- Department of Gastroenterology, The First Affiliated Hospital, Jinan University, Guangzhou, China
Objective: Vitamin D consumption and circulating 25(OH)D level are associated with decreased risk of colorectal cancer (CRC) and colorectal adenoma (CRA), but few studies have assessed their relationship with the incidence and recurrence of CRC precursors. Therefore, we performed this meta-analysis to further evaluate the association.
Methods: We searched PubMed, Web of Science, Scopus and Embase databases in English until August 2021. Studies evaluating the association of vitamin D intake and circulating 25(OH)D level with risk of CRC precursors were included. A random-effects model was used to pool the risk estimates.
Results: A total of 48 studies were selected for inclusion. The CRC precursors incidence was negatively correlated with total vitamin D intake (RR = 0.84 95%CI: 0.80–0.88) and circulating 25(OH)D level (RR = 0.79 95%CI: 0.67–0.92). However, vitamin D intake and circulating 25(OH)D level did not show significant effects on the risk of CRC precursors recurrence. For dose-response analysis, evidence of a linear association was found between CRC precursors incidence and circulating 25(OH)D level, and the risk decreased by 14% per 10 ng/ml increment of circulating 25(OH)D level (RR = 0.86 95% CI: 0.75–0.99).
Conclusion: Vitamin D intake and circulating 25(OH)D level can play an effective role in reducing the risk of incidence of CRC precursors. However, they have not prevented the recurrence of CRC precursors.
Introduction
Colorectal cancer is the third leading cancer in the world, with over 1.8 million new cases and 915,880 deaths worldwide (1). The cause is multifactorial including interaction of age, environmental, and genetic factors (2). Different lifestyles have also been shown to have a clear association with CRC. Obesity, sedentary behavior, smoking, alcohol, red and processed meat are associated with increased risk of CRC, while regular physical activity, fish, fruit and vegetables, fiber, folate, calcium, dairy products and vitamin are associated with decreased risk of CRC (3). This shows the important role of nutrition plays as a causal or protective role in the development of CRC. Therefore, screening for validated nutrients is an important adjuvant strategy in CRC prevention.
Vitamin D is one of the essential nutrients for the human body. With its deficiency, it has been linked to skeletal diseases such as fracture (4), bone metabolism (5), and osteoporosis (6) in the past. Because it affects a broad range of health outcomes, vitamin D has been extensively studied of its importance, vitamin D has been extensively studied. Vitamin D is mainly produced through sun exposure and acquired through dietary products and supplements. As the metabolite of vitamin D in the liver, 25(OH)D is widely used to assessed the status of vitamin D (7). A recent umbrella review of 107 systematic reviews and 74 meta-analyses suggested that higher circulating vitamin D level was associated with decreased risk of cancer (breast/colorectal/rectal/sporadic), cardiovascular disease, hypertension, ischemic disease, stroke, Alzheimer’s disease, depression, tuberculosis, metabolic syndrome, diabetes, fractures, CKD mortality and all-cause mortality (8).
The role of vitamin D in the prevention of CRC was first proposed in 1980 (9). In vitro experiments have demonstrated the anti-tumor effect of vitamin D, especially in CRC (10). Since then, extensive observational (11, 12), RCT (13), and meta-analysis (14–16) studies have been conducted to evaluate the association of vitamin D intake and/or circulating 25(OH)D level in reducing the risk of CRC incidence, recurrence, survival and mortality. A previous study has shown that vitamin D intake plays a protective role in the development of CRC and a 10ng increment in circulating 25(OH)D level may decreased the risk of CRC by 26% (17).
The development of CRC progresses through three pathways, adenoma-carcinoma sequence, serrated pathway and inflammatory pathway (18). Several acquired genetic and epigenetic changes of normal glandular epithelial cells transformed them into invasive colorectal carcinoma, such as chromosomal instabilities (CIN) (19), microsatellite instabilities (MSI) (20), DNA methylation (21), and APC mutation (22). It is estimated that 85–90% of the sporadic CRC progressed from the adenomatous polyp. Another 10–15% from serrated polyp (23). However, hyperplastic polyp, the most prevalent type of serrated polyp, rarely become cancerous (24). Although most of the early stage CRC and CRC precursors can be detected and removed under colonoscopy, nearly half of the patients recurrent within 1 year follow-up (25). So, it is imperative to reduce the incidence and recurrence of CRC precursors to avoid their progression into CRC. Few studies have tried to evaluate the association of vitamin D intake and/or circulating 25(OH)D levels in reducing the risk of CRC precursors. Epidemiologic data suggest that vitamin D intake was associated with decreased risk of colorectal neoplasia. A previous meta-analysis showed that both vitamin D intake and circulating 25(OH)D levels were inversely correlated with colorectal incidence and recurrent adenomas (26). However, recent RCTs have shown that supplementary vitamin D did not significantly reduce the risk of recurrent CRC precursors (27, 28). Some of the present meta-analyses aimed to elucidate the association between vitamin D intake and colorectal adenoma incidence, they did not mention the history of adenoma so they reported the relative risk of incidence and recurrence together (29). In addition, some only assessed the common effects of vitamin D with calcium (25).
To better understand this relationship, we combined all published studies on the association between vitamin D intake/circulating 25(OH)D level and risk of incidence and recurrence of CRC precursors to perform this systematic review and dose-response meta-analysis.
Materials and methods
Design
The protocol of this study was registered in PROSPERO (CRD42021273038). This systematic review and meta-analysis were reported according to PRISMA updated guidelines and PRISMA 2020 27-item checklist (30). We employed the PICO format to answer the research question: “Are vitamin D intake and circulating 25-hydroxyvitamin D level correlated with the risk of the incidence and recurrence of CRC precursors.” Population: Adults with CRA or serrated precursors of CRC (TSA and SSA/P), first-diagnosed or recurrent; adults with only hyperplastic polyps were not included in this study. Intervention: Vitamin D intake (total, dietary and supplementary) or serum/plasma 25(OH)D level. Comparison: Adults without CRA or serrated lesion in the first inspection or follow-up colonoscopy. Outcome: The incidence and recurrence of CRC precursors.
Search strategy
We searched PubMed, Web of Science, Scopus and Embase database in English up to August 2021. The following search terms were used: “vitamin D” or “25(OH)D” or “25-hydroxyvitamin D”; AND: “colorectal adenoma” or “colorectal polyp” or “colorectal lesion” or “colorectal neoplasm” or “colorectal tumor” or “colorectal carcinoma” or “colorectal cancer.” Titles and abstracts were screened independently by two reviewers (Li-liangzi Guo and Si-si Chen) to exclude irrelevant articles. Duplicated articles were excluded and then full texts were retrieved for intensive reading if it met the inclusion criteria, in order to increase the potentially relevant articles.
Study selection
Inclusion criteria: (1) adults >18 years of age; (2) study design of randomized controlled trial (RCT) or observational (cohort studies, case-control studies, or cross-sectional studies) that investigated the association between vitamin D intake, circulating 25(OH)D level and risk of incidence or recurrence of CRC precursors; (3) diagnosis: CRC precursors that were determined by histology (adenomatous polyp, traditional serrated adenoma and sessile serrated adenoma/polyp); (4) studies that reported the risk estimates (RR, OR, or HR) with 95% CIs or calculable original data.
Exclusion criteria: (1) animal and in vitro studies;(2) non-English papers; (3) studies of children, adolescents or pregnant women; (4) including adults with the previous history of adenoma to analyze the incidence of CRC precursors; (5) combined analysis with CRC or hyperplastic polyp; (6) non-original papers (reviews, editorials or commentaries); (7) meta-analysis studies; (8) studies that did not provide enough data on vitamin D intake/circulating 25(OH)D level and risk estimates; (9) duplicate reports and abstracts; (10) studies that investigated the risk, metastasis, survival and prognosis of CRC.
Data extraction
Two investigators (Li-xian Zhong and Kai-yin He) independently extracted the data. Any disagreements between the 2 investigators were resolved through consulting the third investigator (Shao-hui Tang). The following information of included studies was extracted: name of the first author, publication year, country, study design, population characteristics (sample size, age and sex), source of vitamin D intake (total/dietary/supplementary), circulating 25-hydroxyvitamin D level, incidence or recurrence of CRC precursors, type of colorectal lesion (high risk or low risk), the corresponding risk estimates with 95% CIs. High-risk adenomas include large size (≥10 mm), high-grade dysplasia, villous component, or multiple adenomas (≥3). For studies that reported both crude and adjusted estimates, we used the adjusted estimates of the greatest degree for this meta-analysis. For dose-response analysis, the distribution of cases and controls, person-years or non-cases, and risk estimates with 95% CIs for >3 categories. The median level of each category was assigned to the corresponding RR for the study. For open-ended categories, we assumed the length of the interval to be the same as that of the closest interval.
Quality assessment
Two investigators (Yu-ting Li and Wei-wei Chen) independently assessed the study quality. We adopted two different tools to assess the quality of included studies. For observational studies, the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was used and studies were evaluated in seven domains: bias due to confounding, bias in the selection of participants, bias in the measurement of interventions, bias due to departures from intended interventions, bias due to missing data, bias in the measurement of outcomes, bias in the selection of the reported result. Studies with low risk of bias in all seven domains were considered as low risk; studies with low or moderate risk in all domains were considered as moderate risk of bias; studies with serious risk or critical risk in at least one domain were considered as serious risk of bias or critical risk of bias respectively (31).
For RCT studies, the risk of bias was assessed by Cochrane Collaboration’s tool and then exported by Review Manager software (RevMan version 5.3). Studies were judged as high risk, low risk, or unclear risk in the six following domains: sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias (32).
Statistical analysis
The results were expressed in terms of RR and 95% CI for the highest vs. lowest category of vitamin D intake or circulating 25(OH)D level. The natural log-transformed OR/RR/HRs of each study were pooled using the inverse variance method (DerSimonian and Laird) (33) with a random-effects model (34). Studies comparing for the lowest vs. highest category were re-calculated using Jan Hamling et al. methodology (35). To assess the heterogeneity of individual studies, Cochran’s Q-test was used and quantified by I2 statistics. I2 > 50% and P < 0.1 were considered large heterogeneity (36). Sensitivity analysis was conducted to evaluate the stability of the results. Each time one study was omitted to evaluate the risk estimate. Subgroup analyses were further performed according to study design, patient sex, lesion type, and lesion location to explore the potential source of heterogeneity, if data were permitted. Begg’s test, as well as funnel plots, were used to assess publication bias if ≥ 10 studies are available.
If more than 2 observational studies are available, dose-response analysis was conducted to estimate the trend of different categories of vitamin D intake or circulating 25(OH)D level and risk of CRC precursors using generalized least squares (GLST) modeling (37). Both linear and non-linear dose-response analyses were performed. We used restricted cubic spline models with 3 knots at the 10th, 50th, and 90th percentiles of the distribution to construct the non-linear dose-response curve (38).
All statistical analysis was conducted using the STATA software package (version 12.0, Stata Corp., College Station, TX, United States), and the significance difference was defined as P < 0.05 by a two-tailed test.
