- 1Division of Gastroenterology and Hepatology, Department of Medicine, National University Health System, Singapore, Singapore
- 2Yong Loo Lin School of Medicine, National University Singapore, Singapore, Singapore
- 3National University Centre for Organ Transplantation, National University Health System, Singapore, Singapore
- 4Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, National University Hospital Singapore, Singapore, Singapore
- 5Division of Gastroenterology, Hepatology and Nutrition, Department of Internal Medicine, Virginia Commonwealth University, Richmond, VA, United States
- 6Cedars-Sinai Fatty Liver Program, Division of Digestive and Liver Diseases, Department of Medicine, Comprehensive Transplant Centre, Cedars-Sinai Medical Centre, Los Angeles, CA, United States
Patients undergoing liver transplant (LTX) typically confront a challenging postoperative journey. A dysbiotic gut microbiome is associated with the development of complications, including post-LTX allograft rejection, metabolic diseases and de novo or recurrent cancer. A major explanation of this are the bipartite interactions between the gut microbiota and host immunity, which modulates the alloimmune response towards the liver allograft. Furthermore, bacterial translocation from dysbiosis causes pathogenic changes in the concentrations of microbial metabolites like lipopolysaccharides, short-chain fatty acids (SCFAs) and Trimethylamine-N-Oxide, with links to cardiovascular disease development and diabetes mellitus. Gut dysbiosis also disrupts bile acid metabolism, with implications for various post-LTX metabolic diseases. Certain taxonomy of microbiota such as lactobacilli, F.prausnitzii and Bacteroides appear to be associated with these undesired outcomes. As such, an interesting but as yet unproven hypothesis exists as to whether induction of a “beneficial” composition of gut microbiota may improve prognosis in LTX patients. Additionally, there are roles of the microbiome as predictive and prognostic indicators for clinicians in improving patient care. Hence, the gut microbiome represents an exceptionally exciting avenue for developing novel prognostic, predictive and therapeutic applications.
Introduction
Liver transplant (LTX), the only treatment for patients with end-stage liver disease, liver failure and hepatocellular carcinoma (HCC) with a definite long-term survival benefit, experiences relatively high incident rates for postoperative complications (1). Hence, we sought to review how variations in gut microbiota post LTX influences the incidence of rejection, metabolic disease and cancers in the liver to better understand and prevent such complications.
A complex and diverse population of microorganisms, known as the gut microbiome, exists in the human gastrointestinal tract. Through dynamic microbiota-microbiota and microbiota-host interactions, involving a myriad of by-products, the gut microbiome contributes beneficial or pathological influences on host health such as in maintaining metabolic homeostasis, intestinal integrity, and regulating the host immune system.
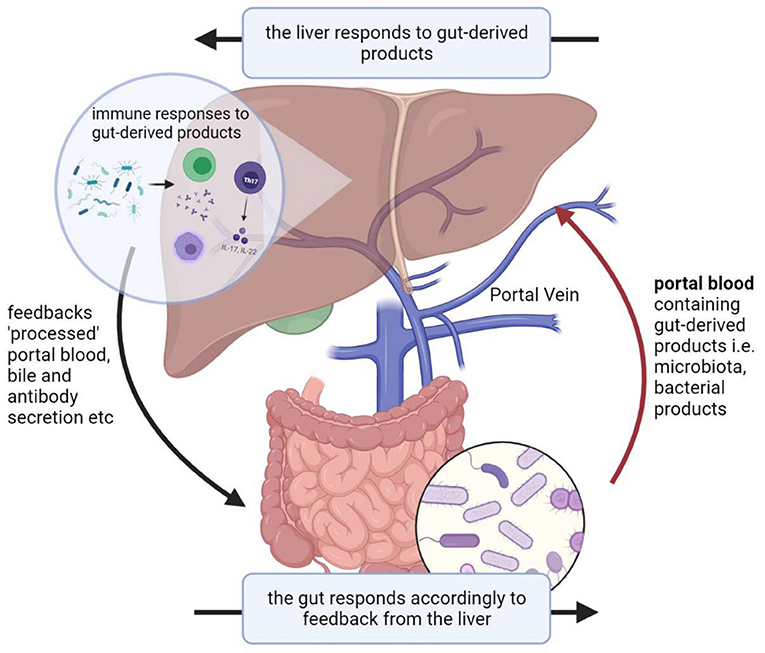
Given its anatomical position, the liver exhibits a bidirectional relationship with the gut and its microbiota, known as the gut-liver axis which exhibits circular causality. The liver thus represents the first line of defense against gut-derived antigens and toxicity factors (2). The liver faces increased pressure during gut microbial dysbiosis, which is defined as any change to the composition of resident commensal microbiota relative to the microbiome found in healthy individuals (3). This increased pressure partly arises from its associations with intestinal epithelial barrier dysfunction which increases exposure of the liver to bacterial components via the gut-liver axis, resulting in hepatic injury (Figure 1). In part, dysbiosis is implicated in the pathogenesis of various liver diseases such as liver cirrhosis, HCC, non-alcoholic fatty liver disease, and other metabolic changes (4).
LTX often corresponds with changes in the composition of the gut microbiome. Such changes can occur due to altered anatomy from surgery, biliary complications post-LTX affecting bile acid secretion into the intestines, use of immunosuppression and antibiotics. Reasons include introduction of donor microbiota to the recipient via the liver allograft. Surgery temporarily increases intestinal permeability, allowing certain opportunistic pathogens to enter the portal or systemic circulation (5–7). Allografts carry donor immune cells that can interact with recipient gut microbiome via the gut-liver axis, resulting in changes in microbial composition. An example of such changes in the gut microbiome of post-LTX patients can include a decrease in Bifidobacterium, Lactobacillus, Faecalibacterum prausnitzii and an increase in Enterobacteriaceae, Enterococcus during a qPCR-based analysis of 111 LTX patients (8).
