- 1Department of Gastroenterology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Clinical Immunology Translational Medicine Key Laboratory of Sichuan Province, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
Background: Ulcerative colitis (UC) is characterized by refractory and recurrent mucosal inflammation, leading to a substantial healthcare burden. Diagnostic biomarkers predicting disease activity and treatment response remain elusive. To evaluate the application value of a novel neutrophil-based index (the neutrophil-to-albumin ratio, NAR) as a novel diagnostic biomarker in patients with UC and a predictive marker for disease activity and response to infliximab (IFX) therapy.
Methods: Clinical characteristics and laboratory parameters of enrolled subjects (patients with UC and healthy controls) were retrieved from the electronic medical record database of our hospital. Serum cytokine and fecal calprotectin levels were measured by enzyme-linked immunosorbent assay (ELISA). Mucosal expression levels of inflammatory agents were measured by quantitative RT-PCR (qRT-PCR).
Results: We found that NAR, which had not yet been explored in UC, was significantly increased in patients with UC (n = 146) compared to that in controls (n = 133) (1.95 ± 0.41 vs. 1.41 ± 0.23, p < 0.0001). NAR showed a positive association with the disease activity and inflammatory load in patients with UC. Pre-treatment NAR was significantly lower in IFX responders than that in non-responders (2.18 ± 0.29 vs. 2.44 ± 0.21, p = 0.0118), showing a significant ability to discriminate initial responders from primary non-responders to IFX induction therapy (AUC = 0.7866, p = 0.0076). Moreover, pre-treatment NAR predicted postinduction serum IFX trough level.
Conclusion: Our study provides evidences to utilize NAR in the diagnosis, activity monitoring, and IFX response prediction in patients with UC.
Introduction
Inflammatory bowel disease (IBD) is characterized by chronic and relapsing inflammation in the gastrointestinal tract, including two eminent forms, ulcerative colitis (UC) and Crohn's disease (CD). The prevalence of IBD is rapidly growing worldwide and rising health expenditures (1–4), leading to a substantial healthcare burden (5). In addition, IBD is medically refractory and has become a great clinical challenge.
Endoscopic biopsies have been recognized as the diagnostic gold standard in IBD. However, endoscopy has drawbacks that limit its application particularly in long-term follow-up, such as invasiveness, high cost, and inter-observer variability (6). In addition, patients may not tolerate endoscopic examinations very well and those who undergo endoscopy may complained embarrassment, discomfort caused by bowel preparation, and increased abdominal pain (7, 8).
Besides endoscopy, a panel of adjunctive serological biomarkers, which are much less-invasive, have been applied at nearly every point in the management of IBD. They have shown practical values to distinguish IBD from gastrointestinal functional disease, differentiate active from inactive status, and predict therapeutic effect, recurrence, prognosis (9).
Blood tests including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), fibrinogen, albumin, bilirubin, uric acid, auto-antibodies (e.g., anti-pTNP, ASCA antibodies) are widely utilized in IBD management (10–14), as well as fecal calprotectin and lactoferrin (15). Given the crucial role of neutrophils in the disease pathogenesis (16, 17), neutrophil-based indexes have been generated and applied in the IBD area (18–23). For example, the neutrophil-to-lymphocyte ratio (NLR) is easily accessible from routine blood tests for both inpatients and outpatients, and it has been strongly suggested as a valuable predictive marker to distinguish patients with IBD from non-IBD controls and differentiate disease activity in IBD (24).
Recently, the neutrophil-to-albumin ratio (NAR) has emerged as a sensitive index which indicates systemic inflammation and has been used in inflammatory, vascular diseases, and cancers (25–27). Since serum biochemical tests are also routine procedures to evaluate the nutritional status and screen renal/hepatobiliary dysfunctions in patients with IBD, NAR can be readily obtained for nearly any patient under care for IBD. However, to our best knowledge, this index has not yet been utilized in UC.
Therefore, we questioned whether NAR could be used as a reliable, non-invasive, and cost-effective biomarkers, regarding disease diagnosis, activity monitoring, or drug response prediction. In this study, we evaluated the application value of NAR as a diagnostic biomarker in patients with UC and a predictive marker for disease activity and response to infliximab (IFX) induction therapy.
Methods
Participants
This prospective study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board for Clinical Research of Sichuan Provincial People's Hospital (No.201685, 2020204).
Patients with UC were consecutively recruited from the Department of Gastroenterology of Sichuan Provincial People's Hospital between January 2017 and December 2021. We also consecutively recruited non-IBD subjects, who underwent routine physical examinations in our hospital during the study. Participants enrolled in the current study were well-informed and signed an informed consent before participation. Patients with UC and controls were gender- and age-matched. Clinical characteristics and laboratory parameters of enrolled subjects were retrieved from the electronic medical record database of our hospital.