Results
Search results and study characteristics
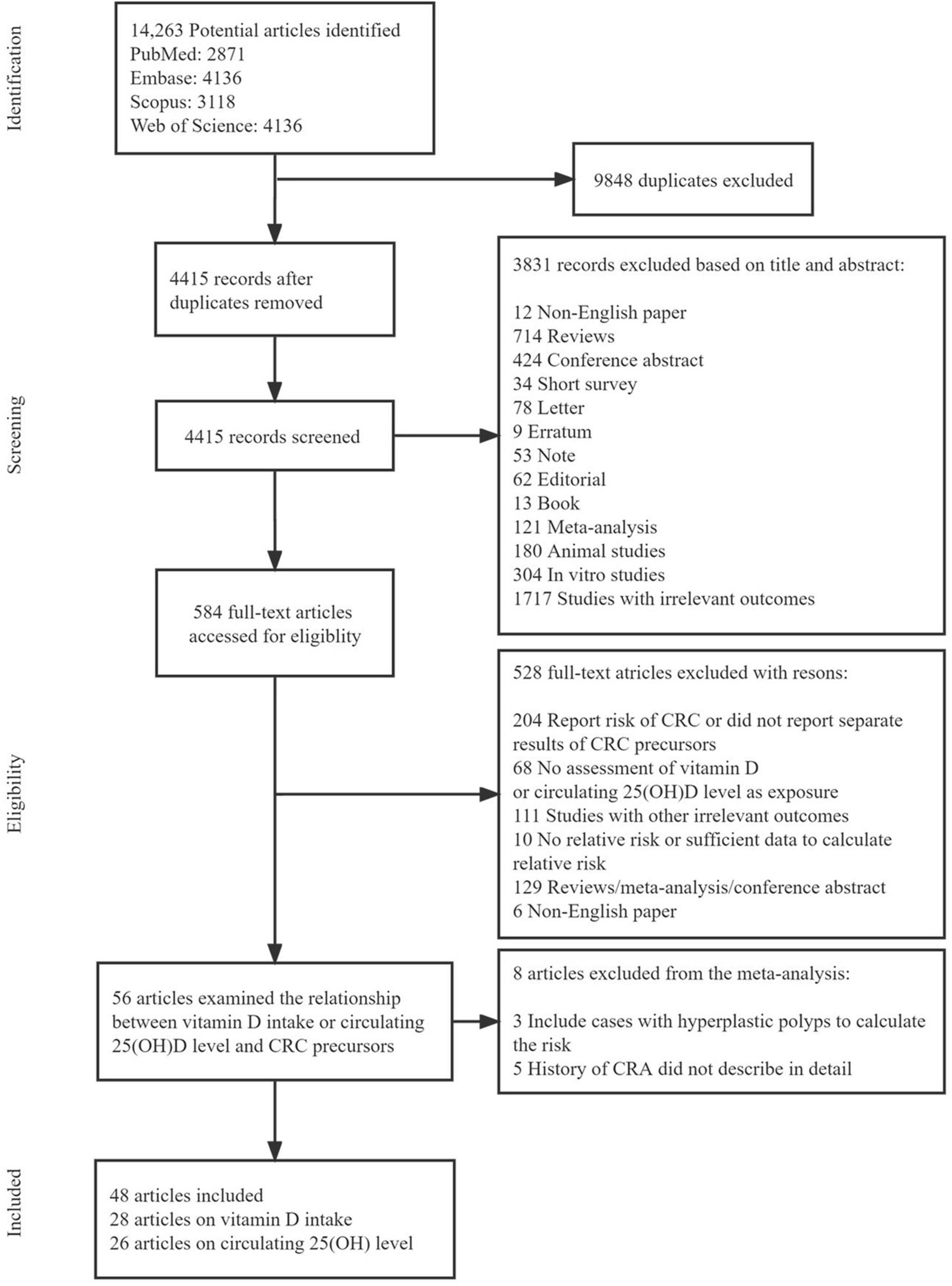
Figure 1 shows the flow diagram of the detailed selection process of the included studies. A total of 14,263 potentially relevant articles were initially retrieved. After excluding 9848 duplicate articles, 4415 articles were screened for title and abstract and then 584 articles remained for full-text review. Among them, 528 articles were excluded (204 only report the risk of CRC or did not report separate results of CRC precursors, 62 had no assessment of vitamin D or circulating 25(OH)D as exposure, 111 studied other irrelevant outcomes, 20 had no relative risk or sufficient data to calculate relative risk, 129 were reviews/meta-analysis/conference abstract, and 6 were non-English paper). Therefore, 56 articles were further assessed for inclusion and 8 articles were excluded (3 included cases with hyperplastic polyps to calculate the risk and 5 did not describe detailed information of CRA history). Finally, 48 eligible articles were included in this systematic review and meta-analysis: 28 articles evaluated the association of vitamin D intake on the risk of CRC precursors occurrence (incidence/recurrence) (27, 28, 39–64), 26 articles evaluated the association between circulating 25(OH)D level and risk of CRC precursors occurrence (incidence/recurrence) (28, 39, 43, 47, 50, 57, 65–84). Supplementary Table 4 summarized the main characteristics of the included studies.
Quality assessment
The quality assessments of the included studies were shown in Supplementary Tables 1, 2. According to the ROBINS-I tool, all 36 observational studies were assessed to have a moderate risk of bias. For 12 RCT studies, all articles were ranked as low risk of bias in randomization, allocation concealment, and selective reporting (Supplementary Figure 1).
Highest vs. lowest category meta-analysis
Vitamin D intake and the risk of colorectal cancer precursors incidence
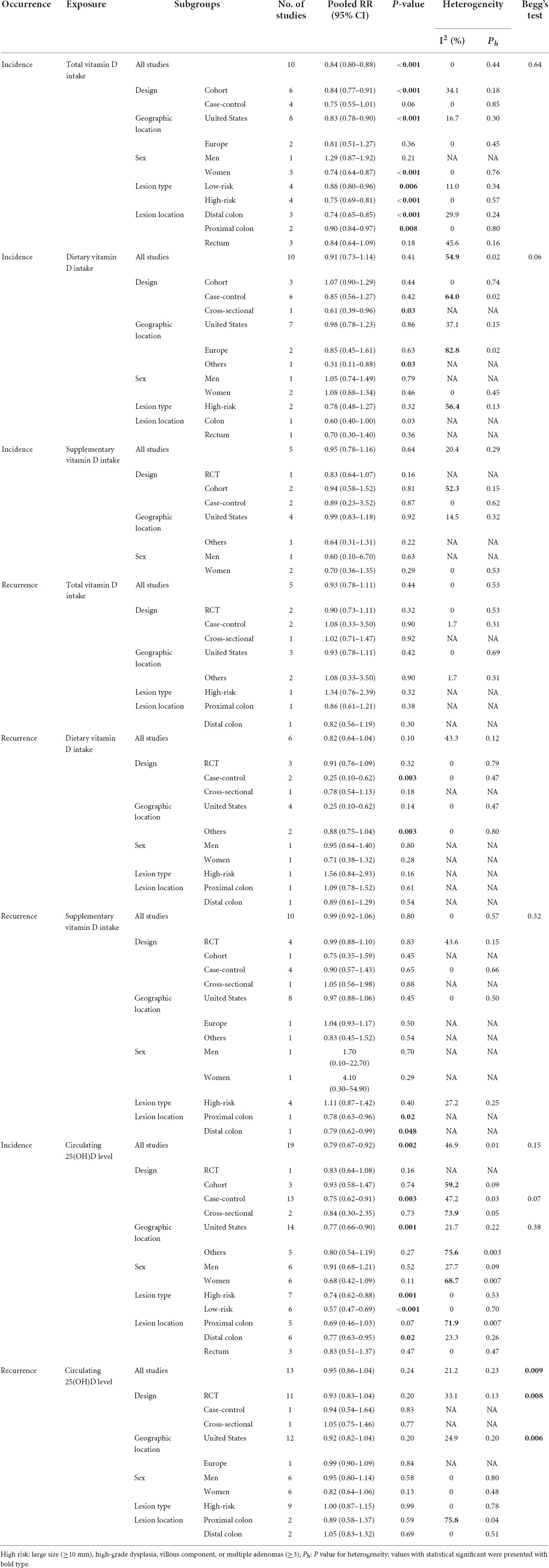
Figures 2–4 showed the risk of CRC precursors incidence and total vitamin D intake (n = 10), dietary vitamin D intake (n = 10), and supplementary vitamin D intake (n = 5), respectively. The pooled summary effect size showed that total vitamin D intake reduced the risk of CRC precursors incidence by 16% (RR = 0.84 95% CI: 0.80–0.88, P < 0.001, I2 = 0%). However, no significant association between dietary vitamin D (RR = 0.91 95% CI: 0.73–1.14, P = 0.41, I2 = 54.9%) and supplementary vitamin D (RR = 0.95 95% CI: 0.78–1.16, P = 0.64, I2 = 20.4%) intake with CRC precursors was observed. The results of subgroup analysis were summarized in Table 1 and shown in Supplementary Figure 2. In the case of incidence of CRC precursors, subgroup analysis showed that total vitamin D intake has similar effects in low-risk (RR = 0.88 95% CI: 0.80–0.96, P = 0.006, I2 = 11%) and high-risk lesion (RR = 0.75 95% CI: 0.69–0.81, P < 0.001, I2 = 0%). When stratified by sex, an inverse association was observed for intake of total vitamin D in women (RR = 0.74 95% CI: 0.64–0.87, P < 0.001, I2 = 0%) but not in men (RR = 1.29 95% CI: 0.87–1.92, P = 0.21). When stratified by lesion location, total vitamin D intake reduced the risk of incidence of CRC precursors in distal (RR = 0.74 95% CI: 0.65–0.85, P < 0.001, I2 = 29.9%) and proximal colon lesion (RR = 0.90 95% CI: 0.84–0.97, P = 0.008, I2 = 0%) more significantly than rectum lesion (RR = 0.84 95% CI: 0.64–1.09, P = 0.18, I2 = 45.6%). When stratified by study design, an inverse association was observed in cohort study (RR = 0.84 95% CI: 0.77–0.91, P < 0.001, I2 = 34.1%) rather than in case-control study (RR = 0.75 95% CI: 0.55–1.01, P = 0.06, I2 = 0%). Furthermore, CRC incidence was inversely associated with total vitamin D intake in American populations (RR = 0.83 95% CI: 0.78–0.90, P < 0.001, I2 = 16.7%) but not in European populations (RR = 0.81 95% CI: 0.51–1.27, P = 0.36, I2 = 0%).