Post-LTX Gut Microbial Variation
Immunology
Tolerance of the Liver Allograft
In general, regulatory T cells (Tregs) contribute toward the tolerative nature of the liver allograft by influencing the development and maintenance of immunological tolerance to self and alloantigens. For example, Tregs inhibit effector T cells by secreting inhibitory cytokines and interacting with CD80/CD86 through immune checkpoint CTLA-4, which downregulates T cell activation (9). Hence, Tregs help prevent the development of acute cellular rejection (ACR) by inducing a tolerogenic environment in LTX patients with an intact immune system.
Post-LTX dysbiosis causes abnormal increases in portal circulation of bacterial products like LPS. Kupffer cells, resident liver macrophages participating in initial immune responses to pathological challenges, respond by increasing concentrations of the anti-inflammatory cytokine IL-10 in the liver (10). Additionally, translocation of gut microbiota induces type 1 interferon and stimulates myeloid cell IL-10 production, thereby increasing hepatic IL-10 concentrations (11). Since Treg expression is dependent on IL-10 signaling (12), alloreactive T cell proliferation is further suppressed, thus promoting allograft tolerance.
LPS-induced local inflammation upregulates costimulatory molecule CD80 in a murine model (13). In humans, CD80 signaling regulates apoptosis and inhibits responses of activated CD8+ T cells via immune checkpoint PD-L1 (14). If the findings of the murine model are applicable to humans, upregulated CD80 from post-LTX dysbiosis can increase apoptosis of CD8+ T cells and inhibit alloimmune responses.
With regards to the specific changes in the composition of the gut microbiome, an increase in gut Bacteroides fragilis and Bacteroides thetaiotaomicron which drive Treg induction and differentiation in post-LTX dysbiosis (15) was found to be correlated with a more tolerant alloimmune response.
Nevertheless, a potential over-suppression of the immune status due to post-LTX dysbiosis, for example, due to excessive upregulation of Tregs from immoderate concentrations of IL-10 that leads to reduced alloreactive T cell proliferation, will increase risk of infections and cancer. This can result in increased mortality rates in LTX patients.
Rejection of the Liver Allograft
Adaptive immune responses via mesenteric lymph nodes, a key immunological component of the gut is transformed by commensal gut bacteria, primarily by stimulating the differentiation of naïve T cells into Tregs (16). This suggests that pre-existing or LTX-induced dysbiosis involving a change in commensal gut bacteria can disrupt the balance of CD4+ T cell subsets in mesenteric lymph nodes. Migration of these altered mesenteric lymph node CD4+ T cells into the liver allograft can promote hepatic injury (17) and accelerate the pathogenesis of early ACR.
Post-LTX hepatic ischemia-reperfusion (I/R) injury is correlated with increased IL-17 levels in portal vein plasma and the small intestine (18). A murine model demonstrated how increased levels of IL-17 significantly suppresses Treg expansion, hence increasing alloreactive T cell action on the allograft (19). This I/R injury-mediated process is hypothesized to occur relatively similarly and accelerate the development of early ACR in humans after LTX. Post-LTX dysbiosis can involve increases in segmented filamentous bacteria, which aid in IL-17 expression (19), alongside increases in lactobacilli. Increased lactobacilli upregulate IL-17 through Peyer's patches' resident T lymphocytes based on another murine model (20). Hence, it is possible for dysbiosis to exacerbate I/R injury-mediated development of early ACR in the human liver if similar interactions occur.
In contrast, the gut microbiome can protect against hepatic I/R injury-associated rejection. For instance, hepatic I/R injury induces Paneth cell degranulation and inhibits their immune response. This is implicated in the worsening of liver injury (18). Degranulation of mast cells during hepatic I/R injury has strong positive correlation to liver damage (21), which deteriorates allograft function. However, alterations in gut microbiota involving butyrate have the potential to improve I/R injury-induced hepatocyte injury by preventing NF-kB activation, upregulate the gut microbial metabolite, 3,4-dihydroxyphenylpropionic acid, which mitigates macrophage pro-inflammatory activity and stimulate the protective effect of nucleotide-binding oligomerization domain-containing protein 2 (NOD2) signaling by gut microbiota, has shown to alleviate hepatic I/R injury (22–24). A study conducted by Ito et al. indicated how increased severity of hepatic I/R injury corresponded to markedly poorer outcomes relating to early allograft dysfunction (25). As such, an altered gut microbiome after LTX that leads to alleviated hepatic I/R injury may be able to play a role in improving early ACR outcomes.
Increased bacterial translocation from the gut into the liver allograft during dysbiosis increases antigen stimulation in the liver. Schurich et al. showed how low-dose antigen stimulation resulted in tolerance whilst high-dose antigen stimulation induced effector T cell differentiation in liver sinusoidal endothelial cell (LSEC) T cell presentation (26). This means increased differentiation of effector T cells, leading to an enhanced alloimmune response and thereby promoting ACR.
Specific cytokines have the potential to act as biomarkers or are involved in the risk of acute rejection after LTX. For example, elevated levels of intracellular cytokine IFN-γ and IL-2 expression in T cells are associated with an increased risk for the development of ACR (27). Dysbiosis influences the host immune response, in turn inducing abnormal production of inflammatory cytokines. In influencing the host immune response, dysbiosis is often associated with dysregulated production of inflammatory cytokines (28). This demonstrates a plausible role of a gut microbiome-based liver immunoassay in the prognoses of ACR, should the specific relationships between dysbiosis and cytokine concentrations be discovered in the future.
In addition, Ren et al. suggested the potential ability of intestinal microbiota variation in predicting early ACR after LTX using a murine model. They conducted real-time quantitative polymerase chain reaction tests which reflected decreased F.prausnitzii and Lactobacillus, whilst Clostridium bolteae increased during ACR. In example, if similar interactions occur in the liver, F.prausnitzii may be able to demonstrate beneficial effects on ACR, where F.prausnitzii endows dendritic cells with properties that promote Treg upregulation, for example via IL-10, alongside reduced effector T cell development in the human colon (29).
