48 hours. However, the study herein might be the first large-scale analysis to show that PPI therapy is significantly associated with the infection including community-and hospital-acquired infection in STEMI patients undergoing PCI. In addition, to the lecture on PPI and infectious diseases, previous studies focus on pneumonia or Clostridium difficile infection, our present findings extended it to the overall infection (24–27).
PPI usage associated with increasing infection is largely driven by pulmonary infection in our study. The mechanisms by which PPI usage increases pulmonary infection remain unknown, especially in patients with AMI. The rational explanation is that PPI adversely lower PH and promotes aerobic bacteria in the stomach, then alters the gut microbiome; it may increase spontaneous bacterial peritonitis or pneumonia (28). This hypothesis implies that these changes facilitate lung infection following minor aspirations, as in aspiration pneumonia (29). It is also reported that PPI might induce hypochlorhydria to damage the defense against ingested bacteria and viruses (30, 31). Additionally, PPI usage was associated with neutrophil function impairment in vitro, which might reduce immune activation on the bacterial challenge (32). On the other hand, PPI usage increasing MACE was partly attributed to the infection events; and may also by alleviating the activity of nitric oxide synthase (33).
The present study results support and confirm previous observational studies that PPI therapy was associated with a high risk of all-cause mortality and MACE (34–37). In a post hoc analysis of the PLATO (Platelet Inhibition and Patient Outcomes) trial, PPI use was independently associated with a higher risk of cardiovascular events for both clopidogrel and ticagrelor (38). Recent meta-analyses, including observational studies, also demonstrated similar results (39, 40). In contrast, randomized clinical trials did not prove that PPI treatment would worsen cardiovascular outcomes (41, 42). This might be explained by the fact that PPI use in the observational studies was a marker rather than a cause of cardiovascular complications. To date, the 2017 ESC guideline recommends PPI use to protect against gastrointestinal hemorrhage in patients who received DAPT, while the 2015 ESC regards combination PPI usage in those at higher risk of bleeding (7, 8). Additionally, the 2014 AHA/ACC suggests that PPI therapy should be considered in patients who required triple antithrombotic therapy although they were without a history of gastrointestinal hemorrhage (9). Despite the conflicting advice, for AMI patients treated with DAPT, the incidence of gastrointestinal hemorrhage (either upper gastrointestinal hemorrhage or any gastrointestinal hemorrhage) was low even in the high-risk patients (10, 43). This provides justification for withholding PPI in routine cases. While our study advances the understanding of PPI in AMI-DAPT subjects, there remains a need for ongoing risk stratification and case-by-case evaluation rather than routine PPI administration, especially in patients considered to be at high risk of infection.
The present study has a number of limitations. First, although we performed multivariable analyses and propensity score analyses to reduce the bias, undetected confounders might still exist for this single-center observational study. Second, we did not assess the effectiveness of PPIs in gastrointestinal hemorrhage prevention and compare the effect of different PPIs, modes of administration and regimens on the results. Future studies are necessary to better understand the net clinical effect of PPI usage and different PPIs on the infection. Third, our cohort was limited to STEMI patients and most of them were treated with clopidogrel. Thus, the findings cannot necessarily be applied to other cardiac patients or those treated with ticagrelor. Forth, the study lacked follow-up data on post-discharge infections. It is important to investigate this to assess the impact of PPI therapy on late occurring infections. And the study also lacked data on cardiovascular mortality. Fifth, according to our current definition of infection, we would miss those patients caused by viruses or include those patients with inappropriate antibiotics applications in clinical practice. Noticeably, some inflammatory indicators, such as C-reactive protein and procalcitonin, were not included in the current study since they were unconventional detection indexes, especially before the PCI procedure. And antibiotic type might also influence the outcome. Future research should include these variables to further evaluate their effects on outcomes.
For patients with STEMI undergoing PCI, PPI usage during hospitalization was related to a higher risk of infection as well as in-hospital all-cause mortality and MACE.