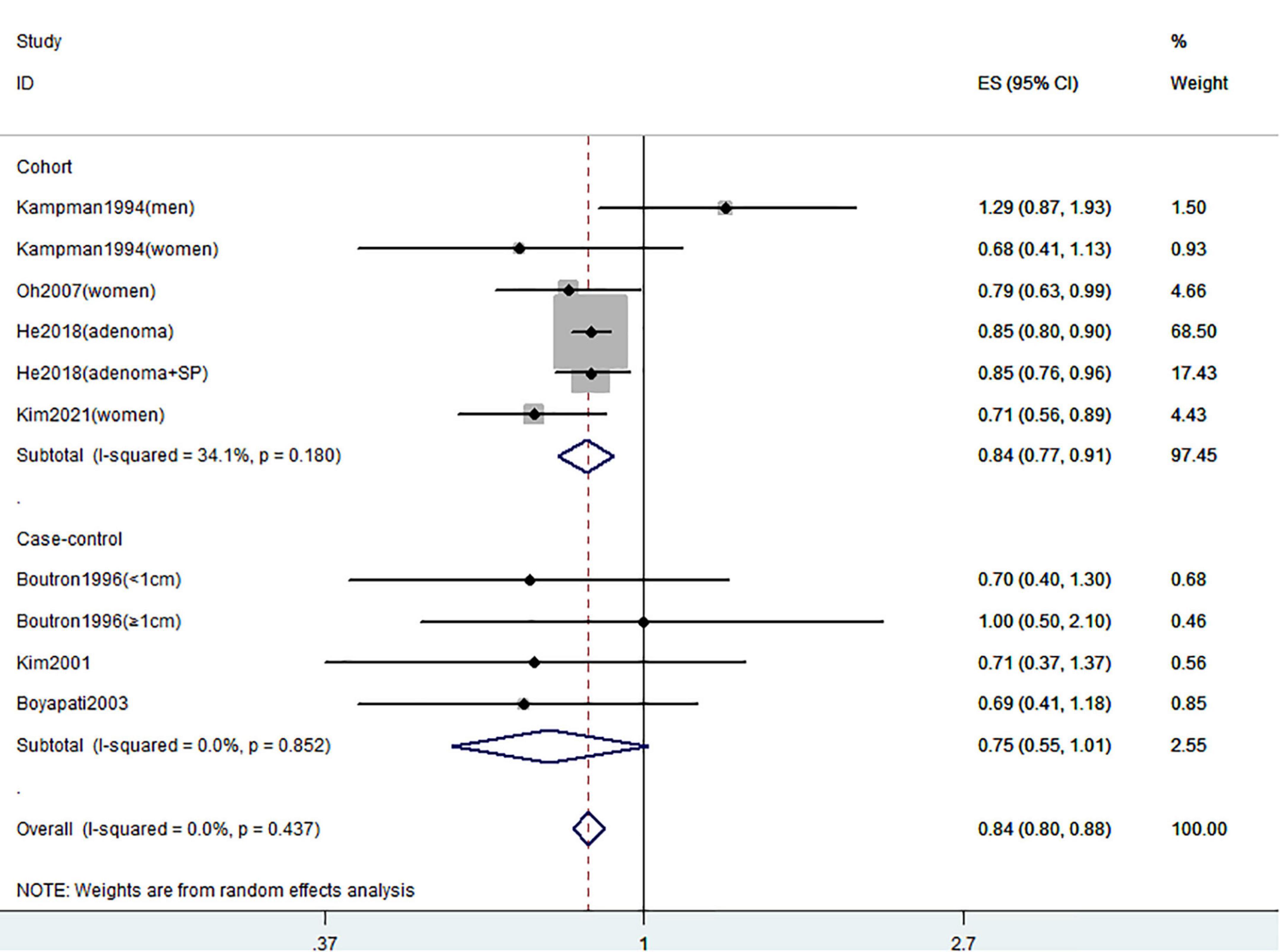
Figure 2. Random-effects meta-analysis of studies that examined total vitamin D intake and risk of CRC precursors incidence. ES, effect size.
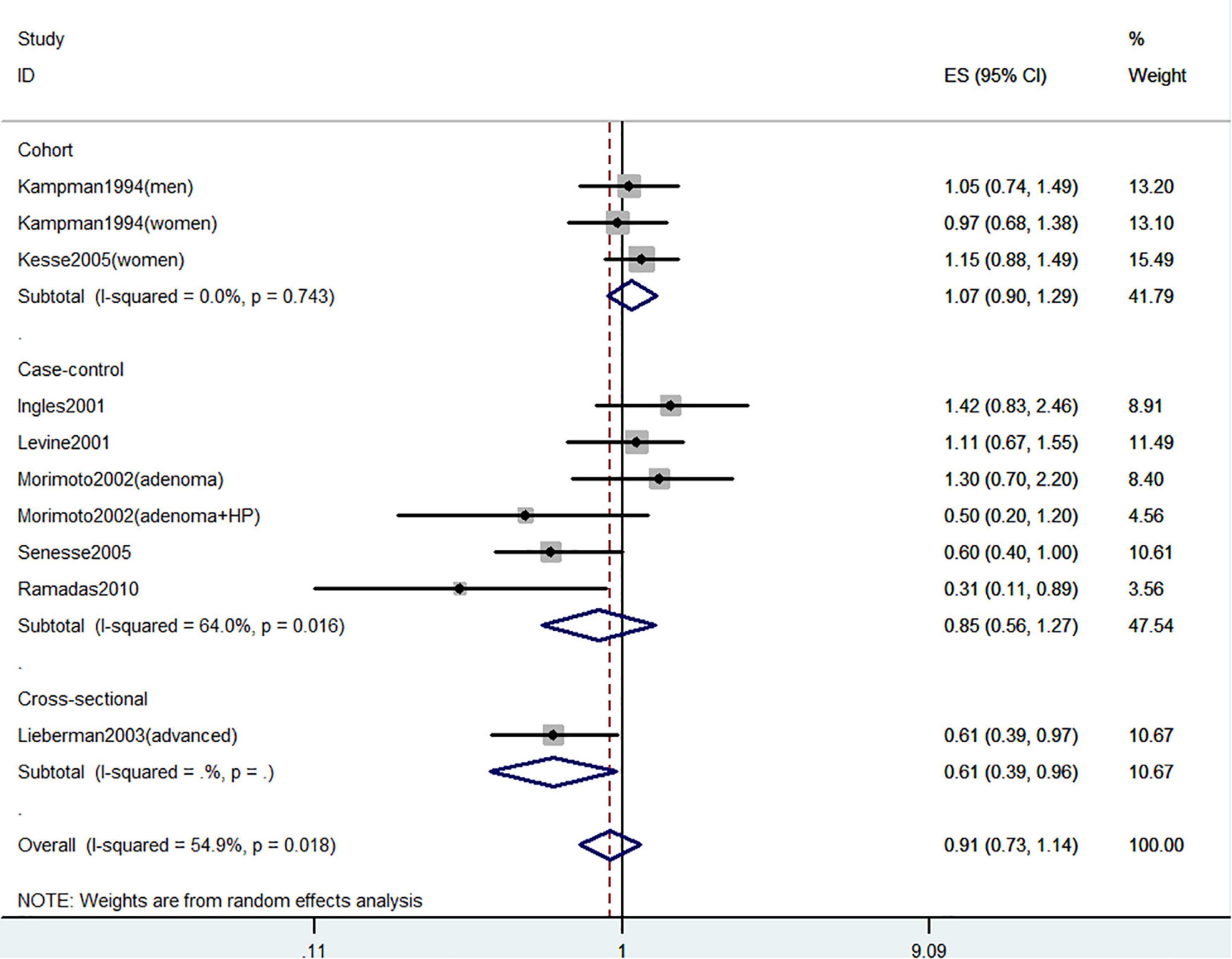
Figure 3. Random-effects meta-analysis of studies that examined dietary vitamin D intake and risk of CRC precursors incidence. ES, effect size.
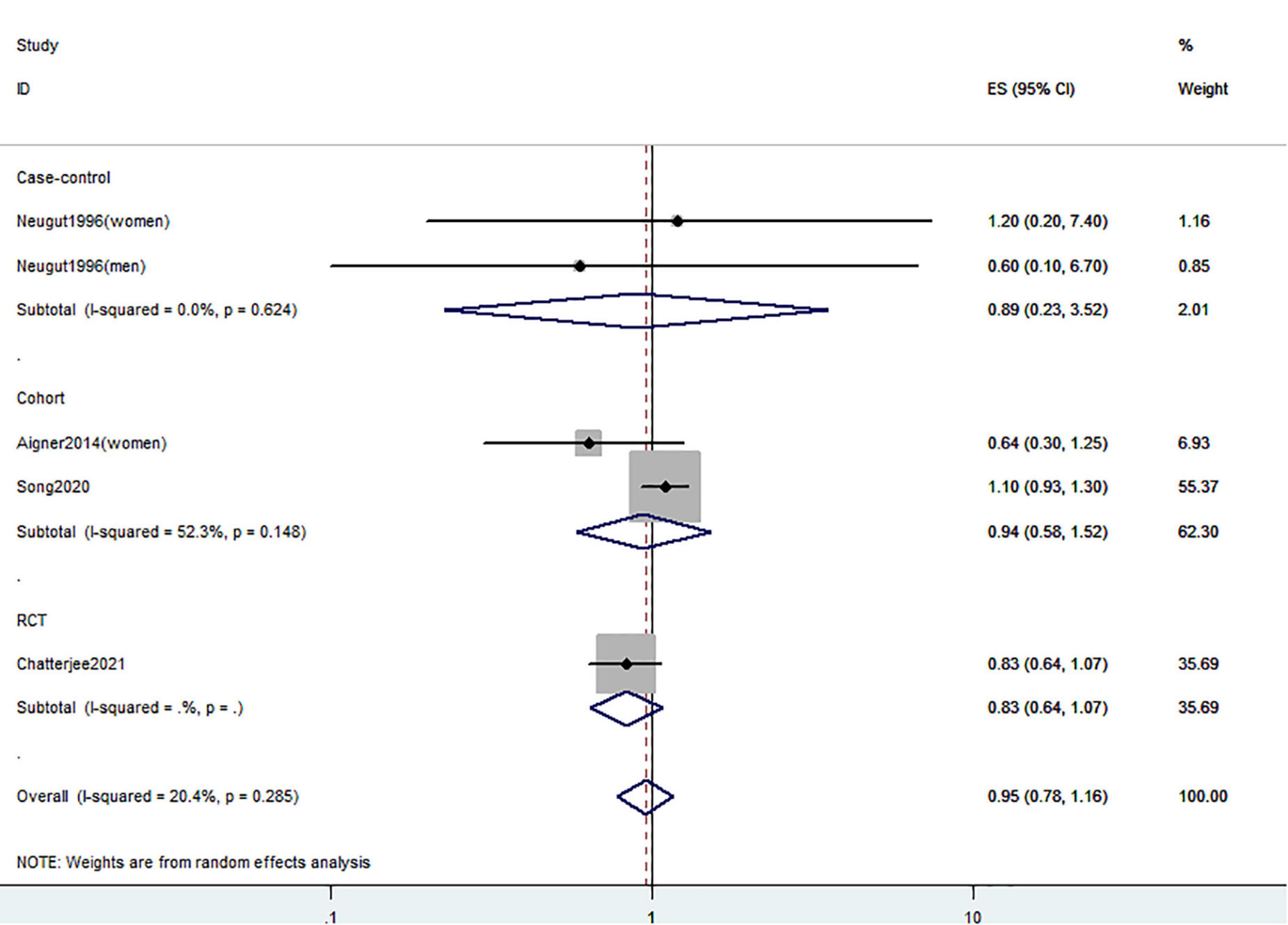
Figure 4. Random-effects meta-analysis of studies that examined supplementary vitamin D intake and risk of CRC precursors incidence. ES, effect size.
