- 1Department of Respiratory and Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Shanghai Jiao Tong University School of Medicine, Shanghai, China
Obstructive sleep apnea (OSA) is a common type of sleep-disordered breathing associated with multiple comorbidities. Continuous positive airway pressure (CPAP) is the first choice for moderate-severe OSA but poor compliance brings a great challenge to its effectiveness. Telehealth interventions ease the follow-up process and allow healthcare facilities to provide consistent care. Fifth-generation wireless transmission technology has also greatly rationalized the wide use of telemedicine. Herein, we review the efficacy of the telehealth system in enhancing CPAP adherence. We recommend applying telemonitoring in clinical practice and advocate the development of a biopsychosocial telemedicine model with the integration of several interventions. Big databases and promising artificial intelligent technologies make clinical decision support systems and predictive models based on these databases possible.
Introduction
Obstructive sleep apnea (OSA) is a common type of sleep-disordered breathing caused by pharyngeal collapse during sleep (1). It is estimated that approximately 1 billion adults aged 30–69 years suffer from OSA worldwide (2). Several studies revealed comorbidities linked with OSA including cardiovascular, cerebrovascular, metabolic diseases, cancer, etc. (3). It is also associated with reduced quality of life (4), excessive daytime somnolence, elevated risk of accidents (5, 6), and sudden cardiac death (7). Since the 1980s, continuous positive airway pressure (CPAP) established as first-line therapy for moderate to severe OSA (8). However, low adherence to CPAP brings a great challenge to clinical practice (9–11). Several strategies are employed to promote adherence and enhance the efficacy of CPAP, i.e., multiple educational interventions, behavioral therapies, CPAP device modifications, etc. (12). During the past two decades, telehealth technology has been applied to the CPAP field, easing the follow-up process and allowing healthcare personnel to deliver more consistent care. Telehealth is defined as the application of telecommunications and digital communication technologies to deliver and facilitate health services (13). Telehealth adopted mobile health, video, audio, digital images, and telemonitoring (TM) to provide clinical and non-clinical services (14). The 2015 guidelines for telemedicine utilization published by the American Academy of Sleep Medicine (AASM) promoted telehealth technology development, and the coronavirus disease (COVID-19) pandemic has expedited internet-based home telemedicine for the diagnosis and treatment of OSA (15). Recent updates from AASM advocated the provision of high-quality sleep care through telehealth interventions and suggested a significant role of telehealth in maintaining the continuity of sleep health (16). Fifth-generation (5G) wireless transmission technology has increased the possibility of wider telemedicine applications. Herein, we aim to review the efficacy of the telehealth system on CPAP adherence and propose the possibility for future developments.
Factors Associated With Continuous Positive Airway Pressure Adherence
Patient characteristics such as age (17, 18), gender (17, 19), race (20), and smoking status (21) could affect adherence, although these factors were not consistent determinants of CPAP adherence (12). For example, the adherence to CPAP positively correlated with age was reported in a retrospective study (17), but a large cohort study showed a negative correlation with age, particularly in those aged >75 years old, which may be due to the body sickness, sleeping time, or sleep quality of the elderly can affect the adherence of CPAP (18). Smokers caused decreased CPAP adherence compared to non-smokers (21), which is attributed to those smokers being more susceptible to upper airway discomfort, the greater severity of OSA, and as a result, less likely to take advice from healthcare providers (21).
The severity of OSA or the symptoms affects CPAP compliance. Apnea-hypopnea index (AHI), oxygen desaturation index (ODI), and the severity of excessive daytime sleepiness are positively related to adherence (21–23). Further, side effects of CPAP may influence adherence, like mucosal drying, difficult nasal breathing, claustrophobia, etc. (24, 25). Additionally, patients’ psychosocial factors such as the internal locus of control (26), mental health, personality (27–29), and availability of social support (30) can affect adherence. Patients with greater locus control can overcome the side effect to achieve better adherence (26). Also, social support and bed partner is vital in improving CPAP compliance. The sleeping partner could give feedback and advice on the patients’ symptomatic improvements thus may help to improve the adherence (30). Moreover, those who are motivated to resolve their problems tend to have better adherence (27–29).
The higher income and education level is associated with increased adherence. The socio-economic status is positively correlated with health knowledge and accessibility to healthcare services (31). Medical cost, insufficient time, and transport issues concerning patients reduce CPAP adherence. Thus, CPAP adherence is determined by multifactor and should be individualized and closely monitored to address non-compliance and tolerance when prescribed (12, 25).
Application of Telemonitoring on the Continuous Positive Airway Pressure Adherence
Effects of Tele-Monitoring on the Obstructive Sleep Apnea Outcome
TM is a subset of telehealth. Real-time feedback reduces clinical care time and ameliorates physiological health account for the positive short-term TM effect significantly (32–36). TM significantly improves psychological health score of Short Form-12 in TM after 6 months (p = 0.05) and also shows a higher positive change in score than standard group (SG) which (9.26 ± 2.09 vs. 0.73 ± 1.78, p = 0.003) (37, 38). Additionally, TM possessed significant clinical improvements and reduced side effects, and improved the tidal volume (TG = 9.4 mL/kg vs. SG = 8.7 mL/kg, p = 0.022) (39) and the blood pressure (systolic blood pressure reduces by 7.4 mmHg and diastolic blood pressure reduces by 4.1 mmHg) (40). It mitigates disease severity shown by improvements on the Epworth Sleepiness Scale (TG ranges from 3.7 to 4.58, SG ranges from 6.05 to 6.1) (38, 40, 41) and reduces residual AHI (TG = 1.3 ± 1.0 vs. SG = 3.2 ± 3.8, p = 0.04) (36). With a combination of patient engagement tools, TM even reduces treatment termination by 2.8–5.6% and reduces mask leakage [(TG = 16.9 L/min vs. SG = 19.4 L/min, p < 0.0001) and (TG = 2.7 ± 4.0 L/min vs. SG = 4.1 ± 5.3 L/min, p < 0.001)] in two respective studies (32, 33, 42).
TM system decreases patients’ burden of visits by enabling flexible timing and reduced traveling times greatly (36). It also reduces costs by €47.32–153.34 compared to inpatient care (43, 44). However, the greater costs of the advanced device and no reimbursement for telemedicine services from most insurance companies are still barriers to patients of lower socioeconomic status (45). Thus, insurance coverage for telemedicine and subsidizing CPAP devices might solve the patients’ financial concerns and secure long-term benefits. TM able to reduce nursing time but there is increased workload in CPAP technicians (36, 44, 46). Since OSA is associated with multiple comorbidities, we notice a distinct lack of studies exploring the associations between TM effects on those with disability.
