
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 11 April 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.879379
This article is part of the Research TopicPrevention and Control of Human T Lymphotropic Viruses 1 and 2 (HTLV-1/2)View all 32 articles
 Miyuna Kimura1,2
Miyuna Kimura1,2 Junji Yamauchi2,3
Junji Yamauchi2,3 Tomoo Sato2,3
Tomoo Sato2,3 Naoko Yagishita2
Naoko Yagishita2 Natsumi Araya2
Natsumi Araya2 Satoko Aratani2,4
Satoko Aratani2,4 Kenichiro Tanabe5
Kenichiro Tanabe5 Erika Horibe6
Erika Horibe6 Toshiki Watanabe6
Toshiki Watanabe6 Ariella Coler-Reilly2
Ariella Coler-Reilly2 Misako Nagasaka2,7
Misako Nagasaka2,7 Yukari Akasu3
Yukari Akasu3 Kei Kaburagi3
Kei Kaburagi3 Takayuki Kikuchi3
Takayuki Kikuchi3 Soichiro Shibata3
Soichiro Shibata3 Hirofumi Matsumoto3
Hirofumi Matsumoto3 Akihito Koseki3,8
Akihito Koseki3,8 Soichiro Inoue1
Soichiro Inoue1 Ayako Takata9
Ayako Takata9 Yoshihisa Yamano2,3*
Yoshihisa Yamano2,3*Background: Human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy (HAM) is a neuroinflammatory disease, causing various neurological symptoms, including motor, sensory, and bladder and bowel dysfunctions. This study was designed to reveal the impact of HAM and related symptoms on health-related quality of life (HRQoL).
Methods: We analyzed the Short Form-36 (SF-36) and clinical data of 538 patients with HAM registered in the HAM-net, a nationwide patient registry for HAM in Japan. HRQoL was evaluated using the SF-6D (a health state utility value calculated from the SF-36) and eight SF-36 subscales. A general liner model was used to estimate the impact of major HAM-related symptoms, including gait dysfunction, sensory disturbance in the legs (pain and numbness), urinary dysfunction, and constipation, on the SF-6D and SF-36 subscale scores.
Results: The mean age and disease duration were 62.0 and 16.5 years, respectively. Of the patients, 73.2% needed walking aid; 42.7 and 67.1% had leg pain and numbness, respectively; 92.1% had urinary dysfunction; and 77.9% had constipation. The mean SF-6D score was 0.565, which was significantly lower than the national average (0.674 in the 60–69 years age group; p < 0.001), exceeding the minimal important difference (0.05–0.1). All the major symptoms were significantly associated with a decrease in the SF-6D score. The SF-36 subscale scores were significantly lower than the national standard of 50 (p ≤ 0.001), except for mental health (MH). Gait dysfunction was associated with lower scores in physical functioning (PF), limitations on role functioning because of physical health, bodily pain, general health perception (GH), vitality (VT), and social functioning; however, no association was observed between gait dysfunction and limitations on role functioning because of emotional problems and MH. Meanwhile, sensory disturbance in the legs was associated with a decrease in scores in all subscales. Urinary dysfunction was associated with worse PF, GH, VT, and MH. Constipation was associated only with PF.
Conclusion: HRQoL of patients with HAM was worse than that of the general population and was associated with all major symptoms. Thus, patients should be comprehensively managed to achieve better HRQoL.
Human T-lymphotropic virus type 1 (HTLV-1) is estimated to infect at least 5–10 million individuals with a heterogeneous global distribution (1). Its endemic regions include the Southwestern part of Japan, South America, the Caribbean area, and sub-Saharan Africa. This retrovirus causes a neuroinflammatory disease, called HTLV-1-associated myelopathy (HAM), in a small proportion of infected individuals (2, 3). Due to the chronic inflammatory destruction of the spinal cord, HAM results in various symptoms, including motor and sensory disturbance in the legs and neurogenic bladder and bowel dysfunctions (4). In severe cases, patients become wheelchair-bound and require urinary catheters and enemas. Although corticosteroids are most widely administered to slow the disease progression, no curative therapy is currently available (5). Thus, symptomatic treatment is the mainstay of management. Therefore, data regarding the burden of complicated symptoms on health-related quality of life (HRQoL) are necessary to enhance medical care and research for patients with HAM. However, information is extremely limited because of the rarity of the disease.
Among the HRQoL measures, the Short Form-36 (SF-36) and the EuroQoL 5-dimension (EQ-5D) are most widely used to measure generic health status in clinical research (6, 7). The SF-36 assesses physical and mental health status using 36 questions. The Short Form 6-dimension (SF-6D), which is a health state utility index, can be calculated from the SF-36 in some languages (8, 9). The EQ-5D also generates a health state utility index using five questions (10). Regarding HRQoL of patients with HAM, several reports from Brazil have been published. Mainly using the SF-36, they have shown that this patient population had poor HRQoL (11–17). Although the SF-6D has not been reported to date, Rosadas et al. have recently demonstrated that the EQ-5D index in patients with HAM living in Brazil and the United Kingdom was significantly lower than that in the general population and HTLV-1 carriers (18). Moreover, these studies have reported that HAM-related symptoms, such as gait dysfunction, pain, and lower urinary tract symptoms, decreased HRQoL by comparing patients with each symptom with those without each symptom (11–18). However, as HAM simultaneously causes multiple symptoms, strictly estimating the effect of each symptom is impossible using univariate analysis performed in these studies with relatively small sample sizes (<60 subjects).
