
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 24 June 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.879527
This article is part of the Research Topic Leprosy Reactions: New Knowledge on Pathophysiology, Diagnosis, Treatment and Prevention View all 11 articles
Patients with leprosy may experience a chronic and severe type II leprosy reaction (ENL) erythema nodosum leprosum that may not respond to thalidomide and systemic immunosuppressants or may even cause serious adverse events. We here present four patients in whom anti-TNF-α therapy was used with successful results and compare our findings with other published cases. Four patients with chronic and severe ENL who did not respond to, at least, thalidomide and steroids (high doses) were followed up at two reference centers in Brazil. A thorough laboratory investigation was performed to exclude tuberculosis and other diseases before the start of immunobiological medication. Three patients were started on etanercept, and one patient was started on adalimumab. Of all patients, three developed severe adverse events resulting from the use of classical immunosuppressants for ENL (cataracts, deep vein thrombosis, diabetes, and osteoporosis). In all cases, a reduction in the number of ENL and, at least half of the immunosuppressant dose between 6 months and 2 years, were observed. Long-term follow-up of one patient revealed a dramatic reduction in hospital admissions due to ENL, from 12 instances in 1 year (before biologic therapy) to none (after biologic therapy), along with an improvement in condyloma acuminatum. In addition, no direct adverse events were observed with biologics. Treatment with anti-TNF-α therapy may be used as an alternative in patients with chronic and severe ENL who do not respond to traditional treatment (e.g., thalidomide, steroids, and other immunosuppressants). This treatment can help reduce the frequency of ENL, the immunosuppressive burden, and the number of hospital admissions.
Leprosy is a disabling disorder that mainly affects the skin and the peripheral nervous system. This disorder may be complicated by immune-mediated inflammatory reactions, such as reversal reaction (type I reaction) and erythema nodosum leprosum (ENL, type II reaction), as well as by peripheral nerve damage (1, 2).
Erythema nodosum leprosum is a clinical feature of multiple systemic symptoms, such as fever, crops of tender erythematous nodules, neuritis, arthritis, orchitis, lymphadenitis, and iritis (1, 2). It is considered an emergency event (3), which may affect 5–10% of patients with borderline leprosy and up to 50% of those with lepromatous leprosy (LL) (4). Given its magnitude and social significance, including the possibility of social stigma and sequelae for the patients and their families, leprosy is still one of the most serious public health problems in Brazil (5).
Among the most common treatments prescribed for ENL are thalidomide, prednisone/prednisolone (systemic steroids), and clofazimine (3). However, using high doses of steroids for prolonged periods of time may lead to well-known side effects, such as diabetes, cataracts, osteoporosis, and obesity (2, 6). Similarly, thalidomide has highly teratogenic effects and must be used with extreme caution, especially for women at childbearing age (2, 3). Both thalidomide and systemic steroids are associated with a risk for deep vein thrombosis and pulmonary embolism, and they even have a synergic risk effect if used in combination (3). Clofazimine is associated with gastrointestinal effects and skin pigmentation and may pose a cosmetic issue (7). Furthermore, systemic immunosuppressants, such as methotrexate, azathioprine, and cyclophosphamide, may increase the risk of infection and hepatic and renal damage (2, 8).
Although thalidomide is the first-line treatment for ENL in many countries, combining it with at least one additional agent, such as pentoxifylline or an immunosuppressant (3, 8), may be necessary for refractory cases (9). Multibacillary patients may experience ENL for many months (3, 8). Therefore, not only can ENL expose its patients to different sequelae and neurological impairments, but also the therapies targeting it may pose a high risk of side effects (7).
Several alternative therapies have been proposed for ENL, such as vaccination with Mycobacterium indicus pranii (10), apremilast (an oral phosphodiesterase IV inhibitor), cyclosporine, and tenidap (an anti-inflammatory drug) (7), as well as anti-TNF-α therapy (1, 2, 6, 11, 12). We here present four patients with severe, recalcitrant, and chronic ENL who did not respond to multiple therapeutic regimens and were treated with anti-TNF-α therapy. We compared these cases to other similar cases and discussed the rationale behind the use of anti-TNF-α therapy.