The data used to support the findings of this study are available from the corresponding author upon request. Requests to access these datasets should be directed to P-CH, Z2RocGMxMDBAMTI2LmNvbQ==.
The studies involving human participants were reviewed and approved by the Guangdong Provincial People’s Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Y-HL, NT, and P-CH: study concept and design. Z-YC, Y-HL, and Y-ND: drafting of the manuscript. C-YD and Z-YC: supervisors of the study and guarantee the study data and accuracy. All authors contributed to the acquisition, analysis, or interpretation of data, critical revision and final approval of the manuscript.
This work was supported by the Shuangqing Talent Program Project of Guangdong Provincial People’s Hospital (Grant No. KJ012019084 to P-CH and Grant No. KJ012019095 to Y-HL), the High-level Hospital Construction Project (Grant No. DFJH2020021), and Outstanding Young Medical Talents in Guangdong Province (KJ012019456).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The content of this manuscript has been presented (abstract) at “Great Wall International Congress of Cardiology 2021 Asian Heart Society Congress 2021”, Cardiovascular Innovations and Applications, Vol. 6. Suppl. 1, 2021.
1. Truffa AA, Granger CB, White KR, Newby LK, Mehta RH, Hochman JS, et al. Serious infection after acute myocardial infarction: incidence, clinical features, and outcomes. JACC Cardiovasc Intervent. (2012) 5:769–76. doi: 10.1016/j.jcin.2012.03.018
2. Piccaro de Oliveira P, Gonzales V, Lopes RD, Schmidt MM, Garofallo S, Santos RP, et al. Serious infections among unselected patients with ST-elevation myocardial infarction treated with contemporary primary percutaneous coronary intervention. Am Heart J. (2016) 181:52–9. doi: 10.1016/j.ahj.2016.08.005
3. Nash MC, Strom JA, Pathak EB. Prevalence of major infections and adverse outcomes among hospitalized. ST-elevation myocardial infarction patients in Florida, 2006. BMC Cardiovasc Disord. (2011) 11:69. doi: 10.1186/1471-2261-11-69
4. Scally B, Emberson JR, Spata E, Reith C, Davies K, Halls H, et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials. Lancet Gastroenterol Hepatol. (2018) 3:231–41. doi: 10.1016/s2468-1253(18)30037-2
5. Wang Y, Ge L, Ye Z, Siemieniuk RA, Reintam Blaser A, Wang X, et al. Efficacy and safety of gastrointestinal bleeding prophylaxis in critically ill patients: an updated systematic review and network meta-analysis of randomized trials. Intens Care Med. (2020) 24:6209–w. doi: 10.1007/s00134-020-06209-w
6. Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O, et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology. (2019) 157:682.e–91.e. doi: 10.1053/j.gastro.2019.05.056
7. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
8. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:267–315. doi: 10.1093/eurheartj/ehv320
9. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr., Ganiats TG, Holmes DR Jr., et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. (2014) 130:2354–94. doi: 10.1161/cir.0000000000000133
10. Sehested TSG, Carlson N, Hansen PW, Gerds TA, Charlot MG, Torp-Pedersen C, et al. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur Heart J. (2019) 40:1963–70. doi: 10.1093/eurheartj/ehz104
11. Marchina S, Doros G, Modak J, Helenius J, Aycock DM, Kumar S. Acid-suppressive medications and risk of pneumonia in acute stroke patients: a systematic review and meta-analysis. J Neurol Sci. (2019) 400:122–8. doi: 10.1016/j.jns.2019.02.041
12. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
13. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. (2011) 124:e574–651. doi: 10.1161/CIR.0b013e31823ba622