Vitamin D intake and the risk of colorectal cancer precursors recurrence
Figures 5–7 showed the risk of CRC precursors recurrence and total vitamin D intake (n = 5), dietary vitamin D intake (n = 6), and supplementary vitamin D intake (n = 9), respectively. The pooled summary effect size showed no significant association between total vitamin D (RR = 0.93 95% CI: 0.78–1.11, P = 0.44, I2 = 0%), dietary vitamin D (RR = 0.82 95% CI: 0.64–1.04, P = 0.10, I2 = 43.3%), and supplementary vitamin D (RR = 0.99 95% CI: 0.92–1.06, P = 0.80, I2 = 0%) intake with the risk of CRC precursors.
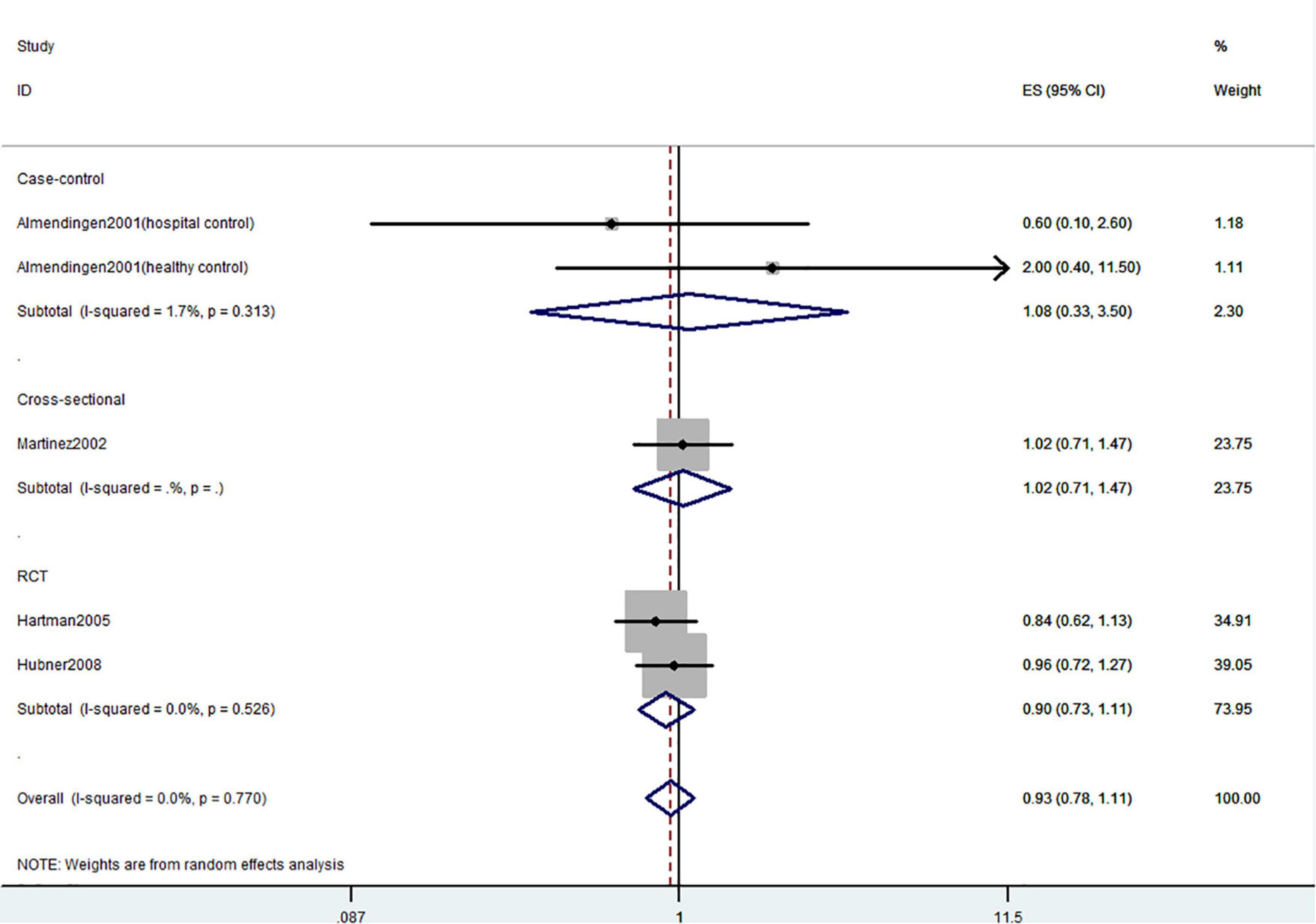
Figure 5. Random-effects meta-analysis of studies that examined total vitamin D intake and risk of CRC precursors recurrence. ES, effect size.
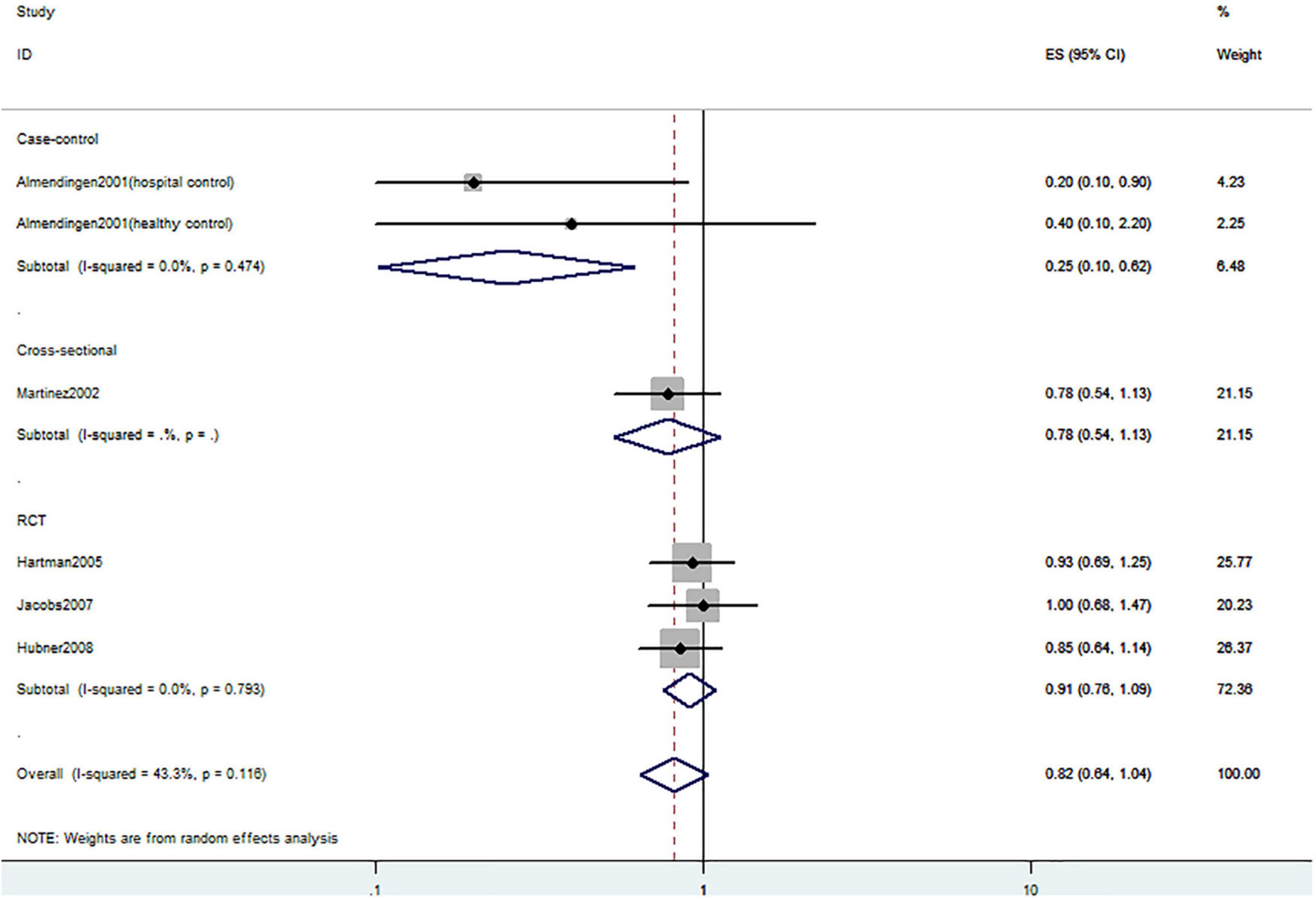
Figure 6. Random-effects meta-analysis of studies that examined dietary vitamin D intake and risk of CRC precursors recurrence. ES, effect size.
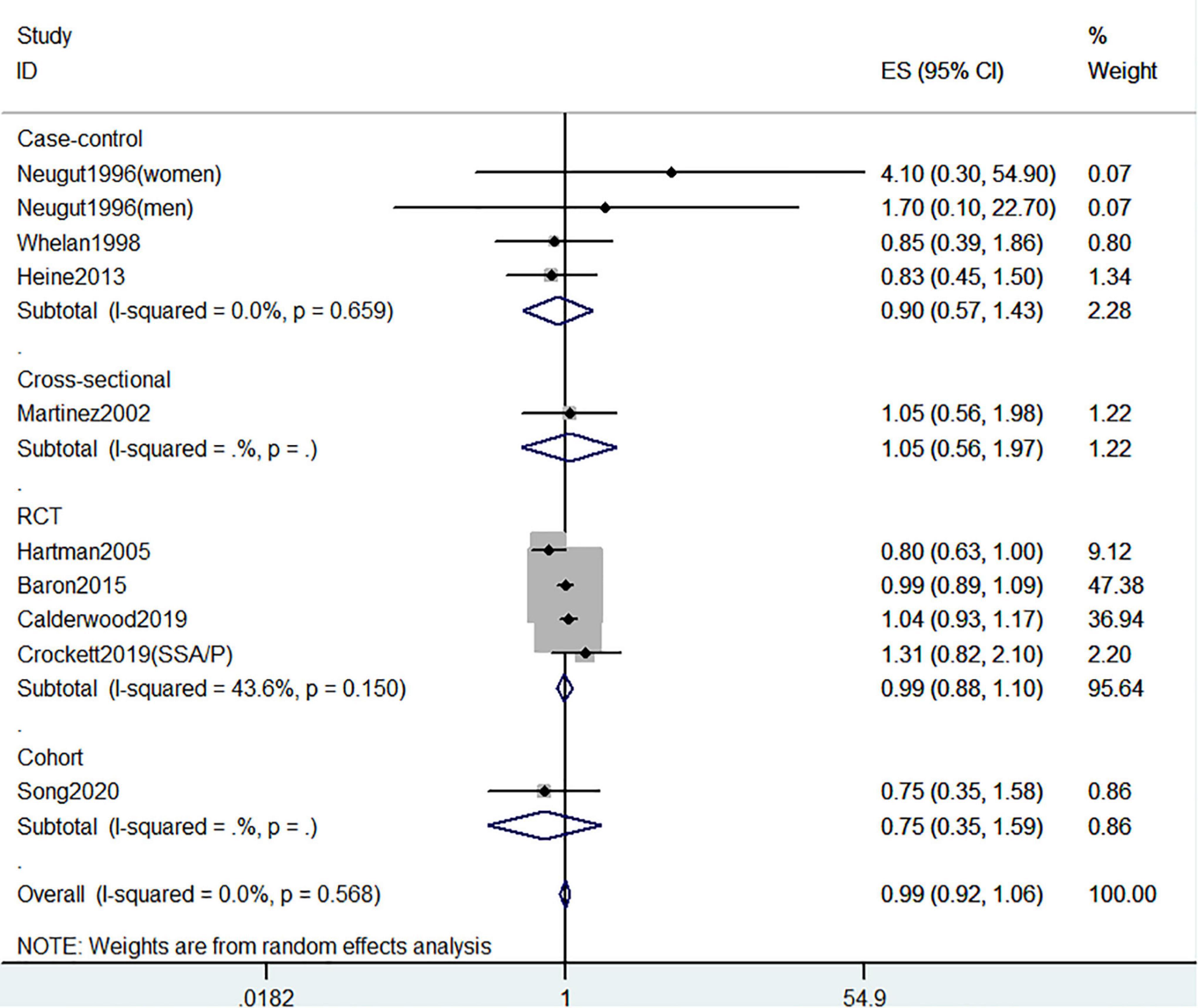
Figure 7. Random-effects meta-analysis of studies that examined supplementary vitamin D intake and risk of CRC precursors recurrence. ES, effect size.
