- 1Division of Endocrinology, Metabolism and Nephrology, Department of Internal Medicine, Tokyo, Japan
- 2Apheresis and Dialysis Center, Keio University School of Medicine, Tokyo, Japan
Since the coronavirus disease 2019 (COVID-19) pandemic continues and a new variant of the virus has emerged, the COVID-19 vaccination campaign has progressed. Rare but severe adverse outcomes of COVID-19 vaccination such as anaphylaxis and myocarditis have begun to be noticed. Of note, several cases of new-onset antineutrophil cytoplasmic antibody-associated vasculitis (AAV) after COVID-19 mRNA vaccination have been reported. However, relapse of AAV in remission has not been recognized enough as an adverse outcome of COVID-19 vaccination. We report, to our knowledge, a first case of renal-limited AAV in remission using every 6-month rituximab administration that relapsed with pulmonary hemorrhage, but not glomerulonephritis, following the first dose of the Pfizer-BioNTech COVID-19 vaccine. The patient received the COVID-19 vaccine more than 6 months after the last dose of rituximab according to the recommendations. However, his CD19+ B cell counts were found to be increased after admission, indicating that our case might have been prone to relapse after COVID-19 vaccination. Although our case cannot establish causality between AAV relapse and COVID-19 mRNA vaccination, a high level of clinical vigilance for relapse of AAV especially in patients undergoing rituximab maintenance therapy following COVID-19 vaccination should be maintained. Furthermore, elapsed time between rituximab administration and COVID-19 mRNA vaccination should be carefully adjusted based on AAV disease-activity.
Introduction
As of February 2022, 390 million cases of COVID-19, with 5.7 million COVID-19 related deaths, have been reported worldwide (1). Large clinical trials have shown that COVID-19 mRNA vaccines were sufficiently safe and effective in preventing COVID-19 (2). Besides the common adverse events following COVID-19 mRNA vaccination, including injection site reactions, fever, fatigue, headaches, and muscle pain (2), rare but severe adverse reactions such as anaphylaxis and myocarditis have been reported (3–5).
