- 1Department of Thoracic and Cardiovascular Surgery, Ajou University School of Medicine, Suwon, South Korea
- 2Department of Radiology and the Research Institute of Radiological Science, Yonsei University Health System, Seoul, South Korea
- 3Department of Thoracic and Cardiovascular Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea
- 4Department of Radiology, Ajou University School of Medicine, Suwon, South Korea
- 5Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, South Korea
Purpose: End-stage lung diseases result in anatomical changes of the thoracic cavity. However, very few studies have assessed changes in the thoracic cavity after lung transplantation (LTx). This study aimed to evaluate the relationships between thoracic cavity volume (TCV) changes after LTx and underlying lung disease.
Methods: We reviewed 89 patients who underwent a pre-LTx pulmonary function test (PFT), chest computed tomography (CT) scan, and 1-year follow-up CT after LTx. These patients were classified into two groups according to pre-LTx PFT as follows: obstructive group [forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio < 70%] and restrictive group (FEV1/FVC ratio > 70%). We measured TCV using CT scan before and at 1 year after LTx and compared the TCV change in the two groups.
Results: In the restrictive group, TCV increased after LTx (preop: 2,347.8 ± 709.5 mL, 1-year postop: 3,224.4 ± 919.0 mL, p < 0.001). In contrast, in the obstructive group, it decreased after LTx (preop: 4,662.9 ± 1,296.3 mL, 1-year postop: 3,711.1 ± 891.7 mL, p < 0.001). We observed that restrictive lung disease, taller stature, lower body mass index, and larger donor lung were independently associated with increased TCV after LTx.
Conclusion: The disease-specific chest remodeling caused by restriction and hyperinflation is at least, in part, reversible. After LTx, the chest remodeling appears to occur in the opposite direction to the disease-specific remodeling caused by the underlying lung disease in recipients.
Introduction
Significant lung size mismatch between the donor and the recipient may cause serious adverse effects during and after lung transplantation (LTx). An oversized graft can lead to inability to close the chest, impaired hemodynamics, persistent atelectasis, and airway complications (1). In contrast, an undersized graft can cause residual space problems, such as persistent pneumothorax and pleural effusions (2, 3). Therefore, lung size matching is an essential component in LTx, and lung transplant surgeons try to achieve it. Various size matching criteria have been proposed to avoid significant size mismatching. Predicted total lung capacity (pTLC), which is calculated using sex and height, is currently the most commonly used criterion (4, 5). Sometimes, transplant surgeons reject organs that are believed to be too large or too small for the patients and resect donor lung parenchyma to reduce the graft volumes (3, 6).
Depending on the type of end-stage lung disease, the patients’ chest wall can change to some extent, and this is referred to as disease-specific chest remodeling (3). Patients with restrictive lung diseases, such as idiopathic pulmonary fibrosis (IPF), typically have decreased lung volume and a smaller thoracic cavity, while patients with obstructive lung diseases, such as chronic obstructive pulmonary disease (COPD), typically have increased lung volume and a larger thoracic cavity, compared to the normal population (7–10). Based on these facts, we assumed that the thoracic cavity could change after LTx either.
In this study, we measured the changes in thoracic cavity volume (TCV) before and after bilateral LTx using chest computed tomography (CT) scans and investigated the factors that were associated with these changes. Such postoperative changes should be considered by surgeons when selecting an appropriate lung graft size.
Materials and Methods
Patients
From January 2007 to September 2017, 212 patients underwent lung transplantation (LTx) at the Yonsei University College of Medicine or Ajou University School of Medicine. Patients were excluded from the study if they met the following criteria: (1) underwent single lung transplantation or combined solid organ transplantation (n = 17); (2) did not have preoperative PFT (PFT) data (n = 34); (3) did not undergo 1-year postoperative chest CT scan (n = 69); and (4) had a significant abnormality, such as pleural effusion (n = 2) or consolidation (n = 2) on 1-year chest CT.
In total, 89 patients who met the aforementioned criteria were reviewed and classified into the obstructive (n = 26) and restrictive (n = 63) groups according to the GOLD criteria based on PFT results (11) (Figure 1). This retrospective study was approved by the Institutional Review Board of each hospital (Severance Hospital: 4-2020-0671 and Ajou University Hospital: AJIRB-MED-MDB-20-245), and the need for informed consent was waived.
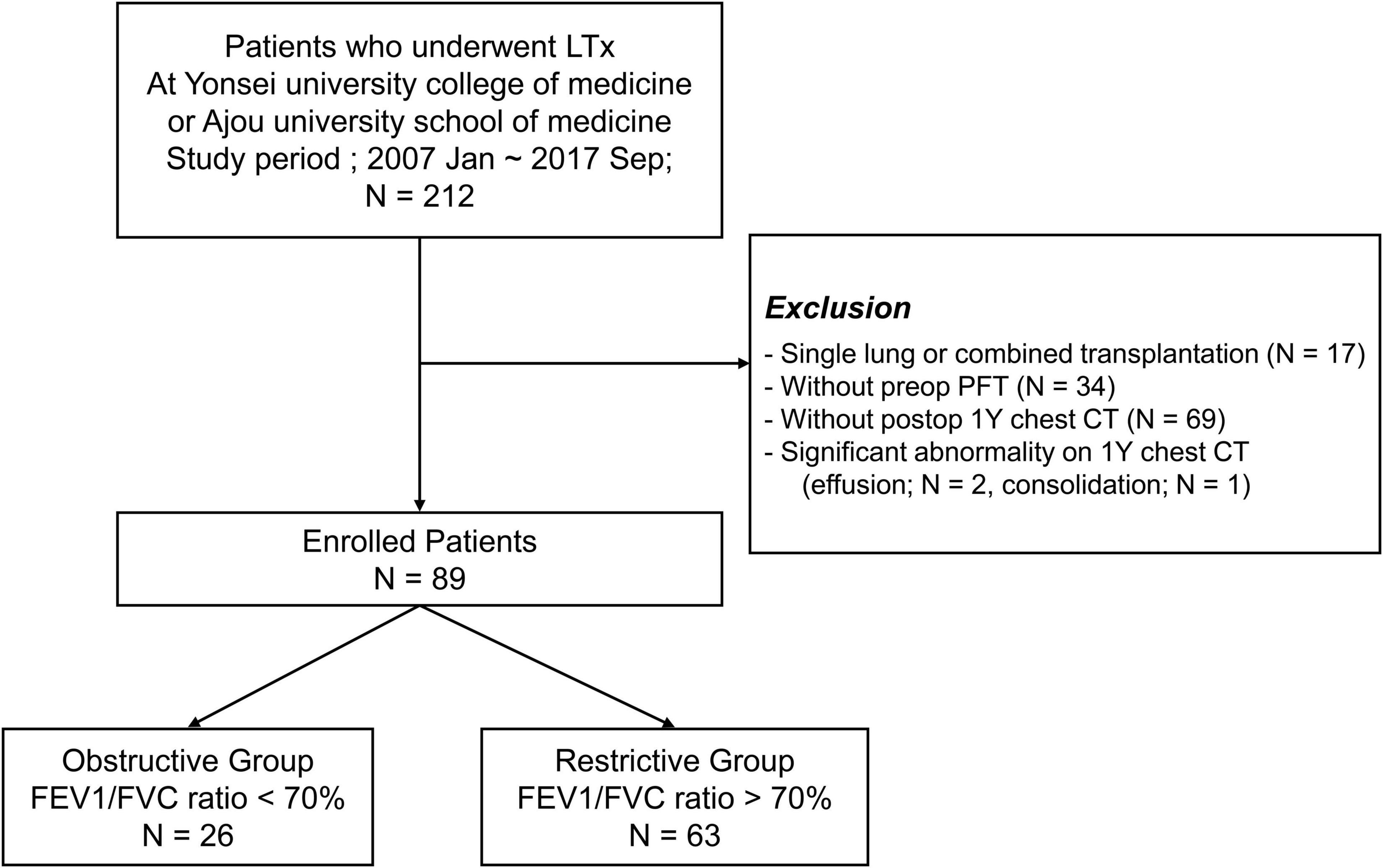
Figure 1. Study population selection. LTx, lung transplantation; preop, pre-operative; postop, postoperative; 1Y, 1 year.