Circulating 25(OH)D level and the risk of colorectal cancer precursors incidence
Figure 8 showed the risk of CRC precursors incidence and circulating 25(OH)D level (n = 19). The results showed that a high level of circulating 25(OH)D decreased 21% risk of CRC precursors (RR = 0.79 95% CI: 0.67–0.92, P = 0.002, I2 = 46.9%). Subgroup analysis showed that circulating 25(OH)D level has similar effects in low-risk (RR = 0.57 95% CI: 0.47–0.69, P < 0.001, I2 = 0%) and high-risk lesion (RR = 0.74 95% CI: 0.62–0.88, P = 0.001, I2 = 0%). When stratified by sex, no significant association was observed in both men (RR = 0.91 95% CI: 0.68–1.21, P = 0.52, I2 = 27.7%) and women (RR = 0.68 95% CI: 0.42–1.09, P = 0.11, I2 = 68.7%). When stratified by lesion location, an inverse association was observed for circulating 25(OH)D level in distal colon (RR = 0.77 95% CI: 0.63–0.95, P = 0.02, I2 = 23.3%) but not in proximal colon (RR = 0.69 95% CI: 0.46–1.03, P = 0.07) and rectum (RR = 0.83 95% CI: 0.51–1.37, P = 0.47). In addition, the protective effect of high circulating 25(OH)D level for CRC precursors risk was found in case-control study (RR = 0.75 95% CI: 0.62–0.91, P = 0.003, I2 = 47.2%) and American populations (RR = 0.77 95% CI: 0.66–0.90, P = 0.001, I2 = 21.7%).
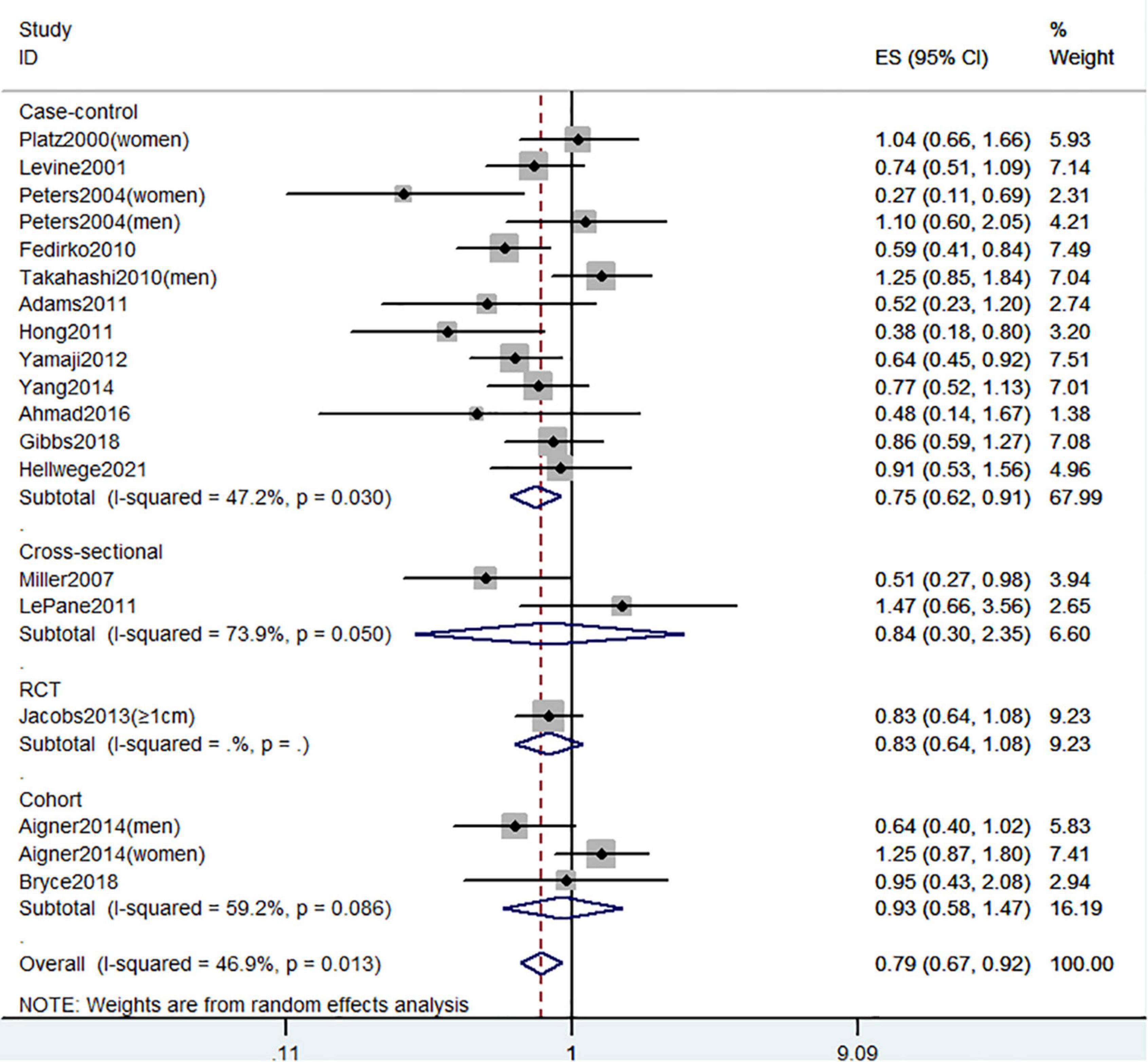
Figure 8. Random-effects meta-analysis of studies that examined circulating 25(OH)D level and risk of CRC precursors incidence. ES, effect size.
Circulating 25(OH)D level and the risk of colorectal cancer precursors recurrence
Figure 9 showed the risk of CRC precursors recurrence and circulating 25(OH)D level (n = 13). However, there was no significant association between circulating 25(OH)D level and risk of CRC precursors (RR = 0.95 95% CI: 0.86–1.04, P = 0.24, I2 = 21.2%).
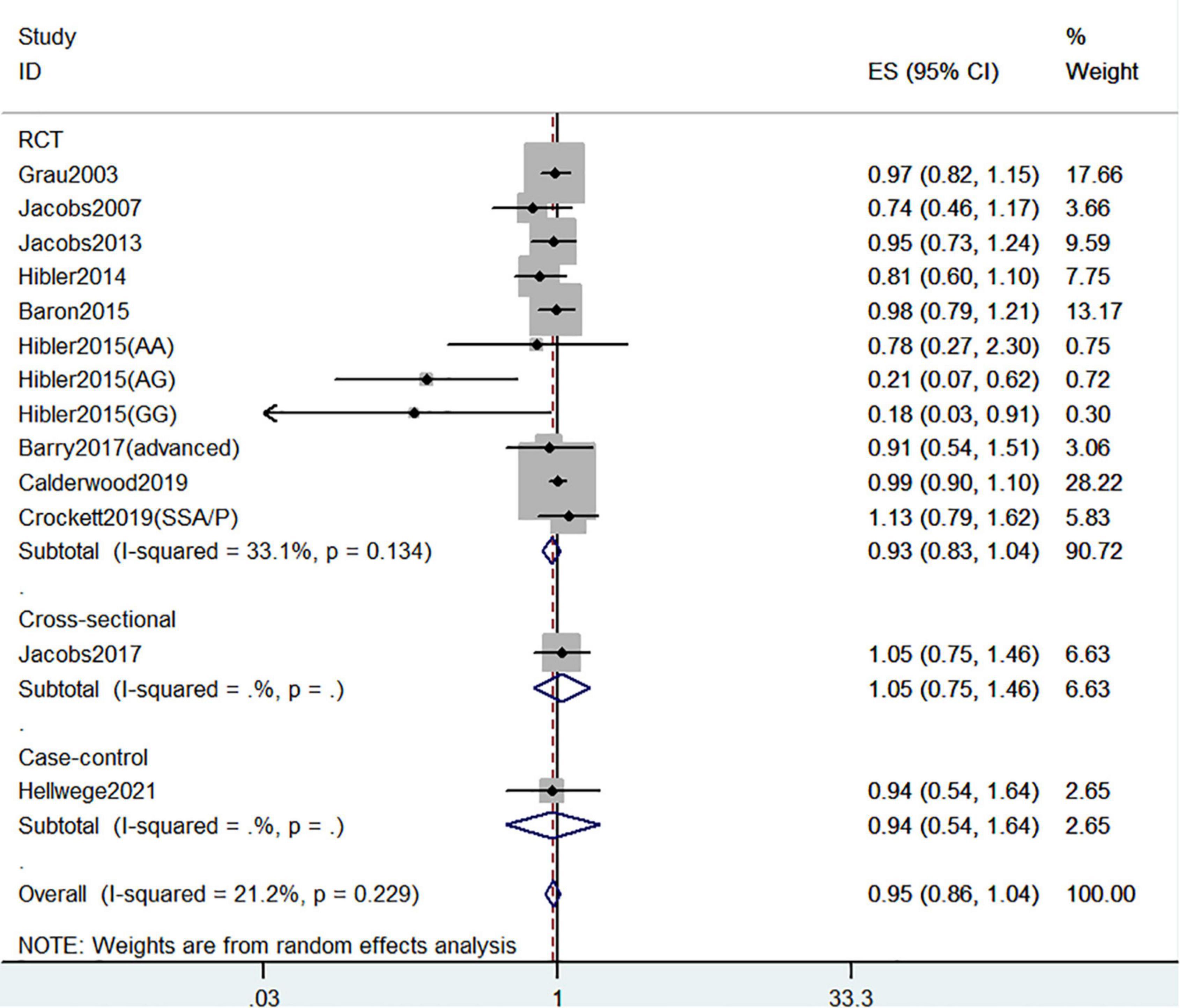
Figure 9. Random-effects meta-analysis of studies that examined circulating 25(OH)D level and risk of CRC precursors recurrence. ES, effect size.