Exclusion criteria for both patients with UC and controls were as follows: smoking, excessive drinking, hematopoietic system disease, hepatobiliary disease, coagulation abnormalities, taking medications that can affect blood cell components or serum biochemistry profiles, hypertension, diabetes, infections, other systemic autoimmune diseases, other gastrointestinal diseases, and cancers. Demographics and clinical parameters of patients with UC (n = 146) and healthy controls (n = 133) were shown in Table 1.
As reported previously (28, 29), the diagnosis of UC was performed according to the comprehensive analysis of medical history, clinical manifestations, radiological, endoscopic, and histological examinations, as well as laboratory tests. The Mayo score system was utilized to determine the disease activity in patients with UC. To detailedly evaluate mucosal disease activity, the ulcerative colitis endoscopic index of severity (UCEIS) system was used. The disease extent of UC was identified based on the Montreal classification.
Infliximab (IFX) induction therapy was administered as reported previously (28). Briefly, patients with UC were infused with IFX (5 mg/kg) at weeks 0, 2 and 6 for induction. Short-term response to IFX were evaluated at 12–14 weeks post the initial infusion, when serum IFX trough levels were determined. Response (complete or partial) or primary non-response was defined by the physicians. To determine the predictive value of NAR in IFX response, patients were subjected to complete blood cell and serum biochemistry tests within 1 week before the first infusion of IFX, and pre-treatment NAR was calculated.
Mucosal Inflammatory Agent Assessment
Mucosal biopsy tissues were collected during endoscopic examinations, immediately frozen in liquid nitrogen, and then sent to our laboratory for extended storage at −80°C. Total RNA was extracted from mucosal tissues using TRIzol reagent (Thermo Scientific). Reverse transcription for mRNA and microRNA (miR) were performed, respectively. Relative expression of cytokines and miR-301a was determined by qRT-PCR using a SYBR Green real-time PCR system (Invitrogen, CA, USA). The GAPDH and U6 expression levels were employed to normalize the expression of mRNA and miR, respectively (28).
Measurement of Serum Cytokine and Fecal Calprotectin
Serum and fecal samples were collected, processed within 1 h, and stored at −80°C. Enzyme-linked immunosorbent assay (ELISA) was performed as described previously (28, 29). All ELISA kits (TNF-α, IFN-γ, calprotectin) were purchased from BioLegend (San Diego, CA, USA) and utilized according to the manufacturer's instructions.
Statistical Analysis
Data are presented as mean ± SD when applicable. Chi-square test was performed to examine the difference of gender between patients with UC and controls. Comparisons between UC and controls regarding age and clinical parameters were performed by unpaired Student's t-test (two-tailed). The difference of NAR between IFX responders and primary non-responders was also examined by unpaired Student's t-test (two-tailed). The discriminating performance of a biomarker in indicated scenarios was determined by receiver operator curves (ROC) analysis. Correlations between two parameters were analyzed using Pearson's correlation analysis. p < 0.05 was set as statistically significant. All statistical analysis was performed using a Prism software Version 8.4 (Graphpad Software, San Diego, California, USA).
Results
Demographics and Clinical Parameters of the Participants
In the current study, 146 patients with UC (female = 52, male = 94) and 133 healthy controls (female = 50, male = 83) were included (Table 1). The mean age of patients with UC and healthy control was 38.5 ± 9.8 and 37.2 ± 10.4 years old, respectively. The duration of disease in patients with UC was 35.4 ± 16.9 months. There were no significant differences between patients with UC and healthy control regarding the age (p = 0.2834) and gender (p = 0.7319). Disease extent of UC was identified according to the Montreal classification system.
Neutrophil percentages were determined by complete blood cell tests, and albumin levels were examined by serum biochemistry tests. In line with existing evidences (11), patients with UC, compared to healthy controls, displayed a significant increase in neutrophil percentages (69.66 ± 11.04 vs. 59.38 ± 8.52 %, p < 0.0001) and a decrease in serum albumin levels (36.80 ± 7.31 vs. 42.40 ± 2.57 g/L, p < 0.0001).
Next, we calculated NAR as the ratio of neutrophil percentages over serum albumin levels and found that NAR was up-regulated by 1.4-fold in patients with UC compared with in healthy controls (1.95 ± 0.41 vs. 1.41 ± 0.23, p < 0.0001).