Size Matching and Lung Transplantation Procedure
pTLC has been used for the donor and recipient size matching at the two institutions, and the equations proposed by Goldman and Becklake were used to calculate pTLC as follows (12):
pTLC ratio (donor pTLC/recipient pTLC) between 0.75 and 1.25 was considered acceptable. However, the donor lung graft was used at the discretion of the attending surgeon considering various factors such as patients’ urgency.
The previously described (13) surgical procedure was nearly identical at the two institutions. Briefly, all lung grafts were recovered en bloc from brain-dead donors. In recipients, a clamshell incision in the fourth intercostal space is the preferred method of surgical incision. Bilateral LTx was performed sequentially. If necessary, graft volume reduction, including wedge resections or lobectomies, was performed due to the excess size of the donor’s lung compared to the size of the recipient’s chest wall. The decision to perform graft volume reduction was made by the attending surgeon.
All organs used for transplantation in this study were provided by the government agency, the Korean Network for Organ Sharing (KONOS). The entire process for transplantation was strictly regulated by the relevant legislation. None of the transplant donors was from a vulnerable population, and all donors or next of kin provided written informed consent that was freely given.
Thoracic Cavity Volume Measurement
All patients underwent chest CT scan before and in the first year after LTx. CT was performed from the lung apices to the adrenal glands during breath holding at the end of inspiration with the subject in the supine position. After acquiring the scout image to determine the field of view, a CT scan was performed using a helical technique at a 1-mm reconstruction interval using the mediastinal window setting. The exposure parameters for the CT scans were as follows: 120 kVp, 50–130 mA, and 1–3-mm slice thickness. The conventional CT images were reconstructed using 1–3-mm reconstruction increment on the scanner workstation. Two radiologists (CHP and SY) reviewed all CT images. Eligible axial CT images were transferred to a commercially available reconstruction program using three-dimensional (3D) reconstruction and measurement (Aquarius iNtuition™ Ver. 4.4.6; TeraRecon, Foster City, CA, United States). TCV was defined as the anatomical volume of both lungs and measured using a threshold-based three-dimensional segmentation technique. First, both lungs were selected and extracted automatically using an automatic segmentation technique. The default threshold was set to −200 to −1,024 HU. Second, the included large airways (trachea and bilateral main bronchi) were carefully selected manually and excluded. Then the extracted three-dimensional lungs were visually confirmed. Finally, the anatomical lung volume was measured and used as TCV (Supplementary Figure 1). ΔTCV was defined as the TCV at the first year after LTx minus the preoperative TCV.
Donor/Recipient Size Discrepancy
Donor Predicted Total Lung Capacity/Recipient Predicted Total Lung Capacity
Because donor lung volume and recipient thoracic cavity volume before the development of pulmonary disease cannot be measured directly, we used donor pTLC/recipient pTLC as a surrogate for donor lung size compared to the non-diseased recipient’s thoracic cavity volume.
Donor Predicted Total Lung Capacity/Recipient Thoracic Cavity Volume
Preoperative recipient TCV (TCVpreop) represents thoracic cavity volume of the recipient with end-stage lung disease. We used donor pTLC/recipient TCVpreop as a surrogate for the lung size of donors compared to the diseased recipient’s thoracic cavity volume.
Statistical Analysis
Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, United States) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) software. Continuous variables were compared using Student’s t-test or the Mann–Whitney U test. Categorical variables were compared using the chi-square test or Fisher’s exact test. A paired-sample t-test was implemented for within-group comparisons between preoperative and 1-year postoperative lung volumes. Analysis of covariance (ANCOVA) was used to compare the groups in terms of preoperative and 1-year postoperative TCV after adjusting for recipient pTLC. The correlations between variables were calculated using the Pearson correlation test. Multivariate logistic regression analysis was performed to identify independent factors associated with ΔTCV. Clinically relevant variables for TCV were included in the model (age, sex, height, body mass index (BMI), donor pTLC/recipient TCV, and lung volume reduction). A p-value of < 0.05 was considered statistically significant.