However, F.prausnitzii can metabolize the immunosuppressant tacrolimus into the M1 metabolite which is 15 times less potent in inhibiting T lymphocyte proliferation, thereby increasing the risk of developing ACR in patients requiring the use of tacrolimus for tolerance of the allograft (30). This highlights upon other potential interactions between gut microbiota and immunosuppressants which can affect the efficacy of post-LTX immunosuppression. Hence, it can be important for clinicians to have a greater understanding and awareness of microbiota-medication interactions to better treat post-LTX complications in their patients.
Persistent dysbiosis leads to persistent bacterial translocation into the liver allograft. This can lead to prolonged upregulation of inflammatory cytokines, which can enhance the development of chronic rejection, particularly if long-term inflammation is observed (31). Knowledge regarding the impact of the post-LTX microbiome on late ACR and CR is highly limited due to sparce research.
This section serves to highlight upon the therapeutic opportunities provided by the gut microbiome with regards to tolerance and rejection of the allograft. Although the liver is more tolerogenic than other organs, there can still be circumstances in which tolerance is not achieved. The ability to remove gut microbiome-related rejection factors is likely beneficial toward reducing overall risk of rejection and maintaining normal allograft function.
Post-transplant Metabolic Disease
Post-transplant Diabetes Mellitus
The gut microbiome is responsible for the physiological homeostasis of bile acids (BAs), in which conjugated bile acids (CBAs) secreted into the duodenum are metabolized by gut microbiota. This involves the hydrolysis of CBAs into secondary bile acids, glycine and taurine via bile salt hydrolases (BSH) produced by intestinal bacteria, such as gram-positive species like Lactobacillus. BSH can carry out 7α-dehydroxylation of cholic acid and chenodeoxycholic acid to form deoxycholic acid and lithocholic acid (LCA). This involves a multistep biochemical pathway found only in anaerobic gut bacteria. BAs serve endocrinal functions mainly by agonizing Farnesoid X receptor (FXR) and G-protein coupled bile acid receptor TGR5. Translocation of bacteria during dysbiosis disrupts BA metabolism, leading to dysregulated agonism of FXR and TGR5. FXR enhances epithelial barrier integrity and plays a crucial role in hepatic triglyceride homeostasis (32). TGR5 improves glucose tolerance by contributing to improvements in hepatic insulin signaling (33). Hence, a lack of FXR and TGR5 signaling from a dysbiosis-mediated dysregulated BA pool can be implicated in the pathogenesis of insulin resistance and thus PTDM. This means that detection of BSH-related bacteria and pathogenic BA modifying bacteria in the postoperative period can aid in the prediction and prevention of PTDM incidence in the LTX patient.
Post-LTX bacterial translocation alongside dysbiosis can lead to low-grade constitutive increase in plasma LPS levels, thereby resulting in LPS-induced metabolic endotoxemia. A related study detected liver insulin resistance in LPS-infused mice (34), and hyperinsulinemia is correlated with the onset of diabetes. Increases in Bacteroides and Prevotella alongside decreases in Firmicutes, Clostridia, and Bifidobacteria (35) can be associated with the development of PTDM given the corresponding decrease in Bifidobacteria post-LTX (8).
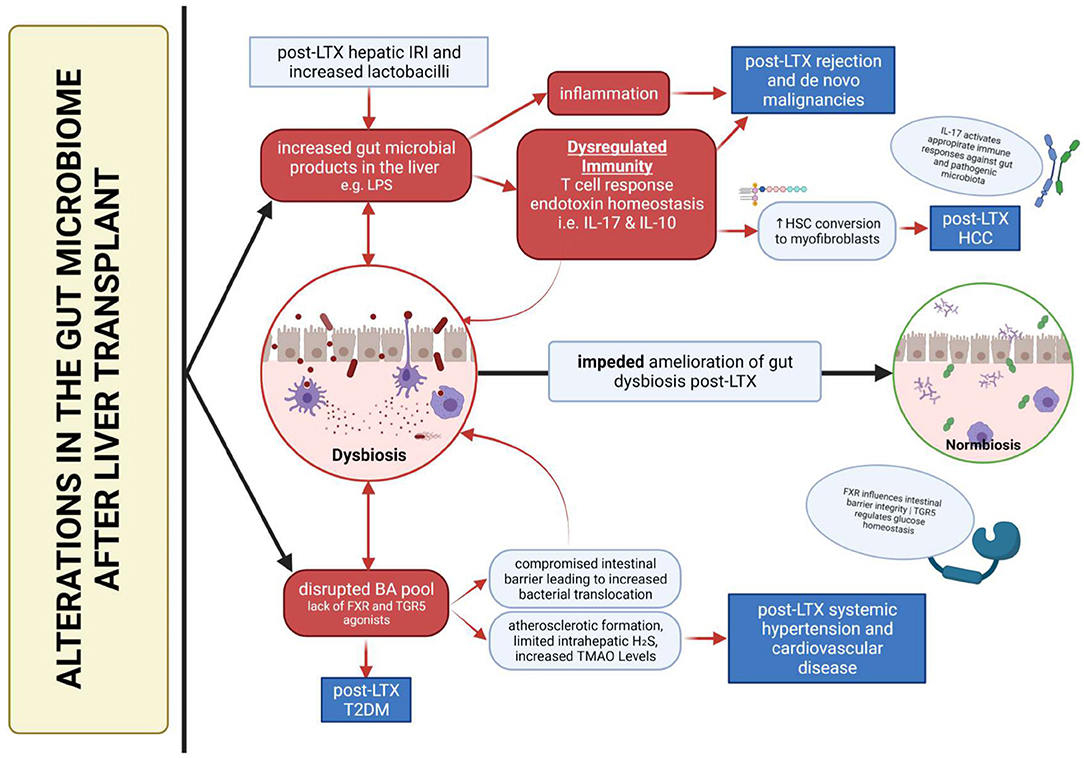
On a separate note, the inevitable modification of the BA pool creates drastic consequences after LTX, particularly through the gut liver axis due to its antimicrobial nature. It allows increased colonization of the microbiome by pathogens, which in turn causes further disruption of the BA pool (36). BAs also modulate a wide range of pro and anti-inflammatory genes induced by LPS (37), a key player in influencing the development of post-LTX complications. This, in tandem with a dysregulated immunity, may result in the creation of a downward spiral, which prevents dysbiosis amelioration and exacerbates the development post-LTX complications (Figure 2).