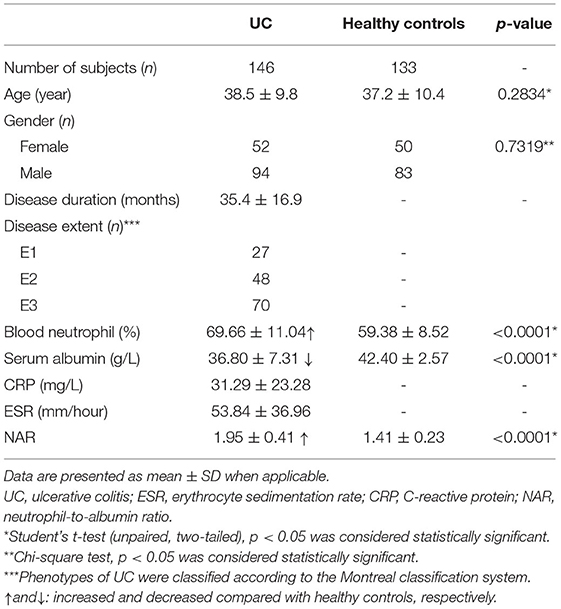
In regard to the ability to discriminate UC from controls, we performed ROC analysis (Figure 1), showing that NAR had larger AUC (AUC = 0.8670) compared to neutrophil (AUC = 0.7750) or albumin (AUC = 0.7569) alone. Taken together, these data suggest that NAR could be a practical, rapid, and easily accessible biomarker for UC diagnosis.
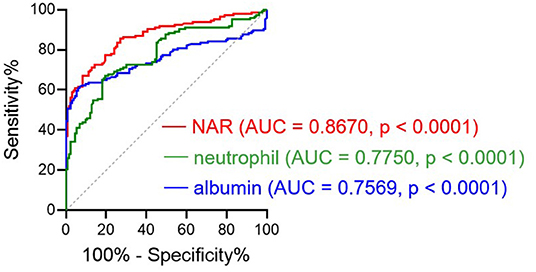
Figure 1. Receiver operating characteristics (ROC) curve analysis. The performances of the neutrophil-to-albumin ratio (NAR), blood neutrophil percentages, and serum albumin levels to discriminate ulcerative colitis (UC) from controls were determined by ROC curve analysis. AUC, area under the ROC curve. p < 0.05 was considered significant. UC, n = 146; Controls, n = 133.
NAR Reflects Disease Activity in UC
Since NAR could be used as a diagnostic biomarker for UC, we moved forward to explore whether this marker was able to distinguish UC activity. We looked at the potential association between NAR and Mayo scores, and patients with higher NAR exhibited more severe disease activity (Figure 2A, Pearson r = 0.7032, p < 0.0001).
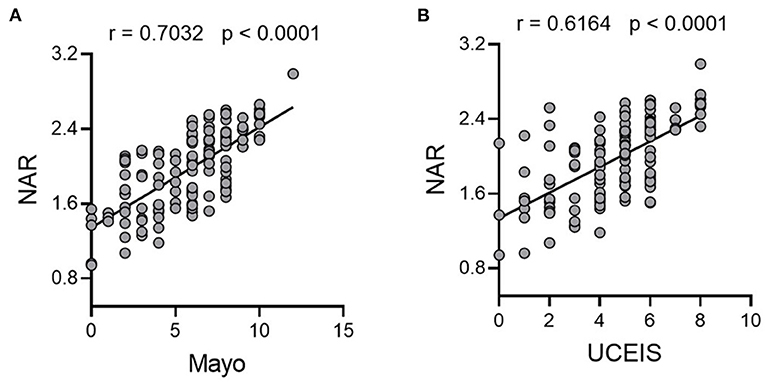
Figure 2. NAR reflects disease activity in UC. All enrolled patients with UC (n = 146) were subjected to disease activity evaluation by the Mayo score and the ulcerative colitis endoscopic index of severity (UCEIS) system. Associations between NAR and (A) Mayo score, and (B) UCEIS were examined. The correlation analysis was performed using Pearson's correlation. p < 0.05 was considered significant.
Additionally, mucosal healing has been highlighted in the management of IBD and amounts of evidences have stressed that patients with mucosal healing display a lower rate of hospitalization and better prognosis (30–33). Therefore, we also employed UCEIS, which had a high interobserver agreement rate (34, 35), as an additional system to detailedly investigate the correlation between NAR and the endoscopic activity of patients with UC. As shown in Figure 2B, NAR showed a positive association with mucosal disease activity (Pearson r = 0.6164, p < 0.0001).
Associations Between NAR and Inflammatory Load in Patients With UC
Both mucosal and systemic inflammatory conditions are related to disease severity in patients with UC, and higher inflammatory responses tend to indicate more severe disease activity and poorer drug responses (36). To this end, we first looked at serum inflammatory factors. CRP and ESR are the most commonly used disease activity biomarkers of IBD and up-regulated serum pro-inflammatory cytokines such as TNF-α and IFN-γ are hallmarks of IBD. We found that NAR was positively correlated with CRP, ESR, serum TNF-α and IFN-γ (Figures 3A–D), suggesting that NAR can be utilized as a marker reflecting the systemic inflammatory load in patients with UC.