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of multisystem autoimmune diseases. It is characterized by production of ANCAs, directed either at proteinase 3 (PR3) or myeloperoxidase (MPO), and small to medium vessel inflammation (6). Rituximab is used as maintenance therapy for AAV and typically administered every 6 months (7). However, it is recommended that patients should wait for COVID-19 vaccination until six months elapse after the last rituximab administration (8). Several cases describing the temporal association between COVID-19 vaccination, and new-onset or relapse of AAV have already been reported (9–14). Herein, we report the first case of renal-limited vasculitis in remission using every 6-month rituximab administration that relapsed with pulmonary hemorrhage, but not glomerulonephritis, following the first dose of Pfizer-BioNTech COVID-19 vaccination.
Case Description
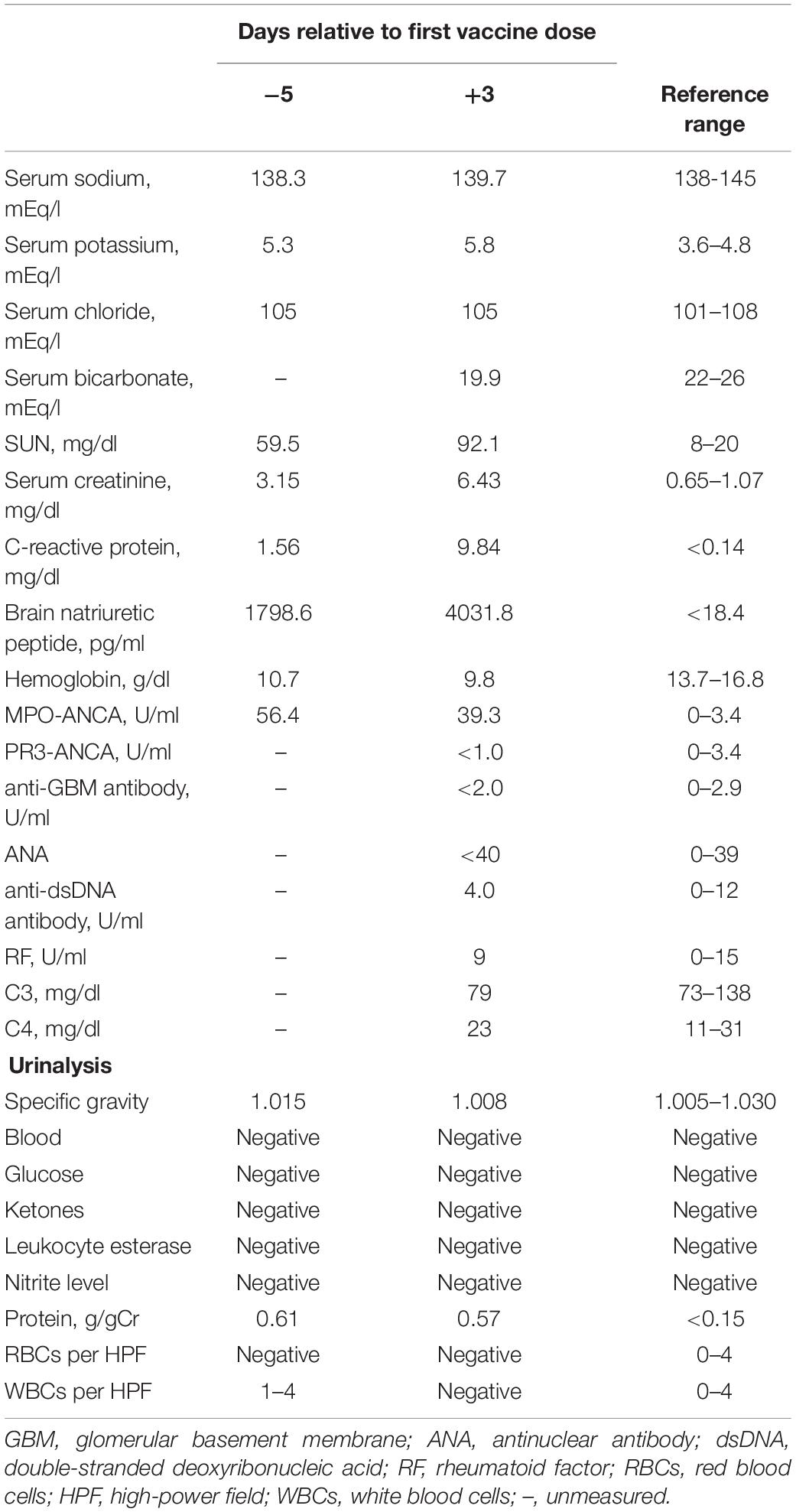
A 74-year-old Japanese man experienced renal-limited vasculitis in 2016, and achieved remission with prednisolone and intravenous cyclophosphamide. Remission was maintained in the patient with prednisolone (10 mg/day) and azathioprine (2 mg/kg/day) until 2020 (15). Although the patient has been in clinical remission, his anti-MPO antibody remained positive. Therefore, we switched his maintenance regimen from azathioprine-based to rituximab-based therapy (375 mg/m2 every 6 months) (7, 16). The patient received his third rituximab dose in mid-April 2021. Eight days before admission in late October, his serum creatinine level was stable at 3.15 mg/dl. Given that urinalysis showed no red blood cells and stable proteinuria at 0.61 g/gCr, his renal-limited vasculitis was confirmed to be in remission. Three days before admission, he received his first dose of the Pfizer-BioNTech COVID-19 vaccine. The patient had no allergies to the drugs or documented history of COVID-19. On the day of the first vaccination, the patient developed cough with productive sputum, and dyspnea. Since dyspnea worsened and hemoptysis appeared over 3 days, he presented to our emergency department. His oxygen saturation was 68% on room air, blood pressure was 90/41 mmHg, and heart rate was 90 beats per min. Reverse transcription-polymerase chain reaction testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA from nasopharyngeal swabs yielded negative results. Laboratory test results on admission showed acute kidney injury in chronic kidney disease with serum creatinine levels of 6.43 mg/dl, high inflammatory reaction with C-reactive protein of 9.84 mg/dl, and brain natriuretic peptide of 4031.8 pg/ml. Urinalysis showed no urinary blood cells and random urinary protein-creatinine ratio was 0.57 g/gCr, which was comparable with the result at