All four patients presented with anesthetic skin patches and/or thickened nerves and acid-fast bacilli on slit skin smears and/or a histopathology compatible with leprosy or ENL (9). Leprosy was classified according to the Ridley–Jopling system using clinical, histological, and bacteriological indices (13). A case definition of ENL was considered if a patient presented with crops of tender cutaneous or subcutaneous erythematous nodules, with or without systemic symptoms, or a suggestive histopathology (9).
The nature of ENL was defined as acute for a single episode lasting less than 24 weeks, recurrent for a patient experiencing a second episode of ENL 28 days or more after stopping the treatment for ENL, and chronic if over 24 weeks or more a patient required ENL treatment either continuously or 27 days or less after the last treatment period (14). In terms of severity, ENL can be classified according to the clinical manifestations as mild if the patient presented with less than 10 nodules per affected body, moderate if the patient presented with 10–20 nodules, and severe if the patient presented with more than 20 nodules (15). This study was approved by the institutional review boards of the original centers (CAAE: 93279318.9.0000.5558), and written consent was obtained from each individual.
Table 1 shows the clinical data of the four patients, along with another five similar published cases. As can be observed, the ratio between the males and females was equal (1:1), with a median age of 44.2 [range: 29–59] years for patients with ENL. The patients were originally from two hospitals at the center of Brazil: Hospital de Doenças Tropicais (Goiás) and Hospital Universitário de Brasília (Distrito Federal). All four patients were diagnosed with LL, according to the Ridley–Jopling classification (13). At the time of diagnosis, they presented with a bacteriological index (BI) of five or more on at least four body sites, and their ENL began when they were started on multidrug therapy (MDT). One of the patients was treated twice with MDT. ENL was classified as severe and chronic with common morphological features compatible with multiple (>50) tender, erythematous papules and nodules, followed by systemic symptoms, such as fever, malaise, arthralgia, joint swelling, and peripheral edema with neurological features of neuritis. No reversal reaction was observed in any of the patients. Thalidomide and high doses of systemic