Results
Patient Characteristics
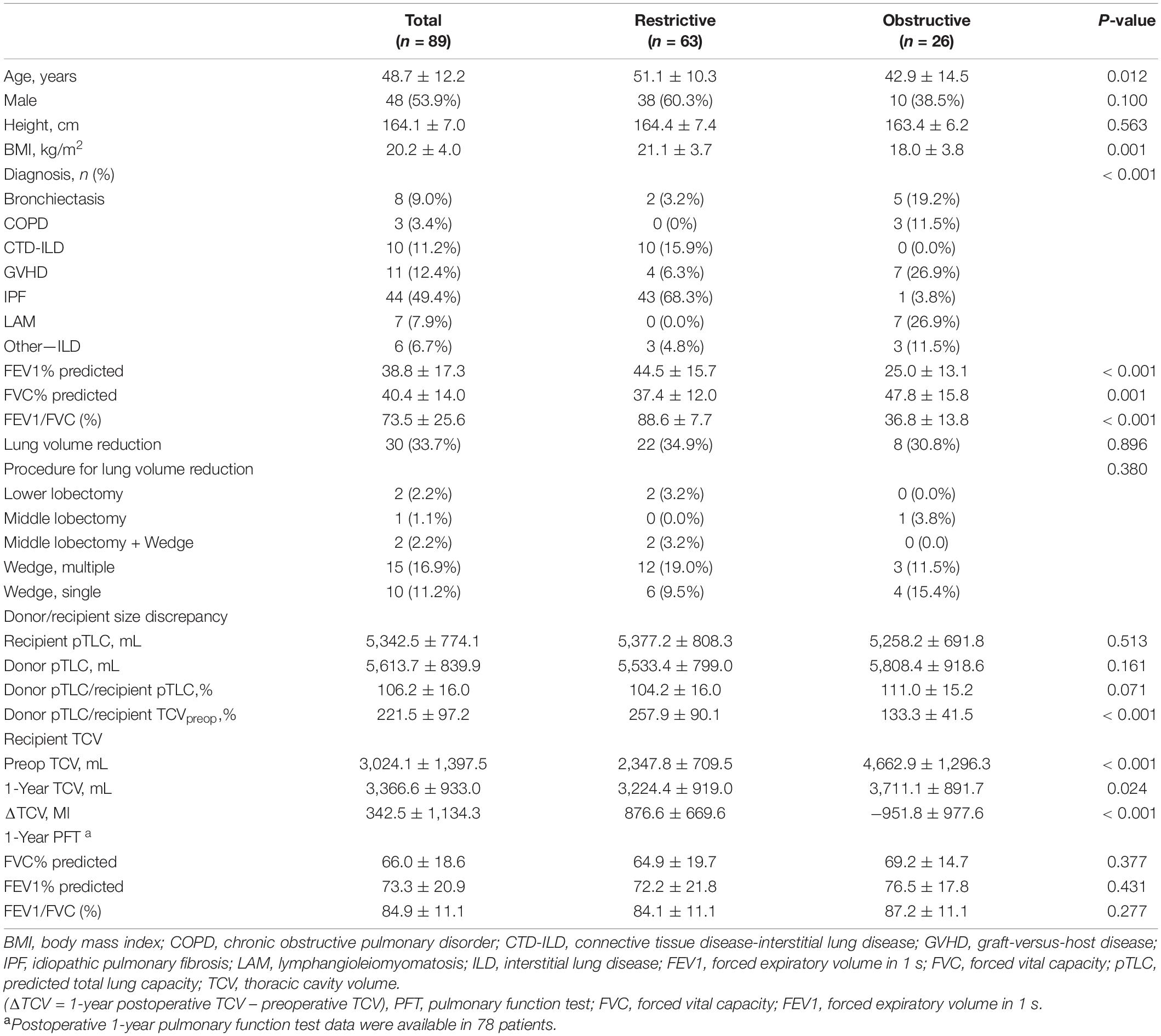
Patient characteristics are summarized in Table 1. The mean age of the 89 participants was 48.7 ± 12.1 years. Forty-eight patients (53.9%) were male. The most common indication for LTx was IPF, which 44 patients (49.4%) had. The restrictive group comprised 63 patients (70.3%), and these patients tended to be older and had a higher BMI than those in the obstructive group.
Predicted Total Lung Capacity and Thoracic Cavity Volume of Donor/Recipient
The donor/recipient pTLC of all patients was 106.2% ± 16.0%. The donor/recipient pTLC was not significantly different between the two groups (restrictive group [104.2% ± 16.0%] vs. obstructive group [111.0% ± 15.2%], p = 0.071). However, the restrictive group had a significantly higher donor pTLC/recipient TCV than the obstructive group [restrictive group (257.9% ± 90.1%) vs. obstructive group (133.3% ± 41.5%), p < 0.001].
Thoracic Cavity Volume Changes Before and After Lung Transplantation
In the restrictive group, TCV increased after LTx (preop: 2,347.8 ± 709.5 mL, 1-year postop: 3,224.4 ± 919.0 mL, p < 0.001). In contrast, in the obstructive group, it decreased after LTx (preop: 4,662.9 ± 1,296.3 mL, 1-year postop: 3,711.1 ± 891.7 mL, p < 0.001, Figure 2).
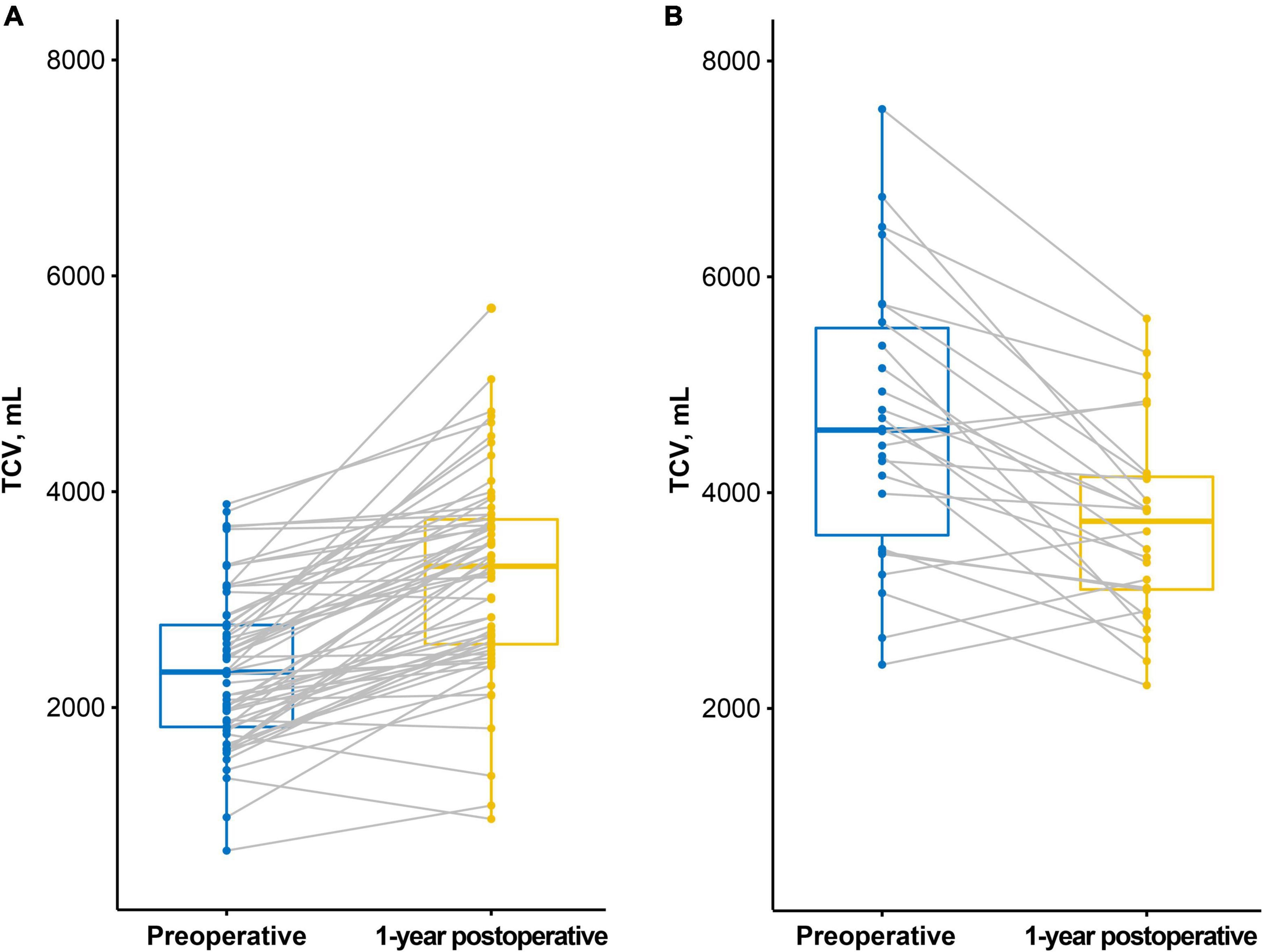
Figure 2. Preoperative and 1-year postoperative thoracic cavity volume (TCV) measured by CT. (A) In the restrictive group, TCV increased after lung transplantation (LTx). (B) In the obstructive group, TCV decreased after LTx.