Dose-response meta-analysis
Seven studies were available for a dose-response analysis of the association between circulating 25(OH)D level and risk of CRC precursors incidence. Both linear and non-linear dose-response analyses were performed. Trend meta-analysis showed a significant negative dose-response relationship in circulating 25(OH)D (P–non–linearity = 0.39) level from linearity. As shown in Figure 10, we found that per 10ng/ml increment of circulating 25(OH)D level could decrease the risk of colorectal cancer precursors by 14% using the fixed-effect model with no heterogeneity (RR = 0.86 95% CI: 0.75–0.99, P = 0.04; Ph = 0.96).
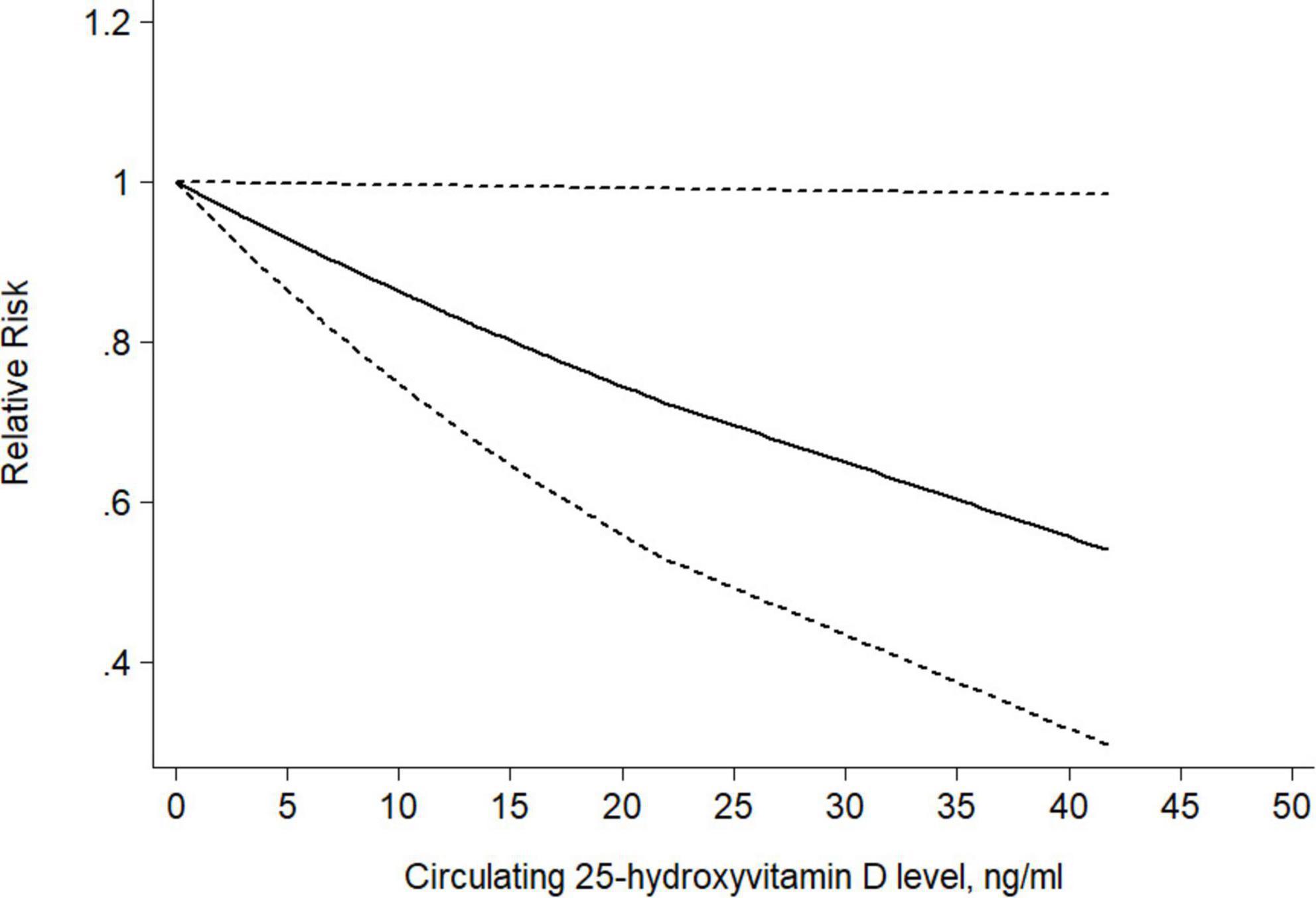
Figure 10. Dose-response relationship between circulating 25(OH)D level and risk of CRC precursors incidence. Weights are from the fixed-effects analysis. Solid line represents the linear trend and short dashes line represent the 95% CI.
Publication bias and sensitivity analysis
The results of Begg’s test showed no publication bias for most of the outcomes but a publication bias for circulating 25(OH)D level and CRC precursors recurrence (Begg’s P = 0.009). The funnel plots were presented in Supplementary Figure 3. We conducted a sensitivity analysis to evaluate the influence of a single study on the overall risk by omitting one study in each turn (Supplementary Table 3). The effect estimated from the sensitivity analyses showed little change which means that the results were stable.
Discussion
The study is the latest and most comprehensive study focused on the association between vitamin D and circulating 25(OH)D level with the incidence and recurrence of CRC precursors. Decreased risk of CRC precursors incidence was observed for total vitamin D intake and 25(OH)D level rather than dietary vitamin D and supplementary vitamin D intake. These discrepant findings may be attributed to different doses, metabolism, bioavailability of vitamin D and other unadjusted potential confounding factors. However, neither vitamin D nor 25(OH)D level play an effective role in preventing the recurrence of CRC precursors.
Our systematic review and meta-analysis updated and expanded upon the eight previous meta-analysis. The first meta-analysis conducted by Wei et al. (26) showed that both circulating 25(OH)D and vitamin D intake was inversely associated with colorectal adenoma incidence and recurrence. The inconsistent result of recurrent risk may be attributed to the small number of included studies. They involved only 4 epidemiologic studies to assess the recurrent risk with vitamin D intake whereas we involved 12 studies, including 6 RCTs and 6 observational studies. Yin et al. (85) and Fedirko et al. (69) reached a similar conclusion as our study, but they did not assess the effect of vitamin D intake. The two most recent meta-analyses of Choi et al. (86) and Huang et al. (29) both included hyperplastic polyps to assess the association and neither of them separated patients with a history of adenoma from those newly diagnosed. Gandini et al. (87) and Zhang et al. (88) included too few studies to reach a reliable result.
Vitamin D belongs to a group of steroids known as secosteroids. Vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) are the most common forms of vitamin D in human body. They can be obtained from the diet and synthesized through ultraviolet B radiation (89). Vitamin D is activated to calcitriol (1,25-dihydroxyvitamin D3) by two cytochrome P450-mediated (CYP450) hydroxylation steps. First, vitamin D was catalyzed by CYP2R1 in the liver to yield circulating 25(OH)D (90). Then, 25(OH)D is metabolized by CYP27B1 in the kidney to yield calcitriol. Calcitriol then performs its biological functions by binding and activating the nuclear vitamin D receptor (VDR), down-regulating the gene transcription of CYP27B1 and parathyroid hormone (PTH), and inducing the expression of CYP24A1 (10, 91–93).
Vitamin D may decrease the risk of CRC precursors incidence through several mechanisms. VDR, present in the majority cells of the human body and is expressed abundantly in intestinal epithelial cells. Previous studies have shown that VDR is a biomarker for the anti-proliferative effect of vitamin D on CRC (94). In vitro studies have shown that calcitriol may paly pro-differentiation effects through increasing the activity of alkaline phosphatase in colorectal cancer cells and colorectal adenoma cell lines (95). Further, it can induce apoptosis in colorectal adenoma and colorectal cancer by up-regulating the expression of pro-apoptotic proteins (96). Moreover, the gut microbiome is believed to be directly involved in colon carcinogenesis (97). Vitamin D has also been reported to regulate the gut microbiome in animal studies (98).
Our systematic review and meta-analysis had several strengths. First, the heterogeneity between studies was low in most of the analyses. Second, we include both RCT and observational studies through a systematic search. Third, an inverse dose-response relationship was observed between circulating 25(OH)D level and CRC precursors incidence, which may strengthen the reliability of the results. Sensitivity analysis was also carried out to investigate the stability of the results. Last, most of the present meta-analyses did not mention the history of adenoma so they report the relative risk of incidence and recurrence together. We conduct this study to analyze the incidence and recurrence of CRC precursors separately. Besides, some meta-analyses assessed the common effects of vitamin D with calcium, but we only assessed the exact effect of vitamin D.
However, there were some limitations to this study. First of all, due to the small number of included studies, further subgroup analyses of lesion type and dose-response analyses of vitamin D intake were not able to be performed. Thus, it is hard to deduce the optimal dosage of vitamin D intake. Second, unpublished researches were not included which may cause potential publication bias. Articles published in non-English language were not included in this study either. Third, although we have included RCT studies that may avoid recall and selection bias, the rest of the case-control studies may not have avoided it. In addition, not all potential confounders were adjusted for in every study, such as sample size, follow-up duration, and study design etc. Fourth, most RCTs were not well-designed according to the guidelines, which vitamin D dose should base on serum 25(OH)D level and should be large enough to increase 25(OH)D concentration (99, 100). Finally, regarding dietary vitamin D intake, because many food frequency tables do not include data on meat, so most of the studies ignored meat as a source of vitamin (101, 102). Furthermore, a recent RCT study suggested that the benefit of supplementary vitamin D intake in preventing advanced colorectal adenomas may vary according to different VDR (65). However, there is little study aimed at this association that data of genetic variants in VDR or vitamin D binding protein (VDBP) were not considered in this study.