Figure 3. Associations of NAR with inflammatory load in patients with UC. Correlation of NAR with (A) C-reactive protein (CRP) and (B) erythrocyte sedimentation rate (ESR). (A,B), n = 146. Serum (C) TNF-α and (D) IFN-γ protein levels were positively associated with NAR. (C,D), n = 122. (E) Fecal calprotectin levels (n = 66), mucosal (F) TNF-α, (G) IFN-γ, and (H) miR-301a relative expression levels positively correlated with NAR. (F–H), UC, n = 110; Controls, n = 75 (for gene relative expression calculation).
Subsequently, we examined associations between NAR and mucosal inflammatory responses. Since neutrophilic accumulation in the inflamed mucosa is intimately associated with the disease activity in UC, it has been suggested that detection of inflammatory proteins secreted by neutrophils in the feces may be a promising procedure for mucosal inflammation assessment (37). Fecal calprotectin is a neutrophil-derived protein that has been applied as a reliable screening tool for intestinal inflammation due to its sensitivity. As shown in Figure 3E, NAR correlated well with fecal calprotectin levels in UC.
Next, we analyzed mucosal expression of TNF- α and IFN- γ mRNA, both of which were also positively associated with NAR (Figures 3F,G). In addition, our previous studies have demonstrated that mucosal miR-301a is a useful biomarker for diagnosis and disease activity in UC (28, 29). Therefore, we employed mucosal expression of miR-301a as an inflammatory factor of patients with UC, which was positively associated with NAR (Figure 3H). Collectively, our findings demonstrate that NAR, which is more convenient and faster than cytokine and fecal protein measurement, might assist to evaluate the systemic and mucosal inflammatory load in patients with UC.
NAR Predicts Response to IFX in Patients With UC
The therapy for IBD has been revolutionized, thanks to biologics, especially anti-TNF agents (such as IFX), which are effective to induce and maintain remission in patients with both UC and CD. However, IFX does not work well in each patient, and about 65% of patients with UC respond to IFX induction therapy and the rate drops down to 45.5% during the maintenance therapy (38). Therefore, efforts have been made to identify reliable predictive biomarkers for both short- and long-term response to IFX.
To this end, we questioned whether NAR played as a predictor in this scenario. We enrolled 34 patients with UC, who were treated with IFX therapy (5 mg/kg at weeks 0, 2 and 6), including 23 responders (both complete or partial) and 11 with primary non-responders (evaluated at 12–14 weeks after the initial infusion). Patients were subjected to complete blood cell and serum biochemistry tests within 1 week before the first infusion of IFX, and pre-treatment NAR was calculated. As shown in Figure 4A, pre-treatment NAR was significantly lower in IFX responders than that in non-responders (2.18 ± 0.29 vs. 2.44 ± 0.21, p = 0.0118).
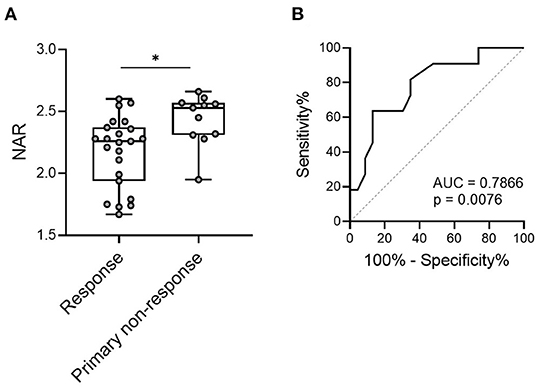
Figure 4. NAR predicts short-term response to infliximab (IFX) in patients with UC. Patients with UC (n = 34) were infused with IFX (5 mg/kg) at weeks 0, 2 and 6 for induction. Short-term response to IFX were evaluated at 12–14 weeks post initial infusion. Patients were subjected to complete blood cell and serum biochemistry tests within 1 week before the first infusion of IFX, and pre-treatment NAR was calculated. (A) Comparison of pre-treatment NAR was performed between responders (complete and partial, n = 23) and primary non-responders (n = 11). *p < 0.05, unpaired Student's t-test (two-tailed). (B) The ability of NAR to discriminate IFX responders from primary non-responders were determined by ROC curve analysis. p < 0.05 was considered significant.
Next, we performed the receiver operating characteristics (ROC) curve analysis to determine the discriminative performance of NAR as predictors for response to IFX induction. NAR exhibited a significant ability to discriminate between initial responders and primary non-responders to IFX induction therapy (Figure 4B, AUC = 0.7866, p = 0.0076).