Therefore, this study analyzed the SF-36 and SF-6D data obtained from more than 500 patients in Japan to reveal the effects of HAM and related symptoms on HRQoL. These patients had worse HRQoL than the general population. Moreover, multivariate analysis demonstrated that not only gait dysfunction but also other symptoms of HAM were significantly associated with poorer HRQoL. To our knowledge, this is the first study that has examined the SF-36 and SF-6D of patients with HAM from Japan.
We used data from the “HAM-net,” a nationwide patient registration system for HAM in Japan (UMIN000028400) (19). The HAM-net recruits patients from all over Japan through the website or leaflets distributed to patients at clinics and patient meetings. Patients can apply to the HAM-net office via telephone, fax, or e-mail. After registration, trained nurses and coordinators uniformly conduct annual telephone interviews with patients to collect their data, including demographic information and medical conditions, such as HAM-related symptoms and medications. This study analyzed data at enrollment in the HAM-net. Of the 558 patients enrolled in the HAM-net between April 1, 2012 and December 31, 2018, 538 patients who had SF-36 data were included in this study (Figure 1). The Bioethics Committee of St. Marianna University School of Medicine approved this study (Approval ID no. 2044). All participants provided written informed consent.
The patients included in this study answered the Japanese standardized version of the SF-36, version 2 (20, 21). The SF-36, which includes 36 questions, comprises the following eight subscales: physical functioning (PF), limitations on role functioning because of physical health (role physical, RP), bodily pain (BP), general health perception (GH), vitality (VT), social functioning (SF), limitations on role functioning because of emotional problems (role emotional, RE), and mental health (MH). We evaluated the subscales of the SF-36 using the norm-based scoring method based on the national standard value. The norm-based score is standardized with a mean value of 50 and standard deviation (SD) of 10, with higher values indicating better HRQoL. Scores of less than 50 indicate that the HRQoL is worse than that of the general Japanese population. Moreover, we calculated the SF-6D score from the SF-36. The SF-6D is a preference-based HRQoL score for economic evaluation, ranging from 0 to 1, with higher values indicating better HRQoL (9).
All data analyzed in this study were collected via telephone interviews conducted at the time of enrollment in the HAM-net. In addition to the SF-36 scores, we extracted data on age and sex as well as medical data regarding the major symptoms of HAM at enrollment, including gait dysfunction, sensory dysfunction in the legs (pain and numbness), urinary dysfunction, and constipation. Gait dysfunction was evaluated using the Osame Motor Disability Score (OMDS), a scale specific to HAM (Table 1) (19). The OMDS ranges from 0 to 13, with higher scores indicating greater walking disability. We divided the OMDS into four levels (i.e., 0–4, 5, 6, and 7–13) to assess the relationship between the OMDS and HRQoL.
• OMDS 0–4: patients who can walk without walking aid.
• OMDS 5: patients who can walk more than 10 m with unilateral support.
• OMDS 6: patients who can walk more than 10 m with bilateral support.
• OMDS 7–13: patients who cannot walk 10 m with bilateral support, including those who cannot walk at all.
Leg pain and numbness were reported by patients as absent, occasional, or persistent. Urinary dysfunction was evaluated using the HAM-bladder dysfunction severity grade (HAM-BDSG) (22). The HAM-BDSG comprises four grades (grades 0–III) according to the dependency on urinary catheters and the presence or absence of lower urinary tract symptoms or medications.
• Grade 0 (HAM-BDSG 0): patients who do not use urinary catheters, have no lower urinary tract symptoms, and take no medications for lower urinary tract symptoms.
• Grade I (HAM-BDSG I): patients who do not use urinary catheters but have lower urinary tract symptoms or take medications.
• Grade II (HAM-BDSG II): patients who require intermittent catheters.
• Grade III (HAM-BDSG III): patients who use indwelling catheters.
The presence or absence of constipation was reported by patients without defining constipation. Data on the use of oral laxatives, manual extraction, enemas, and corticosteroids were also extracted. Of the extracted data, only the HAM-BDSG data of eight patients (1.5%) were missing; they were excluded from the HAM-BDSG analyses.
The outcomes of interest were as follows: (1) HRQoL evaluated using the SF-6D and SF-36 subscales in patients with HAM and (2) impact of HAM-related symptoms on HRQoL.
Patient characteristics were reported as means and SDs or medians with interquartile ranges (IQRs) for continuous variables and frequencies for categorical variables.
We used the unpaired t-test to compare the SF-6D scores of patients with HAM against the national population norm reported by Shiroiwa et al. (23). This report includes SF-6D scores classified according to the age category; however, it did not include an overall population score. Therefore, we compared the SF-6D score of our whole cohort with that of the 60–69 years age category in the general population [mean (SD) score: 0.674 (0.128), n = 199], because the mean age of our patients was 62.0 years. The one-sample t-test was used to compare the SF-36 subscale scores with the national standard of 50.