Effects of the Telemonitoring in Enhancing Continuous Positive Airway Pressure Adherence
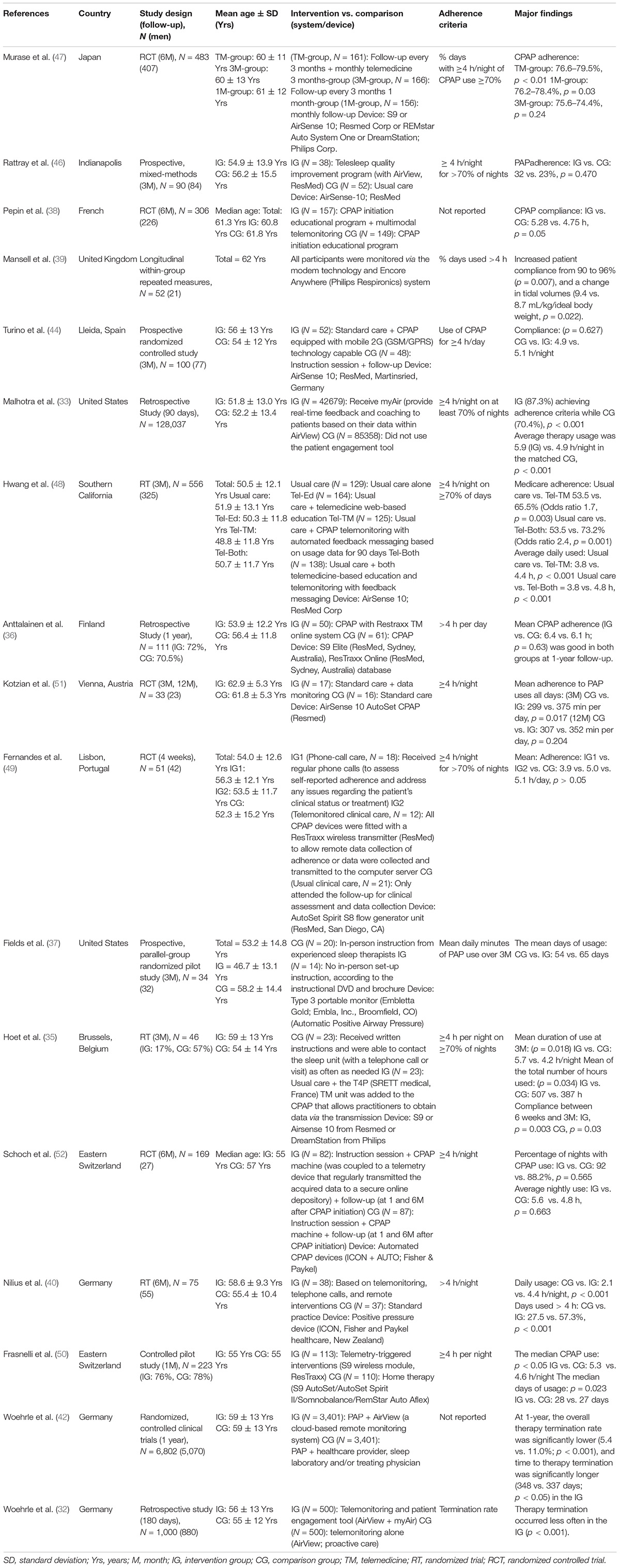
TM system transmits patients’ data to healthcare providers (HPs), enabling HPs to track CPAP usage and adherence at specific time intervals, i.e., 1, 3, and 6 months and more (Table 1) (35, 38, 40, 47–51). Optimally, the HPs will contact the patients through phone calls, messages, email, or by using the automatic feedback system to reinforce knowledge or encourage patients (40, 47, 48). The controlled pilot study and retrospective study demonstrate TM significantly enhanced CPAP mean daily usage hours and median CPAP usage after 1 month (36, 50), although a randomized controlled trial (RCT) finds no significant effect of TM in this 1 month (49).
Randomized trials illustrate CPAP adherence improves after 3-month of TM (35, 48). A shorter time to the first technical intervention might be associated with better compliance (35). Researchers have further confirmed that the combination of TM and education is more effective than TM alone (48). Notably, some prospective studies did not find significant effects at 3 months follow up, which may be attributed to the small sample sizes (37, 44, 46).
TM improves CPAP adherence at 6-month follow-up (38, 40, 47). TM significantly improves CPAP compliance [telemonitor group (TG) = 57.3 ± 34.5% vs. standard group (SG) = 27.5 ± 32.5%, p = 0.00025] and daily CPAP usage time (TG = 4.4 ± 2.5 h/night vs. SG = 2.1 ± 2.2 h/night, p = 0.000063) (40). A RCT observed a positive effect of multimodal TM in CPAP compliance compared to the usual care group (TG = 5.28 ± 2.23 h vs. SG = 4.75 ± 2.50 h, p = 0.05) (38). TM increases the percentage of days with good adherence from 76.6 ± 24.2% to 79.5 ± 22.0% (p < 0.01) after 6 months compared to baseline (47). Contrastingly, a RCT reported that TM did not improve overall adherence after 6 months, which might be due to the “ceiling effect” (52).
Finally, the current evidence has not confirmed the long-term effect on CPAP adherence at 1-year follow-up (36, 51). High dropout rates and telemonitoring during the habituation stage may account for the insignificant results (51). Compared to the titration stage, TM during the habituation stage might be another reason that leads to insignificant results (36). Although there are no significant improvements, TM still possesses a non-inferiority therapeutic effect compared to usual care after 1 year. We postulate that personality traits are a vital factor determining the length of effective TM intervention based on other researches (28) and suggest future research on interventions to secure long-term telemonitoring effects.
It should be noticed that most of the recent findings show equivocal effects similar to a meta-analysis which demonstrates a significantly higher mean difference of 0.79 h of CPAP compliance in the TM group for short-term follow-up (<3 months), but not for long-term follow up (45). Although the long-term effects remain contentious, we still recommend including telemonitoring in current clinical practice due to its multiple benefits and non-inferiority compared to usual care.
Various Telemonitoring Approaches Showed Different Effects for Continuous Positive Airway Pressure Adherence
Several TM approaches are used to improve adherence to CPAP (Table 2).