ANCOVAs were performed to compare the TCV in the two groups adjusting for pTLC. There was a significant difference in preoperative TCV between the groups (F = 89.81, p < 0.001). After transplantation, the difference markedly decreased but was statistically significant (F = 27.85, p < 0.001, Figure 3).
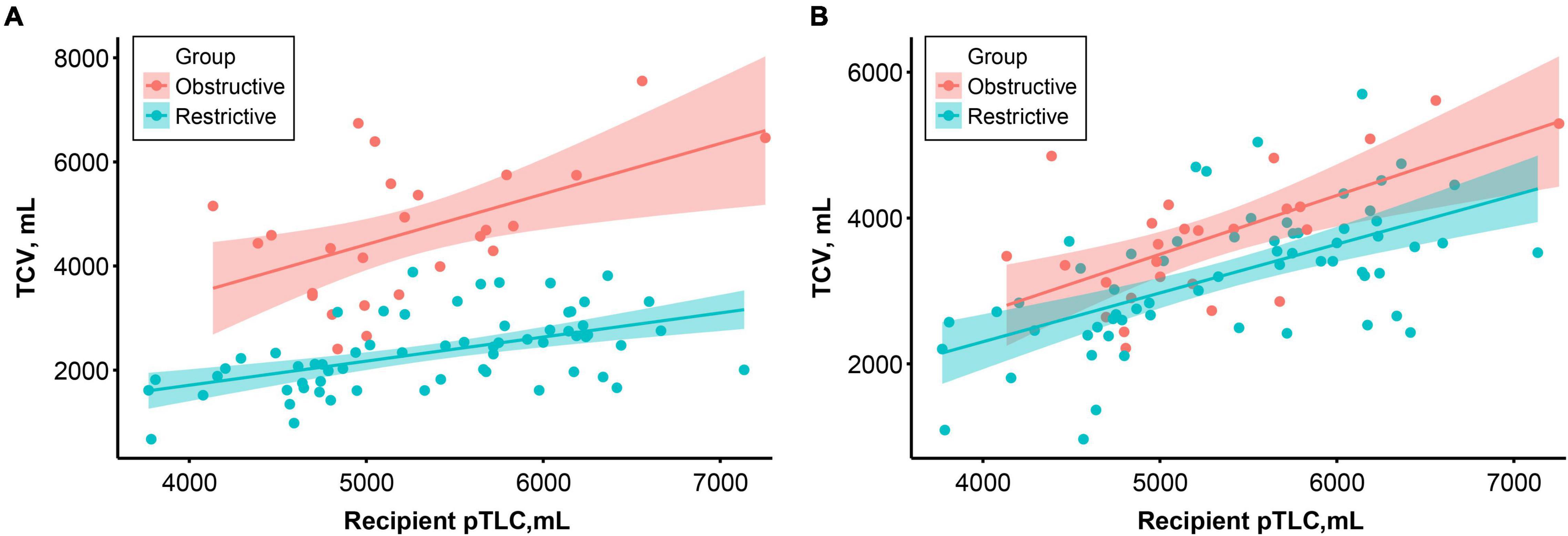
Figure 3. Correlation between recipient predictive total lung capacity (pTLC) and preoperative (A) and 1-year postoperative (B) thoracic cavity volume according to the study groups. Colored lines and areas indicate the regression lines and 95% confidence intervals, respectively.
Correlation Between Postoperative 1-Year Thoracic Cavity Volume and Pulmonary Function Test
Postoperative 1-year TCV was closely correlated with forced vital capacity [Pearson’s correlation coefficient = 0.758, 95% confidence interval (CI) 0.664–0.839, p < 0.001] and forced expiratory volume in 1 s (Pearson’s correlation coefficient = 0.751, 95% CI, 0.635–0.834, p < 0.001, Figure 4).