We estimated the associations of the SF-6D and SF-36 subscale scores with HAM-related symptoms using a general linear model. We included the following predetermined covariates for multivariate adjustment: age (age categories: 39 years or younger, 40–49 years, 50–59 years, 60–69 years, and 70 years or older), sex, OMDS (0–4, 5, 6, and 7–13), leg pain (absent, occasional, and persistent), leg numbness (absent, occasional, and persistent), HAM-BDSG (Grade 0, I, II, and III), and constipation [absent, present (controlled with or without oral laxatives), and present (needs manual extraction or enemas)]. All variables were treated as categorical. No evidence for multicollinearity problems between covariates were found using the variance inflation factor test (less than 5).
All statistical analyses were performed using Statistical Package for the Social Sciences (version 25; IBM Corporation, Armonk, NY, United States). Two-sided p-values of less than 0.05 were used to denote statistical significance.
Table 2 summarizes the patient characteristics according to the OMDS category (n = 538). The mean age and disease duration were 62.0 and 16.5 years, respectively. Among the patients under study, 74.7% were females. The median grade of OMDS was 5 (IQR, 4–6) with a wide distribution from OMDS 0–4 to OMDS 7–13. The prevalence of leg pain was 42.7% (occasional, 20.6%; persistent, 22.1%), and that of leg numbness was 67.1% (occasional, 19.7%; persistent, 47.4%). Regarding bladder dysfunction, 65.5% of the patients had lower urinary tract symptoms and/or medications (HAM-BDSG I). Intermittent (HAM-BDSG II) and indwelling catheters (HAM-BDSG III) were used in 23.6 and 3.0%, respectively. Among the study population, 77.9% had constipation and 9.3% required manual extraction or enemas. The frequency and severity of HAM-related symptoms increased with worsening OMDS.
The SF-6D and SF-36 subscale scores are shown in Figure 2. The mean SF-6D score of 0.565 was significantly lower (p < 0.001) than the Japanese population norm (mean score in the 60–69 years age group: 0.674) (23). The SF-36 subscale scores were significantly lower than the national standard of 50 (p ≤ 0.001), except for the MH score (mean score, 49.8; p = 0.715). While subscales associated with physical health (i.e., PF, RP, BR, and GH) tended to be lower than those associated with mental health (i.e., VT, SF, RE, and MH), the PF score was the lowest (mean score, 18.9).
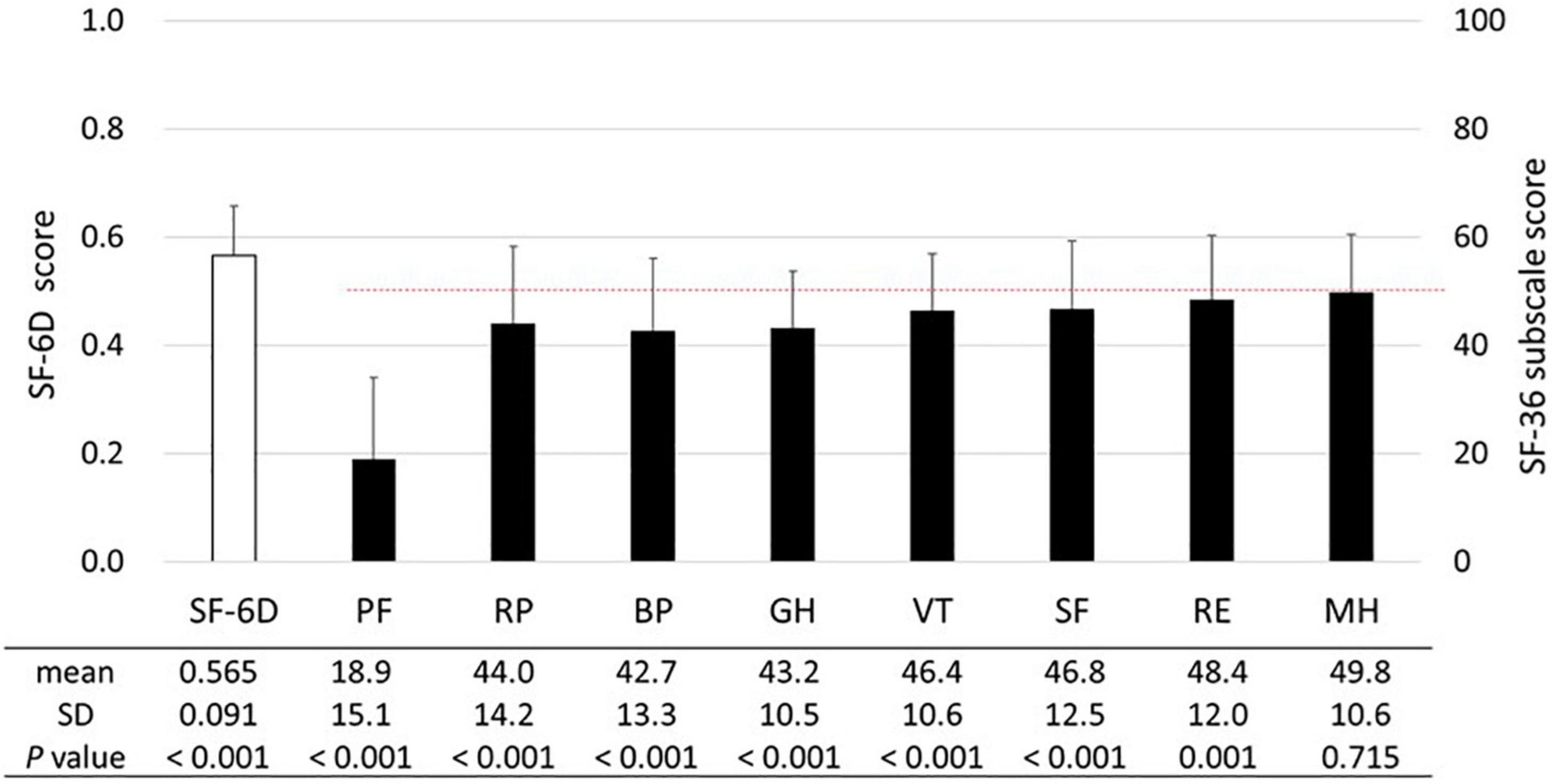
Figure 2. SF-6D and SF-36 subscales. The means and standard deviations (SDs) of the SF-6D and SF-36 subscale scores are presented (n = 538). The dashed line (50 points) represents the mean SF-36 subscale score among the general Japanese population. The unpaired t-test was used to compare the SF-6D score of patients with HAM to the national population norm reported by Shiroiwa et al. [mean (SD) score in the 60–69 years age: 0.674 (0.128)] (23). The SF-36 subscale scores were compared with the national standard of 50 using the one-sample t-test. Abbreviations of SF-36 subscales: BP, bodily pain; GH, general health perception; MH, mental health; PF, physical functioning; RE, role emotional; RP, role physical; SF, social functioning; VT, vitality.