steroids were used in all patients. Methotrexate was used in two patients (Patients 1 and 3). However, in Patient 1, the fourth drug was pentoxifylline. These drugs were used for at least 1 year. Patient 1 was started on high doses of thalidomide, systemic steroids, methotrexate, and pentoxifylline for 4 years. Three patients developed severe adverse events because of the ENL treatment (Patients 1, 2, and 4), including cataracts, deep vein thrombosis (Patient 4), diabetes, obesity, and osteoporosis.
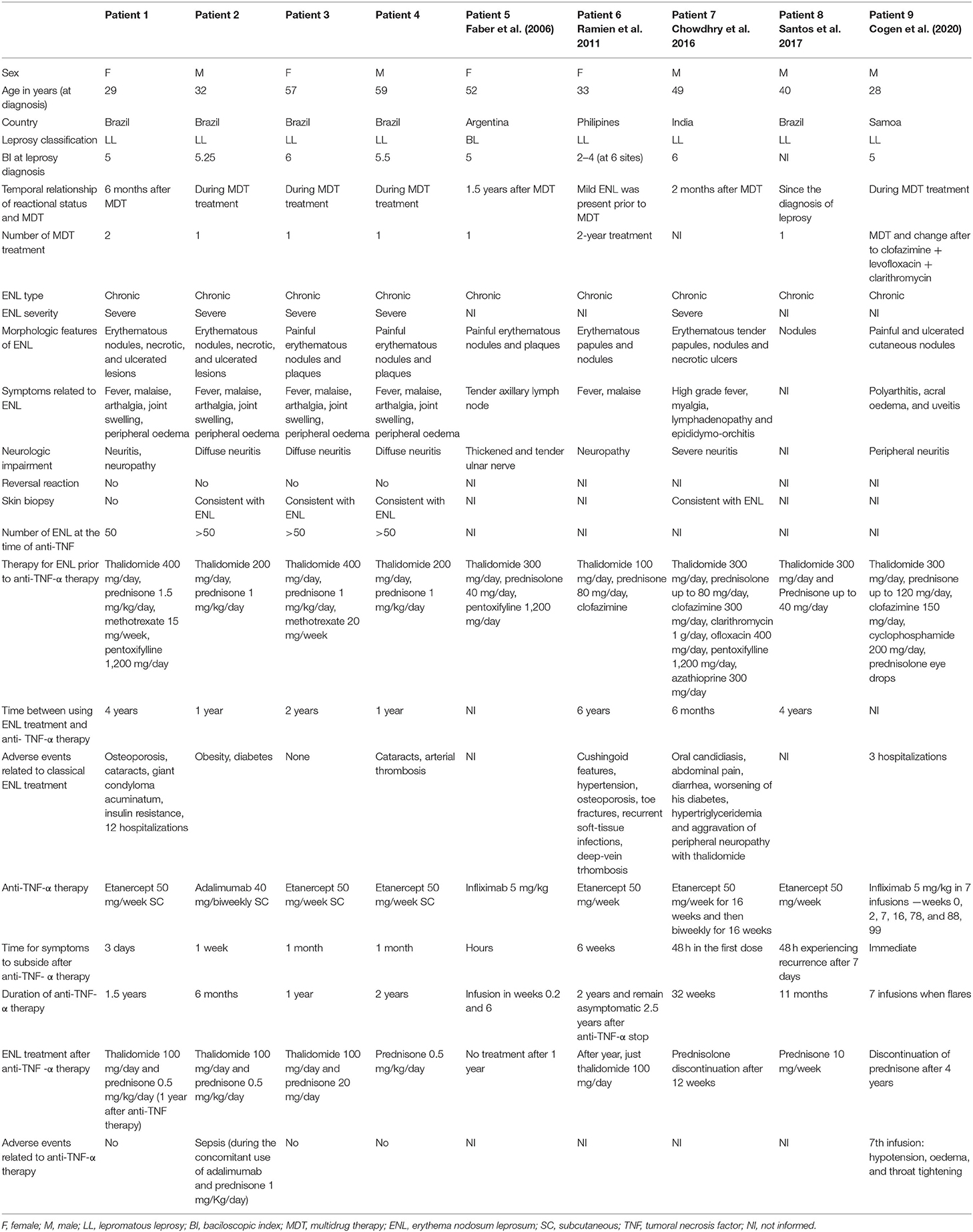
Table 1. Demographic and clinical data of leprosy patients with severe, chronic and refractory ENL (n = 4 patients), and cases already published (n = 5 patients) (2006–2021).
Before anti-TNF-α therapy was started, a complete investigation was performed to rule out latent foci of tuberculosis (Mantoux test and chest X-ray), hepatitis B and C, and HIV. Etanercept was the preferred anti-TNF-α therapy in three patients, with one patient undergoing therapy using adalimumab. Improvements were observed as early as 3 days (Figure 1) to 1 month, and the duration of anti-TNF-α therapy varied from 6 months to 2 years.
The outcomes considered to judge an improvement as a result of anti-TNF-α therapy were as follows: a reduction in the number of ENL cases, a reduction in the rate of hospitalization, and a reduction in the dosage of common treatments for ENL (thalidomide, steroids, methotrexate, pentoxifylline, or clofazimine). All patients exhibited a reduction in ENL, which particularly manifested as a reduction in the number of ENL, in weeks, in Patient 1 (Figure 1). Interestingly, in the year before the anti-TNF-α therapy, Patient 1 was hospitalized 12 times for ENL. However, in the year after therapy, she was hospitalized only once for neuritis. She presented with genital condyloma acuminatum, which was resistant to different treatment modalities. However, after methotrexate was stopped and the dose of systemic steroids was decreased, she improved without a specific treatment for human papillomavirus. Overall, a reduction of at least half in thalidomide and systemic steroids was observed in all four patients on anti-TNF-α therapy over a duration of 6 months−2 years.
Leprosy is a severe, neglected chronic disease. Besides the physical disabilities resulting from the neurological sequelae of this disease, type II reactional states are considered a public health problem in Brazil (12).