NAR Predicts Serum IFX Trough Level (SIFX TL) in Patients With UC
Since one contributing factor of primary non-response is low serum trough levels in IFX-treated patients (39, 40), and IFX clearance is accelerated in patients with high inflammatory load, causing insufficient clinical response (41, 42). As presented above, we demonstrated that NAR was a reflective marker of systemic and mucosal inflammatory conditions and higher NAR indicated lower rate of IFX response. Therefore, we sought to explore whether NAR was associated with postinduction sIFX TL. As shown in Figure 5, all 34 patients who received IFX therapy were grouped by quartiles of NAR and patients in quartiles 1 (≥ 1.67– < 2.11) exhibited significantly higher sIFX TL than those in other 3 quartiles. The lowest sIFX TL was observed in quartiles 4 (≥ 2.55), and no differences was found between quartiles 2 (≥ 2.11– < 2.31) and 3 (≥ 2.31– < 2.55). Collectively, these observations suggest that pre-treatment NAR might be a potential predictor for postinduction sIFX TL.
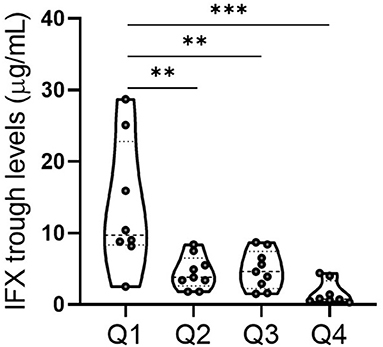
Figure 5. Association between NAR and serum IFX trough level (sIFX TL) in patients with UC. All 34 patients who received IFX induction therapy were grouped by quartiles of NAR. Q1 (quartiles 1, n = 8): 1.67 ≤ NAR < 2.11; Q2 (quartiles 2, n = 9): 2.11 ≤ NAR < 2.31; Q3 (quartiles 3, n = 9): 2.31 ≤ NAR < 2.55; Q4 (quartiles 4, n = 8): 2.55 ≤ NAR. sIFX TL in each group were measured. **p < 0.01, ***p < 0.001. One-way ANOVA followed by Tukey's multiple comparisons test was performed and adjusted p-values were calculated.
Discussion
In the current study, we analyzed the alterations of a novel neutrophil- and albumin-based biomarker in patients with UC, demonstrating that: (1) NAR was significantly up-regulated in patients with UC compared with that in controls; (2) NAR showed significantly positive associations with the disease activity and inflammatory load in UC; (3) higher pre-treatment NAR indicated lower rate of short-term response to IFX therapy in patients with UC; (4) pre-treatment NAR predicted postinduction sIFX TL. These observations prompt us to speculate that NAR may be a promising biomarker in the diagnosis, activity monitoring, and IFX response prediction in patients with UC.
Although endoscopic examinations are the most powerful tool that can directly identify the lesion extent, location, and inflammation activity, several limitations are noteworthy such as high cost, inconvenience, and invasiveness, making this procedure unsuitable for frequent, long-term surveillance of UC. Given the advantage of non-invasiveness, serological and fecal biomarkers that can accurately reflect the gastrointestinal mucosal inflammatory status have become a resurgent interest in the IBD area (10).
To date, a number of such biomarkers have been reported (6, 43). As the most commonly used biomarkers, both CPR and ESR are rapid, easily-measured, and sensitive in the assessment of IBD activity. However, they are not disease specific and rise fast under conditions other than IBD (tissue necrosis, infection, or other causes), which largely limits their practical significance (15).
Fecal calprotectin is now one of the most sensitive biological indicators for discriminating IBD from bowel dysfunction, and the endoscopically active from the inactive (37). However, for some patients, stool-based tests might not be their first choice, probably related to a reluctance to collect and process fecal samples or high cost. Therefore, rapid, convenient, inexpensive, standardized, and reproducible blood-based tests may be a preferable option for most patients who need long-term monitoring.
Complete blood cell and serum biochemical tests are two most common routine examinations for the management of both inpatients and out patients with IBD. In the current study, we employed a newly-identified index calculated as the ratio of neutrophil percentages (from complete blood cell tests) over serum albumin levels (from serum biochemical tests). Since excessive mucosal neutrophil infiltration is a remarkable feature of UC, which plays a crucial role in crypt abscesses and cryptitis, patients with mucosal histologic normalization display better clinical outcomes (44).
On the other hand, patients with IBD usually present nutritional deficiency, which leads to hypoalbuminemia. Moreover, albumin has been reported to have powerful antioxidant properties and could serve as serum biomarkers in oxidative stress/inflammation-associated disorders (45). It has been reported that patients with IBD have lower levels of serum albumin, and hypoproteinemia might reduce therapeutic efficiencies of biologics (46). In the current study, we identified a novel neutrophil- and albumin-based index NAR was significantly increased in patients with UC compared to that in healthy controls. More notably, NAR served as a reliable marker to reflect endoscopic activity and mucosal inflammatory responses in patients with UC.