First, we analyzed the association of the SF-6D score with each HAM-related symptom. In all symptoms, the SF-6D score decreased with worsening (Figure 3). Because patients had multiple symptoms, we estimated the associations using a multivariate model while adjusting for age and sex (Table 3). All symptoms were significantly associated with a decrease in the SF-6D score. The influence of the OMDS (coefficients vs. OMDS 4: OMDS 5, −0.050; OMDS 6, −0.057; and OMDS 7–13, −0.060; p < 0.001 for all) and persistent leg pain (coefficient of persistent pain vs. absent: −0.061; p < 0.001) was greater than that of the other symptoms.
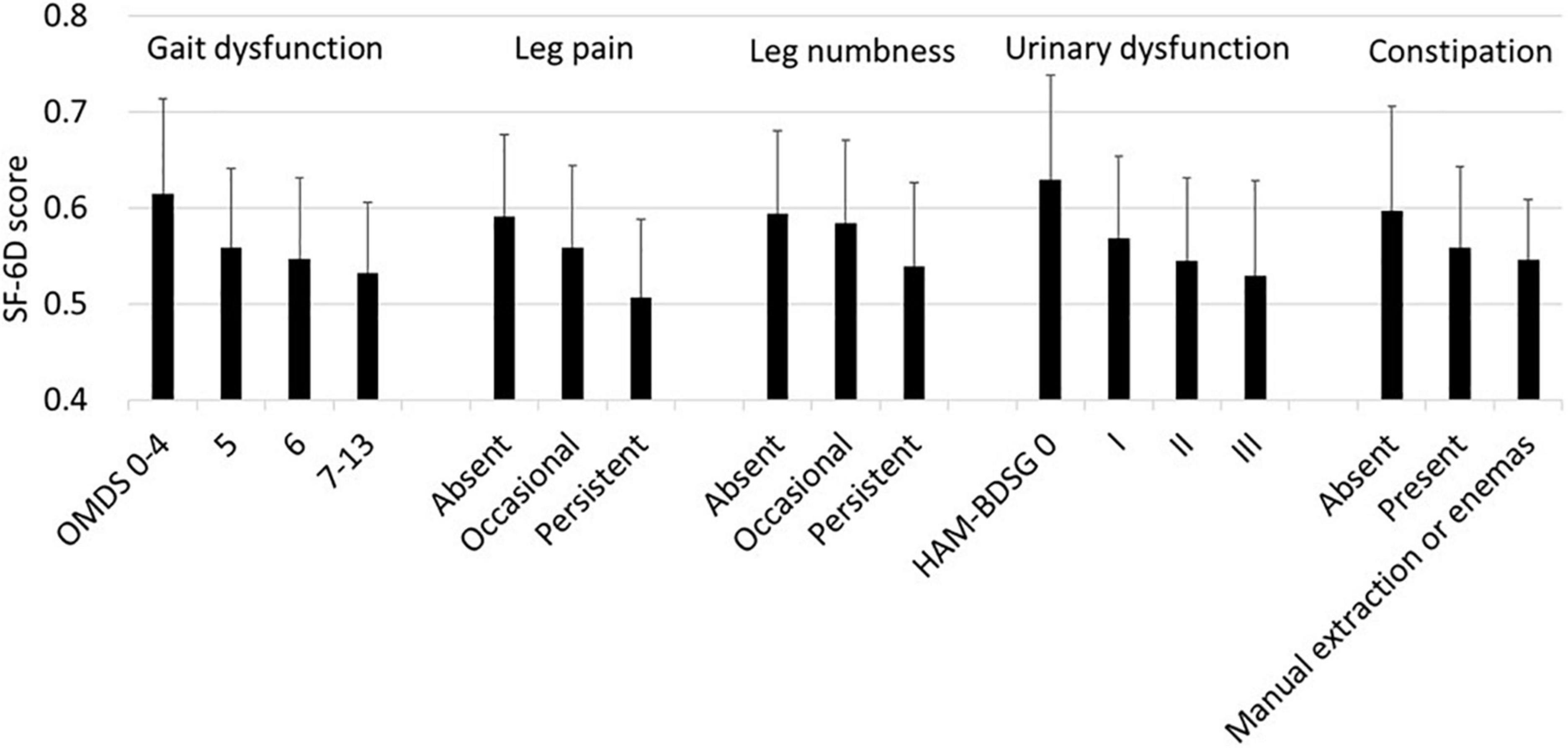
Figure 3. SF-6D according to HAM-related symptoms. The SF-6D score is expressed as mean and standard deviation (n = 538). HAM-BDSG, HAM-bladder dysfunction severity grade; OMDS, Osame Motor Disability Score.