Our cases did not demonstrate a gender-related difference for ENL. In fact, several studies have highlighted that age and gender are not risk factors for ENL (6). Rather, the risk factors for developing ENL include a LL type and a high bacillary index. The relative risk of developing ENL is 3.6 with an LL spectrum and 8.6 with a bacillary index of 6, which is the case for patients with refractory, chronic, and severe ENL (16). Although the onset of ENL has been found to be highest during the first year of MDT (17), some studies claim a higher incidence in the second and third years after MDT is started (7). In three patients, MDT was changed (Patient 9) or repeated (Patients 1 and 6). Although the World Health Organization has estimated the rate of relapse after MDT as 0.77% for multibacillary patients 9 years after treatment (18), ENL may still pose a significant challenge to differentiate from a recurrence of leprosy, especially after many years of MDT (3).
Neurological impairments are common in patients with ENL (8, 19). Recently, Andrade et al. (19) described a link between demyelination and acute neuritis during leprosy. However, it remains unknown whether demyelination is a consequence of the inflammatory process of neuritis or whether it is directly induced by Mycobacterium leprae. Persistent demyelination is associated with axonal damage, which progressively compromises large fibers, leading to motor impairment. Damage to the myelin sheath may be a consequence of the inflammatory process resulting either from humoral immunity or from the release of immune mediators. TNF acts on Schwann cells (SCs) by stimulating the production of IL-6 and IL-8, contributing both directly and indirectly to neuroinflammation (20). Moreover, M. leprae stimulate the secretion of IL-23 in SCs, a cytokine that is believed to be involved in demyelinating processes. Thus, focal demyelination may potentially become a prime target for therapeutic interventions aimed at improving nerve function during leprosy (19). One of the contraindications for the use of anti-TNF-α therapy is demyelinating disorders (21). Since demyelination is regarded as a new discovery in leprosy (19), carefully evaluating patients who undergo this treatment to control their ENL is important. Importantly, none of our cases showed any neurological impairments caused by anti-TNF-α therapy.
We compared all cases with already published ones and found that most of the patients who were started on anti-TNF-α therapy were using at least two medications at high doses for 6 months−6 years (Table 1). Although thalidomide is regarded as the drug of choice for treating ENL, using it at high doses for prolonged periods of time may cause nerve injury and be a confounding factor with the neurological effects of the disease itself (1). Prednisone is added to the treatment regimen when the reactions are difficult to control or in the presence of complications, especially neuritis (22). The data outlined in Table 1 show that prolonged classical treatment is associated with increased side effects (Patients 1, 7, and 8) (1, 12). In addition, the synergic association between systemic steroids and high doses of thalidomide is considered a risk factor for deep vein thrombosis (Patients 4 and 6) and pulmonary thromboembolism (6). Hence, precociously using anti-TNF-α therapy may prevent prolonged exposure to immunosuppressant agents and their side effects (2).
Etanercept was the most used drug for ENL, followed by infliximab and adalimumab. Infliximab is a human-murine chimeric monoclonal antibody against TNF-α, and etanercept is a dimeric fusion protein of the extracellular portion of the p75 TNF receptor coupled to IgG1. Both agents can effectively reduce the levels of TNF-α (7). Although the underlying immunological mechanism of ENL remains unclear, it is important to highlight the role of cytokines. High levels of TNF-α and IL-6 are consistently found in patients with severe disease (23). Moreover, the overexpression of TNF-α and the fact that thalidomide is an important anti-TNF-α agent allowed Farber et al. (11) to treat a patient with an uncontrolled type II reaction using classic systemic therapy with infliximab. After this study, four more cases were described, but only one patient continued to use infliximab (2). Etanercept was chosen instead of infliximab because of the increased risk of reactivation of latent tuberculosis and granulomatous diseases (24). The disadvantage of infliximab is its mode of administration (endovenous), which requires a hospital setting and may lead to adverse events, such as hypotension, edema, and throat tightness (2). Only one study has focused on adalimumab in the context of the reversal reaction, but not in that of the type II reaction. Compared to the administration of prednisolone for 20 weeks, the efficacies of both drugs against skin lesions in the context of the reversal reaction were similar. Interestingly, adalimumab is superior to prednisolone in terms of improving the nerve function and sensory and motor loss (25).