his last outpatient visit. Serum complement levels and immunological workup including rheumatoid factor, anti-glomerular basement (GBM) and anti-double-stranded DNA antibodies were unremarkable (Table 1). High-resolution computed tomography of the chest showed diffuse “ground-glass” opacities and consolidation in both lungs and cardiac enlargement with bilateral pleural effusion. These findings suggested that the patient had alveolar hemorrhage complicated by cardiogenic pulmonary edema (Figures 1A,B). Thus, he was suspected to have vasculitis relapse with diffuse alveolar hemorrhage, but not glomerulonephritis. Particularly, no tumor was detected on computed tomography. Blood and sputum culture results were negative. The patient received mechanical ventilation with positive end-expiratory pressure, and treatment was initiated empirically with intravenous methylprednisolone pulse for 3 days, followed by prednisolone 1 mg/kg daily. His urinary output was minimal, requiring continuous venovenous hemodiafiltration with intravenous catecholamine support. Bronchoscopy with bronchoalveolar lavage revealed hemosiderin-laden macrophages in hemorrhage fluid, leading to a diagnosis of diffuse alveolar hemorrhage (Figures 2A,B). Therefore, plasma exchange was performed thrice every other day, and rituximab was administered weekly at 375 mg/m2 in two doses for remission reinduction of AAV on the basis of clinical presentation of alveolar hemorrhage. His hemoptysis and alveolar hemorrhage on bronchoscopy improved significantly, and he withdrew from mechanical ventilation. Renal biopsy was not performed due to the patient critical state. However, considering that neither urinary red blood cells nor increase in urinary protein was observed over the treatment period, acute deterioration in chronic kidney disease requiring hemodialysis was not likely attributed to AAV relapse but to cardio-renal syndrome (type 5). The patient remained on hemodialysis, whereas his general condition remarkably improved. However, the patient developed urinary tract infection, and rituximab was suspended for infectious control. He experienced recurrent hemoptysis and died of progressive respiratory failure within a day (Figure 3).
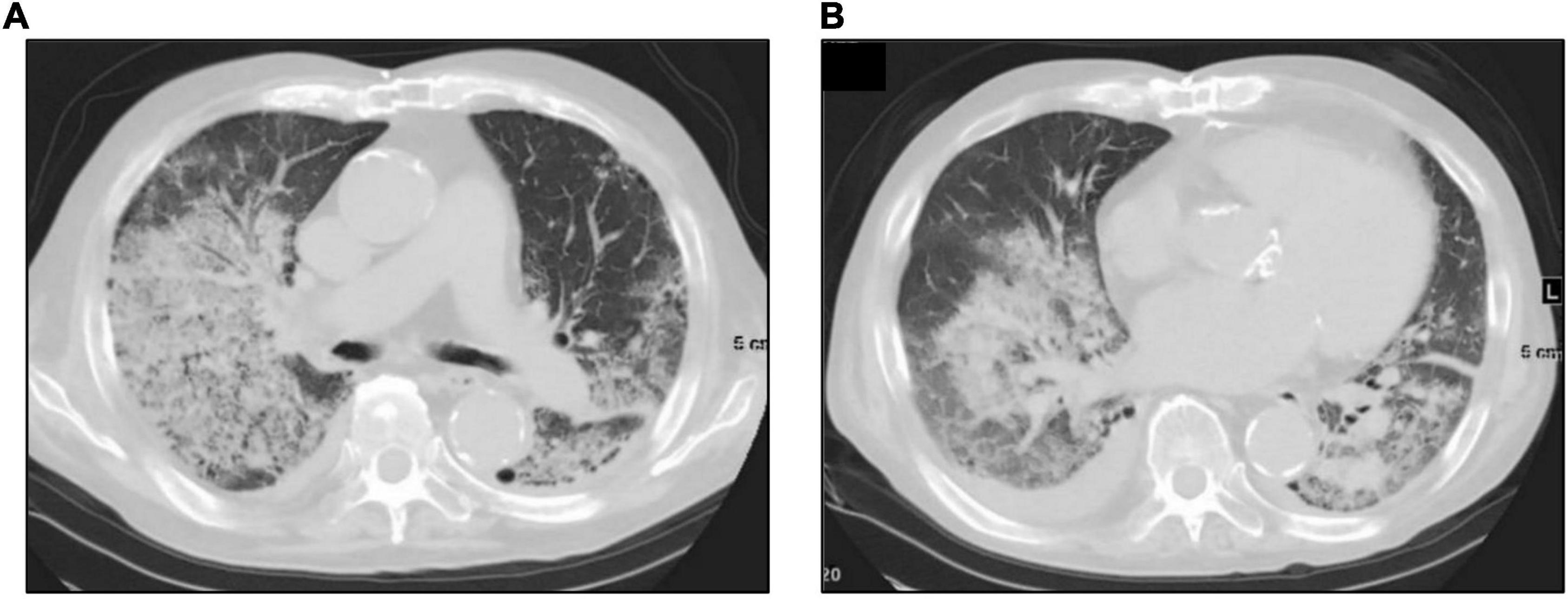
Figure 1. High-resolution computed tomography of the chest on admission. Chest computed tomography on admission shows diffuse “ground-glass” opacities (A), consolidation, cardiac enlargement, and bilateral pleural effusion (B).