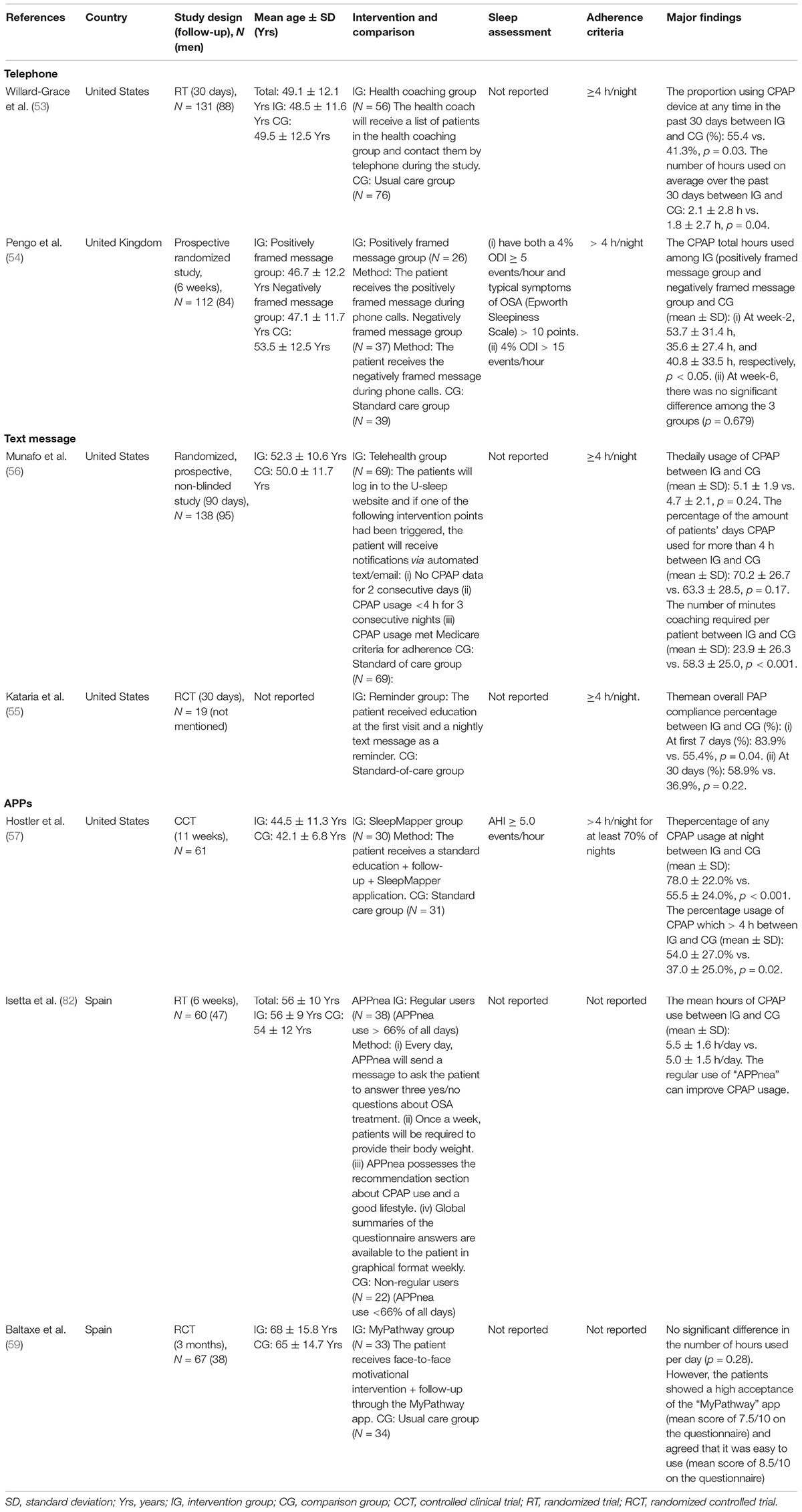
Telephone: Health coaching through the phone improves CPAP usage in the past 30 days (p = 0.03) (53). The researchers call the patients in the intervention group three times in 30 days to identify and assist them in immediate problem-solving (53). The HPs checked CPAP usage, understood the obstacles, and provide patients with different solutions. A designed list of responses used to communicate helps solve issues more effectively in contrast to a study showing the negative result (49, 53). These well-designed interventions might account for the positive effect. In addition, other studies determine that positively framed message increases CPAP total use hours in week-2 but the effect diminishes in week-6 (54).
Text message: Text messages for reminding about CPAP use significantly improve overall compliance in the first 7 days, but the effect fades at 30 days (55). Other research utilizing email and automated message methods shows no significant effect on CPAP adherence (56). The different content of responses from HPs in these studies might account for the differing results. The faded effect might be due to reduced motivation, which could possibly be addressed by a lack of interesting content, videos, or further information on CPAP therapy (54).
Apps: During the past 5 years, a total of three new apps (“SleepMapper”; “MyPathway”; “Appnea”) have been reported in detail (57–59). The apps allow HPs to provide real-time feedback and education, it also allowed patients to self-monitor and receive a personalized prescription for CPAP usage. “SleepMapper” significantly increases any CPAP usage at night (SG = 55.5 ± 24.0% vs. “SleepMapper” group = 78.0 ± 22.0%, p < 0.001) and usage of CPAP > 4 h (SG = 37.0 ± 25.0% vs. “SleepMapper” group = 54.0 ± 27.0%, p = 0.02) compared to the standard care group (57). Compared to new CPAP patients, “Appnea” significantly improves CPAP adherence who frequently used when compared to rarely used patients (5.6 ± 1.4 vs. 4.3 ± 1.3 h/night, p = 0.008) (58). In contrast, although participants were satisfied with the “MyPathway” app, there was no significant modification in PAP adherence. Almost a quarter of patients were 70–79 years and senior patients are perhaps less motivated in learning and utilizing new technology, thus resulting in negative outcomes (59). Limitations of current studies are the small sample sizes and the short study duration (57, 58).
Despite inconclusive results, mobile apps, phone calls, and text messages still possess some advantages. Apps remain the most promising approach. Future app development should continue to focus on user-friendly, online educational programs, troubleshooting models, and real-time access to CPAP usage and AHI data for self-monitoring. They can encourage patients to maintain a healthy lifestyle and adhere to given therapy with minimal effort from HPs (60). The required smartphones or tablet devices are usually affordable (61). For telephone calls and text messages which displayed short-term effects, additional modulations are required to enhance adherence. We postulate that a convenient troubleshooting model and active approach are vital in determining the success of the intervention. Actively assisting users to troubleshoot problems shows better results than passively waiting for calls (59). Maintaining long-term compliance with CPAP therapy is challenging. Adherence to CPAP may decline over time exacerbated by the negative attitudes toward the treatment and insufficient support from family members and the healthcare team (62).
Application of Tele-Education in Improving Continuous Positive Airway Pressure Adherence
Tele-education is the application of technologies to provide distance learning. Patients’ negative perceptions of the benefit and health value of CPAP are the common causes of poor CPAP adherence (63, 64). Patients’ lack of confidence in the therapeutic effect of CPAP results in poor adherence (65, 66). Therefore, education is vital in enhancing patients’ perception of CPAP therapy. Tele-education is a well-known alternative model to educate patients. The modes of tele-education include slide shows, videos, audio, and web-based learning (67). Therefore, patients can watch the educational material as soon as they are available and shorten clinical care time (36). The recent COVID-19 pandemic has also accelerated the development of tele-education.