Similar to the SF-6D, the SF-36 subscale scores decreased as the symptoms worsened (Figure 4). However, the tendency was weaker in the mental health components than in the physical health components.
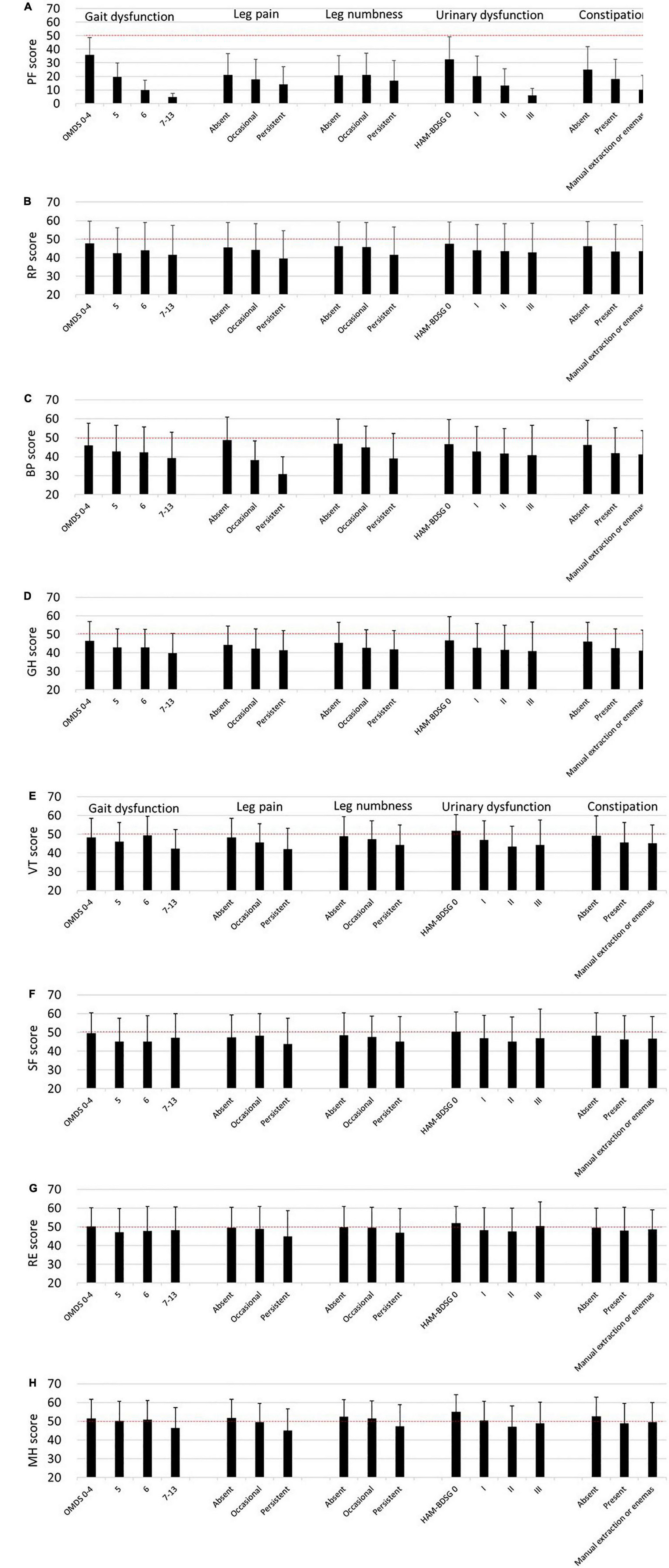
Figure 4. SF-36 subscales according to HAM-related symptoms. The SF-36 subscale scores are expressed as means and standard deviations (n = 538). The dashed lines (50 points) indicate the mean score of the SF-36 subscales among the general Japanese population. (A) Physical functioning (PF), (B) role physical (RP), (C) bodily pain (BP), (D) general health perception (GH), (E) vitality (VT), (F) social functioning (SF), (G) role emotional (RE), (H) mental health (MH). HAM-BDSG, HAM-bladder dysfunction severity grade; OMDS, Osame Motor Disability Score.
Next, we estimated the influence of the symptoms on the SF-36 subscales using multivariate analysis (Table 4). Compared with OMDS 0–4, OMDS 5 or higher was significantly associated with lower scores in PF, RP, GH, and SF. BP and VT scores significantly decreased only in OMDS 7–13. In contrast, RE and MH were not associated with OMDS. Occasional leg pain was significantly associated with lower BP and VT scores. Moreover, persistent pain was associated with a decrease in PF, RP, BP, VT, RE, and MH scores. While occasional numbness was associated only with lower GH scores, persistent numbness was associated with a decrease in RP, GH, VT, SF, and MH scores. Collectively, sensory disturbance in the legs (pain and numbness) was associated with all subscales. HAM-BDSG I was associated with lower PF and VT scores. Furthermore, HAM-BDSG II was associated with a decrease in PF, GH, VT, and MH scores. Constipation was associated only with lower PF scores.