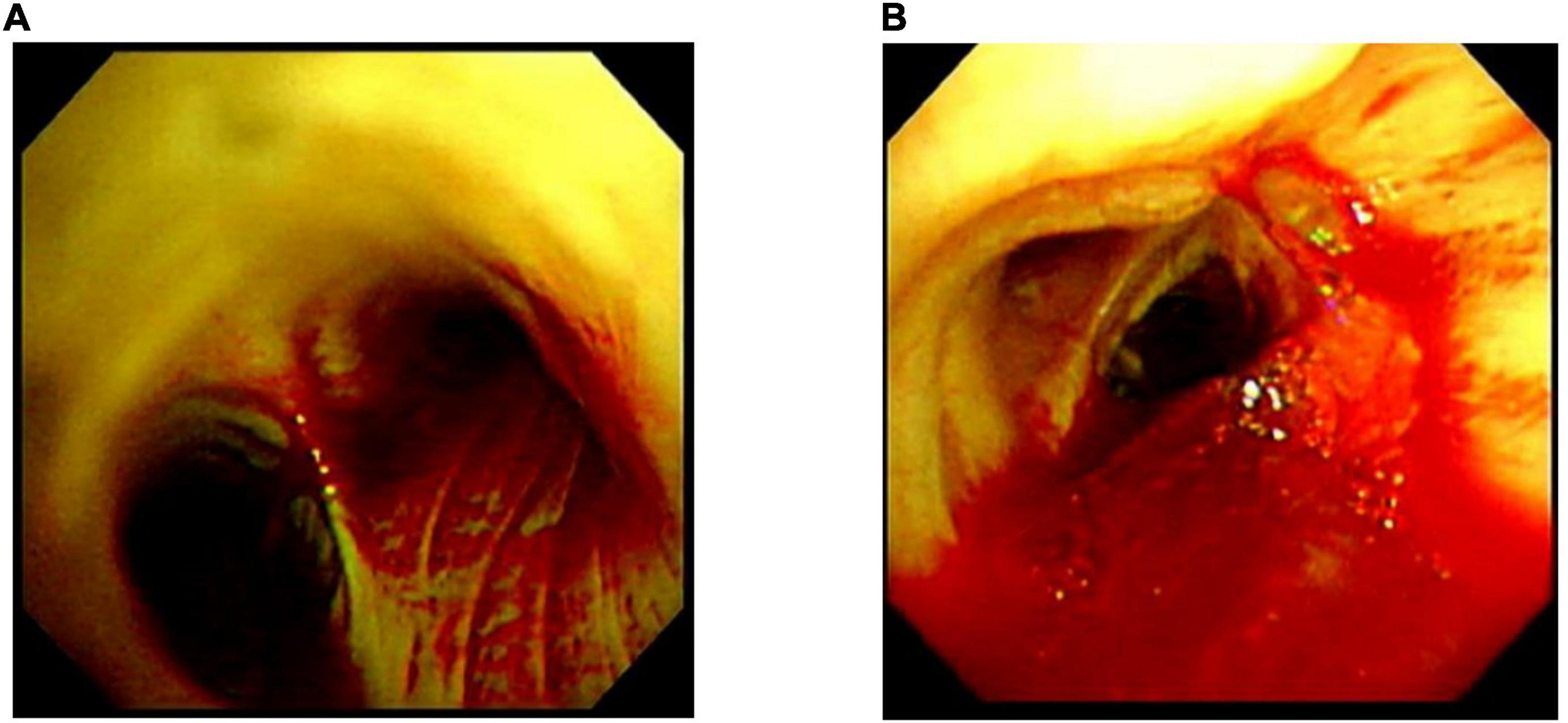
Figure 2. Bronchoscopic images taken prior to the initiation of plasma exchange. Bronchoscopic images taken on the 2nd admission day revealed bleeding from multiple bronchi (A) to vocal cord lesions (B).