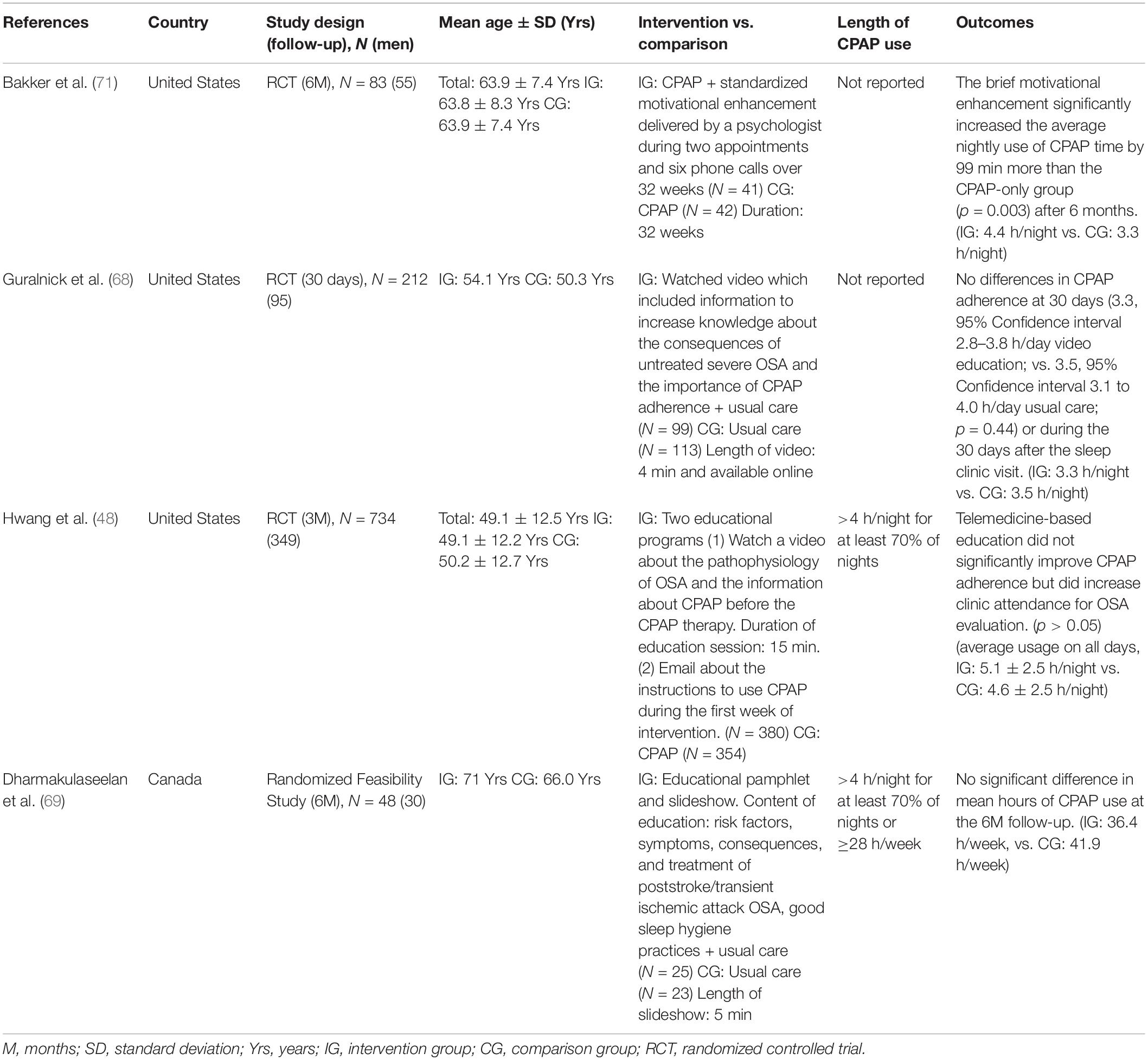
A few studies have investigated the effect of tele-education (Table 3). RCTs found educational videos have no significant improvement in CPAP adherence (48, 68). Although patients understood the given slideshow and educational booklet, there was no significant improvement in mean hours of CPAP (69). The Health Belief Model states that a change in health behavior is primarily due to health perception instead of knowledge (66). Confidence in the therapeutic effect is a key factor that alters health practice (66). Therefore, non-continuous tele-education alone is insufficient (48, 68, 69). Most studies had a small sample size (68, 69) and a short period of intervention (48, 68, 69), which may also influence the outcome. No examination of patients’ knowledge, attitude, and practice pre-and post-study is also a limitation of existing educational studies.
Educational intervention with motivation enhancement significantly improves CPAP adherence (70). Motivation enhancement is a behavioral intervention based on the principle of the motivational interview (71). Patients had a one-on-one conversation session with a psychologist, watched an educational video, and received follow-up phone calls from the psychologist (71). This intervention significantly increased the average nightly use of CPAP time by 99 min more than the CPAP-only group (p = 0.003) after 6 months (71).
The development of tele-technology concerning OSA patients’ psychology and social life remains scarce. Multiple healthcare-related parties should consider the implementation of tele-technology in the advancement of the biopsychosocial model. Since a recent study found group CPAP education enhanced acceptance of therapy, future studies can investigate educational interventions with peer support through social app platforms (72). Integration of continuous tele-education and online motivational interviews might maximize the positive effect on patients’ health behavior by enhancing their confidence level about the therapy (65, 71). We also suggest the inclusion of a polysomnography chart in the educational video, as one RCT found that this increased the mean usage hours (SG = 4.2 ± 2.5 h/night, interventional group = 5.2 ± 2.1 h/night, p = 0.027) and the compliance (SG = 68.3%, interventional group = 86.5%, p = 0.021) (73).
Future of Telehealth Systems for Enhancing Continuous Positive Airway Pressure Adherence
As individual treatments show heterogeneous results, we would like to propose an individualized telehealth model integrating all the interventions shown in Figure 1. Firstly, a new CPAP user should receive educational material in the form of a video, slideshow, or booklet. Then, adherence data should be transmitted to the cloud database, enabling HPs to analyze CPAP adherence. Patients with good compliance will use the apps for self-management and contact HPs when necessary. If HPs review the usage data periodically, then patients with poor adherence could receive the advice and combinations of interventions. Currently available interventions to combine included text message, telephone, motivational enhancement, and patient engagement tools. Developers and researchers could consider including family engagement tools (74), online patient support groups using telecommunication apps, and continuous tele-education in future research.
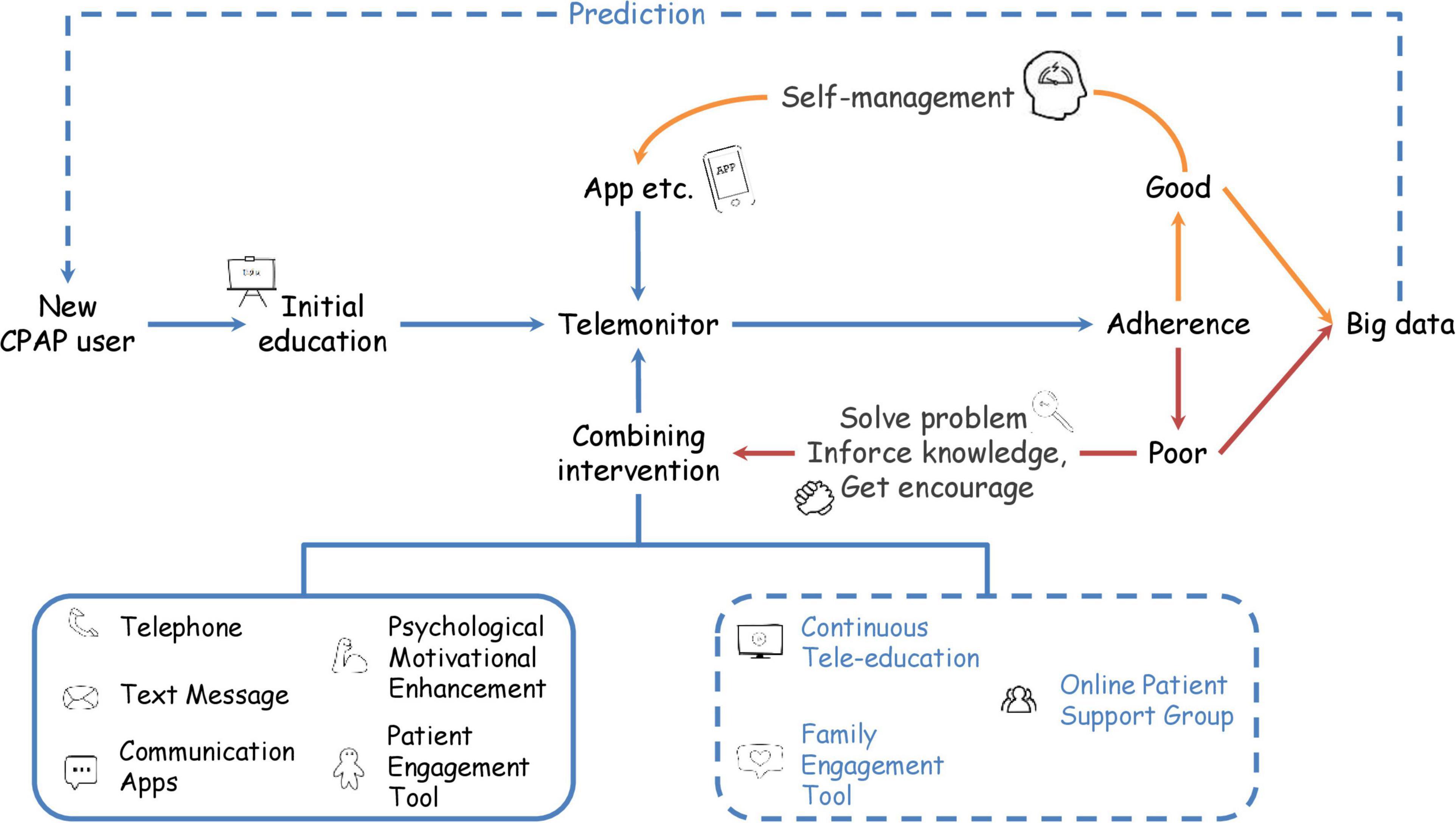
Figure 1. Flowchart for future telehealth application. New CPAP users receive education and initiate a self-management approach initially. Then, healthcare providers remotely monitor CPAP usage data and identify the patient’s adherence occasionally. For the patient with good adherence, healthcare providers administrate an app for the patient to self-manage. Patients with insufficient adherence should join combining interventions. Currently available interventions include telephone, text message, psychological motivational enhancement, and patient engagement tools. Future studies on continuous tele-education, family engagement tools, and online patient support groups are required. Collection of adherence data able to build big data. Training artificial intelligence with big data might help to predict adherence of new CPAP users. Blue words and dotted lines represent future interventions.