Anti-tumor necrosis factor (TNF) therapies have revolutionized the treatment of patients with IBD. Particularly, IFX, as the most-investigated anti-TNF agent, is effective for induction and maintenance of clinical remission and mucosal healing in patients with moderate-to-severe UC, who have lower hospitalization and colectomy rates during follow-up compared to placebo-controls (38). However, about a third of IFX-treated patients with IBD fail to show significant clinical benefit after the induction therapy (14 weeks after the initiation infusion), which is called “primary non-response” (40). Furthermore, secondary non-response during the first year of maintenance therapy can be found in ~40% of patients (47).
Since IFX does not work in all patients and has possibilities of major adverse effects, as well as high expense, it is important to develop predictive biomarkers for the loss of response in patients. Progress has been made in this research field and a panel of biomarkers have been identified, such as patient-related (age, weight, smoke, et al.) and disease-related (disease duration, CRP, albumin, et al.) (46). Regrettably, most markers have not shown practical application and many others remain controversial (46). Here, we found that NAR, which integrated neutrophils and albumin, showed significant abilities to discriminate IFX responders from primary non-responders, providing a new approach for clinical practice.
A number of evidences have suggested that the loss of response to IFX correlates with low levels of serum IFX trough levels (41). In the current study, we observed that patients with higher pre-treatment NAR had lower serum IFX trough concentration. It has been reported that patients in high inflammatory status may have a higher rate of IFX clearance, leading to both insufficient clinical response and development of antibodies toward IFX (ATI) (42, 48, 49). We here demonstrated a role of NAR as an indicator for both systemic and mucosal inflammatory load and patients with more severe UC displayed higher NAR, which might promote IFX clearance and explain their loss of response to this agent.
In summary, we found a novel neutrophil- and albumin-based index NAR was significantly elevated in patients with UC, and showed satisfactory performance in the assessment of disease activity and inflammatory load. In note, NAR displayed predictive values of primary response to IFX induction therapy in patients with UC. Despite these findings, several issues need to be addressed in the following studies: studies with large sample sizes are needed to verify our current observations; whether NAR could be used in long-term follow-up to predict secondary non-response to IFX; the practical significance of NAR for other biologics (such as Vedolizumab and Ustekinumab) need to be investigated. Nevertheless, the current study provides evidences to utilize NAR in the diagnosis, activity monitoring, and IFX response prediction in patients with UC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board for Clinical Research of Sichuan Provincial People's Hospital (No.201685, 2020204). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CH and CG conceptualized, designed the study plan, and edited the manuscript. ZZ, YZ, and XY collected clinical information and samples from enrolled subjects. LL, YP, and CG diagnosed the patients. CH, CG, and YZ analyzed the data and prepare the original draft. ZZ and CH revised the manuscript. All authors discussed, revised the manuscript, and agreed to the published version of the manuscript.
Funding
This work was financially supported by grants from the National Natural Science Foundation of China (82070985 and 82170579) and Foundation of Sichuan Science and Technology Department (2021JDJQ0044).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IBD, inflammatory bowel disease; CD, Crohn's disease; UC, ulcerative colitis; NAR, neutrophil-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; ROC, receiver operating characteristics; PCR, polymerase chain reaction; qRT-PCR, quantitative Real-time PCR; ELISA, enzyme-linked immunosorbent assay; IFX, infliximab; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; TNF, tumor necrosis factor; IFN, interferon; miR, microRNA; UCEIS, ulcerative colitis endoscopic index of severity; AUC, area under the curve.