Post-transplant Dyslipidemia
Dyslipidemia is characterized by abnormal blood lipid profiles. It includes abnormal levels of cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. Its etiology can include additional risk factors like obesity, hypertension and DM. For example, obesity has been associated with a decrease in gut microbiome diversity and elevated circulating LPS levels of patients with metabolic issues (38). Hyperinsulinemia from post-transplant and gut microbial factors leads to increased hepatic uptake of FFA, such that hepatic synthesis of very-low-density lipoprotein (VLDL) is increased (39). Additionally, insulin resistance leads to reduced lipoprotein lipase, which results in lower triglyceride clearance (40). Hence, there is increased VLDL to LDL cholesterol conversion, resulting in increased LDL cholesterol levels and hyperlipidemia.
Post-transplant Systemic Hypertension and Cardiovascular Disease
Accumulating evidence points toward alterations in the gut microbiome playing a role in cardiovascular diseases (CVD). Hypertension (HTN), an established risk factor for CVD was found in patients with a dysbiotic gut microbiome. Additionally, an increase in proportion of HTN-associated pathogenic species of bacteria in the post-LTX microbiome will indicate a more severe occurrence of HTN. Gut-derived SCFAs exhibit protective effects against HTN development (41). This involves its ability to lower blood pressure via activation of G-protein coupled receptors and inhibition of histone deacetylases, according to a murine model (42). As such, reductions in SCFA-producing gut bacteria in post-LTX dysbiosis plays a role in the pathogenesis of post-LTX systemic hypertension.
CVD is also linked to a decrease in the SCFA butyrate-producing gut bacteria such as F. prausnitzii and Roseburia. This may be due to a negative correlation between the concentration of butyrate and C-reactive protein, which is recently implicated in atherosclerosis (43), the most common cause of CVD. Post-LTX dysbiosis mediated inhibition of FXR and TGR5 signaling by bile acids will aggravate atherosclerotic formation whilst inhibiting the anti-atherogenic and lipid-lowering effect of the FXR agonist INT-767 (44), thereby promoting CVD development.
Hydrogen sulfide H2S, which promotes vasodilation by targeting KATP channel protein, is produced via microbiota-driven anaerobic metabolism of sulfur-containing substrates. Liver cystathionine-γ-lyase (CSE) is involved in H2S generation. A deficient H2S/CSE system may be implicated in the development of CVD, particularly HTN due to its role in maintaining basal systolic blood pressure (45). This deficiency can be caused by dysbiosis-driven altered microbial metabolization of bile acids. This is because FXR which regulates CSE expression, is stimulated by the bile acid LCA. However, the overall extent of this impact involving the gut microbiome is not known.
The most researched microbial metabolite for CVD risk and development is Trimethylamine N-oxide (TMAO). The production of TMAO involves the gut microbial-dependent cleavage of dietary trimethylamine-containing compounds like choline to form trimethylamine (TMA), which is oxidized by liver flavin monooxygenase to form TMAO. The concentration of TMAO is influenced by changes in the composition of the gut microbiome after transplant (25). Elevated TMAO levels causes endothelial cell dysfunction through nuclear factor-κB (NF-κB) signaling. This involves the upregulation of inflammatory signals in endothelial cells, elevated oxidative stress and adhesion of leukocytes to endothelial cells, thus promoting vascular inflammation and atherosclerosis (46). TMAO also has a role in upregulating macrophage scavenger receptors, in particular scavenger receptor A1. Increased expression of these receptors promotes cellular accumulation of cholesterol via increased uptake of modified forms of LDLs (47). Hence, elevated TMAO levels due to a post-LTX dysbiotic microbiome can be implicated in the pathogenesis of atherosclerotic CVD and hypothesized to reflect increased risk for major adverse cardiovascular events in LTX patients.
Post-transplant Hepatocellular Carcinoma
Resultant endotoxemia from increased bacterial translocation to the liver allograft during dysbiosis can be implicated in the pathogenesis of recurrent HCC. A mouse model simulating a human-like HCC environment through diethylnitrosamine and hepatotoxin carbon tetrachloride (CCl4) showed how heightened expression of Toll-like receptor 4 (TLR4), due to elevated LPS concentrations in the liver from increased bacterial translocation, promotes hepatocarcinogenesis in a CCl4-dependent manner. This occurs through further upregulation of the hepamitogen epiregulin by HSCs and TLR4 (48). Epiregulin acts on quiescent HSCs, which are the main progenitors for myofibroblasts in the liver. There is increased proliferation and differentiation of quiescent HSCs into myofibroblasts (49). As HCC develops, these myofibroblasts are thought to transform into cancer-associated myofibroblasts that play an important role in inducing HCC (50). Hence, it can be hypothesized that post-LTX dysbiotic microbiomes containing elevated concentrations of LPS-producing gram negative bacteria promotes the pathogenesis of HCC via the LPS-TLR4 pathway. Prolonged exposure of abnormal and increased LPS levels due to persistent post-LTX residual dysbiosis plays a major role in the development of HCC in the long term (48). Alterations in gut microbiota resulting in a modified BA pool also influences HCC development by affecting antitumor surveillance via natural killer T cells and enabling tumor cell proliferation (51). As such, an altered microbiome after LTX can play a role in increasing the risk of recurrent HCC in patients. This scenario may prompt further research post-LTX dysbiosis-related recurrent HCC and relevant therapeutic targets to prevent recurrent HCC development.