Unlike psoriasis, which is also treated using anti-TNF-α therapy, reactive leprosy episodes seem to exhibit a rapid response. The improvement observed in ENL seems to be immediate or within hours with infliximab (Patients 5 and 9) (2, 11), whereas etanercept may take days to a month (Patients 1, 2, 3, 4, 6, 7, and 8) (1, 6, 12). However, since only nine patients underwent anti-TNF-α therapy for ENL, we cannot guarantee the superiority of a specific anti-TNF-α agent. Nevertheless, in psoriasis, another skin disease treated by this class of drugs, infliximab (26) and adalimumab (27) have a faster response and are more efficacious than etanercept, in achieving a clear or almost clear stage.
The dosage of anti-TNF-α therapy used for treating ENL is considered another challenge. Should biologics be administered only during flares or perhaps as a prophylactic therapy? In Patients 5, 7, and 9, the drug was administered at a custom dose, preferably during flares. Since ENL flares decrease over time, specific drugs for ENL treatment are expected to be tapered down (7). However, as systemic steroids are the most detrimental drugs for such patients, it is advisable to stop such drugs in advance and to continue anti-TNF-α therapy along with thalidomide. It is also worth noting that the gaps between the doses of anti-TNF-α therapy may lead to the formation of neutralizing antibodies and, hence, the loss of efficacy (28). Therefore, maintaining a uniform and specific dose is considered a favorable choice.
Overall, no side effects with anti-TNF-α therapy were observed, except in Patient 2, who had sepsis but has also been on systemic steroids for a year. During the early 2000s, when anti-TNF-α therapy was released, the side effects of this type of therapy were strongly debated. In addition, the well-established connection between the inhibition of TNF-α and reactivation of tuberculosis may similarly hold true for leprosy. The rapidity with which patients develop signs of leprosy after receiving the biological agent suggests possible recrudescence of latent leprosy or initial misdiagnosis. Therefore, using anti-TNF-α therapy for patients with leprosy before complete treatment with MDT may reduce the efficacy of antimicrobial agents or promote infection by M. leprae. Although anti-TNF-α therapy is contraindicated in the case of lupus and cardiac congestive failure (stages III–IV) (29), given their target specificity, anti-TNF agents are considered safer than a wide immunological blockade provided by steroids and other immunosuppressants (30, 31).
The indications of anti-TNF-α agents are increasing in many diseases (21). Despite their high cost, more affordable biosimilars are reaching the market (2). It is also worth highlighting that the indication of biologic therapy for ENL may be limited because ENL occurs in ~50% of LL cases (2). Moreover, flares are auto-limited, especially within the first 3 years after MDT, and only 26.8% of patients with ENL require at least one additional agent (16).
Our study has some limitations. For example, it lacks prospective controls (as in all case reports), it lacks laboratory tests aimed at verifying the behavior of anti-inflammatory markers, and it lacks more well-defined and uniform outcome measures for ENL. Hence, some questions remain unanswered: Who is the best patient with ENL for biologic therapy? Is it safe to use biologic therapy along with MDT or is it necessary to wait until treatment is completed? For how long should systemic agents be used until classical treatment of ENL is considered a failure? What is the best anti-TNF-α candidate to treat ENL? What is the best strategy, during flares or as a prophylactic? How does anti-TNF-α therapy affect nerve damage?
In our series, patients who presented with recalcitrant, chronic, and severe ENL were classified as LL patients with a BI higher than five, who had their first episode of ENL within the first year of MDT along with neurological impairments. All patients who received biologic therapy did not respond to high-dose systemic steroids or thalidomide. Anti-TNF-α therapy demonstrates rapid improvements and allows the reduction of ENL, the frequency of hospitalizations, and the doses used of classical drugs. Clinical trials on this population are, therefore, important to determine the role of biologicals in leprosy and neurological impairments and their reactive states.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Comite de Etica do Hospital de Doenças Tropicais - HDT GO and by the Comitê de Ética da Faculdade de Medicina da Universidade de Brasília. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AM: formal analysis, investigation, and resources. CG, PK, and MI: supervision, validation, visualization, writing—original draft, and writing—review and editing. All authors contributed to the article and approved the submitted version.