References
1. Collaborators GBDIBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2020) 5:17–30. doi: 10.1016/S2468-1253(19)30333-4
2. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. (2015) 12:720–7. doi: 10.1038/nrgastro.2015.150
3. Beard JA, Franco DL, Click BH. The burden of cost in inflammatory bowel disease: a medical economic perspective and the future of value-based care. Curr Gastroenterol Rep. (2020) 22:6. doi: 10.1007/s11894-020-0744-z
4. Park KT, Ehrlich OG, Allen JI, Meadows P, Szigethy EM, Henrichsen K, et al. The cost of inflammatory bowel disease: an initiative from the Crohn's & Colitis Foundation. Inflamm Bowel Dis. (2020) 26:1–10. doi: 10.1093/ibd/izz104
5. Rajbhandari R, Blakemore S, Gupta N, Adler AJ, Noble CA, Mannan S, et al. Crohn's disease in low and lower-middle income countries: a scoping review. World J Gastroenterol. (2020) 26:6891–908. doi: 10.3748/wjg.v26.i43.6891
6. Soubieres AA, Poullis A. Emerging biomarkers for the diagnosis and monitoring of inflammatory bowel diseases. Inflamm Bowel Dis. (2016) 22:2016–22. doi: 10.1097/MIB.0000000000000836
7. Navaneethan U, Parasa S, Venkatesh PG, Trikudanathan G, Shen B. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. J Crohns Colitis. (2011) 5:189–95. doi: 10.1016/j.crohns.2010.12.005
8. Buisson A, Gonzalez F, Poullenot F, Nancey S, Sollellis E, Fumery M, et al. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. (2017) 23:1425–33. doi: 10.1097/MIB.0000000000001140
9. Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. (2011) 140:1817–26.e2. doi: 10.1053/j.gastro.2010.11.058
10. Sands BE. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology. (2015) 149:1275–85.e2. doi: 10.1053/j.gastro.2015.07.003
11. Su Q, Li X, Mo W, Yang Z. Low serum bilirubin, albumin, and uric acid levels in patients with Crohn's disease. Medicine. (2019) 98:e15664. doi: 10.1097/MD.0000000000015664
12. Chen XF, Zhao Y, Guo Y, Huang ZM, Huang XL. Predictive value of fibrinogen in identifying inflammatory bowel disease in active stage. BMC Gastroenterol. (2021) 21:472. doi: 10.1186/s12876-021-02040-9
13. Gao H, He Q, Xu C, Pang Z, Feng B, Chen T, et al. The development and validation of anti-paratuberculosis-nocardia polypeptide antibody [anti-pTNP] for the diagnosis of crohn's disease. J Crohns Colitis. (2022) 13:jjac008. doi: 10.1093/ecco-jcc/jjac008
14. Zholudev A, Zurakowski D, Young W, Leichtner A, Bousvaros A. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn's disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol. (2004) 99:2235–41. doi: 10.1111/j.1572-0241.2004.40369.x
15. Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. (2015) 110:802–19; quiz 20. doi: 10.1038/ajg.2015.120
16. Zhou GX, Liu ZJ. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J Dig Dis. (2017) 18:495–503. doi: 10.1111/1751-2980.12540
17. Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. (2013) 7:827–51. doi: 10.1016/j.crohns.2013.06.001
18. Fu W, Fu H, Ye W, Han Y, Liu X, Zhu S, et al. Peripheral blood neutrophil-to-lymphocyte ratio in inflammatory bowel disease and disease activity: a meta-analysis. Int Immunopharmacol. (2021) 101:108235. doi: 10.1016/j.intimp.2021.108235
19. Liu Z, Perry LA, Penny-Dimri JC, Raveendran D, Hu ML, Arslan J, et al. The association of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio with retinal vein occlusion: a systematic review and meta-analysis. Acta Ophthalmol. (2021) 100:e635–e647. doi: 10.1111/aos.14955
20. Han SI, Cha KC, Roh YI, Hwang SO, Jung WJ, Kim TY. Association between novel marker (platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and delta neutrophil index) and outcomes in sudden cardiac arrest patients. Emerg Med Int. (2021) 2021:6650958. doi: 10.1155/2021/6650958
21. Ntalouka MP, Nana P, Kouvelos GN, Stamoulis K, Spanos K, Giannoukas A, et al. Association of neutrophil-lymphocyte and platelet-lymphocyte ratio with adverse events in endovascular repair for abdominal aortic aneurysm. J Clin Med. (2021) 10:1083. doi: 10.3390/jcm10051083
22. Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. (2021) 18:51. doi: 10.1186/s12974-021-02090-6
23. Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. (2020) 18:360. doi: 10.1186/s12916-020-01817-1
24. Torun S, Tunc BD, Suvak B, Yildiz H, Tas A, Sayilir A, et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. (2012) 36:491–7. doi: 10.1016/j.clinre.2012.06.004
25. Peng Y, Xue Y, Wang J, Xiang H, Ji K, Wang J, et al. Association between neutrophil-to-albumin ratio and mortality in patients with cardiogenic shock: a retrospective cohort study. BMJ Open. (2020) 10:e039860. doi: 10.1136/bmjopen-2020-039860