Moreover, the collection of adherence data from CPAP devices into a database also facilitates big data development (35, 38, 40, 47–51). The application of cloud based-data allows an overview of real-world data and investigation of CPAP adherence patterns (75, 76). Besides, big data shows the effectiveness of different interventions with minimal effort required, compared to the traditional observational studies (33). An analysis of AirView alone compares adherence patterns among various countries (N = 4,181,490) and supports the positive effects of a patient engagement tool (77). Big data from German homecare providers also reveals several predictors of poor CPAP adherence and investigated the effect of shifting therapy (78). The current benefits of big data analysis imply the worthiness of building more cloud databases and collecting various types of data.
Recently, some studies have developed artificial intelligence models to predict adherence (79–81). A model predicting the next-30-day adherence phenotype achieved the highest sensitivity (90%), specificity (96%), and accuracy (95%) (79). However, the model for 6-month adherence had a sensitivity ranging between 71 and 77% and a specificity ranging between 69 and 72% (80). These promising results encourage future studies to use big data to develop clinical prediction models to identify patients with likely poor adherence. Additionally, future research could collect various types of data to integrate the biopsychosocial model into clinical prediction models. For example, responses from clinical and psychological questionnaires, the app usage data, and word patterns of patients’ responses can build a database to train more artificial intelligence models. Investigation of knowledge, attitude, and practice of CPAP adherence also assists in the generation of these prediction models (Figure 2).
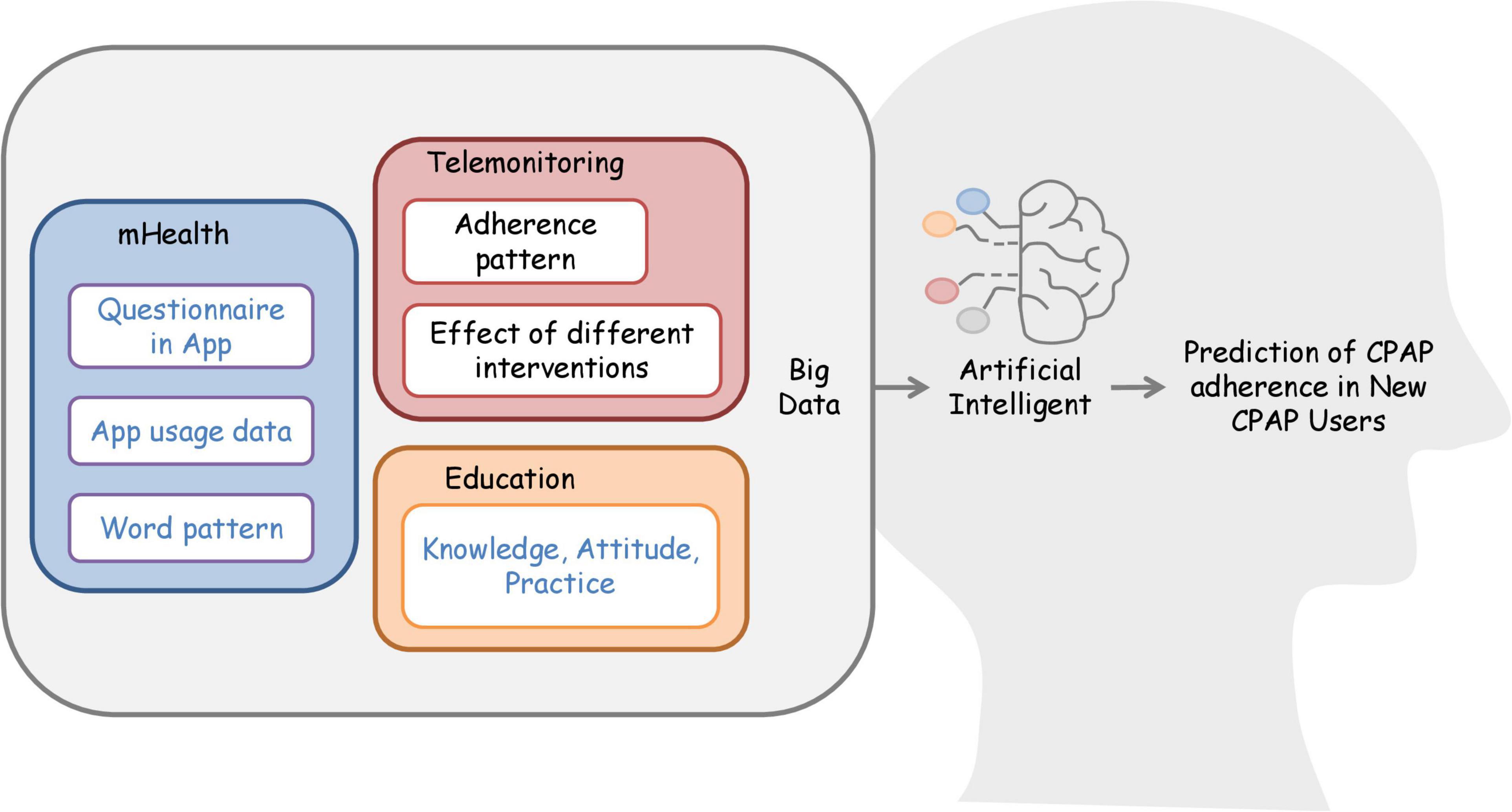
Figure 2. Integration of big data and artificial intelligence for the development of the predictive model. Collection of adherence data from telemonitoring able to generate adherence patterns database. Future studies can use the database to continue work on the artificial intelligence model to predict CPAP adherence. Researchers can also consider building biopsychosocial databases by retrieving data such as psychological, social, and clinical questionnaires in-app. Developers can collect app usage data and word patterns used in telecommunication apps to train the artificial intelligence model to predict CPAP adherence. For education, researchers could work on a prediction model based on the knowledge, attitude, and practice of CPAP users. The blue words represent future intervention.