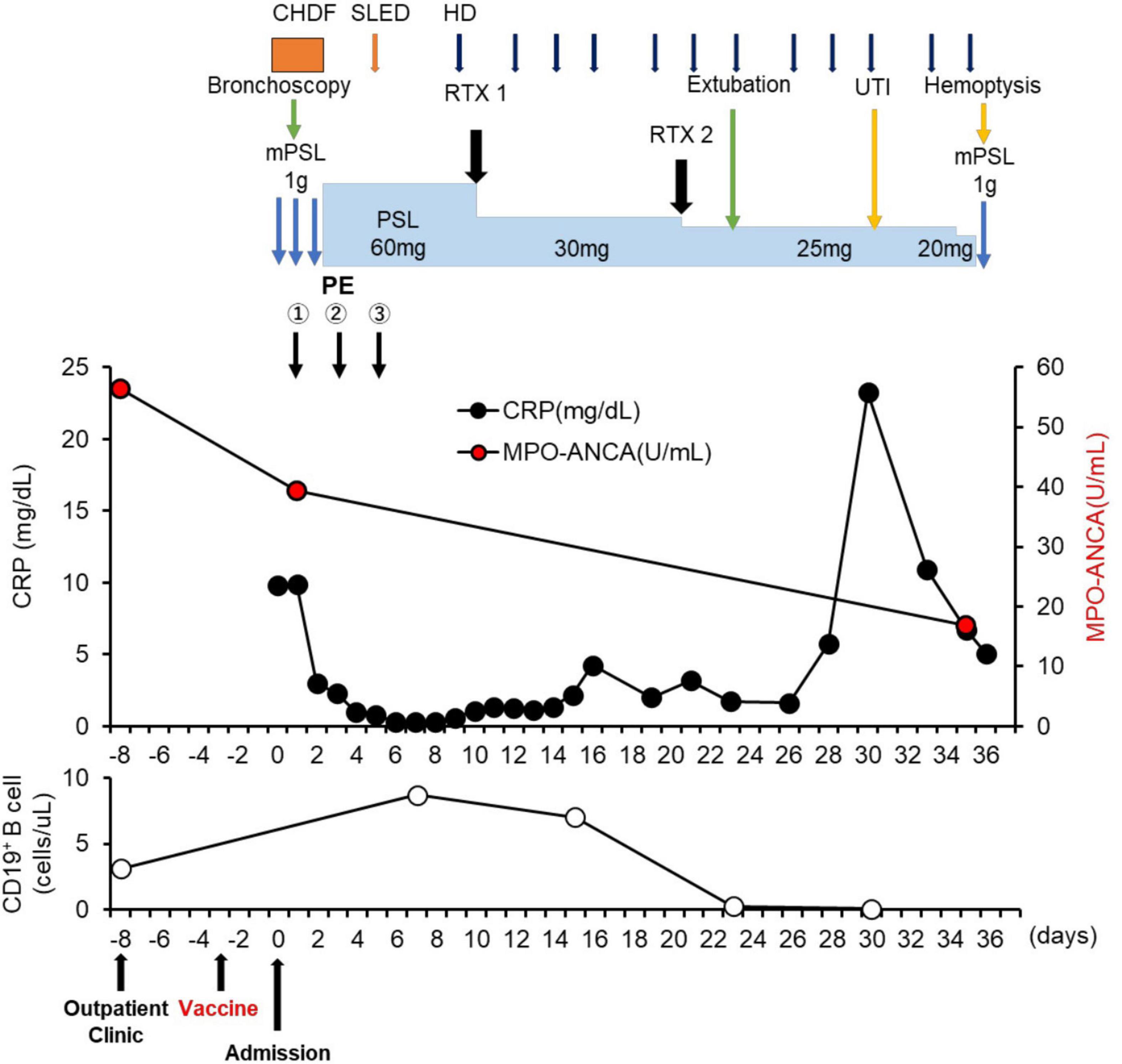
Figure 3. Clinical time course of the patient. CHDF, Continuous hemodiafiltration; SLED, sustained low efficiency dialysis; RTX, rituximab; mPSL, methylprednisolone; PSL, prednisolone; HD, hemodialysis; UTI, urinary tract infection.
Discussion
Herein, we describe the case of AAV relapse with alveolar hemorrhage after the first dose of COVID-19 mRNA vaccination. To date, several cases of de novo ANCA vasculitis and one case of AAV relapse after remission following the second dose of COVID-19 mRNA vaccination have been reported (9–14). Our case was unique in that alveolar hemorrhage, but not glomerulonephritis, developed as a result of renal-limited vasculitis relapse shortly after receiving the first dose of Pfizer-BioNTech mRNA COVID-19 vaccine. This case also represents a dilemma of waiting for COVID-19 mRNA vaccination until 6 months have elapsed after the last dose of rituximab, which might exacerbate AAV activity and increase the risk for AAV relapse.