26. Li R, Sun Z, Song S, He X, Shi X, Li Z, et al. NARFIB: a novel prognostic score based on the neutrophil-to-albumin ratio and fibrinogen can predict the prognosis of gastrointestinal stromal tumors. Cancer Manag Res. (2020) 12:11183–90. doi: 10.2147/CMAR.S281375
27. Tawfik B, Mokdad AA, Patel PM, Li HC, Huerta S. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anticancer Drugs. (2016) 27:879–83. doi: 10.1097/CAD.0000000000000411
28. He C, Shi Y, Wu R, Sun M, Fang L, Wu W, et al. miR-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-alpha in IBD. Gut. (2016) 65:1938–50. doi: 10.1136/gutjnl-2015-309389
29. He C, Yu T, Shi Y, Ma C, Yang W, Fang L, et al. MicroRNA 301A promotes intestinal inflammation and colitis-associated cancer development by inhibiting BTG1. Gastroenterology. (2017) 152:1434–48.e15. doi: 10.1053/j.gastro.2017.01.049
30. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. (2012) 61:1619–35. doi: 10.1136/gutjnl-2012-302830
31. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. (2019) 13:144–64. doi: 10.1093/ecco-jcc/jjy113
32. Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. (2004) 126:451–9. doi: 10.1053/j.gastro.2003.11.010
33. Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. (2004) 126:402–13. doi: 10.1053/j.gastro.2003.11.014
34. Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. (2013) 145:987–95. doi: 10.1053/j.gastro.2013.07.024
35. Samaan MA, Mosli MH, Sandborn WJ, Feagan BG, D'Haens GR, Dubcenco E, et al. A systematic review of the measurement of endoscopic healing in ulcerative colitis clinical trials: recommendations and implications for future research. Inflamm Bowel Dis. (2014) 20:1465–71. doi: 10.1097/MIB.0000000000000046
36. D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. (2007) 132:763–86. doi: 10.1053/j.gastro.2006.12.038
37. D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. (2012) 18:2218–24. doi: 10.1002/ibd.22917
38. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2005) 353:2462–76. doi: 10.1056/NEJMoa050516
39. Cornillie F, Hanauer SB, Diamond RH, Wang J, Tang KL, Xu Z, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. (2014) 63:1721–7. doi: 10.1136/gutjnl-2012-304094
40. Steenholdt C, Bendtzen K, Brynskov J, Thomsen OO, Munck LK, Christensen LA, et al. Changes in serum trough levels of infliximab during treatment intensification but not in anti-infliximab antibody detection are associated with clinical outcomes after therapeutic failure in Crohn's disease. J Crohns Colitis. (2015) 9:238–45. doi: 10.1093/ecco-jcc/jjv004
41. Hemperly A, Vande Casteele N. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet. (2018) 57:929–42. doi: 10.1007/s40262-017-0627-0
42. Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. (2006) 54:3782–9. doi: 10.1002/art.22214
43. Caire MT, Strauss AT, Kumar A, Brady P. Emerging biomarkers for the diagnosis and monitoring of inflammatory bowel diseases. Inflamm Bowel Dis. (2016) 22:E46. doi: 10.1097/MIB.0000000000000973
44. Christensen B, Hanauer SB, Erlich J, Kassim O, Gibson PR, Turner JR, et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol. (2017) 15:1557–64.e1. doi: 10.1016/j.cgh.2017.02.016
45. Das S, Maras JS, Hussain MS, Sharma S, David P, Sukriti S, et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology. (2017) 65:631–46. doi: 10.1002/hep.28897
46. Gisbert JP, Chaparro M. Predictors of primary response to biologic treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis. (2020) 14:694–709. doi: 10.1093/ecco-jcc/jjz195
47. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther. (2011) 33:987–95. doi: 10.1111/j.1365-2036.2011.04612.x
48. Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. (2005) 64:704–7. doi: 10.1136/ard.2004.030452
Keywords: ulcerative colitis, biomarker, inflammatory bowel disease, neutrophil, albumin, neutrophil-to-albumin ratio, infliximab
Citation: Zhou Z, Zhang Y, Pan Y, Yang X, Li L, Gao C and He C (2022) A Novel Neutrophil-Based Biomarker to Monitor Disease Activity and Predict Response to Infliximab Therapy in Patients With Ulcerative Colitis. Front. Med. 9:872831. doi: 10.3389/fmed.2022.872831
Received: 10 February 2022; Accepted: 17 March 2022;
Published: 27 April 2022.
Edited by:
Xiang Xue, University of New Mexico, United StatesReviewed by:
Lorenzo Bertani, University of Pisa, ItalyAndrew S. Day, University of Otago, New Zealand
Copyright © 2022 Zhou, Zhang, Pan, Yang, Li, Gao and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chong He, aGVycmlja2hvb0AxNjMuY29t; Caiping Gao, Z2FvY2FpcEAxMjYuY29t
†These authors have contributed equally to this work
 Zhou Zhou
Zhou Zhou Yinghui Zhang
Yinghui Zhang Yan Pan
Yan Pan Xue Yang
Xue Yang Liangping Li
Liangping Li Caiping Gao
Caiping Gao Chong He
Chong He