Komiyama et al. in a study investigating the role of tumor-related microbiota in the pathogenesis of de novo HCC, identified Firmicutes, Proteobacteria, and Bacteroidetes as dominant tumor-associated microbiota. Moreover, they discovered Ruminococcus gnavus as a signature microbe for hepatitis B and/or hepatitis C virus-related HCC (52). However, the mechanisms at which these microbiotas relate to HCC development is not known. Such microbial characteristics may be indicative of recurrent HCC, if they play out similarly to that of de novo HCC. Further development in this area could enable the use of the gut microbiome as a non-invasive biomarker for diagnosis of recurrent HCC in patients. This may also enable early detection of recurrent HCC since the presence of the tumor-associated microbial characteristics might reflect a predisposition to HCC recurrence. This is clinically relevant since detection of early HCC influences patient prognosis (53).
Post-LTX Extrahepatic de novo Malignancies
LTX recipients are 2-3 times more likely to develop de novo malignancies as compared to the general population (54). Gut microbial dysbiosis arising from LTX contributes toward this risk of malignancy. This is because the gut microbiome influences systemic inflammation and immune homeostasis, thereby increasing host susceptibility to malignancies (55). For instance, variations in the gut microbiome can influence PD-L1, which regulates T cell response to tumor cells (56). Translocation of infectious agents, including opportunistic pathogens from the dysbiotic gut microbiome are implicated in the development of de novo solid organ malignancies (57). However, the specifics and mechanisms by which these microbiome-mediated malignancies occur is not clear due to limited studies.
Discussion
The human gut microbiome plays an important role in the pathogenesis and development of complications after LTX. Hence, gut microbiota presents itself as a very useful predictive tool for post-LTX outcomes. For example, Lu et al. demonstrated how the main difference between LTX recipients and healthy controls lies in the relative abundance of butyrate-producing bacteria. Considering butyrate's capacity to modulate hepatic immunity and mediate suppression of carcinogenesis, such decreases in the gut microbiome can inform clinicians of a LTX recipient's predisposition toward disrupted allograft tolerance and even early HCC development (58). In addition, a murine model indicated the use of microbial profiling in differentiating the cause of liver dysfunction – hepatic I/R injury or ACR. Of note, the study suggested gut microbiota as a more sensitive biomarker than hepatic histology in predicting post-LTX rejection and dysfunction. As such, fecal microbiota sampling can serve as a non-invasive and potentially better biomarker for early detection or prevention of the various post-LTX complications (59).
It is highly advantageous if the presence of reliable indicators, which raise a clinician's concern of complications, increases. This assists clinicians in the deliverance of timely treatment, especially allograft rejection which necessitates prompt therapy. This manuscript has demonstrated the potential roles of the gut microbiome as these predictive and prognostic indicators for clinicians in postoperative care. For example, a clinician can conduct timely analysis of the microbiome at various stages of a patient's liver transplant process. Coupled with knowledge over the impacts of various gut microbiota, a clinician can better predict, detect and asses the risk of various complications, thereby enabling better clinical decisions and prompt treatment.
The clinical significance of the gut microbiome is compounded because of the gut-liver axis – a circular relationship occurs between hepatic disease and dysbiosis. This prompts the consideration of therapeutic alteration of gut microbiota to break this vicious cycle. In addition, given immunosuppression-mediated malignancies and the liver being an immunotolerant organ capable of supporting immunosuppression discontinuation (60), clinicians should consider therapeutic alteration of the microbiome toward a composition that prevents or improves post-LTX rejection and other complications. For example, preventing the inhibition of the antitumor immune response, which arises from constant stimulation by intestinal antigens, can be achieved via alteration of the gut microbiome, hence avoiding HCC progression (61). Such therapeutic alterations of the gut microbiome can be conducted through the administration of probiotics or fecal microbiota transplants (FMT). Although there is limited evidence, existing literature on probiotic usage in LTX recipients suggest some efficacy in reducing post-LTX infection rates (62). On the other hand, FMT has demonstrated to be beneficial toward alcohol-related liver diseases and hepatic encephalopathy, but no studies were found to be conducted in the context of LTX (63, 64). Nonetheless, FMT and probiotics are likely to be effective, given correlations of specific microbiota with improved LTX outcomes. More studies are required to assess their efficacy and feasibility for clinical application. An area for consideration would be the usage of probiotics and FMTs in an immunocompromised LTX recipient, which may increase risk of infectious complications.
On a separate note, immunosuppressants, which play important roles in ensuring allograft tolerance can create unforeseen alterations in the gut microbiome with plausible repercussions. For example, tacrolimus increased Faecalibacterium prausnitzii, Bifidobacterium spp. and decreased Bacteroides-Prevotella, Enterobacteriaceae (65). Of note, Bacteroides-Prevotella and Enterobacteriaceae are the main LPS-producing gram-negative bacteria. The potential reduction of endotoxins suggests an additional ability of immunosuppressants to alter microbial composition, thereby influencing the development of post-LTX complications. Hence, immunosuppressant-microbiome interactions should be another area of consideration for clinicians, particularly if unexpected outcomes occur from immunosuppressant usage in their patients.
As such, the gut microbiome should be seen as a “tool” to improve upon and aid in liver transplant patient care. However, current understandings of the gut microbiome and its alterations after LTX alongside relevant consequences is inadequate for a significant clinical application at present due to limited number of human trials and small sample sizes. Future clinical trials and studies should assess microbial alterations in LTX patients and specific microbial characteristics when considering implicated post-LTX outcomes. Large data pools are required when considering how the same microbiota may exhibit different behaviors in different contexts, where certain species could be beneficial for some but harmful for others. An example of a study that can be conducted is to investigate the microbiota promoting the pathogenesis of PTDM as it is one of the most frequent complications after LTX (66). Greater focus can also be placed into the development of more accurate and efficient forms of metatranscriptomics to greater enable clinician access to and improved incorporation of the microbiome as part of liver transplant patient care.