AAV is an autoimmune disease that could lead to worse COVID-19 outcomes, especially when patients are on 10 mg/day or more of prednisolone, cyclophosphamide, and rituximab (17, 18). Since vaccines appear to prevent SARS-CoV-2 infection and further reduce the risk of disease aggravation and death, the American College of Rheumatology recommends COVID-19 mRNA vaccines for patients with rheumatic and musculoskeletal diseases (19). Our patient was treated with prednisolone 10 mg/day and rituximab for renal-limited vasculitis as maintenance therapy. Therefore, COVID-19 vaccination was strongly recommended for him.
The mechanism for AAV recurrence after vaccination remains enigmatic. However, some reports have described cases of AAV developed after influenza vaccination (20–22), which raised the possibility that an immune response to either the vaccine antigen or one of the excipients in the vaccine might have caused AAV (22). Of note, peripheral blood mononuclear cells extracted from patients in remission of PR3-ANCA positive vasculitis significantly produced more PR3-ANCA in response to influenza vaccines containing RNA in an ex vivo study compared to healthy controls (23).
Billions of people worldwide have received COVID-19 vaccinations (1). Rare but severe adverse events following these vaccinations, such as new-onset or recurrent glomerulonephritis are emerging (24). Similar to influenza vaccines, COVID-19 vaccination may induce dysregulation of immune response to the spike protein or mRNA of SARS-CoV-2 (9–14), giving rise to AAV, as the previously postulated mechanism for SARS-CoV-2 infection (25). It is suggested that antibodies against SARS-CoV-2 spike glycoprotein could cross-react with autoimmune target proteins (26), triggering pre-existing or underlying immune dysregulation in a specific host, ultimately jeopardizing disease flares such as AAV (27). One possibility is that our patient might have had a dormant underlying autoimmune disease in the lungs, which became apparent due to an aberrant immune response provoked by the COVID-19 vaccine. To our best knowledge, there has been only one case of AAV in remission involving the kidney and lung that recurred after COVID-19 vaccination (14). In our case, it is difficult to prove a causal relationship between vaccination and AAV relapse with severe alveolar hemorrhage. Indeed, our patient had no elevated titer of anti-MPO antibody on admission compared with that result of his last outpatient visit (titer on admission: 39.3 U/ml; titer at the last visit: 56.4 U/ml; normal: <3.4 U/ml). Although MPO-ANCAs are known to be pathogenic (28), disease activity of AAV is not always correlated with MPO-ANCA titers, but rather with MPO-ANCA affinity (29, 30). However, after initiating treatment of AAV relapse, the titer of anti-MPO antibody decreased coinciding with improvements in pulmonary hemorrhage (Figure 3), which is similar to a previous case report (31). In addition, Sharma et al. reported a unique case demonstrating that diffuse alveolar hemorrhage occurred 8 h after the first dose of COVID-19 mRNA vaccination without any evidence of immunological complications such as elevation of anti-MPO antibody titers (32). Infection and tumor, two major inciting factors for AAV relapse (33–35) and other immunological underlying diseases for pulmonary-renal syndrome, such as anti-glomerular basement membrane (GBM) disease: Goodpasture syndrome, systemic lupus erythematosus, and dual ANCA and anti-GBM positive disease (36) have been ruled out. Therefore, we suspect that these adverse events in our patient were not mere coincidences. In particular, the time course for onset of vaccine-related symptoms was consistent with these in previous reports that varied from a few hours after the first dose to 6 weeks after the second dose (24). In addition, we cannot exclude the possibility that AAV recurrence might have been related to the elapsed time since the last dose of rituximab (6 months). Importantly, it is recommended that 6 months should be elapsed between rituximab administration and COVID-19 vaccination (8). In our case, CD19+ B cell counts increased from 3.1 cells/μl 5 days before vaccination to 8.7 cells/μl on day 7 after admission (Figure 3). Therefore, AAV in remission with rituximab, as in this case, may be susceptible to relapse after COVID-19 vaccination. Pertinently, the recommendation highlights the possibility that vaccination early after rituximab administration might sufficiently induce protective effects against COVID-19 infection by cellular immunity (8). Therefore, earlier administration of vaccine after rituximab should be carefully considered to prevent CD19+ B cell repletion and AAV relapse.
A limitation to this case report is that we did not demonstrate causality between COVID-19 mRNA vaccination and AAV relapse. This study also did not determine the mechanisms for renal-limited vasculitis recurrence that occurred with alveolar hemorrhage but not glomerulonephritis.