In this study, we evaluated the HRQoL of Japanese patients with HAM for an average disease duration of 16.5 years. Most patients had not only gait dysfunction but also sensory disturbance, urinary dysfunction, and constipation. In this study, the SF-6D and SF-36 subscale scores were lower than the population standards. Multivariate analyses demonstrated that all these symptoms were significantly associated with worse HRQoL.
To our knowledge, no studies have reported on the SF-6D score in patients with HAM; however, the poor scores found in this study were consistent with those reported in a study conducted in Brazil and the United Kingdom, which used the EQ-5D index as a health-related utility value (18). We considered that the decrease in the SF-6D score was clinically meaningful because it was as large as a minimal important difference of 0.05–0.1 (23). Moreover, all symptoms analyzed were significantly associated with lower SF-6D scores in the multivariate analysis. The coefficients in OMDS 5 or higher (vs. OMDS 0–4) and persistent leg pain (vs. absent) were remarkably large and met the minimal important difference, indicating that the management of motor and sensory dysfunctions in the legs has a strong impact on HRQoL. However, caution is required in interpreting the results of this study. Although the SF-36 comprises questions directly reflecting gait function (PF) and pain (BP), no questions focus on other HAM-related symptoms. Hence, compared to gait function and pain, the SF-36 indirectly evaluates the effects of leg numbness, urinary dysfunction, and constipation on HRQoL, which may lead to an underestimation of their burden. Therefore, recognizing that all major symptoms of HAM can significantly affect HRQoL is important.
In this study, the SF-36 subscale scores other than the MH score were below the national average, and the subscales related to physical health were lower than those related to mental health. PF was worst of all the subscales. These findings are consistent with those reported in a Brazilian study (15), suggesting that poor physical health is a characteristic feature of HAM. Regarding the association between OMDS and the subscale scores, PF is naturally and strongly correlated with OMDS because PF mainly reflects mobility. Moreover, OMDS 5 or higher was significantly associated with a decrease in RP, GH, and SF scores; however, the differences in the coefficients between OMDS 5, 6, and 7–13 were relatively small, indicating that there is a large gap in HRQoL between OMDS 0–4 and 5. As a result, maintaining gait function better than OMDS 5 may be important for better HRQoL and can be a therapeutic goal for patients with HAM. In general, gait dysfunction caused by HAM progresses slowly, requiring 5–10 years on average to reach OMDS 5 (19, 24). However, some patients with high inflammatory activity in the spinal cord experience faster deterioration, with OMDS 5 or higher within 2 years after the disease onset (25). Thus, early diagnosis is important for preventing loss of treatment opportunities. Although there is no curative treatment for HAM, evidence suggests that corticosteroid therapy is effective in maintaining gait function (26). The international guideline for HAM recommends the use of corticosteroids based on the rate of disease progression (5). Early diagnosis, evaluation of progression, and treatment initiation at the appropriate time are all essential in the treatment of HAM.
In addition, we found that sensory disturbance and urinary dysfunction affect HRQoL in patients with HAM. First, persistent leg pain and numbness were associated with lower RP scores, indicating that not only gait dysfunction but also sensory disturbance hamper daily activity in patients with HAM. Second, although the OMDS had no significant influence on MH, persistent leg pain and numbness as well as the use of intermittent catheters (HAM-BDSG II) were significantly associated with lower MH scores. These findings are consistent with those reported in recent HRQoL studies in patients with multiple sclerosis and neuromyelitis optica, both of which are neuroinflammatory diseases causing symptoms similar to those in HAM. They reported that not gait dysfunction but chronic pain, including numbness, was associated with depression and mental health satisfaction (27, 28). Third, leg pain, numbness, and urinary dysfunction were also associated with lower GH and VT scores. Intermittent catheterization had the greatest impact on GH and VT of all the symptoms analyzed. In an HRQoL study involving patients who developed neurogenic bladder dysfunction after radical hysterectomy, GH, VT, and MH scores were lower in patients on intermittent catheterization than those in patients with spontaneous voiding, despite similar clinical backgrounds other than the use of intermittent catheters (29). The negative effects of urinary dysfunction on GH, VT, and MH observed in the present study are supported by this report. Collectively, sensory and urinary dysfunctions in patients with HAM should be carefully managed to maintain better HRQoL both physically and mentally.
In this study, the use of indwelling catheters or manual extraction or enemas was not associated with the SF-36 subscale scores despite being the most severe form of bladder and bowel dysfunction. Although the reason is unclear, “response shift” may be one of the reasons. Response shift is a psychological process occurring in individuals with deteriorating health, in which internal standards and values change to adapt to a new situation (30, 31). Longitudinal studies are required to understand the changes in HRQoL over time in patients with HAM.