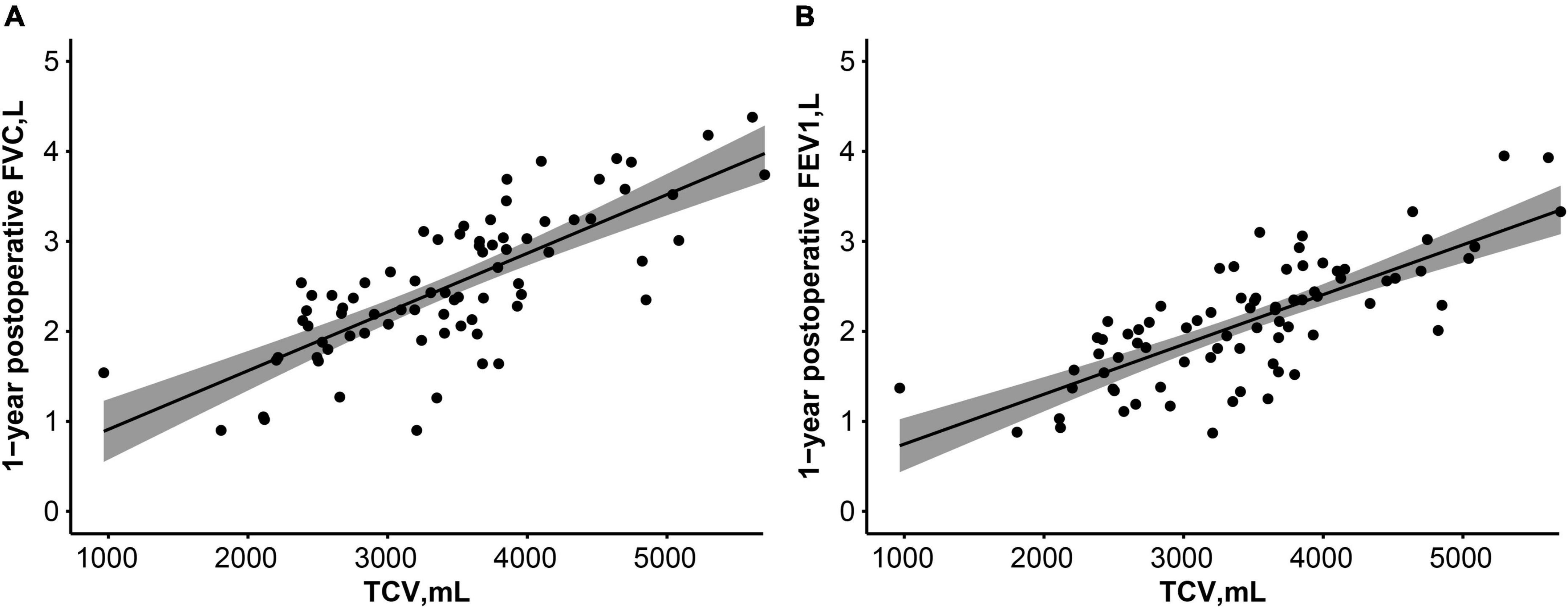
Figure 4. Correlation between postoperative 1-year TCV and pulmonary function test [FVC, L (A), FEV1, L (B)]. Lines and dark areas show the regression lines and 95% confidence interval, respectively. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
Predictive Factors for Thoracic Cavity Volume Change
We constructed a multivariate regression model using clinically relevant variables and lung volume. The restrictive group (vs. the obstructive group), taller recipients, lower recipient BMI, and higher donor pTLC/recipient TCV were found to be independently related to increasing ΔTCV (Table 2).
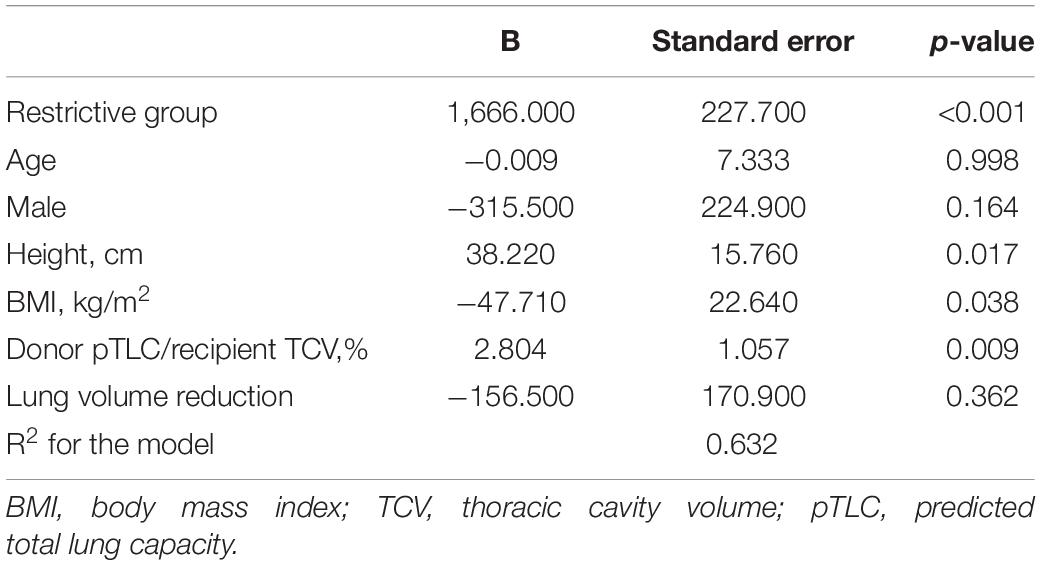
Table 2. Multivariable linear regression model for changes in the thoracic cavity volume after lung transplantation.
Discussion
In this study, we found that the restrictive group had smaller TCV than the obstructive group preoperatively. TCV was found to have increased in the restrictive group and decreased in the obstructive group after LTx. The restrictive group had still smaller TCV than the obstructive group at 1-year post-operation, suggesting that the disease specific chest remodeling—due to restriction and hyperinflation—seems to be partly reversible. In multivariable analysis, disease groups (Restrictive vs. Obstructive), height, BMI, and donor lung size (Donor pTLC/recipient TCV) were associated with the change of TCV (Figure 5).