Conclusion
In conclusion, our study clarified a potentially effective role of total vitamin D intake and circulating 25(OH)D levels in reducing the risk of CRC precursors incidence. Although vitamin D intake and circulating 25(OH)D levels had no positive effect in reducing the recurrence risk of CRC precursors, its function in reducing the incidence of CRC precursors is also important in CRC prevention strategies. More large and precise prospective studies are needed to further verify the association and identify the underlying mechanism.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
L-LG, S-SC, and L-XZ contributed equally to this work. L-LG and S-SC contributed to the conception and design of the study. L-XZ and K-YH extracted the data. Y-TL and W-WC assessed the methodological quality of included studies. L-XZ, S-SC, and Q-TZ contributed to statistical analysis. L-LG drafted the manuscript. S-HT reviewed and revised the manuscript. All authors have read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies, commercial or not-for-profit sectors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.877275/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Wang JL, Chang CH, Lin JW, Wu LC, Chuang LM, Lai MS. Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case-control study in patients with type 2 diabetes mellitus. Int J Cancer. (2014) 135:956–67. doi: 10.1002/ijc.28738
3. Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. (2019) 11:164. doi: 10.3390/nu11010164
4. Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. (2009) 169:551–61. doi: 10.1001/archinternmed.2008.600
5. Holick MF. McCollum Award Lecture, 1994: vitamin D–new horizons for the 21st century. The Am J Clin Nutr. (1994) 60:619–30. doi: 10.1093/ajcn/60.4.619
6. Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. (2007) 370:657–66. doi: 10.1016/S0140-6736(07)61342-7
7. Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. (2014) 14:342–57. doi: 10.1038/nrc3691
8. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. (2014) 348:g2035. doi: 10.1136/bmj.g2035
9. Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. (1980) 9:227–31. doi: 10.1093/ije/9.3.227
10. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. (2007) 7:684–700. doi: 10.1038/nrc2196
11. Wesselink E, Kok DE, Bours MJL, de Wilt JHW, van Baar H, van Zutphen M, et al. Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am J Clin Nutr. (2020) 111:1007–17. doi: 10.1093/ajcn/nqaa049
12. La Vecchia C, Braga C, Negri E, Franceschi S, Russo A, Conti E, et al. Intake of selected micronutrients and risk of colorectal cancer. Int J Cancer. (1997) 73:525–30. doi: 10.1002/(SICI)1097-0215(19971114)73:4<525::AID-IJC12>3.0.CO;2-8
13. Vaughan-Shaw PG, Buijs LF, Blackmur JP, Theodoratou E, Zgaga L, Din FVN, et al. The effect of vitamin D supplementation on survival in patients with colorectal cancer: systematic review and meta-analysis of randomised controlled trials. Br J Cancer. (2020) 123:1705–12. doi: 10.1038/s41416-020-01060-8
14. Boughanem H, Canudas S, Hernandez-Alonso P, Becerra-Tomas N, Babio N, Salas-Salvado J, et al. Vitamin D intake and the risk of colorectal cancer: an updated meta-analysis and systematic review of case-control and prospective cohort studies. Cancers (Basel). (2021) 13:2814. doi: 10.3390/cancers13112814
15. Garland CF, Gorham ED. Dose-response of serum 25-hydroxyvitamin D in association with risk of colorectal cancer: a meta-analysis. J Steroid Biochem Mol Biol. (2017) 168:1–8. doi: 10.1016/j.jsbmb.2016.12.003
16. McCullough ML, Zoltick ES, Weinstein SJ, Fedirko V, Wang M, Cook NR, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst. (2019) 111:158–69.
17. Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. (2011) 29:3775–82. doi: 10.1200/JCO.2011.35.7566
18. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
19. Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. (2010) 138:2059–72. doi: 10.1053/j.gastro.2009.12.065
20. Pritchard CC, Grady WM. Colorectal cancer molecular biology moves into clinical practice. Gut. (2011) 60:116–29. doi: 10.1136/gut.2009.206250
21. Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. (2006) 38:787–93. doi: 10.1038/ng1834
22. Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, et al. APC mutations occur early during colorectal tumorigenesis. Nature. (1992) 359:235–7. doi: 10.1038/359235a0
23. Conteduca V, Sansonno D, Russi S, Dammacco F. Precancerous colorectal lesions (Review). Int J Oncol. (2013) 43:973–84. doi: 10.3892/ijo.2013.2041
25. Emami MH, Salehi M, Hassanzadeh Keshteli A, Mansourian M, Mohammadzadeh S, Maghool F. Calcium and dairy products in the chemoprevention of colorectal adenomas: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2021):1–25. doi: 10.1080/10408398.2021.1911927
26. Wei MY, Garland CF, Gorham ED, Mohr SB, Giovannucci E. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiol Biomarkers Prev. (2008) 17:2958–69. doi: 10.1158/1055-9965.EPI-08-0402
27. Song M, Lee IM, Manson JE, Buring JE, Dushkes R, Gordon D, et al. No association between vitamin D supplementation and risk of colorectal adenomas or serrated polyps in a randomized trial. Clin Gastroenterol Hepatol. (2021) 19:128–135.e6. doi: 10.1016/j.cgh.2020.02.013
28. Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med. (2015) 373:1519–30. doi: 10.1056/NEJMoa1500409
29. Huang D, Lei S, Wu Y, Weng M, Zhou Y, Xu J, et al. Additively protective effects of vitamin D and calcium against colorectal adenoma incidence, malignant transformation and progression: a systematic review and meta-analysis. Clin Nutr. (2020) 39:2525–38. doi: 10.1016/j.clnu.2019.11.012
30. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
31. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
32. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
33. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
34. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
35. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. (2008) 27:954–70. doi: 10.1002/sim.3013
36. Chen B, Benedetti A. Quantifying heterogeneity in individual participant data meta-analysis with binary outcomes. Syst Rev. (2017) 6:243. doi: 10.1186/s13643-017-0630-4
37. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. (2012) 175:66–73. doi: 10.1093/aje/kwr265
38. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. (1989) 8:551–61. doi: 10.1002/sim.4780080504
39. Aigner E, Stadlmayr A, Huber-Schönauer U, Zwerina J, Husar-Memmer E, Niederseer D, et al. Gender- and site-specific differences of colorectal neoplasia relate to vitamin D. Aliment Pharmacol Ther. (2014) 40:1341–8. doi: 10.1111/apt.12981
40. Almendingen K, Hofstad B, Trygg K, Hoff G, Hussain A, Vatn M. Current diet and colorectal adenomas: a case-control study including different sets of traditionally chosen control groups. Eur J Cancer Prev. (2001) 10:395–406. doi: 10.1097/00008469-200110000-00003
41. Boutron MC, Faivre J, Marteau P, Couillault C, Senesse P, Quipourt V. Calcium, phosphorus, vitamin D, dairy products and colorectal carcinogenesis: a French case-control study. Br J Cancer. (1996) 74:145–51. doi: 10.1038/bjc.1996.330
42. Boyapati SM, Bostick RM, McGlynn KA, Fina MF, Roufail WM, Geisinger KR, et al. Calcium, vitamin D, and risk for colorectal adenoma: dependency on vitamin D receptor BSMI polymorphism and nonsteroidal anti-inflammatory drug use? Cancer Epidemiol Biomarkers Prev. (2003) 12:631–7.
43. Calderwood AH, Baron JA, Mott LA, Ahnen DJ, Bostick RM, Figueiredo JC, et al. No evidence for posttreatment effects of vitamin D and calcium supplementation on risk of colorectal adenomas in a randomized trial. Cancer Prev Res. (2019) 12:295–304. doi: 10.1158/1940-6207.CAPR-19-0023
44. Hartman TJ, Albert PS, Snyder K, Slattery ML, Caan B, Paskett E, et al. The association of calcium and vitamin D with risk of colorectal adenomas. J Nutr. (2005) 135:252–9. doi: 10.1093/jn/135.2.252
45. He X, Wu K, Ogino S, Giovannucci EL, Chan AT, Song M. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology. (2018) 155: 355–73.e18. doi: 10.1053/j.gastro.2018.04.019
46. Hubner RA, Muir KR, Liu J-F, Logan RFA, Grainge MJ, Hulston RS, et al. Dairy products, polymorphisms in the vitamin D receptor gene and colorectal adenoma recurrence. Int J Cancer. (2008) 123:586–93. doi: 10.1002/ijc.23536
47. Jacobs ET, Alberts DS, Benuzillo J, Hollis BW, Thompson PA, Martínez ME. Serum 25(OH)D levels, dietary intake of vitamin D, and colorectal adenoma recurrence. J Steroid Biochem Mol Biol. (2007) 103:752–6. doi: 10.1016/j.jsbmb.2006.12.039
48. Kampman E, Giovannucci E, van ’t Veer P, Rimm E, Stampfer MJ, Colditz GA, et al. Calcium, vitamin D, dairy foods, and the occurrence of colorectal adenomas among men and women in two prospective studies. Am J Epidemiol. (1994) 139:16–29. doi: 10.1093/oxfordjournals.aje.a116931
49. Kesse E, Boutron-Ruault MC, Norat T, Riboli E, Clavel-Chapelon F. Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int J Cancer. (2005) 117:137–44. doi: 10.1002/ijc.21148
50. Levine AJ, Harper JM, Ervin CM, Chen YH, Harmon E, Xue S, et al. Serum 25-hydroxyvitamin D, dietary calcium intake, and distal colorectal adenoma risk. Nutr Cancer. (2001) 39:35–41. doi: 10.1207/S15327914nc391_5
51. Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. J Am Med Assoc. (2003) 290:2959–67. doi: 10.1001/jama.290.22.2959
52. Martínez ME, Marshall JR, Sampliner R, Wilkinson J, Alberts DS. Calcium, vitamin D, and risk of adenoma recurrence (United States). Cancer Causes Control. (2002) 13:213–20. doi: 10.1023/A:1015032215779
53. Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. (2002) 11:1012–8.
54. Oh K, Willett WC, Wu K, Fuchs CS, Giovannucci EL. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol. (2007) 165:1178–86. doi: 10.1093/aje/kwm026
55. Whelan RL, Horvath KD, Gleason NR, Forde KA, Treat MD, Teitelbaum SL, et al. Vitamin and calcium supplement use is associated with decreased adenoma recurrence in patients with a previous history of neoplasia. Dis Colon Rectum. (1999) 42:212–7. doi: 10.1007/BF02237131
56. Chatterjee R, Fuss P, Vickery EM, LeBlanc ES, Sheehan PR, Lewis MR, et al. Vitamin D supplementation for prevention of cancer: the D2d cancer outcomes (D2dCA) ancillary study. J Clin Endocrinol Metab. (2021) 106:2767–78. doi: 10.1093/cdn/nzaa067_012
57. Crockett SD, Barry EL, Mott LA, Ahnen DJ, Robertson DJ, Anderson JC, et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: results from a randomised clinical trial. Gut. (2019) 68:475–86. doi: 10.1136/gutjnl-2017-315242
58. Heine-Broring RC, Winkels RM, Botma A, Wahab PJ, Tan ACITL, Nagengast FM, et al. Dietary supplement use is not associated with recurrence of colorectal adenomas: a prospective cohort study. Int J Cancer. (2013) 132:666–75. doi: 10.1002/ijc.27647
59. Ingles SA, Wang J, Coetzee GA, Lee ER, Frankl HD, Haile RW. Vitamin D receptor polymorphisms and risk of colorectal adenomas (United States). Cancer Causes Control. (2001) 12:607–14. doi: 10.1023/A:1011292002475
60. Kim H, Lipsyc-Sharf M, Zong X, Wang X, Hur J, Song M, et al. Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology. (2021) 161:1208.e–17.e. doi: 10.1053/j.gastro.2021.07.002
61. Kim HS, Newcomb PA, Ulrich CM, Keener CL, Bigler J, Farin FM, et al. Vitamin D receptor polymorphism and the risk of colorectal adenomas: evidence of interaction with dietary vitamin D and calcium. Cancer Epidemiol Biomarkers Prev. (2001) 10:869–74.