This study has several limitations. First, HRQoL is influenced by several factors not included in our analysis, such as education, employment status, and comorbidities (23). Moreover, we did not evaluate the intensity of the symptoms; HAM causes many other symptoms, including low back pain and spasticity (4). Therefore, we could not completely adjust symptom severity and potential confounders. Second, the coefficients only indicate associations, and therefore, may not necessarily mean the impact of the symptoms. Third, all symptoms were assessed using information collected through telephone interviews. Although telephone interviews were conducted uniformly by trained nurses and coordinators to collect reliable information, the accuracy of these assessments may be lower than that of a physical examination. In addition, because leg pain, numbness, lower urinary tract symptoms, and constipation are common even in the general population, we could not conclude that these symptoms were caused by HAM. Finally, because we only included Japanese patients, generalizability to other populations may be limited.
We demonstrated that HRQoL of patients with HAM was poorer than that in the general population and was associated with various symptoms, including motor, sensory, and bladder and bowel dysfunctions. Therefore, these patients should be comprehensively monitored and managed to achieve better HRQoL.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Bioethics Committee of St. Marianna University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
MK, JY, TS, NY, NA, SA, KT, AT, and YY contributed to the conception and design of the study. MK, JY, and KT conducted the statistical analysis. MK, JY, TS, NY, NA, SA, KT, EH, TW, AC-R, MN, YA, KK, TK, SS, HM, AK, SI, AT, and YY analyzed and interpreted the results and critically revised the manuscript. MK, JY, and YY drafted the manuscript. All authors read and approved the final version of the manuscript to be submitted.
This study was supported by grant from the Practical Research Project for Rare/Intractable Diseases of the Japan Agency for Medical Research and Development (Grant No. 21ek0109529) and Health and Labour Sciences Research Grant on Rare and Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (Grant No. 19FC1007). The funders played no role in designing the study, collecting and analyzing data, deciding to publish, or preparing the manuscript.
SA was employed by the LSI Medience Co., Tokyo, Japan.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the participants in the HAM-net who contributed to the investigation. Moreover, we thank the staff of the Division of Neurology, Department of Internal Medicine, and the support staff in the laboratory, including Katsunori Takahashi, Yasuo Kunitomo, Yumiko Hasegawa, Mikako Koike, Yuriko Hosokawa, Yumi Saito, and Miho Ishikawa. Furthermore, we want to thank the other members of the HAM-net for their valued assistance. The employees of Accelight, Inc., provided services for data management and analysis, and the employees of Ata Life, Inc. (a site management organization) supported data collection by conducting annual telephone interviews. Accelight, Inc., and Ata Life, Inc. were paid from the grants mentioned in the Funding section but played no role in designing the study, interpreting data, preparing the manuscript or deciding to publish.
1. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. (2012) 3:388. doi: 10.3389/fmicb.2012.00388
2. Gessain A, Vernant JC, Maurs L, Barin F, Gout O, Calender A, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. (1985) 326:407–10. doi: 10.1016/S0140-6736(85)92734-5
3. Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. (1986) 327:1031–2. doi: 10.1016/S0140-6736(86)91298-5
4. Bangham CRM, Araujo A, Yamano Y, Taylor GP. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat Rev Dis Prim. (2015) 1:15012. doi: 10.1038/nrdp.2015.12
5. Araujo A, Bangham CRM, Casseb J, Gotuzzo E, Jacobson S, Martin F, et al. Management of HAM/TSP. Neurol Clin Pract. (2021) 11:49–56. doi: 10.1212/CPJ.0000000000000832
6. Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. (1992) 30:473–83. doi: 10.1097/00005650-199206000-00002
7. Rabin R, De Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med. (2001) 33:337–43. doi: 10.3109/07853890109002087
8. Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. (2002) 21:271–92. doi: 10.1016/S0167-6296(01)00130-8
9. Brazier JE, Fukuhara S, Roberts J, Kharroubi S, Yamamoto Y, Ikeda S, et al. Estimating a preference-based index from the Japanese SF-36. J Clin Epidemiol. (2009) 62:1323–31. doi: 10.1016/J.JCLINEPI.2009.01.022
10. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. (2011) 20:1727–36. doi: 10.1007/S11136-011-9903-X
11. Diniz MSC, Feldner PC, Castro RA, Sartori MGF, Girão MJBC. Impact of HTLV-I in quality of life and urogynecologic parameters of women with urinary incontinence. Eur J Obstet Gynecol Reprod Biol. (2009) 147:230–3. doi: 10.1016/j.ejogrb.2009.07.024
12. Shublaq M, Orsini M, Puccioni-Sohler M. Implications of HAM/TSP functional incapacity in the quality of life. Arq Neuro Psiquiatr. (2011) 69:208–11. doi: 10.1590/S0004-282X2011000200013