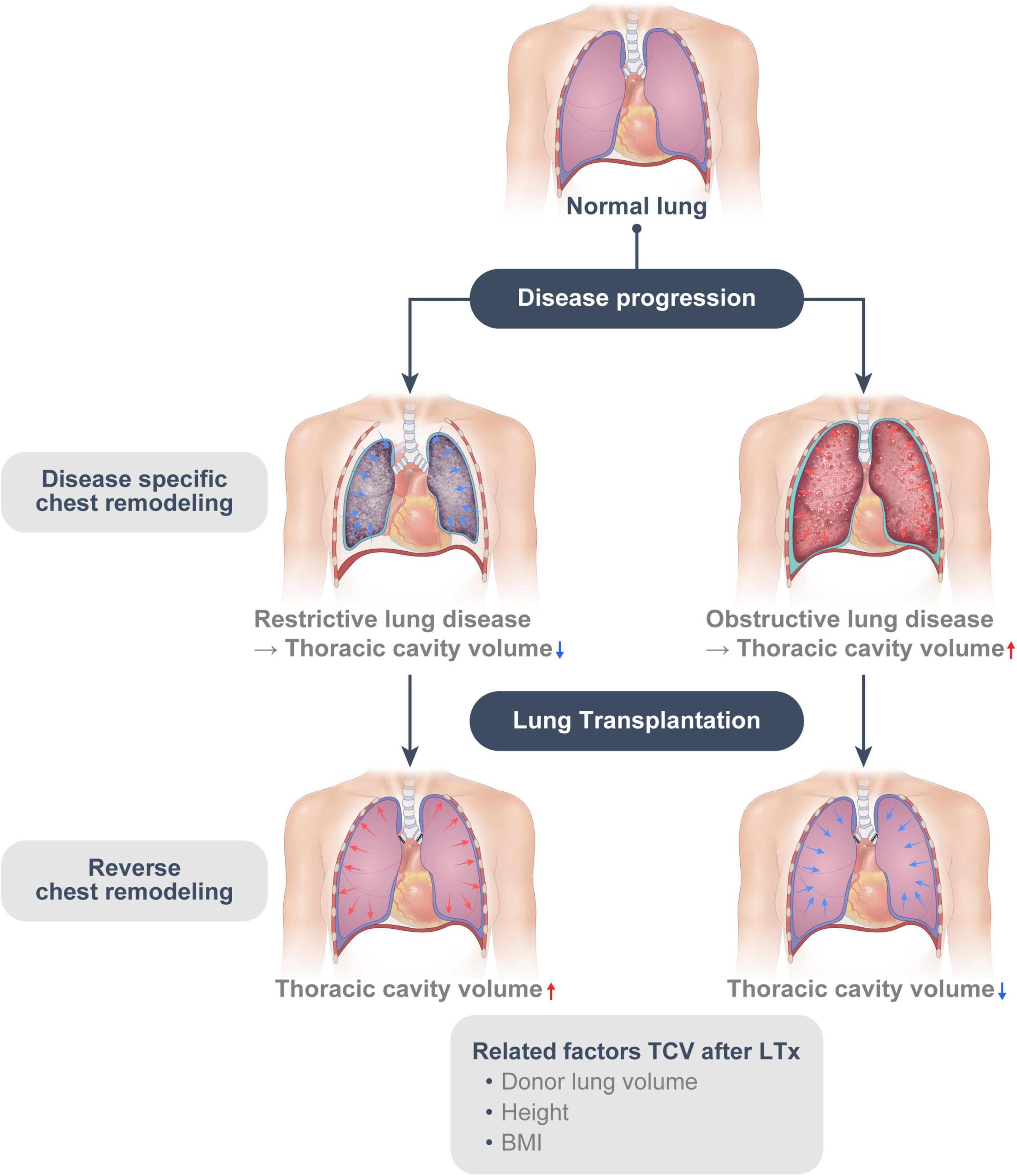
Figure 5. The disease-specific chest remodeling caused by restriction and hyperinflation is at least, in part, reversible. (Reverse chest remodeling).
Due to the unavailability of donor organs, some degree of size mismatching is inevitable in clinical settings; however, there are still controversies about the most suitable size for LTx. Guidelines published in 2003 stated that a donor pTLC between 75% and 125% of the recipient pTLC is considered acceptable and did not cause any adverse clinical or functional effects (14). In this study, we showed that the chest volume changed after LTx and the changes were associated with donor lung size, suggesting that the chest wall can adjust to the newly transplanted lungs. This provides the theoretical background of the wide range of acceptable lung sizes in current guidelines. Post-transplant chest change appeared to proceed in the opposite direction to the preoperative disease-specific chest remodeling. We referred to this phenomenon as “reverse chest remodeling.”
Some studies have implied that disease-specific chest remodeling is reversible after LTx. Wilkens et al. showed reversibility of breathing pattern and chest wall volume during exercise after LTx. They measured breathing patterns and chest wall volumes using opto-electronic plethysmography (OEP) among patients with cystic fibrosis (CF) (n = 9), pulmonary fibrosis (PF) (n = 9), and COPD (n = 21); transplanted patients (n = 16); and healthy participants (n = 10). Patients with CF, PF, or COPD showed distinct breathing patterns. However, after LTx, the breathing pattern of the transplanted patients became similar to those of healthy participants (9). Tamm et al. analyzed perioperative TLC of 82 patients who underwent heart-lung transplantation (HLTx). They found that the postoperative TLC approached the pTLC of the recipient after HLTx, regardless of the underlying disease, preoperative TLC, or donor pTLC (15). Additionally, Chacon et al. investigated patients who underwent either HLTx (n = 6) or bilateral LTx (n = 9) in whom the postoperative TLC was significantly correlated with pTLC (r = 0.724, p = 0.002) but not with preoperative TLC (r = 0.148, p = not significant) (16).
In this study, the restrictive group still had smaller TCV than the obstructive group at 1 year after LTx, and the difference in postoperative 1-year TCV between the groups still existed after adjustment for pTLC, which suggests the incompletion of the reverse remodeling. To investigate the factors associated with reverse remodeling, we performed a multivariable analysis for the factors affecting ΔTCV. Donor pTLC/recipient TCV was positively correlated with ΔTCV in multivariable analysis and the Pearson’s correlation test (Pearson’s correlation coefficient = 0.537, 95% CI, 0.370–0.670, p < 0.001, Supplementary Figure 2). Larger donor pTLC/recipient TCV represents a larger donor lung compared to the diseased thoracic cavity. Therefore, our findings suggest that a constricted thoracic cavity in patients with restrictive lung disease can expand when larger donor lungs are used.
Eberlein et al. investigated 812 bilateral LTx cases from the lung transplant outcome group, a United States National Institutes of Health-sponsored, multicenter, prospective cohort study designed to evaluate risk factors for primary graft dysfunction (PGD). They found that patients with IPF accounted for 43.4% of undersized cohorts (pTLC ratio ≤ 1), and IPF was the most common diagnosis in the undersized cohorts (17). This implies that smaller organs are preferred for patients with restrictive disease because of the risk of complications owing to size mismatch in actual clinical settings. However, in the present study, there was no significant difference in donor/recipient pTLC ratio between the groups. Among patients without lung volume reduction, the restrictive group had significantly lower donor/recipient pTLC ratio compared with that in the obstructive group (98.9 ± 13.9% in restrictive group vs. 109.5 ± 15.7% in obstructive group, Supplementary Table 1). Furthermore, lower lobectomy and multiple resections tended to be performed more frequently in the restrictive group than in the obstructive group. Larger resection of lungs could result in the implantation of smaller lungs, and this might be one of the reasons for smaller TCV in patients with restrictive disease. Some studies have suggested the benefits of an oversized graft, as it delays bronchiolitis obliterans syndrome and has better survival outcomes (18–20). Additionally, Eberlein et al. (1, 17) reported that an oversized graft was not associated with increased postoperative complications. Similarly, we observed a positive correlation between postoperative 1-year TCV and PFT. These suggest an association between the preference of smaller organs in the restrictive group and worse outcomes.