Conclusion
Telemonitoring has been proven to improve the compliance of CPAP, resulting in the reduction of disease severity and side effects, and enhancement of social and psychological support. It also improves the quality of life and slightly alleviates the economic burden. We support the application of telemonitoring in CPAP management and following up. As tele-education alone is insufficient in improving CPAP adherence. Thus, we call for a bio-psycho-social care model integrating multiple interventions to promote better care for CPAP therapy.
Author Contributions
BT, GL, JL, and QL: conception and design of the work. BT, GL, JL, CL, ST, HL, and QL: drafting of the manuscript and final approval for publication. BT, GL, JL, HL, and QL: critically revision for the content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82070089 and 81770084), the Key Research Program of Shanghai Science and Technology Commission (18140903600), and the National Key R&D Program of China (2018YFC1311900).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Turino C, Bertran S, Gavalda R, Teixido I, Woehrle H, Rue M, et al. Characterization of the CPAP-treated patient population in catalonia. PLoS One. (2017) 12:e0185191. doi: 10.1371/journal.pone.0185191
2. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. (2019) 7:687–98. doi: 10.1016/s2213-2600(19)30198-5
3. Bonsignore MR, Baiamonte P, Mazzuca E, Castrogiovanni A, Marrone O. Obstructive sleep apnea and comorbidities: a dangerous liaison. Multidiscip Respir Med. (2019) 14:8. doi: 10.1186/s40248-019-0172-9
4. Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. (2001) 2:477–91. doi: 10.1016/S1389-9457(01)00072-7
5. Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of occupational accidents in workers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. (2016) 39:1211–8. doi: 10.5665/sleep.5834
6. Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. (2009) 5:573–81. doi: 10.5664/jcsm.27662
7. Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. (2013) 62:610–6. doi: 10.1016/j.jacc.2013.04.080
8. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2019) 15:335–43. doi: 10.5664/jcsm.7640
9. Naik S, Al-Halawani M, Kreinin I, Kryger M. Centers for medicare and medicaid services positive airway pressure adherence criteria may limit treatment to many medicare beneficiaries. J Clin Sleep Med. (2019) 15:245–51. doi: 10.5664/jcsm.7626
10. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. (2008) 5:173–8. doi: 10.1513/pats.200708-119MG
11. Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. (2016) 45:43. doi: 10.1186/s40463-016-0156-0
12. Mehrtash M, Bakker JP, Ayas N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung. (2019) 197:115–21. doi: 10.1007/s00408-018-00193-1
14. Physicians AAoF. What’s the Difference Between Telemedicine and Telehealth?. (2022). Available online at: https://www.aafp.org/news/media-center/kits/telemedicine-and-telehealth.html#:~:text=Telehealth%20is%20different%20from%20telemedicine,to%20remote%20non%2Dclinical%20services (accessed July 25, 2021).
15. Singh J, Badr MS, Diebert W, Epstein L, Hwang D, Karres V, et al. American academy of sleep medicine (AASM) position paper for the use of telemedicine for the diagnosis and treatment of sleep disorders. J Clin Sleep Med. (2015) 11:1187–98. doi: 10.5664/jcsm.5098
16. Shamim-Uzzaman QA, Bae CJ, Ehsan Z, Setty AR, Devine M, Dhankikar S, et al. The use of telemedicine for the diagnosis and treatment of sleep disorders: an American academy of sleep medicine update. J Clin Sleep Med. (2021) 17:1103–7. doi: 10.5664/jcsm.9194
17. Woehrle H, Graml A, Weinreich G. Age- and gender-dependent adherence with continuous positive airway pressure therapy. Sleep Med. (2011) 12:1034–6. doi: 10.1016/j.sleep.2011.05.008
18. Martinez-Garcia MA, Valero-Sanchez I, Reyes-Nunez N, Oscullo G, Garcia-Ortega A, Gomez-Olivas JD, et al. Continuous positive airway pressure adherence declines with age in elderly obstructive sleep apnoea patients. ERJ Open Res. (2019) 5:00178–2018. doi: 10.1183/23120541.00178-2018
19. Anttalainen U, Saaresranta T, Kalleinen N, Aittokallio J, Vahlberg T, Polo O. CPAP adherence and partial upper airway obstruction during sleep. Sleep Breath. (2007) 11:171–6. doi: 10.1007/s11325-007-0102-5
20. Billings ME, Auckley D, Benca R, Foldvary-Schaefer N, Iber C, Redline S, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. (2011) 34:1653–8. doi: 10.5665/sleep.1428
21. Jacobsen AR, Eriksen F, Hansen RW, Erlandsen M, Thorup L, Damgard MB, et al. Determinants for adherence to continuous positive airway pressure therapy in obstructive sleep apnea. PLoS One. (2017) 12:e0189614. doi: 10.1371/journal.pone.0189614
22. Campos-Rodriguez F, Martinez-Alonso M, Sanchez-de-la-Torre M, Barbe F, Spanish Sleep N. Long-term adherence to continuous positive airway pressure therapy in non-sleepy sleep apnea patients. Sleep Med. (2016) 17:1–6. doi: 10.1016/j.sleep.2015.07.038
23. McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. (1999) 159:1108–14. doi: 10.1164/ajrccm.159.4.9807111
24. Singhal P, Joshi Y, Singh G, Kulkarni S. Study of factors affecting compliance of continuous positive airway pressure (CPAP) in obstructive sleep apnea-hypopnea syndrome (OSAHS). Eur Respir J. (2016) 48:A2362. doi: 10.1183/13993003.congress-2016.PA2362
25. Ghadiri M, Grunstein RR. Clinical side effects of continuous positive airway pressure in patients with obstructive sleep apnoea. Respirology. (2020) 25:593–602. doi: 10.1111/resp.13808
26. Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J. (2004) 24:461–5. doi: 10.1183/09031936.04.00114603
27. Copur AS, Erik Everhart D, Zhang C, Chen Z, Shekhani H, Mathevosian S, et al. Effect of personality traits on adherence with positive airway pressure therapy in obstructive sleep apnea patients. Sleep Breath. (2018) 22:369–76. doi: 10.1007/s11325-017-1559-5
28. Cayanan EA, Bartlett DJ, Chapman JL, Hoyos CM, Phillips CL, Grunstein RR. A review of psychosocial factors and personality in the treatment of obstructive sleep apnoea. Eur Respir Rev. (2019) 28:190005. doi: 10.1183/16000617.0005-2019
29. Law M, Naughton M, Ho S, Roebuck T, Dabscheck E. Depression may reduce adherence during CPAP titration trial. J Clin Sleep Med. (2014) 10:163–9. doi: 10.5664/jcsm.3444
30. Ye L, Malhotra A, Kayser K, Willis DG, Horowitz JA, Aloia MS, et al. Spousal involvement and CPAP adherence: a dyadic perspective. Sleep Med Rev. (2015) 19:67–74. doi: 10.1016/j.smrv.2014.04.005