Author Contributions
HW: conceptualization, data curation, writing (original draft), and writing (review and editing). WL, CN, and DT: conceptualization, data curation, and writing (review and editing). GB, AK, DH, MS, and MN: writing (review and editing) and supervision. NS and MM: conceptualization, writing (review and editing), and supervision. All authors contributed to the article and approved the submitted version.
Funding
NS is supported by the Dean's Research Development Award (DRDA) awarded by Yong Loo Lin School of Medicine in National University of Singapore, Wong Hock Boon Society Fellowship awarded by Yong Loo Lin School of Medicine in National University of Singapore, and Junior Research Award (JRA) awarded by National University Hospital (NUH) in National University Health System, Singapore (NUHS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACR, Acute cellular rejection; BA, Bile acids; BSH, Bile salt hydrolase; CVD, Cardiovascular disease; FMT, Fecal microbiota transplant; HBV, Hepatitis B virus; HCC, Hepatocellular carcinoma; HSC, Hepatic stellate cells; HTN, Hypertension; I/R, Ischemia-reperfusion; LPS, Lipopolysaccharide; LTX, Liver transplant; SCFA, Short-chain fatty acid; TLR, Toll-like receptor; TMAO, Trimethylamine N-oxide; Tregs, Regulatory T cells.
References
1. Samstein B, Smith A, Freise C, Zimmerman M, Baker T, Othloff K, et al. Complications and their resolution in recipients of deceased and living donor liver transplants: findings from the A2ALL cohort study. Am J Transplant. (2016) 16:594. doi: 10.1111/ajt.13479
2. Giannelli V, Di Gregorio V, Lebba V, Giusto M, Schippa S, Merli M, et al. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis. World J Gastroenterol. (2014) 20:16795–810. doi: 10.3748/wjg.v20.i45.16795
3. Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. (2014) 16:1024–33. doi: 10.1111/cmi.12308
4. Fukui H. Role of gut dysbiosis in liver diseases: what have we learned so far? Diseases. (2019) 7:58. doi: 10.3390/diseases7040058
5. Bajaj JS, Kakiyama G, Cox IJ, Nittono H, Takei H, White M, et al. Alterations in gut microbial function following liver transplant. Liver Transpl. (2018) 24:752. doi: 10.1002/lt.25046
6. Kriss M, Verna EC, Rosen HR, Lozupone CA. Functional microbiomics in liver transplantation: identifying novel targets for improving allograft outcomes. Transplantation. (2019) 103:668–78. doi: 10.1097/TP.0000000000002568
7. Ponziani FR, Valenza V, Nure E, Bianco G, Marrone G, Grieco A, et al. Effect of liver transplantation on intestinal permeability and correlation with infection episodes. PLoS ONE. (2020) 15:e0235359. doi: 10.1371/journal.pone.0235359
8. Wu ZW, Ling ZX, Lu HF, Zuo J, Sheng JF, Zheng SS, et al. Changes of gut bacteria and immune parameters in liver transplant recipients. Hepatobiliary Pancreat Dis Int. (2012) 11:40–50. doi: 10.1016/S1499-3872(11)60124-0
9. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am J Clin Oncol. (2016) 39:98–106. doi: 10.1097/COC.0000000000000239
10. Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Büschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. (1995) 22:226–9. doi: 10.1016/0168-8278(95)80433-1
11. Hackstein CP, Assmus LM, Welz M, Klein S, Schwandt T, Schultze J, et al. Gut microbial translocation corrupts myeloid cell function to control bacterial infection during liver cirrhosis. Gut. (2017) 66:507–18. doi: 10.1136/gutjnl-2015-311224
12. Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich J-M, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. (2011) 34:566. doi: 10.1016/j.immuni.2011.03.018
13. Taddio MF, Jaramillo CAC, Runge P, Blanc A, Keller C, Talip Z, et al. In vivo imaging of local inflammation: monitoring LPS-induced CD80/CD86 upregulation by PET. Mol Imaging Biol. (2021) 23:196. doi: 10.1007/s11307-020-01543-3
14. Rollins MR, Johnson RMG. CD80 expressed by CD8+ T cells contributes to PD-L1-induced apoptosis of activated CD8+ T cells. J Immunol Res. (2017) 2017:7659462. doi: 10.1155/2017/7659462
15. Wegorzewska MM, Glowacki RWP, Hsieh SA, Donermeyer DL, Hickey CA, Horvath SC, et al. Diet modulates colonic T cell responses by regulating the expression of a Bacteroides thetaiotaomicron antigen. Sci Immunol. (2019) 4:eaau9079. doi: 10.1126/sciimmunol.aau9079
16. Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. (2011) 478:250–4. doi: 10.1038/nature10434
17. Su L, Wu Z, Chi Y, Song Y, Xu J, Tan J, et al. Mesenteric lymph node CD4 + T lymphocytes migrate to liver and contribute to non-alcoholic fatty liver disease. Cell Immunol. (2019) 337:33–41. doi: 10.1016/j.cellimm.2019.01.005
18. Park SW, Kim M, Brown KM, D'agati VD, Lee HT. Paneth cell-derived interleukin-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology. (2011) 53:1662–75. doi: 10.1002/hep.24253
19. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. (2009) 139:485–98. doi: 10.1016/j.cell.2009.09.033
20. Ren W, Chen S, Zhang L, Liu G, Hussain T, Hao X, et al. Interferon tau affects mouse intestinal microbiota and expression of IL-17. Med Inflammation. (2016) 2016:2839232. doi: 10.1155/2016/2839232