The National Emphysema Treatment Trial (NETT) showed that lung volume reduction (LVR) surgery could improve lung function, exercise capacity, and survival in selected patients with COPD (21, 22). In COPD, the diaphragm is flattened and straightened, which leads to asynchronous movement of the chest wall (23). Zoumot et al. assessed 26 patients who underwent LVR [surgical (n = 9) or bronchoscopic (n = 7)] or a sham/unsuccessful bronchoscopic treatment (control subjects, n = 10) (24). Patients who underwent LVR showed significant radiologic evidence of decreased lung volume and improved chest wall asynchrony. This study showed that TCV decreased after LTx in the obstructive group, which suggests that COPD patients who undergo LTx might have improved respiratory mechanics.
Height was positively correlated with ΔTCV. Taller patients are likely to have a larger TCV before a lung disease develops and may have the potential to have increased TCV after LTx. In contrast, BMI was negatively correlated with ΔTCV. Obese patients are known to have decreased functional residual capacity (FRC) (25, 26). One study showed that obesity affects the shape of the diaphragm, and the diaphragm was displaced cranially in obese patients (26). Those results are consistent with the result of a separate study showing that BMI was negatively correlated with the height of the diaphragm as measured by posteroanterior chest radiography (27). Such results show that obesity can increase abdominal pressure, displace the diaphragm cranially, and restrict TCV recovery after LTx.
Our study has some limitations. This was a retrospective study conducted in two Korean transplantation centers. The patients included in this study were all Koreans. Lung volumes or TCV varies among races, and pTLC is not specifically calculated for Asians only. Therefore, the donor pTLC could be different from the actual TLC of donors and TCV measured by CT in our study population. In the study period, LVR was performed quite frequently (33.7%). Also, we did not employ the delayed sternum closure strategy that is used for oversized grafts. In case of oversized grafts, graft volume reductions are frequently needed to close the chest. However, some centers recently reported that delayed chest closure may be beneficial in those situations (28, 29). Delayed chest wall closure provides time for the patient to recover from ischemic-reperfusion injury and allows the chest wall to become more compliant. This might make transplantation of larger lungs without graft reduction possible. Nevertheless, further studies are needed to reveal the mechanism underlying the effect of delayed chest wall closure on postoperative TCV and LTx outcomes.
In conclusion, disease-specific chest remodeling caused by restriction and hyperinflation is at least partly reversible. After LTx, the chest remodeling appears to occur in the opposite direction to the disease-specific remodeling caused by the underlying lung disease in recipients. Additionally, this “reverse chest remodeling” might be affected by donor lung size, height, and BMI; furthermore, surgeons prefer smaller organs for restrictive disease and larger organs for obstructive disease; this could restrict “reverse chest remodeling” and be associated with impaired lung function and respiratory mechanics. Therefore, transplant surgeons should consider this remodeling for size matching and donor LVR.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
This retrospective study was approved by the Institutional Review Board of each hospital (Severance Hospital: 4-2020-0671 and Ajou University Hospital: AJIRB-MED-MDB-20-245). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
WY, CP, and SH contributed to conception and design of the study. WY, CP, SY, and JJ organized the database. WY and JS performed the statistical analysis. WY wrote the first draft of the manuscript. CP, HP, JL, SY, JS, JJ, and SH wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors admire and are grateful to Dr. Joo Han Song (Division of Pulmonology, Department of Internal Medicine, Severance Hospital) for his efforts and sacrifice in caring for patients who had undergone a lung transplant in the ICU, and for his help in this study. We also hope he recovers soon.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.881119/full#supplementary-material
Supplementary Figure 1 | Thoracic cavity volume measurement using a threshold-based three-dimensional segmentation technique. (A) A chest CT image of a 56-year-old male patient with IPF before lung transplantation shows subpleural interstitial fibrosis and honeycombing appearances. (B) Both lungs are selected using a threshold-based automatic segmentation technique ranging from −200 to −1,024 HU. The selected area is displayed in green. (C) To excluded large airways, trachea and main bronchi are carefully selected manually. Selected bilateral main bronchi are shown in pink. (D) Anatomical lung volume is measured after careful visual inspection to confirm three-dimensional lung enucleation. The thoracic cavity volume of this patient is 2,890 cm3 before lung transplantation. (E) After lung transplantation, the thoracic cavity volume was measured in the same way in the chest CT images. A chest CT images 1 year after lung transplantation of this patient shows no evidence of pulmonary fibrosis. (F) The thoracic cavity volume measured by chest CT is 3,985 cm3.
Supplementary Figure 2 | Correlation between ΔTCV and Donor pTLC/recipient TCV (Pearson’s correlation coefficient = 0.537, 95% CI, 0.370–0.670; p < 0.001). Lines and dark areas show the regression lines and 95% confidence interval, respectively. TCV, Thoracic cavity volume; pTLC, predicted total lung capacity.
Supplementary Table 1 | Patient characteristics with/without volume reduction.