31. Simon-Tuval T, Reuveni H, Greenberg-Dotan S, Oksenberg A, Tal A, Tarasiuk A. Low socioeconomic status is a risk factor for CPAP acceptance among adult OSAS patients requiring treatment. Sleep. (2009) 32:545–52. doi: 10.1093/sleep/32.4.545
32. Woehrle H, Arzt M, Graml A, Fietze I, Young P, Teschler H, et al. Effect of a patient engagement tool on positive airway pressure adherence: analysis of a German healthcare provider database. Sleep Med. (2018) 41:20–6. doi: 10.1016/j.sleep.2017.07.026
33. Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest. (2018) 153:843–50. doi: 10.1016/j.chest.2017.11.005
34. Pépin JL, Tamisier R, Hwang D, Mereddy S, Parthasarathy S. Does remote monitoring change OSA management and CPAP adherence? Respirology. (2017) 22:1508–17. doi: 10.1111/resp.13183
35. Hoet F, Libert W, Sanida C, Van den Broecke S, Bruyneel AV, Bruyneel M. Telemonitoring in continuous positive airway pressure-treated patients improves delay to first intervention and early compliance: a randomized trial. Sleep Med. (2017) 39:77–83. doi: 10.1016/j.sleep.2017.08.016
36. Anttalainen U, Melkko S, Hakko S, Laitinen T, Saaresranta T. Telemonitoring of CPAP therapy may save nursing time. Sleep Breath. (2016) 20:1209–15. doi: 10.1007/s11325-016-1337-9
37. Fields BG, Behari PP, McCloskey S, True G, Richardson D, Thomasson A, et al. Remote ambulatory management of veterans with obstructive sleep apnea. Sleep. (2016) 39:501–9. doi: 10.5665/sleep.5514
38. Pepin JL, Jullian-Desayes I, Sapene M, Treptow E, Joyeux-Faure M, Benmerad M, et al. Multimodal remote monitoring of high cardiovascular risk patients with OSA initiating CPAP a randomized trial. Chest. (2019) 155:730–9. doi: 10.1016/j.chest.2018.11.007
39. Mansell SK, Cutts S, Hackney I, Wood MJ, Hawksworth K, Creer DD, et al. Using domiciliary non-invasive ventilator data downloads to inform clinical decision-making to optimise ventilation delivery and patient compliance. BMJ Open Respir Res. (2018) 5:e000238. doi: 10.1136/bmjresp-2017-000238
40. Nilius G, Schroeder M, Domanski U, Tietze A, Schafer T, Franke KJ. Telemedicine improves continuous positive airway pressure adherence in stroke patients with obstructive sleep apnea in a randomized trial. Respiration. (2019) 98:410–20. doi: 10.1159/000501656
41. Aalaei S, Rezaeitalab F, Tabesh H, Amini M, Afsharisaleh L, Mostafavi SM, et al. Factors affecting patients’ adherence to continuous positive airway pressure therapy for obstructive sleep apnea disorder: a multi-method approach. Iran J Med Sci. (2020) 45:170–8. doi: 10.30476/ijms.2019.45785
42. Woehrle H, Ficker JH, Graml A, Fietze I, Young P, Teschler H, et al. Telemedicine-based proactive patient management during positive airway pressure therapy: impact on therapy termination rate. Somnologie (Berl). (2017) 21:121–7. doi: 10.1007/s11818-016-0098-9
43. Lugo VM, Garmendia O, Suarez-Girón M, Torres M, Vázquez-Polo FJ, Negrín MA, et al. Comprehensive management of obstructive sleep apnea by telemedicine: clinical improvement and cost-effectiveness of a virtual sleep unit. A randomized controlled trial. PLoS One. (2019) 14:e0224069. doi: 10.1371/journal.pone.0224069
44. Turino C, de Batlle J, Woehrle H, Mayoral A, Castro-Grattoni AL, Gómez S, et al. Management of continuous positive airway pressure treatment compliance using telemonitoring in obstructive sleep apnoea. Eur Respir J. (2017) 49:1601128. doi: 10.1183/13993003.01128-2016
45. Chen C, Wang J, Pang L, Wang Y, Ma G, Liao W. Telemonitor care helps CPAP compliance in patients with obstructive sleep apnea: a systemic review and meta-analysis of randomized controlled trials. Ther Adv Chronic Dis. (2020) 11:2040622320901625. doi: 10.1177/2040622320901625
46. Rattray NA, Khaw A, McGrath M, Damush TM, Miech EJ, Lenet A, et al. Evaluating the feasibility of implementing a telesleep pilot program using two-tiered external facilitation. BMC Health Serv Res. (2020) 20:357. doi: 10.1186/s12913-020-05164-y
47. Murase K, Tanizawa K, Minami T, Matsumoto T, Tachikawa R, Takahashi N, et al. A randomized controlled trial of telemedicine for long-term sleep apnea continuous positive airway pressure management. Ann Am Thorac Soc. (2020) 17:329–37. doi: 10.1513/AnnalsATS.201907-494OC
48. Hwang D, Chang JW, Benjafield AV, Crocker ME, Kelly C, Becker KA, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence. The tele-osa randomized trial. Am J Respir Crit Care Med. (2018) 197:117–26. doi: 10.1164/rccm.201703-0582OC
49. Fernandes M, Antunes C, Martinho C, Carvalho J, Abreu T, Oliveira A, et al. Evaluation of telemonitoring of continuous positive airway pressure therapy in obstructive sleep apnoea syndrome: telepap pilot study. J Telemed Telecare. (2019) 27:353–8. doi: 10.1177/1357633x19875850
50. Frasnelli M, Baty F, Niedermann J, Brutsche MH, Schoch OD. Effect of telemetric monitoring in the first 30 days of continuous positive airway pressure adaptation for obstructive sleep apnoea syndrome – a controlled pilot study. J Telemed Telecare. (2016) 22:209–14. doi: 10.1177/1357633x15598053
51. Kotzian ST, Saletu MT, Schwarzinger A, Haider S, Spatt J, Kranz G, et al. Proactive telemedicine monitoring of sleep apnea treatment improves adherence in people with stroke– a randomized controlled trial (hopes study). Sleep Med. (2019) 64:48–55. doi: 10.1016/j.sleep.2019.06.004
52. Schoch OD, Baty F, Boesch M, Benz G, Niedermann J, Brutsche MH. Telemedicine for continuous positive airway pressure in sleep apnea. A randomized, controlled study. Ann Am Thorac Soc. (2019) 16:1550–7. doi: 10.1513/AnnalsATS.201901-013OC
53. Willard-Grace R, Wolf J, Huang B, Lewis E, Su G. Pilot of brief health coaching intervention to improve adherence to positive airway pressure therapy. Jt Comm J Qual Patient Saf. (2020) 46:631–9. doi: 10.1016/j.jcjq.2020.08.011
54. Pengo MF, Czaban M, Berry MP, Nirmalan P, Brown R, Birdseye A, et al. The effect of positive and negative message framing on short term continuous positive airway pressure compliance in patients with obstructive sleep apnea. J Thorac Dis. (2018) 10:S160–9. doi: 10.21037/jtd.2017.07.110
55. Kataria LV, Sundahl CA, Skalina LM, Shah M, Pfeiffer MH, Balish MS, et al. Annie: the veterans health administration’s personalized text message application promotes compliance with positive airway pressure. Sleep. (2017) 40:A196–A. doi: 10.1093/sleepj/zsx050.525