Clinicians should be aware of a possible causal relationship between AAV relapse and COVID-19 mRNA vaccination and might have to carefully adjust elapsed time between rituximab administration and COVID-19 vaccination based on AAV disease-activity.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
KN and SY wrote the manuscript. KN, SY, IY, NY, DK, KK, MA, TN, EY, KU, TT, JY, TY, TK, and HI took clinical care of the patient. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the treating medical staff for their skillful care of this patient and Editage (www.editage.com) for English language editing.
References
1. Johns Hopkins University Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Available online at: https://coronavirus.jhu.edu/map.html (accessed February 11, 2022).
2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
3. Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. (2021) 325:780–1. doi: 10.1001/jama.2021.0600
4. CDC Covid-19 Response Team, Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of moderna COVID-19 vaccine – United States, december 21, 2020-january 10, 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:125–9. doi: 10.15585/mmwr.mm7004e1
5. Fragkou PC, Dimopoulou D. Serious complications of COVID-19 vaccines: a mini-review. Metabol Open. (2021) 12:100145. doi: 10.1016/j.metop.2021.100145
6. Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol. (2017) 12:1680–91. doi: 10.2215/cjn.02500317
7. Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. (2014) 371:1771–80. doi: 10.1056/NEJMoa1404231
8. Kronbichler A, Anders HJ, Fernandez-Juárez GM, Floege J, Goumenos D, Segelmark M, et al. Recommendations for the use of COVID-19 vaccines in patients with immune-mediated kidney diseases. Nephrol Dial Transplant. (2021) 36:1160–68. doi: 10.1093/ndt/gfab064
9. Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. (2021) 78:611–3. doi: 10.1053/j.ajkd.2021.06.016
10. Hakroush S, Tampe B. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after Pfizer-BioNTech COVID-19 mRNA vaccination. Front Immunol. (2021) 12:762006. doi: 10.3389/fimmu.2021.762006
11. Dube GK, Benvenuto LJ, Batal I. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int Rep. (2021) 6:3087–9. doi: 10.1016/j.ekir.2021.08.012
12. Anderegg MA, Liu M, Saganas C, Montani M, Vogt B, Huynh-Do U, et al. De novo vasculitis after mRNA-1273 (moderna) vaccination. Kidney Int. (2021) 100:474–6. doi: 10.1016/j.kint.2021.05.016
13. Sekar A, Campbell R, Tabbara J, Rastogi P. ANCA glomerulonephritis after the moderna COVID-19 vaccination. Kidney Int. (2021) 100:473–4. doi: 10.1016/j.kint.2021.05.017
14. Conticini E, d’Alessandro M, Bergantini L, Bargagli E, Gentili F, Mazzei MA, et al. Relapse of microscopic polyangiitis after vaccination against COVID-19: a case report. J Med Virol. (2021) 93:6439–41. doi: 10.1002/jmv.27192
15. Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniené J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. (2003) 349:36–44. doi: 10.1056/NEJMoa020286
16. Terrier B, Pagnoux C, Perrodeau É, Karras A, Khouatra C, Aumaître O, et al. Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis. (2018) 77:1150–6. doi: 10.1136/annrheumdis-2017-212768
17. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. (2020) 584:430–6. doi: 10.1038/s41586-020-2521-4
18. Sattui SE, Conway R, Putman MS, Seet AM, Gianfrancesco MA, Beins K, et al. Outcomes of COVID-19 in patients with primary systemic vasculitis or polymyalgia rheumatica from the COVID-19 global rheumatology alliance physician registry: a retrospective cohort study. Lancet Rheumatol. (2021) 3:e855–64. doi: 10.1016/s2665-9913(21)00316-7
19. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol. (2021) 73:e60–75. doi: 10.1002/art.41928
20. Birck R, Kaelsch I, Schnuelle P, Flores-Suárez LF, Nowack R. ANCA-associated vasculitis following influenza vaccination: causal association or mere coincidence? J Clin Rheumatol. (2009) 15:289–91. doi: 10.1097/RHU.0b013e3181b55fe4