21. He Z, Li Y, Ma S, Yang M, Ma Y, Ma C, et al. Degranulation of gastrointestinal mast cells contributes to hepatic ischemia-reperfusion injury in mice. Clin Sci. (2018) 132:2241–59. doi: 10.1042/CS20180662
22. Perez-Chanona E, Mühlbauer M, Jobin C. The microbiota protects against ischemia/reperfusion-induced intestinal injury through nucleotide-binding oligomerization domain-containing protein 2 (NOD2) signaling. Am J Pathol. (2014) 184:2965–75. doi: 10.1016/j.ajpath.2014.07.014
23. Ren J, Hu D, Mao Y, Yang H, Liao W, Xu W, et al. Alteration in gut microbiota caused by time-restricted feeding alleviate hepatic ischaemia reperfusion injury in mice. J Cell Mol Med. (2019) 23:1714. doi: 10.1111/jcmm.14069
24. Li R, Xie L, Li L, Chen X, Yao T, Tian Y, et al. The gut microbial metabolite, 3,4-dihydroxyphenylpropionic acid, alleviates hepatic ischemia/reperfusion injury via mitigation of macrophage pro-inflammatory activity in mice. Acta Pharm Sin B. (2021) 12:182–96. doi: 10.1016/j.apsb.2021.05.029
25. Ito T, Naini BV, Markovic D, Aziz A, Younan S, Lu M Hirao H, et al. Ischemia-reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am J Transplant. (2021) 21:614–25. doi: 10.1111/ajt.16219
26. Schurich A, Berg M, Stabenow D, Böttcher J, Kern M, Schild HJ, et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J Immunol. (2010) 184:4107–14. doi: 10.4049/jimmunol.0902580
27. Millán O, Rafael-Valdivia L, Torrademé E, López A, Fortuna V, Sánchez-Cabus S, et al. Intracellular IFN-γ and IL-2 expression monitoring as surrogate markers of the risk of acute rejection and personal drug response in de novo liver transplant recipients. Cytokine. (2013) 61:556–64. doi: 10.1016/j.cyto.2012.10.026
28. Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. (2016) 167:1125–36.e8. doi: 10.1016/j.cell.2016.10.020
29. Alameddine J, Godefroy E, Papargyris L, Sarrabayrouse G, Tabiasco J, Bridonneau C, et al. Faecalibacterium prausnitzii skews human DC to prime IL10-producing T cells through TLR2/6/JNK signaling and IL-10, IL-27, CD39, and IDO-1 induction. Front Immunol. (2019) 10:143. doi: 10.3389/fimmu.2019.00143
30. Guo Y, Crnkovic CM, Won KJ, Yang X, Lee JR, Orjala J, et al. Commensal gut bacteria convert the immunosuppressant tacrolimus to less potent metabolites. Drug Metab Dispos. (2019) 47:194–202. doi: 10.1124/dmd.118.084772
31. Won S-M, Park E, Jeong J-J, Ganesan R, Gupta H, Gebru YA, et al. The gut microbiota-derived immune response in chronic liver disease. Int J Mol Sci. (2021) 22:8309. doi: 10.3390/ijms22158309
32. Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. (2015) 3:5. doi: 10.3978/j.issn.2305-5839.2014.12.06
33. Holter MM, Chirikjian MK, Briere DA, Maida A, Sloop KW, Schoonjans K, et al. Compound 18 improves glucose tolerance in a hepatocyte TGR5-dependent manner in mice. Nutrients. (2020) 12:2124. doi: 10.3390/nu12072124
34. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. (2007) 56:1761–72. doi: 10.2337/db06-1491
35. Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. (2013) 8:e0071108. doi: 10.1371/journal.pone.0071108
36. Cheng SL, Li X, Lehmler HJ, Phillips B, Shen D, Cui JY. Gut microbiota modulates interactions between polychlorinated biphenyls and bile acid homeostasis. Toxicol Sci. (2018) 166:269–87. doi: 10.1093/toxsci/kfy208
37. Wammers M, Schupp A-K, Bode JG, Ehlting C, Wolf S, Deenen R, et al. Reprogramming of pro-inflammatory human macrophages to an anti-inflammatory phenotype by bile acids. Sci Rep. (2018) 8:255. doi: 10.1038/s41598-017-18305-x
38. Harsch IA, Konturek PC. The role of gut microbiota in obesity and type 2 and type 1 diabetes mellitus: new insights into “old” diseases. Med Sci. (2018) 6:32. doi: 10.3390/medsci6020032
39. Hays AP, Hill RBJ. Enzymes of lipid synthesis in the liver of the cortisone-treated rat. Biochim Biophys Acta. (1965) 98:646–8. doi: 10.1016/0005-2760(65)90164-5
40. Ibels SL, Reardon MF, Nestel PJ. Plasma post-heparin lipolytic activity and triglyceride clearance in uremic and hemodialysis patients and renal allograft recipients. J Lab Clin Med. (1976) 87:648–58.
41. Yang F, Chen H, Gao Y, An N, Li X, Pan X, et al. Gut microbiota-derived short-chain fatty acids and hypertension: mechanism and treatment. Biomed Pharmacother. (2020) 130:110503. doi: 10.1016/j.biopha.2020.110503
42. Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks C, et al. HDAC inhibition attenuates inflammatory, hypertrophic and hypertensive responses in spontaneously hypertensive rats. Hypertension. (2010) 56:437. doi: 10.1161/HYPERTENSIONAHA.110.154567
43. Trøseid M, Andersen GØ, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. EBioMedicine. (2020) 52:102649. doi: 10.1016/j.ebiom.2020.102649
44. Miyazaki-Anzai S, Masuda M, Kohno S, Levi M, Shiozaki Y, Keenan AL, et al. Simultaneous inhibition of FXR and TGR5 exacerbates atherosclerotic formation. J Lipid Res. (2018) 59:1709–13. doi: 10.1194/jlr.M087239
45. Pearson RJ, Wilson T, Wang R. Endogenous hydrogen sulfide and the cardiovascular system-what's the smell all about? Clin Invest Med. (2006) 29:146–50.