56. Munafo D, Hevener W, Crocker M, Willes L, Sridasome S, Muhsin M. A telehealth program for CPAP adherence reduces labor and yields similar adherence and efficacy when compared to standard of care. Sleep Breath. (2016) 20:777–85. doi: 10.1007/s11325-015-1298-4
57. Hostler JM, Sheikh KL, Andrada TF, Khramtsov A, Holley PR, Holley AB. A mobile, web-based system can improve positive airway pressure adherence. J Sleep Res. (2017) 26:139–46. doi: 10.1111/jsr.12476
58. Isetta V, Torres M, González K, Ruiz C, Dalmases M, Embid C, et al. A new mhealth application to support treatment of sleep apnoea patients. J Telemed Telecare. (2017) 23:14–8. doi: 10.1177/1357633x15621848
59. Baltaxe E, Embid C, Aumatell E, Martínez M, Barberan-Garcia A, Kelly J, et al. Integrated care intervention supported by a mobile health tool for patients using noninvasive ventilation at home: randomized controlled trial. JMIR Mhealth Uhealth. (2020) 8:e16395. doi: 10.2196/16395
60. Rathbone AL, Prescott J. The use of mobile apps and SMS messaging as physical and mental health interventions: systematic review. J Med Internet Res. (2017) 19:e295. doi: 10.2196/jmir.7740
61. Clough BA, Casey LM. The smart therapist: a look to the future of smartphones and mhealth technologies in psychotherapy. Prof Psychol Res Pr. (2015) 46:147–53. doi: 10.1037/pro0000011
62. Brostrom A, Nilsen P, Johansson P, Ulander M, Stromberg A, Svanborg E, et al. Putative facilitators and barriers for adherence to CPAP treatment in patients with obstructive sleep apnea syndrome: a qualitative content analysis. Sleep Med. (2010) 11:126–30. doi: 10.1016/j.sleep.2009.04.010
63. Scharf SM, Seiden L, DeMore J, Carter-Pokras O. Racial differences in clinical presentation of patients with sleep-disordered breathing. Sleep Breath. (2004) 8:173–83. doi: 10.1007/s11325-004-0173-5
64. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (Sahs). Sleep Med Rev. (2003) 7:81–99. doi: 10.1053/smrv.2001.0197
65. Chuang SC, Cheng YH, Chang CJ, Chiang YT. The impact of self-confidence on the compromise effect. Int J Psychol. (2013) 48:660–75. doi: 10.1080/00207594.2012.666553
66. Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q. (1988) 15:175–83. doi: 10.1177/109019818801500203
68. Guralnick AS, Balachandran JS, Szutenbach S, Adley K, Emami L, Mohammadi M, et al. Educational video to improve CPAP use in patients with obstructive sleep apnoea at risk for poor adherence: a randomised controlled trial. Thorax. (2017) 72:1132–9. doi: 10.1136/thoraxjnl-2017-210106
69. Dharmakulaseelan L, Kirolos N, Kamra M, Armesto-Heys A, Bouthillier C, Runions S, et al. Educating stroke/Tia patients about obstructive sleep apnea after stroke: a randomized feasibility study. J Stroke Cerebrovasc Dis. (2019) 28:104317. doi: 10.1016/j.jstrokecerebrovasdis.2019.104317
70. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine systematic review, meta-analysis, and grade assessment. J Clin Sleep Med. (2019) 15:301–34. doi: 10.5664/jcsm.7638
71. Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, et al. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest. (2016) 150:337–45. doi: 10.1016/j.chest.2016.03.019
72. Lettieri CJ, Walter RJ. Impact of group education on continuous positive airway pressure adherence. J Clin Sleep Med. (2013) 9:537–41. doi: 10.5664/jcsm.2742
73. Saraç S, Afçar G, Oruç Ö, Topçuoğlu ÖB, Saltürk C, Peker Y. Impact of patient education on compliance with positive airway pressure treatment in obstructive sleep apnea. Med Sci Monit. (2017) 23:1792–9. doi: 10.12659/msm.902075
74. Ventola CL. Mobile devices and apps for health care professionals: uses and benefits. P T. (2014) 39:356–64.
75. Cistulli PA, Armitstead J, Pepin JL, Woehrle H, Nunez CM, Benjafield A, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. (2019) 59:114–6. doi: 10.1016/j.sleep.2019.01.004
76. Patel SR, Bakker JP, Stitt CJ, Aloia MS, Nouraie SM. Age and sex disparities in adherence to CPAP. Chest. (2021) 159:382–9. doi: 10.1016/j.chest.2020.07.017
77. Drager LF, Malhotra A, Yan Y, Pépin JL, Armitstead JP, Woehrle H, et al. Adherence with positive airway pressure therapy for obstructive sleep apnea in developing versus developed countries: a big data study. J Clin Sleep Med. (2020) 17:703–9. doi: 10.5664/jcsm.9008
78. Woehrle H, Arzt M, Graml A, Fietze I, Young P, Teschler H, et al. Predictors of positive airway pressure therapy termination in the first year: analysis of big data from a German homecare provider. BMC Pulm Med. (2018) 18:186. doi: 10.1186/s12890-018-0748-8
79. Hevener W, Beine B, Woodruff J, Munafo D, Fernandez C, Rusk S, et al. Using AI to predict future CPAP adherence and the impact of behavioral and technical interventions. Sleep. (2020) 43:A243–A. doi: 10.1093/sleep/zsaa056.632
80. Araujo M, Kazaglis L, Bhojwani R, Iber C, Khadanga S, Srivastava J. Machine learning to predict pap adherence and compliance in tele-health management. Sleep. (2018) 41:A400–1. doi: 10.1093/sleep/zsy061.1077
81. Araujo M, Kazaglis L, Iber C, Srivastava J. A data-driven approach for continuous adherence predictions in sleep apnea therapy management. In: Baru C, Huan J, Khan L, Hu XT, Ak R, Tian Y, editors. 2019 IEEE International Conference on Big Data. (Piscataway, NJ: IEEE) (2019). p. 2716–25. doi: 10.1109/BigData47090.2019.9006476
Keywords: sleep apnea syndromes, eHealth, telemedicine, compliance, CPAP, telemonitoring
Citation: Thong BKS, Loh GXY, Lim JJ, Lee CJL, Ting SN, Li HP and Li QY (2022) Telehealth Technology Application in Enhancing Continuous Positive Airway Pressure Adherence in Obstructive Sleep Apnea Patients: A Review of Current Evidence. Front. Med. 9:877765. doi: 10.3389/fmed.2022.877765
Received: 17 February 2022; Accepted: 05 April 2022;
Published: 03 May 2022.
Edited by:
Zhongxing Zhang, Clinic and Care Center Barmelweid, SwitzerlandReviewed by:
Corrado Pelaia, Magna Graecia University, ItalyTarja Saaresranta, University of Turku, Finland
Copyright © 2022 Thong, Loh, Lim, Lee, Ting, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Yun Li, bGlxaW5neXVuNjhAaG90bWFpbC5jb20=
 Benjamin Ka Seng Thong
Benjamin Ka Seng Thong Grace Xin Yun Loh
Grace Xin Yun Loh Jia Jan Lim
Jia Jan Lim Christina Jia Liang Lee
Christina Jia Liang Lee Shu Ning Ting
Shu Ning Ting Hong Peng Li1
Hong Peng Li1 Qing Yun Li
Qing Yun Li