14. Thiele H, Kurz T, Feistritzer HJ, Stachel G, Hartung P, Lurz P, et al. General versus local anesthesia with conscious sedation in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Circulation. (2020) 142:1437–47. doi: 10.1161/circulationaha.120.046451
15. Allen-Bridson K, Gross C, Hebden JN, Morrell GC, Wright MO, Horan T. Healthcare-associated infections studies project: an american journal of infection control and national healthcare safety network data quality collaboration-ventilator-associated event 1, 2013. Am J Infect Control. (2013) 41:1085–6. doi: 10.1016/j.ajic.2013.05.010
16. Hermos JA, Young MM, Fonda JR, Gagnon DR, Fiore LD, Lawler EV. Risk of community-acquired pneumonia in veteran patients to whom proton pump inhibitors were dispensed. Clin Infect Dis. (2012) 54:33–42. doi: 10.1093/cid/cir767
17. Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. (2009) 301:2120–8. doi: 10.1001/jama.2009.722
18. MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med. (2014) 174:564–74. doi: 10.1001/jamainternmed.2013.14673
19. Wang Y, Ye Z, Ge L, Siemieniuk RAC, Wang X, Wang Y, et al. Efficacy and safety of gastrointestinal bleeding prophylaxis in critically ill patients: systematic review and network meta-analysis. BMJ. (2020) 368:l6744. doi: 10.1136/bmj.l6744
20. Ye Z, Reintam Blaser A, Lytvyn L, Wang Y, Guyatt GH, Mikita JS, et al. Gastrointestinal bleeding prophylaxis for critically ill patients: a clinical practice guideline. BMJ. (2020) 368:l6722. doi: 10.1136/bmj.l6722
21. Markwart R, Saito H, Harder T, Tomczyk S, Cassini A, Fleischmann-Struzek C, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intens Care Med. (2020) 46:1536–51. doi: 10.1007/s00134-020-06106-2
22. Xie J, Yang Y, Huang Y, Kang Y, Xu Y, Ma X, et al. The current epidemiological landscape of ventilator-associated pneumonia in the intensive care unit: a multicenter prospective observational study in China. Clin Infect Dis. (2018) 67:S153–61. doi: 10.1093/cid/ciy692
23. Wu H, Jing Q, Wang J, Guo X. Pantoprazole for the prevention of gastrointestinal bleeding in high-risk patients with acute coronary syndromes. J Crit Care. (2011) 26:.e1–6. doi: 10.1016/j.jcrc.2010.12.007
24. Selvanderan SP, Summers MJ, Finnis ME, Plummer MP, Ali Abdelhamid Y, Anderson MB, et al. Pantoprazole or placebo for stress ulcer prophylaxis (POP-UP): randomized double-blind exploratory study. Crit Care Med. (2016) 44:1842–50. doi: 10.1097/ccm.0000000000001819
25. Alhazzani W, Guyatt G, Alshahrani M, Deane AM, Marshall JC, Hall R, et al. Withholding pantoprazole for stress ulcer prophylaxis in critically Ill patients: a pilot randomized clinical trial and meta-analysis. Crit Care Med. (2017) 45:1121–9. doi: 10.1097/ccm.0000000000002461
26. Krag M, Marker S, Perner A, Wetterslev J, Wise MP, Schefold JC, et al. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med. (2018) 379:2199–208. doi: 10.1056/NEJMoa1714919
27. El-Kersh K, Jalil B, McClave SA, Cavallazzi R, Guardiola J, Guilkey K, et al. Enteral nutrition as stress ulcer prophylaxis in critically ill patients: a randomized controlled exploratory study. J Crit Care. (2018) 43:108–13. doi: 10.1016/j.jcrc.2017.08.036
28. Lanas-Gimeno A, Hijos G, Lanas A. Proton pump inhibitors, adverse events and increased risk of mortality. Expert Opin Drug Safety. (2019) 18:1043–53. doi: 10.1080/14740338.2019.1664470
29. Mandell LA, Niederman MS. Aspiration Pneumonia. N Engl J Med. (2019) 380:651–63. doi: 10.1056/NEJMra1714562
30. Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterology. (2017) 153:35–48. doi: 10.1053/j.gastro.2017.04.047