62. Neugut AI, Horvath K, Whelan RL, Terry MB, Garbowski GC, Bertram A, et al. The effect of calcium and vitamin supplements on the incidence and recurrence of colorectal adenomatous polyps. Cancer. (1996) 78:723–8. doi: 10.1002/(SICI)1097-0142(19960815)78:4<723::AID-CNCR5>3.0.CO;2-F
63. Ramadas A, Kandiah M. Nutritional status and the risk for colorectal adenomas: a case-control study in Hospital Kuala Lumpur, Malaysia. Pak J Nutr. (2010) 9:269–78. doi: 10.3923/pjn.2010.269.278
64. Senesse P, Touvier M, Kesse E, Faivre J, Boutron-Ruault MC. Tobacco use and associations of β-carotene and vitamin intakes with colorectal adenoma risk. J Nutr. (2005) 135:2468–72. doi: 10.1093/jn/135.10.2468
65. Barry EL, Peacock JL, Rees JR, Bostick RM, Robertson DJ, Bresalier RS, et al. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: a randomized clinical trial. JAMA Oncol. (2017) 3:628–35. doi: 10.1001/jamaoncol.2016.5917
66. Adams SV, Newcomb PA, Burnett-Hartman AN, White E, Mandelson MT, Potter JD. Circulating 25-hydroxyvitamin-D and risk of colorectal adenomas and hyperplastic polyps. Nutr Cancer. (2011) 63:319–26. doi: 10.1080/01635581.2011.535960
67. Ahmad II, Trikudanathan G, Feinn R, Anderson JC, Nicholson M, Lowe S, et al. Low serum vitamin D: a surrogate marker for advanced colon adenoma? J Clin Gastroenterol. (2016) 50:644–8. doi: 10.1097/MCG.0000000000000497
68. Bryce C. Association of 25-OH vitamin D status with findings on screening colonoscopy. Mil Med. (2018) 183:547–51. doi: 10.1093/milmed/usx152
69. Fedirko V, Bostick RM, Goodman M, Flanders WD, Gross MD. Blood 25-hydroxyvitamin D3 concentrations and incident sporadic colorectal adenoma risk: a pooled case-control study. Am J Epidemiol. (2010) 172:489–500. doi: 10.1093/aje/kwq157
70. Gibbs DC, Fedirko V, Um C, Gross MD, Thyagarajan B, Bostick RM. Associations of circulating 25-Hydroxy vitamin D3 concentrations with incident, sporadic colorectal adenoma risk according to common vitamin D-binding protein isoforms. Am J Epidemiol. (2018) 187:1923–30. doi: 10.1093/aje/kwy102
71. Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. (2003) 95:1765–71. doi: 10.1093/jnci/djg110
72. Hong SN, Kim JH, Choe WH, Lee SY, Seol DC, Moon HW, et al. Circulating vitamin D and colorectal adenoma in asymptomatic average-risk individuals who underwent first screening colonoscopy: a case-control study. Digest Dis Sci. (2012) 57:753–63. doi: 10.1007/s10620-011-1926-1
73. Jacobs ET, Hibler EA, Lance P, Sardo CL, Jurutka PW. Association between circulating concentrations of 25(OH)D and colorectal adenoma: a pooled analysis. Int J Cancer. (2013) 133:2980–8. doi: 10.1002/ijc.28316
74. Miller EA, Keku TO, Satia JA, Martin CF, Galanko JA, Sandler RS. Calcium, dietary, and lifestyle factors in the prevention of colorectal adenomas. Cancer. (2007) 109:510–7. doi: 10.1002/cncr.22453
75. Peters U, Hayes RB, Chatterjee N, Shao W, Schoen RE, Pinsky P, et al. Circulating vitamin D metabolites, polymorphism in vitamin D receptor, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. (2004) 13:546–52. doi: 10.1158/1055-9965.546.13.4
76. Platz EA, Hankinson SE, Hollis BW, Colditz GA, Hunter DJ, Speizer FE, et al. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and adenomatous polyps of the distal colorectum. Cancer Epidemiol Biomarkers Prev. (2000) 9:1059–65.
77. Takahashi R, Mizoue T, Otake T, Fukumoto J, Tajima O, Tabata S, et al. Circulating vitamin D and colorectal adenomas in Japanese men. Cancer Sci. (2010) 101:1695–700. doi: 10.1111/j.1349-7006.2010.01575.x
78. Yamaji T, Iwasaki M, Sasazuki S, Sakamoto H, Yoshida T, Tsugane S. Association between plasma 25-hydroxyvitamin D and colorectal adenoma according to dietary calcium intake and vitamin D receptor polymorphism. Am J Epidemiol. (2012) 175:236–44. doi: 10.1093/aje/kwr295
79. Hellwege JN, Zhu X, Huang X, Shrubsole MJ, Fan L, Li B, et al. Blunted PTH response to vitamin D insufficiency/deficiency and colorectal neoplasia risk. Clin Nutr. (2021) 40:3305–13. doi: 10.1016/j.clnu.2020.10.057
80. Hibler EA, Klimentidis YC, Jurutka PW, Kohler LN, Lance P, Roe DJ, et al. CYP24A1 and CYP27B1 polymorphisms, concentrations of vitamin D metabolites, and odds of colorectal adenoma recurrence. Nutr Cancer. (2015) 67:1131–41. doi: 10.1080/01635581.2015.1068818
81. Hibler EA, Sardo Molmenti CL, Lance P, Jurutka PW, Jacobs ET. Associations between circulating 1,25(OH)2D concentration and odds of metachronous colorectal adenoma. Cancer Causes Control. (2014) 25:809–17. doi: 10.1007/s10552-014-0382-6
82. Jacobs ET, Haussler MR, Alberts DS, Kohler LN, Lance P, Martínez ME, et al. Association between circulating vitamin D metabolites and fecal bile acid concentrations. Cancer Prev Res. (2016) 9:589–97. doi: 10.1158/1940-6207.CAPR-16-0033
83. LePane CA, Singh G, Spanier-Stiasny JA, Svinarich DM, Rasansky RJ, Hoffman SMJ. Implications of serum 25-hydroxyvitamin d on the prevalence of neoplastic polyps: a cross-sectional study. Gastroenterol Res. (2011) 4:43–50. doi: 10.4021/gr291e
84. Yang B, Thyagarajan B, Gross MD, Fedirko V, Goodman M, Bostick RM. No evidence that associations of incident, sporadic colorectal adenoma with its major modifiable risk factors differ by chromosome 8q24 region rs6983267 genotype. Mol Carcinog. (2014) 53:E193–200. doi: 10.1002/mc.22086
85. Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and colorectal adenoma risk. Prev Med. (2011) 53:10–6. doi: 10.1016/j.ypmed.2011.05.013
86. Choi YJ, Kim YH, Cho CH, Kim SH, Lee JE. Circulating levels of vitamin D and colorectal adenoma: a case-control study and a meta-analysis. World J Gastroenterol. (2015) 21:8868–77. doi: 10.3748/wjg.v21.i29.8868
87. Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. (2011) 128:1414–24. doi: 10.1002/ijc.25439
88. Zhang L, Zou H, Zhao Y, Hu C, Atanda A, Qin X, et al. Association between blood circulating vitamin D and colorectal cancer risk in Asian countries: a systematic review and dose-response meta-analysis. BMJ Open. (2019) 9:e030513. doi: 10.1136/bmjopen-2019-030513
89. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Investig. (2006) 116:2062–72. doi: 10.1172/JCI29449
90. Ponchon G, DeLuca HF. The role of the liver in the metabolism of vitamin D. J Clin Investig. (1969) 48:1273–9. doi: 10.1172/JCI106093
91. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. (2016) 96:365–408. doi: 10.1152/physrev.00014.2015
92. Kim MS, Fujiki R, Murayama A, Kitagawa H, Yamaoka K, Yamamoto Y, et al. 1Alpha,25(OH)2D3-induced transrepression by vitamin D receptor through E-box-type elements in the human parathyroid hormone gene promoter. Mol Endocrinol (Baltimore, Md). (2007) 21:334–42. doi: 10.1210/me.2006-0231
93. Pike JW, Meyer MB. Regulation of mouse Cyp24a1 expression via promoter-proximal and downstream-distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D(3) action. Arch Biochem Biophys. (2012) 523:2–8. doi: 10.1016/j.abb.2011.12.003
94. Shabahang M, Buras RR, Davoodi F, Schumaker LM, Nauta RJ, Evans SR. 1,25-Dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res. (1993) 53:3712–8.
95. Gaschott T, Steinmeyer A, Steinhilber D, Stein JZK. 156718, a low calcemic, antiproliferative, and prodifferentiating vitamin D analog. Biochem Biophys Res Commun. (2002) 290:504–9. doi: 10.1006/bbrc.2001.6213
96. Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. (2000) 60:2304–12.
97. Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. (2015) 6:6528. doi: 10.1038/ncomms7528
98. Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. (2013) 143:1679–86. doi: 10.3945/jn.113.180794
99. Munoz A, Grant WB. Vitamin D and cancer: an historical overview of the epidemiology and mechanisms. Nutrients. (2022) 14:1448. doi: 10.3390/nu14071448
100. Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev. (2014) 72:48–54. doi: 10.1111/nure.12090
101. Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, Key TJ. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Public Health Nutr. (2011) 14:340–6. doi: 10.1017/S1368980010002454
Keywords: Vitamin D, 25-hydroxyvitamin D, colorectal adenoma, sessile serrated adenoma/polyp, meta-analysis
Citation: Guo L-l, Chen S-s, Zhong L-x, He K-y, Li Y-t, Chen W-w, Zeng Q-t and Tang S-h (2022) Vitamin D intake as well as circulating 25-hydroxyvitamin D level and risk for the incidence and recurrence of colorectal cancer precursors: A meta-analysis. Front. Med. 9:877275. doi: 10.3389/fmed.2022.877275
Received: 16 February 2022; Accepted: 02 August 2022;
Published: 25 August 2022.
Edited by:
Nora L. Nock, Case Western Reserve University, United StatesReviewed by:
William B. Grant, Sunlight Nutrition and Health Research Center, United StatesXuan Qin, Baylor College of Medicine, United States
Copyright © 2022 Guo, Chen, Zhong, He, Li, Chen, Zeng and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shao-hui Tang, dGFuZ3NoYW9odWkyMDZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Li-liangzi Guo
Li-liangzi Guo Si-si Chen
Si-si Chen Li-xian Zhong
Li-xian Zhong Kai-yin He
Kai-yin He Yu-ting Li
Yu-ting Li Wei-wei Chen
Wei-wei Chen Qiu-ting Zeng
Qiu-ting Zeng Shao-hui Tang
Shao-hui Tang