This study was financed in part by Fundo de Apoio à Dermatologia (FUNADERM)—Sociedade Brasilleira de Dermatologia (SBD).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all of the professionals at the Hospital Universitário de Brasília, Brazil, who are involved in the support of leprosy patients.
1. Chowdhry S, Shukla A, D'souza P, Dhali T, Jaiswal P. Treatment of severe refractory erythema nodosum leprosum with tumor necrosis factor inhibitor etanercept. Int J Mycobacteriol. (2016) 5:223–5. doi: 10.1016/j.ijmyco.2016.02.002
2. Cogen AL, Lebas E, De Barros B, Harnisch JP, Faber WR, Lockwood DN, et al. Biologics in leprosy: a systematic review and case report. Am J Trop Med Hyg. (2020) 102:1131–6. doi: 10.4269/ajtmh.19-0616
3. Maymone MBC, Venkatesh S, Laughter M, Abdat R, Hugh J, Dacso MM, et al. Leprosy: treatment and management of complications. J Am Acad Dermatol. (2020) 83:17–30. doi: 10.1016/j.jaad.2019.10.138
4. Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. (2017) 8:233. doi: 10.3389/fimmu.2017.00233
5. Penna GO, Pinheiro AM, Nogueira LSC, Carvalho LR, Oliveira MBB, Carreiro VP. Clinical and epidemiological study of leprosy cases in the University Hospital of Brasília: 20 years−985–2005. Rev Soc Bras Med Trop. (2008) 41:575–80. doi: 10.1590/S0037-86822008000600006
6. Ramien ML, Wong A, Keystone JS. Severe refractory erythema nodosum leprosum successfully treated with the tumor necrosis factor inhibitor etanercept. Clin Infect Dis an Off Publ Infect Dis Soc Am. (2011) 52:e133–5. doi: 10.1093/cid/ciq213
7. Bhat RM, Vaidya TP. What is new in the pathogenesis and management of erythema nodosum leprosum. Indian Dermatol Online J. (2020) 11:482–92. doi: 10.4103/idoj.IDOJ_561_19
8. Costa PSS, Fraga LR, Kowalski TW, Daxbacher ELR, Schuler-Faccini L, Vianna FSL. Erythema nodosum leprosum: update and challenges on the treatment of a neglected condition. Acta Trop. (2018) 183:134–41. doi: 10.1016/j.actatropica.2018.02.026
9. Walker SL, Balagon M, Darlong J, Doni SN, Hagge DA, Halwai V, et al. ENLIST 1: an international multi-center cross-sectional study of the clinical features of erythema nodosum leprosum. PLoS Negl Trop Dis. (2015) 9:e0004065. doi: 10.1371/journal.pntd.0004065
10. Gupta SK, Kumari S. Chronic recalcitrant erythema nodosum leprosum: therapeutic dilemma and role of mycobacterium indicus pranii vaccine. An Bras Dermatol. (2022) 97:49–53. doi: 10.1016/j.abd.2020.08.032
11. Faber WR, Jensema AJ, Goldschmidt WFM. Treatment of recurrent erythema nodosum leprosum with infliximab. N Engl J Med. (2006) 355:739. doi: 10.1056/NEJMc052955
12. Santos JRS, Vendramini DL, Nery JADC, Avelleira JCR. Etanercept in erythema nodosum leprosum. An Bras Dermatol. (2017) 92:575–7. doi: 10.1590/abd1806-4841.20175471
13. Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr other Mycobact Dis Off organ Int Lepr Assoc. (1966) 34:255–73.
14. Walker SL, Lebas E, Doni SN, Lockwood DNJ, Lambert SM. The mortality associated with erythema nodosum leprosum in Ethiopia: a retrospective hospital-based study. PLoS Negl Trop Dis. (2014) 8:e2690. doi: 10.1371/journal.pntd.0002690
15. Gomes GJ, Penna GO, Miranda CLC, Martelli CMT. Erythema nodosum leprosum: clinical and therapeutic up-date. An Bras Dermatol. (2002) 77:389–407.