References
1. Eberlein M, Arnaoutakis GJ, Yarmus L, Feller-Kopman D, Dezube R, Chahla MF, et al. The effect of lung size mismatch on complications and resource utilization after bilateral lung transplantation. J Heart Lung Transplant. (2012) 31:492–500. doi: 10.1016/j.healun.2011.12.009
2. Backhus LM, Sievers EM, Schenkel FA, Barr ML, Cohen RG, Smith MA, et al. Pleural space problems after living lobar transplantation. J Heart Lung Transplant. (2005) 24:2086–90. doi: 10.1016/j.healun.2005.06.013
3. Mason DP, Batizy LH, Wu J, Nowicki ER, Murthy SC, McNeill AM, et al. Matching donor to recipient in lung transplantation: how much does size matter? J Thorac Cardiovasc Surg. (2009) 137:1234–40.e1. doi: 10.1016/j.jtcvs.2008.10.024
4. Barnard JB, Davies O, Curry P, Catarino P, Dunning J, Jenkins D, et al. Size matching in lung transplantation: an evidence-based review. J Heart Lung Transplant. (2013) 32:849–60. doi: 10.1016/j.healun.2013.07.002
5. Ouwens JP, van der Mark TW, van der Bij W, Geertsma A, de Boer WJ, Koëter GH. Size matching in lung transplantation using predicted total lung capacity. Eur Respir J. (2002) 20:1419–22. doi: 10.1183/09031936.02.00294402
6. Shigemura N, Bermudez C, Hattler BG, Johnson B, Crespo M, Pilewski J, et al. Impact of graft volume reduction for oversized grafts after lung transplantation on outcome in recipients with end-stage restrictive pulmonary diseases. J Heart Lung Transplant. (2009) 28:130–4. doi: 10.1016/j.healun.2008.11.003
7. Smith V, Pizzorni C, Riccieri V, Decuman S, Brusselle G, De Pauw M, et al. Stabilization of microcirculation in patients with early systemic sclerosis with diffuse skin involvement following rituximab treatment: an open-label study. J Rheumatol. (2016) 43:995–6. doi: 10.3899/jrheum.151018
8. Shin JM, Kim TH, Haam S, Han K, Byun MK, Chang YS, et al. The repeatability of computed tomography lung volume measurements: comparisons in healthy subjects, patients with obstructive lung disease, and patients with restrictive lung disease. PLoS One. (2017) 12:e0182849. doi: 10.1371/journal.pone.0182849
9. Wilkens H, Weingard B, Lo Mauro A, Schena E, Pedotti A, Sybrecht GW, et al. Breathing pattern and chest wall volumes during exercise in patients with cystic fibrosis, pulmonary fibrosis and COPD before and after lung transplantation. Thorax. (2010) 65:808–14. doi: 10.1136/thx.2009.131409
10. Baratella E, Ruaro B, Giudici F, Wade B, Santagiuliana M, Salton F, et al. Evaluation of correlations between genetic variants and high-resolution computed tomography patterns in idiopathic pulmonary fibrosis. Diagnostics (Basel). (2021) 11:762. doi: 10.3390/diagnostics11050762
11. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. (2013) 187:347–65. doi: 10.1164/rccm.201204-0596PP
12. Goldman HI, Becklake MR. Respiratory function tests; normal values at median altitudes and the prediction of normal results. Am Rev Tuberc. (1959) 79:457–67. doi: 10.1164/artpd.1959.79.4.457
13. Yu WS, Paik HC, Haam SJ, Lee CY, Nam KS, Jung HS, et al. Transition to routine use of venoarterial extracorporeal oxygenation during lung transplantation could improve early outcomes. J Thorac Dis. (2016) 8:1712–20. doi: 10.21037/jtd.2016.06.18
14. Orens JB, Boehler A, de Perrot M, Estenne M, Glanville AR, Keshavjee S, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. (2003) 22:1183–200. doi: 10.1016/s1053-2498(03)00096-2
15. Tamm M, Higenbottam TW, Dennis CM, Sharples LD, Wallwork J. Donor and recipient predicted lung volume and lung size after heart-lung transplantation. Am J Respir Crit Care Med. (1994) 150:403–7. doi: 10.1164/ajrccm.150.2.8049822
16. Chacon RA, Corris PA, Dark JH, Gibson GJ. Respiratory mechanics after heart-lung and bilateral lung transplantation. Thorax. (1997) 52:718–22. doi: 10.1136/thx.52.8.718
17. Eberlein M, Reed RM, Bolukbas S, Diamond JM, Wille KM, Orens JB, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant. (2015) 34:233–40. doi: 10.1016/j.healun.2014.09.030
18. Eberlein M, Diehl E, Bolukbas S, Merlo CA, Reed RM. An oversized allograft is associated with improved survival after lung transplantation for idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. (2013) 32:1172–8. doi: 10.1016/j.healun.2013.06.011
19. Eberlein M, Permutt S, Brown RH, Brooker A, Chahla MF, Bolukbas S, et al. Supranormal expiratory airflow after bilateral lung transplantation is associated with improved survival. Am J Respir Crit Care Med. (2011) 183:79–87. doi: 10.1164/rccm.201004-0593OC
20. Eberlein M, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Shlobin OA, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. (2012) 141:451–60. doi: 10.1378/chest.11-0767
21. Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, Pepper J, et al. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med. (2000) 343:239–45. doi: 10.1056/nejm200007273430402
22. Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. (2003) 348:2059–73. doi: 10.1056/NEJMoa030287
23. Gilmartin JJ, Gibson GJ. Abnormalities of chest wall motion in patients with chronic airflow obstruction. Thorax. (1984) 39:264–71. doi: 10.1136/thx.39.4.264
24. Zoumot Z, LoMauro A, Aliverti A, Nelson C, Ward S, Jordan S, et al. Lung volume reduction in emphysema improves chest wall asynchrony. Chest. (2015) 148:185–95. doi: 10.1378/chest.14-2380
25. Jenkins SC, Moxham J. The effects of mild obesity on lung function. Respir Med. (1991) 85:309–11. doi: 10.1016/s0954-6111(06)80102-2
26. Boriek AM, Lopez MA, Velasco C, Bakir AA, Frolov A, Wynd S, et al. Obesity modulates diaphragm curvature in subjects with and without COPD. Am J Physiol Regul Integr Comp Physiol. (2017) 313:R620–9. doi: 10.1152/ajpregu.00173.2017
27. Bellemare JF, Cordeau MP, Leblanc P, Bellemare F. Thoracic dimensions at maximum lung inflation in normal subjects and in patients with obstructive and restrictive lung diseases. Chest. (2001) 119:376–86. doi: 10.1378/chest.119.2.376
28. Shigemura N, Orhan Y, Bhama JK, D’Cunha J, Zaldonis D, Pilewski JM, et al. Delayed chest closure after lung transplantation: techniques, outcomes, and strategies. J Heart Lung Transplant. (2014) 33:741–8. doi: 10.1016/j.healun.2014.03.003
Keywords: lung transplantation, thoracic cavity volume, restrictive lung disease, obstructive lung disease, chest wall remodeling
Citation: Yu WS, Park CH, Paik HC, Lee JG, You S, Shin J, Jung J and Haam S (2022) Changes in Thoracic Cavity Volume After Bilateral Lung Transplantation. Front. Med. 9:881119. doi: 10.3389/fmed.2022.881119
Received: 22 February 2022; Accepted: 10 May 2022;
Published: 26 May 2022.
Edited by:
Michel Gonzalez, Center Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Mark O. Wielpütz, Heidelberg University, GermanyBarbara Ruaro, University of Trieste, Italy
Copyright © 2022 Yu, Park, Paik, Lee, You, Shin, Jung and Haam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chul Hwan Park, park_chulhwan@yuhs.ac; Seokjin Haam, haamsj@aumc.ac.kr
 Woo Sik Yu
Woo Sik Yu Chul Hwan Park2*
Chul Hwan Park2* Jaeyong Shin
Jaeyong Shin Seokjin Haam
Seokjin Haam