21. Uji M, Matsushita H, Iwata S. Microscopic polyangiitis after influenza vaccination. Intern Med. (2005) 44:892–6. doi: 10.2169/internalmedicine.44.892
22. Duggal T, Segal P, Shah M, Carter-Monroe N, Manoharan P, Geetha D. Antineutrophil cytoplasmic antibody vasculitis associated with influenza vaccination. Am J Nephrol. (2013) 38:174–8. doi: 10.1159/000354084
23. Jeffs LS, Nitschke J, Tervaert JW, Peh CA, Hurtado PR. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin Rheumatol. (2016) 35:943–51. doi: 10.1007/s10067-015-3073-0
24. Klomjit N, Alexander MP, Fervenza FC, Zoghby Z, Garg A, Hogan MC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. (2021) 6:2969–78. doi: 10.1016/j.ekir.2021.09.008
25. Uppal NN, Kello N, Shah HH, Khanin Y, De Oleo IR, Epstein E, et al. De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. (2020) 5:2079–83. doi: 10.1016/j.ekir.2020.08.012
26. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. (2020) 217:108480. doi: 10.1016/j.clim.2020.108480
27. Akinosoglou K, Tzivaki I, Marangos M. Covid-19 vaccine and autoimmunity: awakening the sleeping dragon. Clin Immunol. (2021) 226:108721. doi: 10.1016/j.clim.2021.108721
28. Schlieben DJ, Korbet SM, Kimura RE, Schwartz MM, Lewis EJ. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. Am J Kidney Dis. (2005) 45:758–61. doi: 10.1053/j.ajkd.2005.01.001
29. Suzuki K, Kobayashi S, Yamazaki K, Gondo M, Tomizawa K, Arimura Y, et al. Analysis of risk epitopes of anti-neutrophil antibody MPO-ANCA in vasculitis in Japanese population. Microbiol Immunol. (2007) 51:1215–20. doi: 10.1111/j.1348-0421.2007.tb04017.x
30. Yoshida M, Sasaki M, Nakabayashi I, Akashi M, Tomiyasu T, Yoshikawa N, et al. Two types of myeloperoxidase-antineutrophil cytoplasmic autoantibodies with a high affinity and a low affinity in small vessel vasculitis. Clin Exp Rheumatol. (2009) 27:S28–32.
31. Chen CC, Chen HY, Lu CC, Lin SH. Case report: anti-neutrophil cytoplasmic antibody-associated vasculitis with acute renal failure and pulmonary hemorrhage may occur after COVID-19 vaccination. Front Med (Lausanne). (2021) 8:765447. doi: 10.3389/fmed.2021.765447
32. Sharma A, Upadhyay B, Banjade R, Poudel B, Luitel P, Kharel B. A case of diffuse alveolar hemorrhage with COVID-19 vaccination. Cureus. (2022) 14:e21665. doi: 10.7759/cureus.21665
33. Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. (1994) 120:12–7. doi: 10.7326/0003-4819-120-1-199401010-00003
34. Hutton HL, Holdsworth SR, Kitching AR. ANCA-associated vasculitis: pathogenesis, models, and preclinical testing. Semin Nephrol. (2017) 37:418–35. doi: 10.1016/j.semnephrol.2017.05.016
35. Mahr A, Heijl C, Le Guenno G, Faurschou M. ANCA-associated vasculitis and malignancy: current evidence for cause and consequence relationships. Best Pract Res Clin Rheumatol. (2013) 27:45–56. doi: 10.1016/j.berh.2012.12.003
Keywords: renal-limited ANCA-associated vasculitis, relapse, alveolar hemorrhage, COVID-19 mRNA vaccination, rituximab
Citation: Nishioka K, Yamaguchi S, Yasuda I, Yoshimoto N, Kojima D, Kaneko K, Aso M, Nagasaka T, Yoshida E, Uchiyama K, Tajima T, Yoshino J, Yoshida T, Kanda T and Itoh H (2022) Development of Alveolar Hemorrhage After Pfizer-BioNTech COVID-19 mRNA Vaccination in a Patient With Renal-Limited Anti-neutrophil Cytoplasmic Antibody-Associated Vasculitis: A Case Report. Front. Med. 9:874831. doi: 10.3389/fmed.2022.874831
Received: 13 February 2022; Accepted: 16 March 2022;
Published: 08 April 2022.
Edited by:
Min Chen, Peking University First Hospital, ChinaReviewed by:
Vladimir Tesar, Charles University, CzechiaChien-Chou Chen, Tri-Service General Hospital, Taiwan
Copyright © 2022 Nishioka, Yamaguchi, Yasuda, Yoshimoto, Kojima, Kaneko, Aso, Nagasaka, Yoshida, Uchiyama, Tajima, Yoshino, Yoshida, Kanda and Itoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shintaro Yamaguchi, eWFtYTEwMDVAYTYua2Vpby5qcA==
 Ken Nishioka
Ken Nishioka Shintaro Yamaguchi
Shintaro Yamaguchi Itaru Yasuda
Itaru Yasuda Norifumi Yoshimoto1
Norifumi Yoshimoto1 Jun Yoshino
Jun Yoshino