31. Almario CV, Chey WD, Spiegel BMR. Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors. Am J Gastroenterol. (2020) 115:1707–15. doi: 10.14309/ajg.0000000000000798
32. Wandall JH. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut. (1992) 33:617–21. doi: 10.1136/gut.33.5.617
33. Ghebremariam YT, LePendu P, Lee JC, Erlanson DA, Slaviero A, Shah NH, et al. Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation. (2013) 128:845–53. doi: 10.1161/circulationaha.113.003602
34. Zou JJ, Chen SL, Tan J, Lin L, Zhao YY, Xu HM, et al. Increased risk for developing major adverse cardiovascular events in stented Chinese patients treated with dual antiplatelet therapy after concomitant use of the proton pump inhibitor. PLoS One. (2014) 9:e84985. doi: 10.1371/journal.pone.0084985
35. Bhurke SM, Martin BC, Li C, Franks AM, Bursac Z, Said Q. Effect of the clopidogrel-proton pump inhibitor drug interaction on adverse cardiovascular events in patients with acute coronary syndrome. Pharmacotherapy. (2012) 32:809–18. doi: 10.1002/j.1875-9114.2012.01112.x
36. Weisz G, Smilowitz NR, Kirtane AJ, Rinaldi MJ, Parvataneni R, Xu K, et al. Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: the ADAPT-DES study. Circulat Cardiovasc Intervent. (2015) 8:1952. doi: 10.1161/circinterventions.114.001952
37. Burkard T, Kaiser CA, Brunner-La Rocca H, Osswald S, Pfisterer ME, Jeger RV. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. (2012) 271:257–63. doi: 10.1111/j.1365-2796.2011.02423.x
38. Goodman SG, Clare R, Pieper KS, Nicolau JC, Storey RF, Cantor WJ, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. (2012) 125:978–86. doi: 10.1161/circulationaha.111.032912
39. Batchelor R, Kumar R, Gilmartin-Thomas JFM, Hopper I, Kemp W, Liew D. Systematic review with meta-analysis: risk of adverse cardiovascular events with proton pump inhibitors independent of clopidogrel. Aliment Pharmacol Ther. (2018) 48:780–96. doi: 10.1111/apt.14955
40. Serbin MA, Guzauskas GF, Veenstra DL. Clopidogrel-proton pump inhibitor drug-drug interaction and risk of adverse clinical outcomes among PCI-treated ACS patients: a meta-analysis. J Manag Care Spec Pharm. (2016) 22:939–47. doi: 10.18553/jmcp.2016.22.8.939
41. O’Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. (2009) 374:989–97. doi: 10.1016/s0140-6736(09)61525-7
42. Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation. (2011) 123:474–82. doi: 10.1161/circulationaha.110.965640
43. Jensen BES, Hansen JM, Larsen KS, Junker AB, Lassen JF, Jensen SE. Schaffalitzky de Muckadell OB. Randomized clinical trial: the impact of gastrointestinal risk factor screening and prophylactic proton pump inhibitor therapy in patients receiving dual antiplatelet therapy. Eur J Gastroenterol Hepatol. (2017) 29:1118–25. doi: 10.1097/meg.0000000000000934
Keywords: proton pump inhibitor, infection, myocardial infarction, percutaneous coronary intervention, mortality
Citation: Liu Y-H, Cao Z-Y, Dai Y-N, Zeng L-H, Zhang Y-S, Fan H-L, Duan C-Y, Tan N and He P-C (2022) Association of Proton Pump Inhibitor and Infection and Major Adverse Clinical Events in Patients With ST-Elevation Myocardial Infarction: A Propensity Score Matching Analysis. Front. Med. 9:882341. doi: 10.3389/fmed.2022.882341
Received: 23 February 2022; Accepted: 07 April 2022;
Published: 04 May 2022.
Edited by:
Surasak Saokaew, University of Phayao, ThailandReviewed by:
Sukrit Kanchanasurakit, University of Phayao, ThailandCopyright © 2022 Liu, Cao, Dai, Zeng, Zhang, Fan, Duan, Tan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Tan, Z2R0YW5uaW5nQDEyNi5jb20=; Peng-Cheng He, Z2RocGMxMDBAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.