13. Netto EC, Brites C. Characteristics of chronic pain and its impact on quality of life of patients with HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Clin J Pain. (2011) 27:131–5. doi: 10.1097/AJP.0b013e3181f195d3
14. Martins JVP, Baptista AF, Araújo Ade Q. Quality of life in patients with HTLV-I associated myelopathy/tropical spastic paraparesis. Arq Neuro Psiquiatr. (2012) 70:257–61. doi: 10.1590/S0004-282X2012005000006
15. Caiafa RC, Orsini M, Felicio LR, Puccioni-Sohler M. Muscular weakness represents the main limiting factor of walk, functional independence and quality of life of myelopathy patients associated to HTLV-1. Arq Neuro Psiquiatr. (2016) 74:280–6. doi: 10.1590/0004-282X20160019
16. Macêdo MC, Mota Rde S, Patrício NA, Santos AP, Mendes SM, Dias CM, et al. Quality of life and pain multidimensional aspects in individuals with HTLV-1. Braz J Infect Dis. (2016) 20:494–8. doi: 10.1016/J.BJID.2016.05.010
17. Gascón MRP, Mellão MA, Mello SH, Negrão RM, Casseb J, Oliveira ACP. The impact of urinary incontinence on the quality of life and on the sexuality of patients with HAM/TSP. Braz J Infect Dis. (2018) 22:288–93. doi: 10.1016/J.BJID.2018.07.003
18. Rosadas C, Assone T, Yamashita M, Adonis A, Puccioni-Sohler M, Santos M, et al. Health state utility values in people living with HTLV-1 and in patients with HAM/TSP: the impact of a neglected disease on the quality of life. PLoS Negl Trop Dis. (2020) 14:e0008761. doi: 10.1371/journal.pntd.0008761
19. Coler-Reilly ALG, Yagishita N, Suzuki H, Sato T, Araya N, Inoue E, et al. Nation-wide epidemiological study of Japanese patients with rare viral myelopathy using novel registration system (HAM-net). Orphanet J Rare Dis. (2016) 11:69. doi: 10.1186/s13023-016-0451-x
20. Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. J Clin Epidemiol. (1998) 51:1037–44. doi: 10.1016/S0895-4356(98)00095-X
21. Fukuhara S, Ware JE, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J Clin Epidemiol. (1998) 51:1045–53. doi: 10.1016/S0895-4356(98)00096-1
22. Yamakawa N, Yagishita N, Matsuo T, Yamauchi J, Ueno T, Inoue E, et al. Creation and validation of a bladder dysfunction symptom score for HTLV-1-associated myelopathy/tropical spastic paraparesis. Orphanet J Rare Dis. (2020) 15:175. doi: 10.1186/s13023-020-01451-3
23. Shiroiwa T, Fukuda T, Ikeda S, Igarashi A, Noto S, Saito S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res. (2016) 25:707–19. doi: 10.1007/s11136-015-1108-2
24. Olindo S, Cabre P, Lézin A, Merle H, Saint-Vil M, Signate A, et al. Natural history of human T-lymphotropic virus 1–associated myelopathy: a 14-year follow-up study. Arch Neurol. (2006) 63:1560. doi: 10.1001/archneur.63.11.1560
25. Sato T, Yagishita N, Tamaki K, Inoue E, Hasegawa D, Nagasaka M, et al. Proposal of classification criteria for HTLV-1-associated myelopathy/tropical spastic paraparesis disease activity. Front Microbiol. (2018) 9:1651. doi: 10.3389/fmicb.2018.01651
26. Yamauchi J, Tanabe K, Sato T, Nakagawa M, Matsuura E, Tsuboi Y, et al. Efficacy of corticosteroid therapy for HTLV-1-associated myelopathy: a randomized controlled trial (HAMLET-P). Viruses. (2022) 14:136. doi: 10.3390/V14010136
27. Gustavsen S, Olsson A, Søndergaard HB, Andresen SR, Sørensen PS, Sellebjerg F, et al. The association of selected multiple sclerosis symptoms with disability and quality of life: a large Danish self-report survey. BMC Neurol. (2021) 21:317. doi: 10.1186/S12883-021-02344-Z
28. Ayzenberg I, Richter D, Henke E, Asseyer S, Paul F, Trebst C, et al. Pain, depression, and quality of life in neuromyelitis optica spectrum disorder: a cross-sectional study of 166 AQP4 antibody-seropositive patients. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e985. doi: 10.1212/NXI.0000000000000985
29. Sekido N, Takaoka E, Nishiyama H, Ochi H, Satoh T. Impact of clean intermittent catheterization on quality of life of patients with neurogenic lower urinary tract dysfunction due to radical hysterectomy: a cross-sectional study. Low Urin Tract Symptoms. (2021) 13:168–76. doi: 10.1111/LUTS.12350
30. Sprangers MAG, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. (1999) 48:1507–15. doi: 10.1016/S0277-9536(99)00045-3
Keywords: HTLV-1, SF-36, SF-6D, quality of life, HTLV-1-associated myelopathy
Citation: Kimura M, Yamauchi J, Sato T, Yagishita N, Araya N, Aratani S, Tanabe K, Horibe E, Watanabe T, Coler-Reilly A, Nagasaka M, Akasu Y, Kaburagi K, Kikuchi T, Shibata S, Matsumoto H, Koseki A, Inoue S, Takata A and Yamano Y (2022) Health-Related Quality of Life Evaluation Using the Short Form-36 in Patients With Human T-Lymphotropic Virus Type 1-Associated Myelopathy. Front. Med. 9:879379. doi: 10.3389/fmed.2022.879379
Received: 19 February 2022; Accepted: 15 March 2022;
Published: 11 April 2022.
Edited by:
Antonio C. R. Vallinoto, Federal University of Pará, BrazilReviewed by:
Cintia Yolette Aben-Athar, Federal University of Pará, BrazilCopyright © 2022 Kimura, Yamauchi, Sato, Yagishita, Araya, Aratani, Tanabe, Horibe, Watanabe, Coler-Reilly, Nagasaka, Akasu, Kaburagi, Kikuchi, Shibata, Matsumoto, Koseki, Inoue, Takata and Yamano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshihisa Yamano, eXlhbWFub0BtYXJpYW5uYS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.