16. Saunderson P, Gebre S, Byass P. ENL reactions in the multibacillary cases of the AMFES cohort in central Ethiopia: incidence and risk factors. Lepr Rev. (2000) 71:318–24. doi: 10.5935/0305-7518.20000035
17. da Silva SF, Griep RH. Leprosy reaction in patients of health centers from the planning area 3.2 of Rio de Janeiro municipality. Hansen Int. (2007) 32:155–62.
19. Andrade PR, Jardim MR, Da Silva ACC, Manhaes PS, Antunes SLG, Vital R, et al. Inflammatory cytokines are involved in focal demyelination in leprosy neuritis. J Neuropathol Exp Neurol. (2016) 75:272–83. doi: 10.1093/jnen/nlv027
20. Santos Morais Junior G, Shu Kurizky P, Penha Silva Cerqueira SR, Holanda Barroso D, Schulte HL, Pires de Albuquerque C, et al. Enhanced IL-6 and IL-12B gene expression after SARS-CoV-2 infection in leprosy patients may increase the risk of neural damage. Am J Trop Med Hyg. (2021) 1–5104:2190–4. doi: 10.4269/ajtmh.21-0034
21. Putinatti MS, de MA, Lastória JC, Padovani CR. Prevention of repeated episodes of type 2 reaction of leprosy with the use of thalidomide 100 mg/day. An Bras Dermatol. (2014) 89:266–72. doi: 10.1590/abd1806-4841.20142037
22. Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present, and future. Int Immunol. (2015) 27:55–62. doi: 10.1093/intimm/dxu102
23. Kahawita IP, Lockwood DNJ. Toward understanding the pathology of erythema nodosum leprosum. Trans R Soc Trop Med Hyg. (2008) 102:329–37. doi: 10.1016/j.trstmh.2008.01.004
24. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis an Off Publ Infect Dis Soc Am. (2004) 38:1261–5. doi: 10.1086/383317
25. Mitra D. A randomized controlled trial of anti-TNF alpha bio-similar adalimumab vs. prednisolone in the management of leprosy patients with new type 1 lepra reaction. Exp Dermatol. (2018) 27:4. doi: 10.1093/ofid/ofx163.1805 Conference: 3rd Inflammatory Skin Disease Summit-The Translational Revolution. Austria. 4. Available online at: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01707212/full
26. Sbidian E, Chaimani A, Afach S, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane database Syst Rev. (2020) 1:CD011535. doi: 10.1002/14651858.CD011535.pub3
27. Signorovitch JE, Wu EQ, Yu AP, Gerrits CM, Kantor E, Bao Y, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. (2010) 28:935–45. doi: 10.2165/11538370-000000000-00000
28. Roda G, Jharap B, Neeraj N, Colombel J-F. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. (2016) 7:e135. doi: 10.1038/ctg.2015.63
29. Kamata M, Tada Y. Safety of biologics in psoriasis. J Dermatol. (2018) 45:279–86. doi: 10.1111/1346-8138.14096
30. Saurat J-H, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne J-P, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. (2008) 158:558–66. doi: 10.1111/j.1365-2133.2007.08315.x
Keywords: leprosy, dermatology, immunosuppression therapy, autoimmunity, leprosy reaction
Citation: Mendes AFM, Gomes CM, Kurizky PS and Ianhez M (2022) Case Report: A Case Series of Immunobiological Therapy (Anti-TNF-α) for Patients With Erythema Nodosum Leprosum. Front. Med. 9:879527. doi: 10.3389/fmed.2022.879527
Received: 19 February 2022; Accepted: 06 June 2022;
Published: 24 June 2022.
Edited by:
John S. Spencer, Colorado State University, United StatesReviewed by:
Veronica Schmitz, Oswaldo Cruz Foundation (FIOCRUZ), BrazilCopyright © 2022 Mendes, Gomes, Kurizky and Ianhez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mayra Ianhez, aWFuaGV6QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.