46. Chou R-H, Chen C-Y, Chen I-C, Huang H-L, Lu Y-W, Kuo C-S, et al. Trimethylamine N-oxide, circulating endothelial progenitor cells, and endothelial function in patients with stable angina. Sci Rep. (2019) 9:4249. doi: 10.1038/s41598-019-40638-y
47. Mohammadi A, Najar AG, Yaghoobi MM, Jahani Y, Vahabzadeh Z. Trimethylamine-N-oxide treatment induces changes in the ATP-binding cassette transporter a1 and scavenger receptor A1 in murine macrophage J774A.1 cells. Inflammation. (2016) 39:393–404. doi: 10.1007/s10753-015-0261-7
48. Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. (2012) 21:504–16. doi: 10.1016/j.ccr.2012.02.007
49. Tomita K, Haga H, Mizuno K, Katsumi T, Sato C, Okumoto K, et al. Epiregulin promotes the emergence and proliferation of adult liver progenitor cells. Am J Physiol Gastrointest Liver Physiol. (2014) 307:50–7. doi: 10.1152/ajpgi.00434.2013
50. Yavuz BG, Pestana RC, Abugabal YI, Krishnan S, Chen J, Hassan MM, et al. Origin and role of hepatic myofibroblasts in hepatocellular carcinoma. Oncotarget. (2020) 11:1186. doi: 10.18632/oncotarget.27532
51. Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. (2018) 360:5931. doi: 10.1126/science.aan5931
52. Komiyama S, Yamada T, Takemura N, Kokudo N, Hase K, Kawamura YI. Profiling of tumour-associated microbiota in human hepatocellular carcinoma. Sci Rep. (2021) 11:10589. doi: 10.1038/s41598-021-89963-1
53. Setsu T, Tsuchiya A, Watanabe T, Nagoya T, Ikarashi S, Hayashi K, et al. Early detection of hepatocellular carcinoma recurrence using the highly sensitive fucosylated fraction of alpha-fetoprotein. Case Rep Gastroenterol. (2017) 11:142–7. doi: 10.1159/000462969
54. Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl. (2012) 18:1277–89. doi: 10.1002/lt.23531
55. Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. (2018) 33:570–80. doi: 10.1016/j.ccell.2018.03.015
56. Routy B, Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
57. Liu X, Cheng Y, Zang D, Zhang M, Li X, Liu D, et al. The role of gut microbiota in lung cancer: from carcinogenesis to immunotherapy. Front Oncol. (2021) 11:720842. doi: 10.3389/fonc.2021.720842
58. Lu HF, Ren ZG, Li A, Zhang H, Xu SY, Jiang JW, et al. Fecal microbiome data distinguish liver recipients with normal and abnormal liver function from healthy controls. Front Microbiol. (2019) 10:1518. doi: 10.3389/fmicb.2019.01518
59. Ren Z, Jiang J, Lu H, Chen X, He Y, Zhang H, et al. Intestinal microbial variation may predict early acute rejection after liver transplantation in rats. Transplantation. (2014) 98:844. doi: 10.1097/TP.0000000000000334
60. Moini M, Schilsky ML, Tichy EM. Review on immunosuppression in liver transplantation. World J Hepatol. (2015) 7:1355. doi: 10.4254/wjh.v7.i10.1355
61. Ponziani FR, Nicoletti A, Gasbarrini A, Pompili M. Diagnostic and therapeutic potential of the gut microbiota in patients with early hepatocellular carcinoma. Ther Adv Med Oncol. (2019) 11:1758835919848184. doi: 10.1177/1758835919848184
62. Jorgenson MR, Descourouez JL, Siodlak M, Tjugum S, Rice JP, Fernandez LA. Efficacy and safety of probiotics and synbiotics in liver transplantation. Pharmacotherapy. (2018) 38:758–68. doi: 10.1002/phar.2130
63. Shasthry SM. Fecal microbiota transplantation in alcohol related liver diseases. Clin Mol Hepatol. (2020) 26:294. doi: 10.3350/cmh.2020.0057
64. Hassouneh R, Bajaj JS. Gut microbiota modulation and fecal transplantation: an overview on innovative strategies for hepatic encephalopathy treatment. J Clin Med. (2021) 10:330. doi: 10.3390/jcm10020330
65. Jiang J-W, Ren Z-G, Lu H-F, Zhang H, Li A, Cui G-Y, et al. Optimal immunosuppressor induces stable gut microbiota after liver transplantation. World J Gastroenterol. (2018) 24:3871. doi: 10.3748/wjg.v24.i34.3871
Keywords: liver transplantation, gut microbiome, immunity, rejection, cancer, metabolic disease, prevention
Citation: Wong HJ, Lim WH, Ng CH, Tan DJH, Bonney GK, Kow AWC, Huang DQ, Siddiqui MS, Noureddin M, Syn N and Muthiah MD (2022) Predictive and Prognostic Roles of Gut Microbial Variation in Liver Transplant. Front. Med. 9:873523. doi: 10.3389/fmed.2022.873523
Received: 10 February 2022; Accepted: 06 April 2022;
Published: 10 May 2022.
Edited by:
Roberto Gramignoli, Karolinska Institutet (KI), SwedenReviewed by:
Juan Pablo Roblero, Clinical Hospital of the University of Chile, ChileFrancesco Ravaioli, University of Bologna, Italy
Copyright © 2022 Wong, Lim, Ng, Tan, Bonney, Kow, Huang, Siddiqui, Noureddin, Syn and Muthiah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark D. Muthiah, bWRjbWRtQG51cy5lZHUuc2c=; orcid.org/0000-0002-9724-4743; Nicholas Syn, bmljaG9sYXNzeW5AZ21haWwuY29t
 Hon Jen Wong
Hon Jen Wong Wen Hui Lim
Wen Hui Lim Cheng Han Ng
Cheng Han Ng Darren Jun Hao Tan2
Darren Jun Hao Tan2 Alfred W. C. Kow
Alfred W. C. Kow Mazen Noureddin
Mazen Noureddin Nicholas Syn
Nicholas Syn Mark D. Muthiah
Mark D. Muthiah